Abstract
Toxic cyanobacteria became more widely recognized as a potential health hazard in the 1990s, and in 1998 the World Health Organization (WHO) first published a provisional Guideline Value of 1 μg L−1 for microcystin-LR in drinking-water. In this publication we compare risk assessment and risk management of toxic cyanobacteria in 17 countries across all five continents. We focus on the three main (oral) exposure vehicles to cyanotoxins: drinking-water, water related recreational and freshwater seafood. Most countries have implemented the provisional WHO Guideline Value, some as legally binding standard, to ensure the distribution of safe drinking-water with respect to microcystins. Regulation, however, also needs to address the possible presence of a wide range of other cyanotoxins and bioactive compounds, for which no guideline values can be derived due to insufficient toxicological data. The presence of microcystins (commonly expressed as microcystin-LR equivalents) may be used as proxy for overall guidance on risk management, but this simplification may miss certain risks, for instance from dissolved fractions of cylindrospermopsin and cyanobacterial neurotoxins. An alternative approach, often taken for risk assessment and management in recreational waters, is to regulate cyanobacterial presence – as cell numbers or biomass – rather than individual toxins. Here, many countries have implemented a two or three tier alert level system with incremental severity. These systems define the levels where responses are switched from Surveillance to Alert and finally to Action Mode and they specify the short-term actions that follow. Surface bloom formation is commonly judged to be a significant risk because of the elevated concentration of microcystins in a scum. Countries have based their derivations of legally binding standards, guideline values, maximally allowed concentrations (or limits named otherwise) on very similar scientific methodology, but underlying assumptions such as bloom duration, average body size and the amount of water consumed while swimming vary according to local circumstances. Furthermore, for toxins with incomplete toxicological data elements of expert judgment become more relevant and this also leads to a larger degree of variation between countries’ thresholds triggering certain actions. Cyanobacterial blooms and their cyanotoxin content are a highly variable phenomenon, largely depending on local conditions, and likely concentrations can be assessed and managed best if the specific conditions of the locality are known and their impact on bloom occurrence are understood. Risk Management Frameworks, such as for example the Water Safety Plan concept of the WHO and the ‘bathing water profile’ of the European Union are suggested to be effective approaches for preventing human exposure by managing toxic cyanobacteria from catchment to consumer for drinking water and at recreational sites.
Keywords: Algal blooms, Drinking-water, Eutrophication, Guideline-values, Microcystins, Recreation
1. Introduction
Cyanobacteria cause problems worldwide, and the major cause for the global occurrence of nuisance blooms is eutrophication of surface water, in particular through excessive use of fertilizer and manure in agriculture as well as through sewage discharges. Climate warming also seems to play a role (Paerl and Huisman, 2009), whether through direct effects of warming or earlier, prolonged or higher water column stability (Carey et al., 2012). Eutrophication and climate may act together in supporting cyanobacterial blooms (Brookes and Carey, 2011; Carey et al., 2012), although the evidence for synergistic interactions seems strongly dependent on trophic state and the cyanobacterial taxa involved (Rigosi et al., 2014). Blooms have been reduced successfully in a large number of lake restoration programs, which almost invariably include abatement of nutrient loading in the catchment (Schindler, 2006; Schindler et al., 2008), sometimes in combination with additional measures like biomanipulation to interrupt the hysteresis of the turbid stable state (Jeppesen et al., 2007). In some cases internal measures like artificial mixing of lakes have been successful in removing nuisance blooms even in the absence of nutrient reduction (Visser et al., 1996).
Toxic cyanobacteria started to be more widely recognized as a potential health hazard in the 1980s, a number of case studies were published attributing illness to cyanobacterial toxins (see Kuiper-Goodman et al., 1999; Chorus et al., 2000), and numerous cases of animal deaths along water courses afflicted with cyanobacterial blooms were calling public attention to the issue. Progress in the elucidation of the chemical structures of a number of cyanotoxins and in the availability of chemical detection methods suitable for routine analyses picked up speed in the mid 1980s, and by the late 1990s a wider understanding of both their modes of action and their occurrence was available (Chorus and Bartram, 1999). The accumulating data suggested that, among the chemicals found in water or used for drinking or recreation, cyanotoxins may well be among the substances occurring most frequently at potentially harmful concentrations. In 1998 the World Health Organization (WHO; see Box 1 for all abbreviations) first published a provisional drinking-water Guideline Value of 1 μg L−1 for one very common cyanotoxin, microcystin-LR (MCYST-LR), in its Addendum to Volume 2 of the Guidelines for Drinking-water Quality (see Chorus and Bartram, 1999). Since then, the number of countries which have addressed the cyanotoxin hazard has increased and further countries are currently discussing the most appropriate regulatory approach for their respective conditions. The primarily hepatotoxic micro-cystins – a family of more than 80 different congeners, commonly measured and expressed as total MCYST-LR equivalents – are probably the most widespread and best studied group of cyanotoxins (Dittmann et al., 2013; Ferrao-Filho and Kozlowsky-Suzuki, 2011; Ibelings and Havens, 2008; Kozlowsky-Suzuki et al., 2012). Data on the occurrence of other cyanotoxins are increasingly becoming available, particularly for cylindrospermopsin (CYN), neurotoxins like saxitoxin (STX) or anatoxins (ATX) (Metcalf et al., 2008; Seifert et al., 2007; van Apeldoorn et al., 2007; van der Merwe et al., 2012) and information on new classes arising (e.g. jamaicamides, Neilan et al., 2013). Regulations and guidelines, however, have been struggling with the multitude of cyanobacterial toxins that might occur, be it other microcystins or different classes of toxins, particularly as for most of them, toxicological data are insufficient for the derivation of concentration limits.
Box 1. Abbreviations.
| ATX | Anatoxin(s)-a/a(s) |
| BMAA | Beta-N-methylamino-l-alanine |
| Chl-a | Chlorophyll-a |
| CYN | Cylindrospermopsin |
| ELISA | Enzyme linked immunosorbent assay |
| EU BWD | European Union Bathing Water Directive |
| EU DWD | European Union Drinking Water Directive |
| EU WFD | European Union Water Framework Directive |
| GDWQ | WHO Guideline Values for Drinking Water |
| GV | Guideline value |
| HACCP | Hazard analysis and critical control points |
| (H)AL | (Health) Alert level |
| i.p. | Intraperitonial (injection in body cavity) |
| IARC | International agency for research on cancer |
| MCYST | Microcystin(s) |
| N(L)OAEL | No (lowest) observed adverse effect level |
| NOD | Nodularin |
| OATP | Organic anion transporting polypetides |
| PHRMP | Public Health Risk Management Plans |
| (P)MAC | (Provisional) Maximum concentration |
| (P)MAV | (Provisional) Maximum value |
| PST | Paralytic shellfish toxins |
| RMF | Risk management framework |
| S | Standard value |
| STX | Saxitoxin(s) |
| TDI | Tolerable daily intake |
| TWQR | Target water quality range |
| UF | Uncertainty factors |
| US | EPA USA Environmental Protection Agency |
| WHO | World Health Organization |
| WSP | Water Safety Plan |
To some extent toxin levels respond to environmental conditions so that the toxin content per cell may vary several fold (Neilan et al., 2013; van der Merwe et al., 2012; Wiedner et al., 2003); also the proportion of different MCYST congeners may change with changes in the environment (Tonk et al., 2005). Maximal cyanotoxin concentrations in a given waterbody, however, largely depend on the concentrations of cyanobacterial biomass – modified by the ratio of toxic to non-toxic strains, currently or previously present. In particular, concentration via scum formation (i.e. the accumulation of floating cyanobacteria at the lake surface during periods of calm weather) may increase toxin levels by orders of magnitude Therefore the amount of cyanobacteria observed can serve as a basis for alert level frameworks and risk assessment well before, or even without toxin analysis. Accordingly, some countries are implementing alert level frameworks and risk-based approaches on basis of cyano-bacterial cell numbers or biovolume in their national guidance or regulations, sometimes complementary to regulating maximum cyanotoxin concentrations.
In principle, regulatory approaches differ for the main three exposure routes to cyanotoxins, i.e. oral, pulmonary and dermal. Dermal symptoms caused by freshwater cyanobacteria are typically mild and self-limiting, thus requiring some public education and guidance, but not necessarily regulation. Concern regarding pulmonary exposure to date is based on two early studies, i.e. one exposing guinea pigs experimentally (Falconer and Humpage, 2005) and one evaluating atypical pneumonia of army cadets submersed during their training (Lawton and Codd, 1991). However, more recent studies confirming this exposure route to be relevant are lacking, and uptake through aspiration usually also involves swallowing, thus at least partially occurring via the oral pathway. Accordingly, regulations and guidelines to date focus on the main vehicles of oral exposure, i.e. ingestion of toxins via drinking-water, recreation or consumption of fish, molluscs and crayfish from freshwater bodies, which we term ‘freshwater seafood’. The literature on exposure through drinking-water (e.g. Falconer and Humpage, 2005; Hitzfeld et al., 2000; Zamyadi et al., 2012) is more extensive than that for other possible exposure vehicles, notably recreational exposure (Backer et al., 2010; Chorus et al., 2000) and uptake via food (Ibelings and Chorus, 2007). The focus on drinking-water may be attributed to its exceptional role as basis for life, with daily consumption in the range of liters and little means for individuals to avoid exposure when it is contaminated. On the other hand in countries where drinking water is usually well treated while eutrophication is still widespread and blooms are common, recreation may be the major exposure vehicle (Codd, pers. comm.).
