Abstract
This study evaluated molecular characteristics that are potentially prognostic in cats with oral squamous cell carcinoma (SCC) that underwent stereotactic radiation therapy (SRT). Survival time (ST) and progression-free interval (PFI) were correlated with mitotic index, histopathological grades, Ki67 and epidermal growth factor receptor expressions, tumour microvascular density (MVD), and tumour oxygen tension (pO2). Median ST and PFI were 106 and 87 days, respectively (n=20). Overall response rate was 38.5% with rapid improvement of clinical symptoms in many cases. Patients with higher MVD or more keratinized SCC had significantly shorter ST or PFI than patients with lower MVD or less keratinized SCC (P=0.041 and 0.049, respectively). Females had significantly longer PFI and ST than males (P≤0.016). Acute toxicities were minimal. However, treatment-related complications such as fractured mandible impacted quality of life. In conclusion, SRT alone should be considered as a palliative treatment. MVD and degree of keratinization may be useful prognostic markers.
Keywords: feline oral squamous cell carcinoma, microvascular density, stereotactic radiation therapy
Introduction
Feline oral squamous cell carcinoma (SCC) is one of the most devastating veterinary cancers due to its high rate of treatment failure. Although traditional and novel treatment modalities have been evaluated, there has been no major improvement in tumour control or survival time (ST).1–9 Given that almost all patients with oral SCC that undergo any treatment type eventually succumb to local tumour recurrence or tumour progression, primary tumour control is the most critical treatment goal. However, lack of our knowledge about the biology of SCC limits development of more effective treatment modalities.
In human SCC and feline SCC, possible prognostic markers have been evaluated and those include histopathological grading, Ki67, epidermal growth factor receptor (EGFR) and microvascular density (MVD). Histopathological grading has been used to evaluate and prognosticate human oral SCC and parameters include degree of keratinization, mitotic index and stage of invasion.10–13 Ki67 is a protein specifically expressed in the nucleus of actively proliferating cells.14 One study evaluated the correlation between immunohistochemically evaluated Ki67 expression and treatment outcome after electron radiation therapy (XRT) in feline cutaneous SCC.15 In this study, patients with higher Ki67 expression had better treatment outcome compared to patients with lower Ki67 expression. EGFR is a transmembrane receptor coupled with tyrosine kinase. This receptor has been shown to play key roles in multiple cellular events including cell proliferation, apoptosis and migration in human tumours.16,17 High EGFR immunoreactivity has been shown to be prognostic for shorter survival and local control after fractionated XRT in human head and neck (H&N) SCC.18 Our previous study revealed that EGFR expression may be a prognostic factor in feline oral SCC.19 MVD is a technique to evaluate tumour angiogenesis that has been identified as a prognostic factor in some human malignancies including breast carcinoma, ovarian carcinoma, and H&N SCC.20–23
XRT plays an important role in management of human and veterinary cancer patients. Stereotactic radiation therapy (SRT) is a novel technique recently introduced in veterinary medicine. This new technique allows increasing dose to the target yet spares adjacent normal structures because of the steep dose drop-off outside the treatment target and higher delivery accuracy. It has been suggested that the biological responses/events occur in the human tumour and its microenvironment after SRT is different from those after conventional fractionated XRT.24,25 Previous studies with xenograft models have shown that significant number of tumour vasculature endothelial cells undergo apoptosis when tumours are treated with a larger dose/fraction such as >10 Gy/fraction.25 In addition, tumours irradiated with a larger dose/fraction (8–10 Gy) have been shown to undergo mitotic death following G2 phase-arrest and those receiving an extremely large dose/fraction (>15–20 Gy) have been shown to undergo interphase death regardless of the cell cycle phase.25 Clinically, SRT has been evaluated as a primary treatment modality for human H&N SCC.26–28 In these studies, most patients showed clinical responses and the authors concluded that SRT is a useful treatment option for human patients with H&N SCC although its long-term treatment outcome was not as favourable as that of more aggressive combination treatment of surgery, XRT and chemotherapy. However, because SRT is a completely new XRT technique in veterinary medicine, knowledge about its efficacy for a variety of veterinary malignancies including feline oral SCC is scarce.
Oxygen status in tissue is a well-known factor that affects the biological response of cells to photon irradiation.29 Human cancer contains hypoxic regions due to abnormal tumour vascular function, structure and organization.30 Also, hypoxic tumours are known to have less apoptotic potential.31,32 In human H&N SCC and cervical carcinoma, tumour hypoxia, which is typically defined as median or mean oxygen tension (pO2) < 5–10 mm Hg, predicts tumour control and ST after fractionated XRT.2,33–40 Fractionated XRT has been advocated to increase post-treatment tumour pO2 theoretically.29 One study has shown an improvement in tumour pO2 in human patients with cervical SCC after treatments including fractionated XRT although results of clinical studies are not consistent.34,41,42 Post-SRT in vivo oxygen measurement in spontaneous cancer patients has not been reported in human or veterinary medicine.
The main purpose of this study was to prospectively evaluate the prognostic significance of variety of different markers (histopathological grades, Ki67, EGFR, MVD, tumour pO2 and patient parameters) for the patients with feline oral SCC treated by SRT.
Materials and methods
Patient population
Twenty feline oral SCC patients who were referred to the Flint Animal Cancer Center, Colorado State University (CSU-ACC) between January 2010 and July 2011 were enrolled into the study at owner’s consent. All patients underwent diagnostic evaluations including chest radiographs, complete blood count, serum chemistry profile and urinalysis. All patients were clinically staged using an established WHO clinical staging scheme (Table 1).43 This study protocol was approved by institutional animal care and use committee (IACUC). All biopsy samples were confirmed to be SCC by a boarded veterinary pathologist (E. J. E.).