The 1998 WHO provisional Guideline Value (GV) of 1 μg L−1 for the concentration of MCYST-LR in drinking-water was based upon laboratory studies with mice which resulted in a No Observed Adverse Effect Level (NOAEL) of 40 μg kg−1 bodyweight d−1; the GV was derived using the following equation:
where 60 kg is the average bodyweight of an adult, 0.8 the proportion of daily intake of MCYST attributed to drinking-water, 2 L the average consumption per day, and the factor of 1000 to account for intra- and interspecies variation (a factor of 10 each) and for uncertainties in the data, i.e. lack of data on lifetime exposure and carcinogenicity of MCYST-LR (a further factor of 10). The Tolerable Daily Intake (TDI) is calculated from the NOAEL divided by the factor of 1000, i.e. 0.04 μg kg− bw d− . This TDI was also used as one basis for WHO Guidelines for safe recreational exposure (Chorus et al., 2000).
In addition to their production of toxins, cyanobacteria owe their reputation as the ‘scourge of water management’ (Visser et al., 2005) to a further specific trait, the production of intracellular gas-vacuoles (Kinsman et al., 1991; Walsby et al., 1991). These gas-vacuoles provide cyanobacteria with buoyancy, so that in the absence of water-body mixing cyanobacterial cells and colonies suspended in the water may float to the lake surface and accumulate in dense surface scums. Since microcystins are predominately intracellular, when biomass accumulates microcystin concentrations increase manifold and risk assessment needs to take this into account. Scums often drift toward the shore, where the risk of human or animal contact with such high concentrations of cyanobacteria and the toxins they contain or release when cells lyse is considerably higher (Ibelings et al., 2003). While this applies to cyanotoxins which are pre-dominantly intracellular, in particular to microcystins, this accumulation mechanism may be less pronounced for other cyanotoxins, such as saxitoxins or cylindrospermopsin, of which a larger fraction often occurs extracellularly – more than 50% or even almost all of the toxin (Saker and Griffiths, 2000).
Countries have implemented cyanotoxin guidelines or standards and procedures to assess and manage the risk of cyanotoxins. These standardized guidelines and regulations address the concentrations of cyanobacteria or their toxins that should not be exceeded. Usually together with immediate, short-term actions to take if these concentrations are exceeded in order to prevent or minimize exposure to harmful cyanobacteria and their toxins. Herein we report these national guidelines, distinguishing regulations for the major oral exposure vehicles, i.e. drinking-water and recreation, and including those for food from those few countries which have implemented regulations. We assess the progress in regulatory approaches toward cyanotoxins and discuss why countries choose to implement different guidelines and regulations, based upon the same scientific underpinnings. We further show how this progress has moved from a focus on monitoring based on guideline values to more comprehensive approaches of risk assessment and risk management, in particular with reference to the WHO Water Safety Plan concept (see Ibelings & Chorus, 2007) and the Bathing Water Directive (BWD) of the European Union.
2. Methods
During the VI and the VIII International Conference on Toxic Cyanobacteria, held in Norway (2004) and Turkey (2010), scientists and regulators reported on regulations aimed at controlling the risks of exposure to toxic cyanobacteria in their respective countries, and all conference participants were invited to submit a summary of regulations in their country for a report entitled “Current approaches to cyanotoxin risk assessment, risk management and regulations in different countries”, compiled and edited by Chorus (2005, 2012). A wide variety of contributions was submitted from countries in Africa, Australasia, Europe and the Americas, although neither compilation attempted to be comprehensive or globally balanced. From both reports we have amassed data to summarize current regulations for drinking-water (Supplementary materials Table S1), recreational waterbody use (Table S2) and freshwater food (Table S3). A priori it appears valuable to compare regulatory approaches taken in various countries who greatly differ in lake types (e.g. depth or trophic state), regional climate, lake use (drinking-water production, recreational intensity, etc.), public awareness of the issue, legal status of the guidelines and regulations, governance structure (federal vs. de-centralized) and presence or absence of overarching, even international frameworks like the BWD in European countries.
We emphasize that none of the contributions to Chorus (2005, 2012) which are used as a basis for this manuscript represent formal government contributions, rather these are inputs from scientists or managers working in their respective countries. Nevertheless for ease of understanding we will refer to countries by their names in Sections 3 and 4.
3. Results
Most countries that regulate cyanotoxins define limits for concentrations in finished drinking-water and, albeit less widely, in water used for recreational purposes. Such limits are defined for MCYST in general or specifically for MCYST-LR, while other cyanotoxins are rarely explicitly regulated. The status of limits varies between countries, as is reflected in terminology such as guideline value, standard maximum acceptable value, maximum acceptable concentration or health alert level, some of which are explicitly labeled ‘provisional’ (see Box 1 for abbreviations). In some countries regulations or guidance for toxin concentrations in water are supplemented and supported by the implementation of alert level frameworks to indicate the risk of cyanotoxin occurrence. For drinking-water this is the case in e.g. Australia, France, Finland, New Zealand, and Singapore. For recreational waterbody use, most guidance or regulations are based on some measure of cyanobacterial bloom intensity, using parameters such as cell number, biovolume or pigment concentration (Chl-a or – more specifically for cyanobacteria–phycocyanin/phycoerythrin, both detectable in situ by fluorometry probes) to reflect the concentration of cyanobacterial biomass. Such alert level frameworks typically define 2 or 3 levels of cyanobacterial biomass to guide responses (e.g. intensified monitoring) or interventions (e.g. upgrading treatment or temporarily banning site use).
The full results of the international survey are presented in Supplementary Material Tables S1 (drinking-water), S2 (recreation) and S3 (food), with summary data and typical examples from some countries presented in graphs and discussed in the text.
3.1. Drinking-water
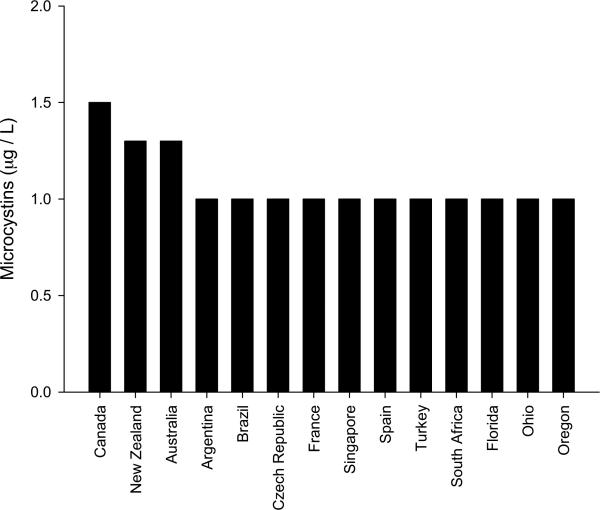
Most countries with regulations or guidance on cyanotoxin in drinking-water base their limit for the concentration in finished drinking-water on the provisional WHO Guideline Value for MCYST-LR of 1 μg L−1 or they use the underlying TDI of 0.04 μg per kg body-weight with some minor national adaptations (for amount of water consumed and/or average body-weight). In consequence, variations between the limits thus derived by different countries are minor, i.e. limits range from 1.0 to 1.5 μg L−1 (Fig. 1). A number of countries define a legally binding standard value (S) of 1 μg L−1 MCYST-LR in the final product (tap water) e.g. Brazil, Uruguay, The Czech Republic, France and Spain (Azevedo, Vidal and Britos, Marsalek et al., Griscya, Quesada et al., in Chorus, 2005, 2012). Some countries like Canada, the Czech republic or Singapore (Giddings et al., Marsalek et al., Wong et al., in Chorus, 2012) explicitly refer only to MCYST-LR, more often microcystins are expressed as MCYST-LR equivalents (sum of all variants), e.g. in Australia, France or Finland (Mulvenna and Orr, Griscya, Rapala et al., in Chorus, 2012). Other toxins for which some countries give guidance (but not legally binding standards) include CYN (1 μg L−1 in Australia and New Zealand or 15 μg L−1 in Brazil), STX (3 μg L−1 in Australia, New Zealand and Brazil) or ATX-a (3.7 μg L −1 in Canada, 6 μg L−1 in New Zealand) (Burch and Humpage, Wood and Williamson, in Chorus, 2005, 2012).
Fig. 1.
Guideline values for microcystin in drinking-water compared internationally.
Health Canada assessed MCYST-LR as potentially carcinogenic, based upon limited evidence in experimental animals; Canada has implemented a provisional Maximum Acceptable Concentration (PMAC) for MCYST-LR of 1.5 μg L−1 in treated drinking-water, aimed to protect against the sum of all MCYST (Giddings et al., in Chorus, 2012). The value is slightly higher than the provisional WHO GV because Health Canada assumes a somewhat greater bodyweight and smaller intake of drinking-water (as the bloom season lasting only 3–4 months). The US federal government has not established regulations or guidelines to define acceptable levels of cyanotoxins in drinking-water (or recreational water), although cyanobacteria and their toxins are on the Drinking-Water Contaminant Candidate List and assessments on whether or not to regulate them are ongoing; however, three of the fifty states of the USA have regulations or health advisory levels for them in drinking-water (Hudnell et al. in Chorus, 2012).
New Zealand has regulations for the largest range of toxins in drinking-water: MCYST-LR eq., STX-eq., ATX (a + a(s)), NOD, and Homoanatoxin-a. Cyanotoxins are regulated through Provisional Maximum Acceptable Values (PMAVs), since there are no WHO guidelines for most of them – other than the provisional WHO guideline for MCYST-LR (see Table S2 for PMAVs for the respective toxins). PMAVs are informed by current scientific understanding, even if incomplete. Cyanobacteria and cyanotoxins do not fall in the highest priority class (which contains infectious microorganisms), but are listed as Priority Class 2 determinants, together with chemical pollutants. Of interest is the remark that unlike other Class 2 contaminants cyanotoxins can increase rapidly, as the source in cyanobacterial blooms and scums fluctuates with environmental conditions. New Zealand, like Australia has a Risk Management Framework (see Section 4) in place, and the authors report this as having been effective in preventing concentrations exceeding the PMAVs in drinking-water reservoirs from having reached consumers (Wood and Williamson in Chorus, 2012).
In the European Union, national drinking-water legislation is required to be based on the Drinking Water Directive. This stipulates an overall objective “to protect human health from the adverse effects of any contamination of water intended for human consumption by ensuring that it is wholesome and clean” and that it is “free from any micro-organisms and parasites and from any substances which, in numbers or concentrations, constitute a potential danger to human health”. However, it does not explicitly address cyanobacterial toxins. For Poland, Mankiewicz-Boczek et al. (in Chorus, 2012) explain that while a GV of 1 μg L−1 for MCYST-LR had been included in Polish legislation in 2002, this was repealed in 2012 since the DWD does not specify MCYST. Also Denmark, Germany, Greece, Hungary and the Netherlands (Christoffersen and Warming, Chorus, Kagalou et al., Törökne, Ibelings et al., in Chorus, 2005, 2012) have no specific regulations for cyanobacteria and their toxins in drinking-water. For Denmark, Germany and Hungary the authors explain that one reason why this is not considered necessary is that direct use of surface water contributes only a minor percentage of the drinking-water (Christoffersen and Warming 2012 in Chorus, 2005; Törökné 2005, in Chorus, 2005, 2012). The German Drinking Water Ordinance, however, explicitly requires that no substances may occur in hazardous concentrations, and this means that if MCYST is found, operators or health authorities would turn to the provisional WHO GV to assess whether the concentration found would qualify as “hazardous”.
3.2. Recreation
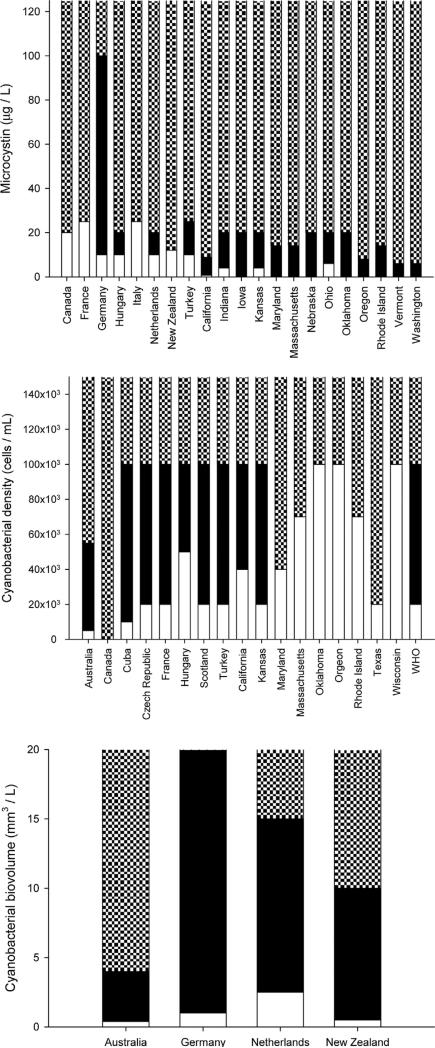
Many countries apply a two or three tier alert level framework with incremental severity, based upon the immediate, short term assessment of cyanobacterial cell numbers or biovolume at a recreational site. The lowest level ‘Surveillance Mode’ typically does not result in any public action, it merely leads authorities to continue or intensify their monitoring efforts. The next level ‘Alert Mode’ is a consequence of an indicator like microcystin (Fig. 2, top panel) or cyanobacterial cell counts/biovolume (Fig. 2 middle and lower panel) exceeding a threshold value. At Alert Mode, typically the public is warned via on site signs, information via the Internet, telephone hotlines, etc. Warning signs may inform the public about the increased risks for skin irritation and gastro-intestinal illness. The highest level ‘Action Mode’ is based upon one of several conditions: (i) the presence of persistent cyanobacterial blooms or surface scums, (ii) surpassing the next level threshold in cyanobacterial biomass (as pigment concentration, cell counts or biovolume), or (iii) any report of toxic effects attributed to cyanobacteria. In this mode the public is typically advised to refrain from recreation involving water contact (particularly with the risk of oral uptake), and authorities may actually temporarily close waterbodies for primary contact recreation like swimming. Australia (Burch, 2008, Mulvenna and Orr in Chorus, 2012) distinguishes two further levels of ‘Action Mode’: level one is based upon adverse health effects from ingestion of known toxins, based upon toxicity data for MCYST-LR; level two is based on the likelihood of adverse health effects resulting from exposure to high cell densities, irrespective of the presence of known toxins.
Fig. 2.
Alert levels for toxic cyanobacteria in recreational waters compared internationally on basis of microcystins (top panel), cell numbers (middle panel) and biovolume of cyanobacteria (lower panel). White means Surveillance mode, black Alert mode and hatched Action mode. See text for details. The upper end of the scale is “open”, meaning that for instance any level of microcystin exceeding the upper boundary of Alert mode automatically falls into Action mode, but concentrations (in particular in scums) clearly can be higher than the maximum value of 150 μg L 1 on the y-axis of the top panel a (analogous for middle and lower panels).
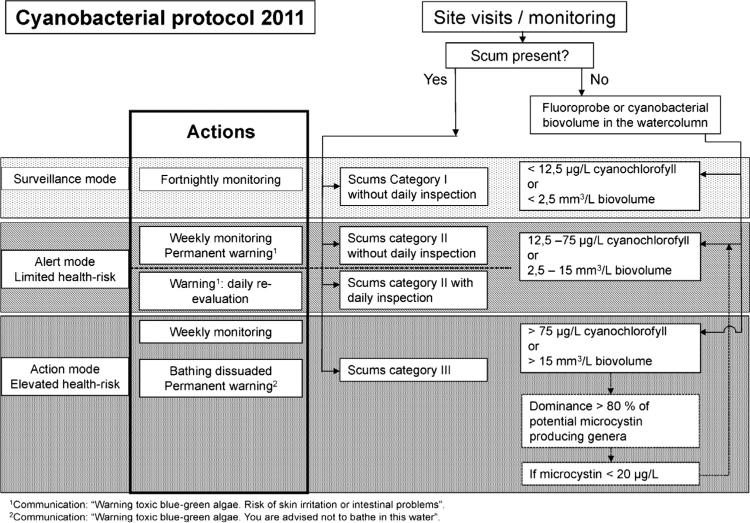
The three tier based cyanobacterial protocol for use in recreational waters shown in Fig. 3, as used in the Netherlands (Ibelings et al., in Chorus, 2012) is a typical example of an alert levels framework for recreational water-body use: bathing sites in this country are monitored on at least a fortnightly basis. Professionals take samples at representative locations for subsequent analysis of cyanobacterial biomass using a microscope or DNA analysis (qPCR). They are also equipped with pictures of surface scums of increasing intensity as well as with a FluoroProbe (BBE Moldaenke GmbH) that measures cyanobacterial pigment (phycocyanin) concentration to assist their immediate on-site assessment. Scums of categories I and II lead to up-scaling from Surveillance to Alert Mode, and so does cyano-chlorophyll-a > 12.5 μg L−1 or cyano-biovolume > 2.5 mm3 L−1. At this alert level, where health risks are deemed limited, the monitoring frequency increases from fortnightly to weekly, and a warming is posted: “Warning toxic blue-green algae, risk of skin irritation or intestinal problems”. The warning may be evaluated on a daily basis if on-site inspection is available. In case of scums of the highest category III or cyano-chlorophyll-a > 75 μg L−1 or cyano-biovolume > 15 mm3 L−1 the alert level is raised to Action Mode, and bathing is dissuaded by the following warning: “Warning toxic blue-green algae. You are advised not to bathe in this water”. Local authorities have the right to close the site for recreation.
Fig. 3.
Example of a cyanobacterial protocol used for uniform risk assessment and management of recreational waters in the Netherlands.
Canada (Giddings et al., in Chorus, 2012) applies only a single level as guideline value for recreation, a GV of 20 μg L−1 MCYSTLR-eq., based upon the NOAEL of 40 μg kg −1 bodyweight (Fawell et al., 1999) and an uncertainty factor of 100. In this case no additional uncertainty factor for life time exposure is taken into account, given that recreation is short lived and episodic. Moreover the GV was developed with children in mind as through their lower bodyweight and behavior they are more at risk than adults (assumption of a 13 kg child ingesting 0.25 L of lake water). Singapore (Wong et al., in Chorus, 2012) also uses a single Chl-a based Alert/Action level of >50 μg L−1: over a 3 year period water quality must comply with this standard during 95% of the time. If not, freshwater systems can be judged to be unsuitable for swimming, and the public would be notified of this. In the US, there are no federal guidelines, water quality criteria, or regulations concerning the management of harmful algal blooms, either in drinking water or recreational waters. However, 21 of the fifty states have implemented some form of guidance on cyanobacterial or cyanotoxin occurrence in recreational waters (Hudnell et al. in Chorus, 2012). For example, Ohio has a regulatory limit of 1 μg L−1 of microcystin in drinking water. In addition, a health advisory or “no contact” advisory is posted when recreational water concentrations of microcystin are ≥6 μg L−1 or ≥20 μg L−1, respectively.
New Zealand (Wood and Williamson, in Chorus, 2012) is one of only two countries where benthic mats of cyanobacteria are also part of the classification (see Table S2). Similar to planktonic cyanobacteria three alert levels – corresponding to Surveillance, Alert and Action Mode –are distinguished. Photographs to assist in sampling and risk assessment are provided. Surveillance Mode is based upon up to 20% coverage of the sediment with potentially toxigenic benthic cyanobacteria and results in fortnightly surveys at representative locations in the water body. In Alert Mode (20– 50% coverage) the sampling frequency and intensity is increased, the public health unit is informed and toxicity testing is recommended. When coverage of benthic, potentially toxigenic cyanobacteria exceeds 50% (or when up to 50% coverage, but benthic cyanobacteria are visibly detaching from their substrate) the alert level is raised to Action Mode, amongst others resulting in notification of the general public.
In Finland (Rapala et al., 2012 in Chorus, 2012) cyanobacteria are monitored weekly on 300 predetermined sites. Four levels of algal (including cyanobacteria) abundance are based upon visual inspection by environmental or public health professionals or trained volunteers. The observations are ranked in four classes, and levels increase from no algae visible (Level 0) to greenish flakes or narrow stripes (Level 1), to strong coloration or small surface scums (Level 2) and widespread and dense surface scums or aggregates of floating cyanobacteria at the lake shore (Level 3). Although the system is based upon visual inspection, microscopic analysis of cyanobacteria or analysis of toxin concentrations is advised. Regional authorities make the information available via LakeWiki. A weekly report summarizing the information for Finland as a whole is created and published, including a color coded map and a cyanobacterial “abundance barometer”. The LakeWiki database in addition contains basic information of all 55 821 lakes larger than 1 ha in Finland. Citizens are invited to update information, amongst others with pictures of cyanobacterial blooms.
The German system is also based upon visual assessment of sites, combined with assessment of cyanobacterial occurrence; MCYST analyses are included as optional element intended to avoid unnecessary restrictions on recreation. Secchi disk readings of less than 1 m and Chl-a exceeding 40 mg L 1 with a dominance of cyanobacteria result in bathing being discouraged, while presence of scums and MCYST above 100 μg L−1 result in the recommendation that bathing sites are temporarily closed.
The proposed procedure for risk assessment in Polish bathing waters (Mankiewicz-Boczek et al., in Chorus, 2012) is specific in that it is the only protocol at present to include molecular detection of toxigenic cyanobacterial strains (PCR amplification of mcy genes). When positive, the detection of strains capable of producing MCYST is followed by actual screening of MCYST concentration in the surface water. When concentrations measured with ELISA exceed 2.5 μg L−1 a more quantitative and qualitative analysis using HPLC follows. The Polish Water-law of 2010 states that when bathing water does not meet the required standards, authorities must determine the cause of the pollution and take action to improve water quality and protect human health. In line with EU Bathing Water Directive (BWD) it does not specify the actions to be taken but rather their required outcome.
For member states of the European Union (EU) the BWD provides the basis for regulating recreational water use. Its revision in 2006 first introduced a risk-based framework for freshwater and coastal recreational sites – the “bathing water profiles”. These require the responsible authorities to assess potentially contaminating conditions influencing the site to enable timely identification of health risks, and amongst others this includes describing the potential risk of cyanobacterial proliferation for individual bathing waters (see Table S2). While the BWD describes risk assessment and regulations in general terms, most EU member states specify this through more specific regulations, in many cases based upon quantitative analysis of cyanobacterial density or biomass. Interestingly, while the two- or three-tiered alert level frameworks used by many countries show similarities, the thresholds they use to increase alerts from one level to the next are much more variable than the guideline values or standards for drinking-water. For moving the alert level from basic (Monitoring/ Surveillance Mode) to the next level (Alert Mode), thresholds span two orders of magnitude: in terms of cells mL−1 from 500 in New Zealand, over 5000 in Australia and 20 000 in the Czech Republic, France and Turkey 20 000–50 000 cells mL−1 in Hungary. Likewise whereas many countries change to the highest alert level, Action Mode, at 100 000 cells mL−1, Australia applies a cut-off at ca. half this cell density and certain US states (e.g. Texas, Maryland) at yet much lower levels.
Alert levels defined on basis of biovolume show similar variation between countries. In New Zealand for example Monitoring Mode is below 0.5 mm3 L , Alert Mode between 0.5 and <1.8 mm3 L−1 and Action Mode is implemented when cyanobacterial biovolume is ≥1.8 mm3 L−1 (if potentially toxic cyanobacteria dominate or ≥10 mm3 L−1 for all cyanobacteria). In Australia these values are >0.04 3 to <0.4 mm3 L−1 for Surveillance Mode, respectively ≥0.4 to <4 mm3 L−1 for Alert Mode and ≥4 mm3 L−1 (when known toxin producers are present) for Action Mode. In the Netherlands >2.5 3 and >15 mm3 L−1 are used for raising the alert levels from Monitoring to Alert and Action Mode respectively (in the Netherlands dominant cyanobacteria almost invariably belong to toxin producing genera, so that values for biovolume in the Netherlands can be compared to the more strict values for Australia and New Zealand). Germany recommends temporary closure of waterbodies when blooms are conspicuous and particularly if MCYST levels exceed 100 μg L−1, Italy prohibits bathing at 25 μg L−1, while at this level Turkey discourages recreation. In the US California applies a low MCYST based alert level of 0.8 μg L−1 for Action Mode, Oklahoma in comparison uses 20 μmg L−1.
3.3. Food
There are far fewer regulations for exposure via food as compared to exposure via drinking-water. The reason for this difference is unclear. It may be unintentional, or it may be that jurisdictions are prioritizing the drinking-water pathway (see however Ibelings and Chorus, 2007 who demonstrate that in some cases intake through food may exceed that through drinking-water). Of the US states only California provides guidelines and regulations on cyanotoxins in fish (Hudnell et al. in Chorus, 2012). California limits cyanotoxins in fish for human consumption as follows: for MCYST 1 (LA, LR, RR, YR) up to 10 ng g−1 wet weight (ww), for CYN 66 ng g−1 , and for ATX 1100 ng g− ww (Table S3). With the exception of STX for marine shellfish Australia (Mulvenna and Orr, in Chorus, 2012) does not provide GV on safe levels of cyanotoxins in freshwater seafood on a national level; Victoria, however, does provide Health Alert Levels (HAL; see Table S3 in Supplementary material): for CYN and deoxy-CYN the HAL is 18– 39 μg kg−1 ww of whole organism while for MCYST-LR or equivalent hepatotoxins it is 24–51 μg kg−1 ww and for STX (800 μg kg−1) of whole organism.
Denmark (Christoffersen and Warming 2012, in Chorus, 2012) has no specific regulations for cyanotoxins, but harvesting and distribution of mussels is only allowed if no algal toxins – of all known types – are detected in water and animals and standards for food hygiene regulations are met. A new order has been put in place in 2011 – implementation of EU Regulations on food hygiene and control of products from animal origin – targeted specifically at monitoring algae and their toxins for sites where breeding and harvesting of mussels takes place, hence aimed at marine environment (Mytilus edulis and marine toxins). At present this specific EU regulation does not appear to be used for protection against cyanotoxins in freshwater seafood. France also recognizes the primary focus on marine seafood but does acknowledge that based on a chronic TDI of 0.04 μg kg−1 bw d−1 a daily consumption of fish of 86 g and an intake of 2 L drinking-water per day (at 1 μg L−1), the limit for adults consuming freshwater species would be 5.6 μg MCYST per kg fish (for edible parts of fish). For children with a lower bodyweight and lower consumption, the limit value would be 1.4 μg kg−1 of fish. Similarly, Greece acknowledges that a 300 g serving of carp would exceed the TDI for lifelong consumption 14-fold. Also consumption of Astacus astacus and Rana epirotica would exceed the TDI approximately six-fold.
4. Discussion
4.1. Guideline values
A conspicuous outcome of the comparison of regulatory approaches is the homogeneity of the values used for microcystins in drinking-water: 11 countries use the provisional WHO Guideline value for Microcystin-LR of 1 μg L−1 in their national approaches to cyanotoxins. This reflects a high level of acceptance of the WHO Guidelines for Drinking-water Quality (WHO GDWQ). As pointed out by WHO, (WHO, 2009), “the GDWQ provide the scientific point of departure for standard setting and Regulation“; they are “based upon the best available evidence and scientific consensus” ... and “are derived so as to take account of the needs of an individual through a normal lifetime, including changes in sensitivity that may occur between life stages”; in particular they “are the collective product of many experts and of extensive recovered experience“. However, WHO also emphasizes that it is up to national governments to decide for which chemicals to set legally binding national standards and whether or not to set these at the level suggested by the WHO GDWQ. Countries have indeed taken into account their respective conditions and circumstances, some with setting somewhat higher values, some with including all micro-cystins, and some with giving values also for further cyanotoxins, though not as legally binding standard, but rather in advisory fashion, i.e. as guideline values, provisional maximum values or concentrations, or health alert levels.
Where guideline values (GV) or preliminary maximum concentrations (PMAC) are given for CYN or neurotoxins, they vary more than the relatively fixed GV for MCYST-LR, due to incomplete toxicological data and in consequence a need to fill gaps with assumptions – and assumptions are more variable. Regardless of how many cyanotoxins are regulated (New Zealand lists a total of seven different toxins) it is clear that some risk of exposure through drinking-water may remain regarding further unknown and possibly harmful metabolites produced by cyanobacteria.
4.2. Microcystin as guidance or lead toxin in drinking water
A number of countries implicitly use the toxicological assessment of MCYST-LR to define maximum accepted concentrations for all microcystins, based on the assumption that as no other variant is known to have a higher acute i.p. toxicity this approach is likely to be conservative, i.e. reflecting a worst-case scenario. There are indications that this may not be the case. Results of a study by Fischer et al. (2010) on the activity of specific organic anion transporting polypeptides (OATPs) indicate enhanced cellular uptake of the more hydrophobic MCYST-LW and -LF, which could result in higher toxicity of these congeners, however results obtained with cellular assays cannot be quantitatively extrapolated to whole organisms. Microcystins are often measured as the sum of all variants and then expressed as MCYST-LR equivalents. Furthermore, where cyanobacterial numbers or biomass are used, their tolerable levels are set on the basis of ratios of microcystins to biomass or chlorophyll-a, or on the basis of content per cell. This adds a further assumption, i.e. that ratios or cellular content will be in the same range for other cyanotoxins. The question thus is: how justified is the use of a value for MCYST-LR as general ‘indicator’ of cyanotoxin risk?
A justification implicitly or explicitly encountered is the frequency of MCYST occurrence, which particularly at high concentrations in scums or high density of dispersed filamentous cyanobacteria in hypertrophic lakes carries potential sub-acute and even acute health risks (see Ibelings and Chorus, 2007). Moreover, indeed the maximum reported ratios of toxins to cyanobacterial biomass so far published do not tend to be higher for any of the other known cyanotoxins. Thus, an alert level based on a maximum ratio of MCYST-LR to biomass is likely to provide a similar level of protection if the toxin is not MCYST-LR but e.g. CYN or ATX. The more critical issue may be that the underlying assumption of oral toxicity being in a similar range as that of MCYST-LR is not well understood. For microcystin-congeners other than MCYST-LR, the above mentioned recent findings by Fischer et al. (2010) on toxicokinetics of MCYST-LW and MCYST-LF have led to a call for a MCYST congener specific approach in risk assessment (Faassen and Lurling, 2013).
A relevant – though implicit – justification for orienting cyanotoxin guidance along MCYST-LR is that measures taken to mitigate exposure against MCYST-LR or microcystins will also be effective against other cyanotoxins. In drinking-water treatment this applies to the removal of cell-bound cyanotoxins, but it is important to keep in mind that this does not work for cyanotoxins dissolved in water – while microcystins are largely cell-bound, this is not the case for most other cyanotoxins. Measures taken in the catchment or the water-body address the proliferation of cyanobacterial cells rather than specific toxins. In consequence, if they are successful against microcystin-producing cyanobacteria, they are likely to mitigate the biomass of further cyanobacterial taxa that produce other cyanotoxins. This assumption holds in many cases. Chorus and Niesel (2011) show a very clear dependency of cyanobacterial dominance on the concentrations of total phosphorus from a statistical analysis of data from 3000 samples collected from 210 waterbodies throughout Europe: cyanobacterial biovolumes above 0.1 mm3 L−1 proved highly unlikely at total phosphorus concentrations below 25 μmg L−1.
4.3. The role of national and local experience in setting guideline values or standards
A further factor influencing national approaches to setting guideline values or standards is experience. Brownson et al. (2009) explain that evidence based public healthcare relies upon quantitative (e.g. epidemiological, laboratory animal or ecological experiments) as well as qualitative (e.g. narrative accounts) information. Narrative and anecdotal information, however, varies between countries on basis of national experience with for instance severity of eutrophication and possible calamities with harmful cyanobacteria like the Palm Island, Australia or Caruaru, Brazil incidents (Carmichael et al., 2001) which caused the hospitalization or death, respectively, of dozens of people (see Chorus et al., 2000). In contrast, in many regions of northern Germany with eutrophication and blooms having been widely prevalent for several decades without human illness having become conspicuous, in spite of intensive recreational water-body use, the first discussions in the early 1990s of the need to address cyanobacterial toxins faced considerable skepticism, and it was dog deaths in the media that convinced the public in the regions where they occurred that actions were required. In contrast, regulators were soon convinced by the strong weight of the scientific evidence in relation to the criteria well accepted for other toxins; moreover, once convinced of the necessity to address the issue, the majority in the commission drafting Germany's first advisory for recreational use in 1992 voted in favor of more strict measures than mere information and warning, i.e. including closure of bathing sites during blooms. The argument at the time was that in the German regulatory culture a strong signal like lake closure is important for such to be taken seriously unless more drastic measures like temporary closure are a real option. In addition the role played by individual scientists, managers and policy makers in countries, as well as their personal view on cyanobacterial risks have an effect on which guidelines and regulations are implemented.
Thus although procedures for risk assessment in different countries are likely to share common scientific elements, the assumptions being made will vary according to political, social and economic context (Chorus and Bartram, 1999), and this is fully in line with the aims of the WHO Guidelines for Drinking-water Quality, which emphasize that “The judgment of safety – or what is a tolerable risk in particular circumstances – is a matter in which society as a whole has a role to play. The final judgment as to whether the benefit resulting from the adoption of any of the guidelines given in the GDWQ justifies the cost is for each country to decide” (WHO, 2009).
4.4. Cyanobacterial biomass in recreational waters
As discussed in Section 4.2, the toxicity of Microcystin-LR is widely used as rough orientation for defining action levels to protect bathers (Backer et al., 2010; Chorus et al., 2000; Falconer and Humpage, 2005; Fromme et al., 2000; Pilotto et al., 1997), but these levels are then defined in terms of cyanobacterial biomass, cell numbers or both. These are preferred over cyanotoxins in order to facilitate monitoring and immediate interventions in response to the results and to account for the potential presence of further cyanobacterial metabolites causing for instance the irritating and inflammatory symptoms reported. A few countries (e.g. Germany, Italy) include the option of a direct measurement of MCYST concentration to assess the risk due to toxic cyanobacteria in their recreational waters. Others like the Netherlands have abandoned such an approach (cf. contributions Ibelings et al. to Chorus, 2005, 2012) because of the potential relevance of toxins or irritants other than MCYST. Indeed many other metabolites have been identified as products produced by cyanobacteria (Sivonen et al., 2010; Welker et al., 2012), but there are very few data on their toxicity. It is well known, however, that crude extracts of cyanobacterial blooms are more toxic than the purified toxins extracted from them (Ibelings and Havens, 2008), which may be due to unidentified toxic substances or due to synergistic effects between known toxins (Pires et al., 2011). As indicator of biomass, cyanobacterial cell densities have been commonly used, but biovolume or cyano-Chl-a are increasingly preferred (see Table S2) because they are better indicators for potential toxin levels, as these relate more directly to biomass than to cell numbers (as cell size differs widely between species). Box 2 gives an overview of the advantages and disadvantages of different assessment methods used by various countries for quantification of cyanobacterial biomass.
Box 2. Abundance and biomass of cyanobacteria.
Cyanobacterial protocols for recreational waters, like the example from the Netherlands shown in Fig. 3, commonly focus on cyanobacterial abundance and biomass rather than on microcystin concentrations. This avoids a focus on a single toxin. The disadvantage of this approach is that no clear relation between a biomass signal and toxin concentrations exists because all cyanobacteria as well as planktonic algae contribute biomass or contain pigments, while not all cyanobacteria produce toxins. Thus, measures of cyanobacterial abundance or biomass provide only an indication of the upper limit of cyanotoxin concentrations to be expected. Nonetheless this information is useful for risk assessment and management if either a worst-case assumption for toxins per biomass is used or if this relationship can be gleaned from experience within the given water-body. Different methods to estimate the abundance or biomass can be applied:
Chlorophyll-a
Method based on spectrophotometric analysis after extraction of the pigments with e.g. ethanol. Advantage of this method is the ease of use. The parameter can be routinely measured and is therefore relatively fast and cheap. Disadvantage is that the method is not specific for cyanobacteria; all phytoplankton contains chlorophyll a. Extraction and analysis of specific cyanobacterial pigments like phycocyanin is less straightforward. However, the analysis of chlorophyll-a can be supplemented by qualitative microscopy, i.e. estimating (without cell counting, usually within 10 min or less) whether the phytoplankton largely consists of cyanobacteria or not.
Fluorescence
Part of the light which is used for photosynthesis by phytoplankton cells may be re-emitted as fluorescence and this can be used as an indicator of biomass. Modern sensors measure not only chlorophyll-a fluorescence, but also cyanobacteria specific pigments which differ in their absorption maximum, i.e. phycocyanin or phycoerythrin, and thus allow the distinction of cyanobacteria from other phytoplankton. Advantage of the method is the instantaneous results of the measurements, which can even be obtained in situ. The parameter can be routinely measured and is therefore relatively fast and cheap. The fluorescence signal, however, is not always straight forward to interpret since quenching of fluorescence depends on environmental conditions and the physiological state of the cells.
Microscopic counts/estimates
Method based on the analysis of concentrated cells, preferably in a sedimentation chamber and counted under an inverted microscope (Uthermohl method). Advantage of this method is the direct insight into the composition of the phytoplankton population in general and the possible abundance of potential toxin producing genera/species of cyanobacteria. Disadvantage is that the method is time consuming (and therefore relatively expensive) and a specialist job. The quantification is complicated by growth forms of cyanobacteria, i.e. colonies or filaments, the latter sometimes coiled or twisted. Cell counts compared between laboratories may show a high level of variation. A further disadvantage is the variation in cell size: some picoplanktonic cyanobacteria are extremely small, and this may result in very high cell counts even if the water appears clear.
Biovolume
Like above (microscopic counts), but in addition the cell numbers are multiplied by the mean cell volume of each genus/species (either obtained from measuring key geometric dimensions of 10–20 cells per sample, or from literature) to provide an estimate of biomass. Advantage of using this method is that the relative biomass of cyanobacteria in relation to other phytoplankton genera can be estimated, and toxin concentrations relate to biomass much more tightly than to cell numbers. Cell sizes are incorporated in the measurements, dominance of phytoplankton genera and species can be more reliably evaluated. Disadvantage of the method is equal to that of the microscopic counts. Furthermore, this method introduces an additional parameter, the mean cell volume of each taxon, with a certain distribution and variation and hence additional scope for uncertainty.
DNA-copy detection
Method based on the extraction of DNA and the multiplication of certain gene targets. Targets are either on the phycocyanin genes or on the genes encoding for the toxin production. Advantage of the method is the objective quantification and that the targets are genus specific. The method is relatively fast (result within hours) and sensitive, so that low concentrations of cells can be detected. Disadvantage is the need for a well-equipped laboratory and pcr-expertise. Besides, the relation between cell numbers and DNA copies can be a source of variation. Overestimation of the cell densities may occur. Detection of toxic genes may not relate to actual toxin production and concentrations.
Although alert levels in Netherlands are distinctively higher than in most other countries the application of these alert levels in risk management still results in warnings or even closure of waterbodies on a fairly extensive scale by the responsible authorities. This is a country known for the highly eutrophic state of its lakes (despite some successful restoration efforts, Gulati and Van Donk, 2002), where stricter alert levels might result in extended closure of many lakes. In the two densely populated western provinces (North- and South-Holland) warnings were issued 34 and 90 times, respectively in summer of 2010, whilst bathing was dissuaded in 9 and 15 cases. A bathing prohibition was given 2 and 7 times. The alert levels in the Netherlands were developed as the outcome of intensive discussions between scientists, lake managers and policy makers. The chief objective of a process of cyanobacterial risk assessment and management, as formalized in the protocols, is the prevention of the public from being exposed to harmful concentrations of cyanobacterial toxins, while at the same time avoiding unnecessary costs and carbon footprints (e.g. in production of drinking water), restrictions on recreational activities and consumption of freshwater seafood (in particular where this may be an important source of nutrition – see Ibelings and Chorus, 2007). The protocol used in the Netherlands – in addition to health risks – takes into account the promotion of outdoor activities, feasibility, complexity and costs of monitoring or risk control of cyanobacteria, as well as the ease of communication to the public. Likewise, the approach in Germany suggests MCYST measurements in order to avoid undue restrictions on recreational use of waters where blooms occur, but their toxin content is low.
This quite wide variability in approaches to toxic cyanobacteria in water-bodies used for recreation reflects not only the differences between the realities of settings, but also implicit differences in the acceptance of risk. The recently risk-based approaches to regulation have adopted the view that it is important to make differences in the acceptance of risk explicit and transparent.
4.5. Risk-based approaches
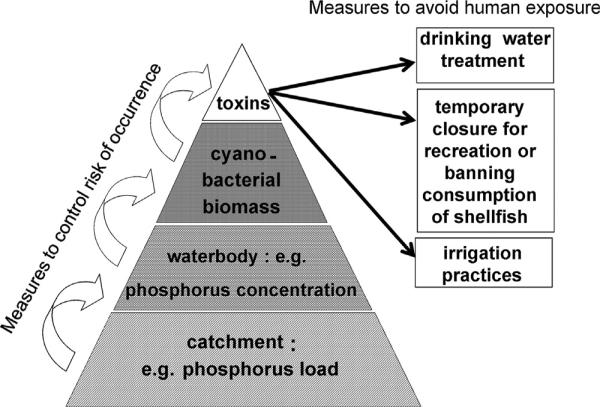
A broader concept of risk assessment (widely used in many sectors besides public health, e.g. also in the insurance business), while making use of the available information on the generic properties of the hazard, sets the focus on the setting or situation, i.e. it calls for estimating how large the risk caused by a certain hazard is likely to be in that specific case (within this concept the generic toxicity assessment discussed above would be “hazard characterization” rather than risk assessment). This concept estimates the likelihood of the hazard to occur in relation to the severity of its impact – e.g. on human health. This is particularly useful for prioritizing hazards and – in consequence – measures to abate them. Risk-based approaches focus on understanding the potentially occurring hazards in specific, individual supply systems or recreational settings, the system's efficacy in controlling them, the development of management plans to ensure controls are working, emergency and contingency strategies, clear lines of communication, and on documentation of the risk assessments as well as records of system performance. In such a comprehensive management system guideline values and standards take on an additional role: they still serve as measure for overall verification that the system is working, i.e. the water to which people may be exposed should not contain concentrations exceeding these values. However, in the context of risk-based approaches guideline values or standards are also important for defining targets for the system’s performance in managing the risks. For cyanotoxins, a target for MCYST-LR or MCYST-LR equivalents translates to targets for cyanobacterial biomass. The target for cyanobacterial biomass in turn translates to a concentration of total phosphorus which, if met, renders higher biomass levels quite unlikely. Last but not least, the target concentration for total phosphorus determines the target for phosphorus loading to the water-body (see Fig. 4). If these targets cannot be met and this results in a risk of cyanotoxin occurrence in raw water used for drinking-water abstraction, barriers need to be in place for off-take schemes and treatment methods that mitigate cell and toxin concentrations. Risk-based approaches include an assessment of how effectively the whole set of measures and barriers control cyanobacterial occurrence and/or remove and reduce cyanotoxin concentrations.
Fig. 4.
Schematic representation of risk management of toxic cyanobacteria, conforming to the Water Safety Plan concept of the WHO (protection from catchment to consumer).
4.6. Water Safety Plans
A truly risk-based regulatory approach to cyanotoxins requires such a comprehensive system assessment and management concept for the individual water use system. It can use an audit as tool to demonstrate compliance in the sense of an independent assessment of the quality of the risk assessment and management system designed for the specific water supply or recreational site. Risk assessment and management is being increasingly widely used. The Public Health Risk Management Plans in New Zealand are one example. For drinking-water the WHO Guidelines for Drinking-water Quality (http://www.who.int/water_sanitation_health/dwq/guidelines/en/) have introduced the concept of developing situation-specific Water Safety Plans (http://www.who.int/water_sanitation_health/publication_9789241562638/en/) related to the Codex Alimentarius Arius HACCP concept. This Hazard Assessment and Critical Control Points concept emphasizes that monitoring the end product only (e.g. tap water) will not guarantee safety. A comprehensive risk assessment of the whole chain involved in production of drinking-water or food should identify the critical control points or measures to be implemented and closely monitored to ensure that they are working at all times. For WSP this means risk assessment and control from “catchment to consumer”. Control of eutrophication, the most sustainable way to tackle the problem of harmful algal blooms, usually requires management measures at the catchment scale.
The key advantage of the WHO Water Safety Plan concept for regulating the occurrence of toxic cyanobacteria is that it encourages and facilitates tackling the problem at its source. As preliminary step to develop a Water Safety Plan, the concept calls for the formation of a team of stakeholders well familiar with the local circumstances, including those in the catchment, possibly including external expertise. This provides an excellent platform for cyanobacterial management, which can rarely be achieved solely by the water supplier, the public health authority or the authority responsible for water-body management. The Water Safety Plan team can bring together the expertise and local system understanding to assess the given system, i.e. the factors causing eutrophication, to plan measures to control the conditions leading to cyanobacterial blooms – and/or to implement barriers against human exposure if blooms cannot be prevented.
4.7. Overarching legislation with implications for cyanotoxins
For member states of the European Union, three European Directives are relevant for the hazards due to cyanobacterial blooms: The aim of the Water Framework Directive (http://ec.europa.eu/environment/water/water-framework/index_-en.html) is that all European waters meet criteria for good ecological status by 2015. Criteria are of hydromorphological, physical, chemical and biological nature (e.g. Allan et al., 2006), and this is to be achieved by management plans for the specific River Basin Districts, i.e. natural geographical and hydrological units. Eutrophication control in these River Basin Districts is a necessary means to achieve the goals of the WFD, to which all EU member states are committed. A good ecological status of waterbodies should greatly benefit the aims of EU Bathing Water Directive (http://ec.europa.eu/environment/water/water-bathing/) and the EU Drinking Water Directive (http://ec.europa.eu/environment/water/water-drink/index_en.html). The BWD lays down provisions for developing a bathing water profile. This calls for assessing potential sources of contamination of the bathing site, i.e. assessing risks, and it defines the classification of bathing water quality, its management and the provision of information to the public. By 2015 all bathing waters are required have an acceptable minimum water quality. With respect to cyanobacteria the BWD stipulates that if a bathing water profile for an individual site indicates the potential for cyanobacterial proliferation, appropriate monitoring shall be carried out to enable timely identification of health risks. If proliferation does occur and risks have been identified adequate measures shall be taken immediately to prevent exposure, including information to the public. The DWD provides a general degree of protection, without mentioning cyanobacteria specifically: it is intended to protect human health by ensuring that drinking-water does not contain microorganisms, parasites or substances in concentrations which constitute a potential health risk. Member states shall take any action which is required to guarantee and purity of water intended for human consumption.
On a similar note the Clean Water Act in the USA(http://www2.epa.gov/laws-regulations/summary-clean-water-act) aims at restoration and maintenance of the chemical, physical and biological integrity of the Nation`s waters. The US EPA designated numerical criteria for phosphorous and nitrogen as a priority for controlling freshwater eutrophication, which has rapidly increased over the past few decades (EPA, National Lake Assessment; http://water.epa.gov/type/lakes/lakessurvey_index.cfm). To reduce harmful algal blooms a policy of watershed management is the basis, in some cases effectively supplemented by manipulations within water-bodies.
4.8. Remaining challenges
Regardless as to whether regulatory approaches chiefly call for compliance to standards or for a risk assessment and management system, it is difficult to set targets or define tolerable levels without an understanding of the toxicity of cyanobacterial compounds. For this purpose, gaps in data on chronic oral toxicity are a substantial issue for assessing the risks from exposure to most cyanotoxins. While the potential carcinogenicity of microcystin or nodularin has been categorized by the International Agency for Research on Cancer (IARC, 2010), quantitative data are lacking for re-assessing whether taking carcinogenicity into account would change the provisional WHO Guideline value for MCYST-LR. Using epidemio-logical data for setting guidelines or standards is notoriously difficult due to the many confounders, and where they are used, this is often from workplace exposure where cause-effect relationships become clearer – an approach which is scarcely viable for cyanotoxins. New toxicological studies providing data for the derivation of guidelines or standards have scarcely been published in recent years, as producing such data still requires animal experiments which, however, are expensive and increasingly unpopular. Alternative concepts based on sets of suborganismic assays testing a range of endpoints and toxic mechanisms are currently being developed, but are not yet at the point of providing data on the basis of a widely harmonized concept that would be accepted by regulators as a basis for defining tolerable concentrations.
A challenge scarcely addressed to date is that of benthic cyanobacteria, which are increasingly linked to animal deaths, caused by neurotoxins in benthic cyanobacterial (Phormidium) mats that become detached and float to the surface. While these are difficult to quantify, New Zealand has taken the pragmatic approach of using percentage coverage of the stream bottom together with river flow as an indicator of this risk.
Representative sampling of cyanobacteria is widely perceived as a challenge when monitoring compliance with standards or guideline values, as their distribution can be highly patchy with strong temporal variation. However, this situation is not necessarily different for other hazards. Many contaminants typically occur sporadically rather than on a regular basis, i.e. due to storm water inflows, prolonged dry periods or to activities in the catchment taking place at irregular intervals. A basic principle for developing a Water Safety Plan therefore is to consider not only the hazard and its human health impact, but particularly the hazardous events that cause it to occur in a water course. This approach moves the regulatory focus away from endpoint monitoring toward understanding the respective system and casting this understanding into a management plan.
At the technological or methodological level these are scarcely conceptual challenges, as approaches to preventing and reducing nutrient loading to water courses are well understood, as are methods for managing water-bodies to create conditions less favorable for cyanobacteria and techniques to remove cells and toxins in drinking-water treatment. The primary challenge here would be to choose the best technological approach and methods, taking duration of toxin occurrence, costs and carbon footprints of removal through treatment into account and balancing this against the – usually longer-term – success chances and costs for measures in the catchment that would effectively control eutrophication and thus mitigate blooms.
In many regions of the world, in spite of all we know about eutrophication and how to control it, the perhaps biggest challenge of all is to preserve water resources of high quality, in face of increasing pressures from changes in land-use, climate change and a still expanding human population.
4.9. Impact of cyanotoxin regulations
The regulatory approaches in place have been developed to be precautionary, i.e. to safely prevent illness or even death, as is standard for regulating concentrations of chemicals in drinking-water. The primary outcome of public health concern associated with exposure to cyanotoxins is liver cancer (from exposure to microcystins and possibly cylindrospermopsin). However, even for those toxins, causal relationships are difficult to glean from epidemiologic studies because there are many other possible causative factors. Individual responses are not measured or estimated and other biological or chemical contaminants in surface waters typically have not been ruled out (USEPA, 2006). For example, in the studies specifically designed to assess the association between microcystin exposure and subsequent liver cancer in China (IARC, 2010) the study population was also exposed to aflatoxin and hepatitis, other potential causes of liver cancer. For cyanotoxins, as well as for many of the other chemicals found in drinking water, the effects from reducing exposure through regulations specifying maximum allowable concentrations cannot easily be measured in terms of disease prevention.
4.10. Summary and conclusions
Given that eutrophication is still a growing problem in many countries, possibly acerbated by climate warming, cyanobacterial blooms are here to stay for quite a while yet. It is widely accepted that cyanobacterial blooms pose a threat to the safe production of drinking water, the consumption of freshwater seafood and recreational activities, and an increasing number of countries worldwide are responding to this threat with regulations or guidance for drinking water and recreation, and occasionally for food. Almost invariably countries base their guidance on the WHO provisional Guideline Value for drinking-water of 1 μg L−1 MCYSTLR. While authorities struggle to regulate toxins other than MCYSTLR, given the paucity of toxicological data, we argue that MCYST-LR can serve as orientation or ‘guidance toxin’ for risks from other microcystins and even other classes of toxins, provided the limitations of this approach are kept in mind, particularly with respect to dissolved cyanotoxins in drinking-water treatment. For recreational water-body use, the majority of countries choose to base their risk assessment and management on cyanobacterial cell numbers or biomass, rather than toxin concentrations, and alert level frameworks are a widespread approach. In stark contrast to the uniformity of standards or guideline values for drinking water, different countries apply very different values as thresholds in their alert level systems, with for example California scaling up to Action level at less than 1% of the MCYST concentration used in Germany, or New Zealand scaling up to the highest intervention level at only 7% of the cyanobacterial biovolume used in the Netherlands. We do not feel that it is up to the authors of this publication to judge which country has the more evidence-based approach, since local conditions and experiences play an important role in such decisions. In each country there are knowledgeable scientists and managers to develop the concept that works best for their local or national situation.
A key conclusion from this overview of different countries’ approaches and the uncertainties involved might be that it is difficult to employ regulatory approaches for cyanotoxin concentrations that focus on compliance. A possible way forward would be the implementation of a comprehensive framework for risk assessment and management. The concept outlined by the WHO of developing a Water Safety Plan for the specific drinking-water supply system offers a suitable approach for implementing such a framework. For recreational water-body use, the concept of developing bathing water profiles, as required by the EU Bathing Water Directive, follows a similar line of thinking. Both partially evade the issue of toxicological uncertainties by focusing on developing management plans that control cyanobacterial proliferation and/or remove toxins, thus directing regulatory attention to mitigating the problem at its source. Nonetheless, toxicological assessments can still serve two important roles; setting health-based targets to guide such management plans and to provide a measure to verify that the water is safe to use for drinking or recreation. The lacking chronic toxicity data therefore appear to be the most important knowledge gap to close in order to improve the basis for cyanotoxin regulations.
Supplementary Material
Acknowledgement
BWI is grateful for numerous discussions on cyanobacteria and how to monitor or model them in the EU COST Actions CyanoCOST and NETLAKE[SS].
Footnotes
Author declaration
B.W. Ibelings wrote substantial parts of the manuscript. L. Backer provided substantial contributions to compilation of the international data set on which the manuscript is based and provided substantial input to the text. E. Kardinaal provided substantial contributions to the structure and content of the manuscript through many rounds of revision, authored Box 2 and prepared the graphs. I. Chrous edited 2 international reports published by the German Environment Agency and which provided the input for the current manuscript. She also wrote substantial parts of the manuscript.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.hal.2014.10.002.
References
- Allan IJ, Mills GA, Vrana B, Knutsson J, Holmberg A, Guigues N, Laschi S, Fouillac AM, Greenwood R. Strategic monitoring for the European Water Framework Directive. TRAC – Trends Anal. Chem. 2006;25(7):704–715. [Google Scholar]
- Backer LC, McNeel SV, Barber T, Kirkpatrick B, Williams C, Irvin M, Zhou Y, Johnson TB, Nierenberg K, Aubel M, LePrell R, Chapman A, Foss A, Corum S, Hill VR, Kieszak SM, Cheng Y-S. Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon. 2010;55(5):909–921. doi: 10.1016/j.toxicon.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Brookes JD, Carey CC. Resilience to blooms. Science. 2011;334(6052):46–47. doi: 10.1126/science.1207349. [DOI] [PubMed] [Google Scholar]
- Brownson RC, Fielding JE, Maylahn CA. Evidence-based public health: a fundamental concept for public health practice. Annu. Rev. Public Health. 2009;30:175–201. doi: 10.1146/annurev.publhealth.031308.100134. [DOI] [PubMed] [Google Scholar]
- Burch MD. Effective doses, guidelines & regulations. In: Hudnell HK, editor. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Springer; 2008. pp. 831–853. [Google Scholar]
- Carey CC, Ibelings BW, Hoffmann EP, Hamilton DP, Brookes JD. Ecophysiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012;46(5):1394–1407. doi: 10.1016/j.watres.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Carmichael WW, Azevedo S, An JS, Molica RJR, Jochimsen EM, Lau S, Rinehart KL, Shaw GR, Eaglesham GK. Human fatalities from cyanobacteria: chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001;109(7):663–668. doi: 10.1289/ehp.01109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorus I, editor. Current Approaches to Cyanotoxin Risk Assessment, Risk Management and Regulations in Different Countries. Umweltbundesamt; Berlin: 2005. [Google Scholar]
- Chorus I, editor. Current Approaches to Cyanotoxin Risk Assessment, Risk Management and Regulations in Different Countries. Umweltbundesamt; Berlin: 2012. [Google Scholar]
- Chorus I, Bartram J, editors. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management. E & FN Spoon; 1999. p. 400. [Google Scholar]
- Chorus I, Falconer IR, Salas HJ, Bartram J. Health risks caused by freshwater cyanobacteria in recreational waters. J. Toxicol. Environ. Health-Part B – Crit. Rev. 2000;3(4):323–347. doi: 10.1080/109374000436364. [DOI] [PubMed] [Google Scholar]
- Chorus I, Niesel V. Steps towards a statistical model to predict phytoplankton responses to changes in trophic state. In: Chorus I, Schauser I, editors. Oligotrophication of Lake Tegel and Schlachtensee, Berlin. Analysis of System Components, Causalities and Response Thresholds Compared to Responses of Other Waterbodies. Federal Environment Agency; Dessau-Rosslau, Germany: 2011. p. 157. [Google Scholar]
- Dittmann E, Fewer DP, Neilan BA. Cyanobacterial toxins: biosynthetic routes and evolutionary roots. FEMS Microbiol. Rev. 2013;37(1):23–43. doi: 10.1111/j.1574-6976.2012.12000.x. [DOI] [PubMed] [Google Scholar]
- Faassen EJ, Lurling M. Occurrence of the microcystins MC-LW and MC-LF in Dutch surface waters and their contribution to total microcystin toxicity. Mar. Drugs. 2013;11:2643–2654. doi: 10.3390/md11072643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer IR, Humpage AR. Health risk assessment of cyanobacterial (blue-green algal) toxins in drinking water. Int. J Environ. Res. Public Health. 2005;2(1):43–50. doi: 10.3390/ijerph2005010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell JK, Mitchell RE, Everett DJ, Hill RE. The toxicity of cyanobacterial toxins in the mouse: I Microcystin-LR. Hum. Exp. Toxicol. 1999;18(3):162–167. doi: 10.1177/096032719901800305. [DOI] [PubMed] [Google Scholar]
- Ferrao-Filho A. da S., Kozlowsky-Suzuki K. Cyanotoxins: bioaccumulation and effects on aquatic anmials. Mar. Drigs. 2011;9:2729–2772. doi: 10.3390/md9122729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Hoeger SJ, Stemmer K, Feurstein DJ, Knobeloch D, Nussler A, Dietrich DR. The role of organic anion transporting polypeptides (OATPs/SLCOs) in the toxicity of different microcystin congeners in vitro: a comparison of primary human hepatocytes and OATP-transfected HEK293 cells. Toxicol. Appl. Pharmacol. 2010;245:9–20. doi: 10.1016/j.taap.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Fromme H, Kohler A, Krause R, Fuhrling D. Occurrence of cyanobacterial toxins – Microcystins and anatoxin-a – in Berlin water bodies with implications to human health and regulations. Environ. Toxicol. 2000;15(2):120–130. [Google Scholar]
- Gulati RD, Van Donk E. Lakes in the Netherlands, their origin, eutrophication and restoration: state of the art review. Hydrobiologia. 2002;478:73–106. [Google Scholar]
- Hitzfeld BC, Hoger SJ, Dietrich DR. Cyanobacterial toxins: removal during drinking water treatment, and human risk assessment. Environ. Health Perspect. 2000;108:113–122. doi: 10.1289/ehp.00108s1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibelings BW, Chorus I. Accumulation of cyanobacterial toxins in freshwater seafood and its consequences for public health: a review. Environ. Pollut. 2007;150(1):177–192. doi: 10.1016/j.envpol.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Ibelings BW, Havens KE. Cyanobacterial toxins: a qualitative meta-analysis of concentrations, dosage and effects in freshwater, estuarine and marine biota. In: Hudnell HK, editor. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Springer; 2008. pp. 675–732. [DOI] [PubMed] [Google Scholar]
- Ibelings BW, Vonk M, Los HFJ, van der Molen DT, Mooij WM. Fuzzy modeling of cyanobacterial surface waterblooms: validation with NOAA-AVHRR satellite images. Ecol. Appl. 2003;13(5):1456–1472. [Google Scholar]
- Jeppesen E, Sondergaard M, Meerhoff M, Lauridsen TL, Jensen JP. Shallow lake restoration by nutrient loading reduction - some recent findings and challenges ahead. Hydrobiologia. 2007;584:239–252. [Google Scholar]
- Kinsman R, Ibelings BW, Walsby AE. Gas vescile collapse by turgor pressure and its role in buoyancy regulation by Anabaena flos aquae. J. Gen. Microbiol. 1991;137:1171–1178. [Google Scholar]
- Kozlowsky-Suzuki B, Wilson AE, Ferrao-Filho A.d.S. Biomagnification or biodilution of microcystins in aquatic foodwebs? Meta-analyses of laboratory and field studies. Harmful Algae. 2012;18:47–55. [Google Scholar]
- Kuiper-Goodman T, Falconer I, Fitzgerald J. Human health aspects. In: Chorus I, Bartram J, editors. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management. E & FN Spoon; London: 1999. pp. 113–153. [Google Scholar]
- Lawton LA, Codd GA. Cyanobacterial (blue-green algal toxins) and their significance in UK and European waters. J. Inst. Water Environ. Manage. 1991;5(4):460–465. [Google Scholar]
- Metcalf JS, Banack SA, Lindsay J, Morrison LF, Cox PA, Codd GA. Cooccurrence of beta-N-methylamino-l-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990–2004. Environ. Microbiol. 2008;10(3):702–708. doi: 10.1111/j.1462-2920.2007.01492.x. [DOI] [PubMed] [Google Scholar]
- Neilan BA, Pearson LA, Muenchhoff J, Moffitt MC, Dittmann E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 2013;15(5):239–1253. doi: 10.1111/j.1462-2920.2012.02729.x. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Huisman J. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009;1(1):27–37. doi: 10.1111/j.1758-2229.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- Pilotto LS, Douglas RM, Burch MD, Cameron S, Beers M, Rouch GJ, Robinson P, Kirk M, Cowie CT, Hardiman S, Moore C, Attewell RG. Health effects of exposure to cyanobacteria (blue-green algae) during recreational water-related activities. Aust. N. Z. J. Public Health. 1997;21(6):562–566. doi: 10.1111/j.1467-842x.1997.tb01755.x. [DOI] [PubMed] [Google Scholar]
- Pires LMD, Sarpe D, Brehm M, Ibelings BW. Potential synergistic effects of microcystins and bacterial lipopolysaccharides on life history traits of Daphnia galeata raised on low and high food levels. Aquat. Toxicol. 2011;104(3–4):230–242. doi: 10.1016/j.aquatox.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Rigosi A, Carey CC, Ibelings BW, Brookes JD. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr. 2014;59(1):99–114. [Google Scholar]
- Saker ML, Griffiths DJ. The effect of temperature on growth and cylindrospermopsin content of seven isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from water bodies in northern Australia. Phycologia. 2000;39(4):349–354. [Google Scholar]
- Schindler DW. Recent advances in the understanding and management of eutrophication. Limnol. Oceanogr. 2006;51(1):356–363. [Google Scholar]
- Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ, Beaty KG, Lyng M, Kasian SEM. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. U. S. A. 2008;105(32):11254–11258. doi: 10.1073/pnas.0805108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert M, McGregor G, Eaglesham G, Wickramasinghe W, Shaw G. First evidence for the production of cylindrospermopsin and deoxy-cylindrospermop sin by the freshwater benthic cyanobacterium, Lyngbya wollei (Farlow ex Gornont) Speziale and Dyck. Harmful Algae. 2007;6(1):73–80. [Google Scholar]
- Sivonen K, Leikoski N, Fewer DP, Jokela J. Cyanobactins-ribosomal cyclic peptides produced by cyanobacteria. Appl. Microbiol. Biotechnol. 2010;86(5):1213–1225. doi: 10.1007/s00253-010-2482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonk L, Visser PM, Christiansen G, Dittmann E, Snelder E, Wiedner C, Mur LR, Huisman J. The microcystin composition of the cyanobacterium Planktothrix agardhii changes toward a more toxic variant with increasing light intensity. Appl. Environ. Microbiol. 2005;71(9):5177–5181. doi: 10.1128/AEM.71.9.5177-5181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Environmental Protection Agency (USEPA) Toxicological reviews of cyanobacterial toxins: microcystins LR, RR, YR and LA (External review draft, November 2006) US Environmental Protection Agency; Washington: 2006. (EPA/66/R-06/139) [Google Scholar]
- van Apeldoorn ME, van Egmond HP, Speijers GJA, Bakker GJI. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007;51(1):7–60. doi: 10.1002/mnfr.200600185. [DOI] [PubMed] [Google Scholar]
- van der Merwe D, Sebbag L, Nietfeld JC, Aubel MT, Foss A, Carney E. Investigation of a Microcystis aeruginosa cyanobacterial freshwater harmful algal bloom associated with acute microcystin toxicosis in a dog. J. Vet. Diagn. Invest. 2012;24(4):679–687. doi: 10.1177/1040638712445768. [DOI] [PubMed] [Google Scholar]
- Visser P, Ibelings B, Mur L, Walsby A. The ecophysiology of the harmful cyanobacterium Microcystis. Harmful Cyanobact. 2005:109–142. [Google Scholar]
- Visser PM, Ibelings BW, vanderVeer B, Koedood J, Mur LR. Artificial mixing prevents nuisance blooms of the cyanobacterium Microcystis in Lake Nieuwe Meer, the Netherlands. Freshw. Biol. 1996;36(2):435–450. [Google Scholar]
- Walsby AE, Kinsman R, Ibelings BW, Reynolds CS. Highly buoyant colonies of the cyanobacterium Anabaena lemmermanni form persistent surface waterblooms. Arch. Fur Hydrobiol. 1991;121(3):261–280. [Google Scholar]
- Welker M, Dittmann E, von Doehren H. Hopewood DA, editor. Cyanobacteria as a source of natural products. Natural Product Biosynthesis by Microorganisms and Plants, Pt C. 2012:23–46. doi: 10.1016/B978-0-12-404634-4.00002-4. [DOI] [PubMed] [Google Scholar]
- World Health Organisation . Public Health and the Environment. WHO; Geneva: 2009. Guidelines for drinking water quality policies and procedures used in updating the WHO Guidelines for Drinking water Quality. p. 39. [Google Scholar]
- Wiedner C, Visser PM, Fastner J, Metcalf JS, Codd GA, Mur LR. Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl. Environ. Microbiol. 2003;69(3):1475–1481. doi: 10.1128/AEM.69.3.1475-1481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamyadi A, MacLeod SL, Fan Y, McQuaid N, Dorner S, Sauve S, Prevost M. Toxic cyanobacterial breakthrough and accumulation in a drinking water plant: A monitoring and treatment challenge. Water Res. 2012;46(5):1511–1523. doi: 10.1016/j.watres.2011.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.