Table 1.
Clinical staging scheme for feline oral tumours
| Primary Tumour (T) |
|||
|
| |||
| T1 | Tumour<2cm in diameter at greatest dimension, without evidence of bone invasion (a) or with evidence of bone invasion (b) |
||
| T2 | Tumour 2–4 cm in diameter at greatest dimension, without evidence of bone invasion (a) or with evidence of bone invasion (b) |
||
| T3 | Tumour >4 cm in diameter at greatest dimension, without evidence of bone invasion (a) or with evidence of bone invasion (b) |
||
|
| |||
| Regional lymph nodes (N) |
|||
| NO | No regional lymph node metastasis | ||
| N1 | Movable ipsilateral lymph nodes, without evidence of lymph node metastasis (a) or with evidence of lymph node metastasis (b) |
||
| N2 | Movable contralateral lymph nodes, without evidence of lymph node metastasis (a) or with evidence of lymph node metastasis (b) |
||
| N3 | Fixed lymph nodes | ||
|
| |||
| Distant metastasis (M) | |||
| M0 | Distant metastasis not detected | ||
| M1 | Distant metastasis detected | ||
|
| |||
| Stage grouping | Tumour (T) | Nodes (N) | Metastasis (M) |
|
| |||
| I | T1 | N0, N1a, N2a | M0 |
| II | T2 | N0, N1a, N2a | M0 |
| III | T3 | N0, N1a, N2a | M0 |
| Any T | N1b | M0 | |
| IV | Any T | N2b, N3 | M0 |
| Any T | Any N | M1 | |
CT or PET/CT examination and stereotactic radiation therapy
All patients underwent either CT or 18F-FDG PET/CT examination for radiation therapy planning, using an integrated PET/CT scanner (Philips Gemini TF Big Bore 16-slice scanner, Philips Medical Systems, Andover, MA, USA). After inducing general anaesthesia (typically with atropine, methadone or hydromorphone, and ketamine or propofol induction, followed by maintenance with oxygen/isoflurane or sevoflurane admixture), patients were positioned on the CT couch in either ventral or dorsal recumbency using custom-made immobilization devices reported in our previous study.44,45 Patients who underwent PET/CT examination were injected intravenously (IV) with 0.17 mCi kg−1 ± 10% 18F-FDG and the time recorded. A detailed PET/CT protocol is described in our previous report.45 For the patients who did not undergo PET/CT, regular pre- and post-contrast CT studies were performed with the same patient setup apparatus.
Post-contrast simulation CT images were imported into the Eclipse treatment planning workstation (version 8.6.0, Varian Medical Systems, Palo Alto, CA, USA). Normal organs at risk (OAR) such as eyes, lenses, brain, skin, oral mucosa, bones, trachea, oesophagus, mandibular salivary glands, spinal cord, optic chiasm and tongue were identified and contoured. Mandibular and retropharyngeal lymph nodes were also contoured. Grossly identifiable tumour was delineated as the gross tumour volume (GTV) based on contrast enhancement and PET avidity. No expansion for potential subclinical diseases [GTV-to-clinical target volume (CTV) margin] was used. This is because SRT delivers a higher dose per fraction to the target and irradiating the surrounding normal tissues (that could be included in the CTV) with high dose per fraction increases the risk of late radiation toxicities. A uniform planning target volume (PTV) expansion (2 mm) was added to the GTV, lymph nodes, and all OARs. Computerized, three-dimensional image-based treatment planning using an inverse planning algorithm, tissue heterogeneity correction and intensity modulation (sliding leaf technique with multi-leaf collimator) was used for all patients. Photon energies (6 MV and/or 10 MV) were used for all patients and typical plans consisted of equally spaced 6–10 co-planar beams. Isocentric (100 cm) technique was used for all patients. Tissue-equivalent bolus was used when required. Plans were evaluated by visual inspection of dose colour-wash and dose volume histograms and approved by an American College of Veterinary Radiology board-certified veterinary radiation oncologist (S. M. L.). The regional lymph nodes were irradiated either prophylactically or with curative intent depending on the result of physical examination, CT or PET/CT images, and cytological examination. Quality assurance of the plan and dose delivery was performed for each case by an American Board of Radiology certified therapeutic medical physicist.
On the day of treatment, patients were anaesthetized and positioned on the treatment couch with the immobilization devices made at the time of CT/PET-CT examination. A Varian Trilogy linear accelerator (Varian Medical Systems, Palo Alto, CA, USA) was used to administer SRT. Alignment of patient positioning was done by comparing digitally reconstructed radiographs that are created in the Eclipse workstation and/or original planning CT to two orthogonal kV images and/or cone-beam CT that were obtained with on-board imaging device.44 Using an image comparison tool, appropriate couch shifts (X, Y, and Z directions, as well as couch rotation) were applied.
In this study, the proposed SRT protocol was 10 Gy×3 fx (3 consecutive days) to the PTV but this was modified to 20 Gy×1 fx because many patients had concurrent medical issues that precluded repeated anaesthesia. This change was also approved by the IACUC.
Patient follow-up and evaluation of treatment response, outcome and toxicities
Patients were checked 2 weeks after the SRT either at the CSU-ACC or at the referring animal hospitals. All patients were followed-up every 2–3 months thereafter until their death or as needed. A follow-up CT or PET/CT examination was performed to evaluate treatment response 4 weeks after SRT. Only contrast-enhanced CT images were used for this purpose to maintain consistency between patients. Complete remission (CR), partial response (PR), progressive disease (PD) and stable disease (SD) were defined as follows; CR=disappearance of detectable diseases, PR≥30% reduction in the largest tumour dimension, PD≥20% increase in the largest tumour dimension, SD=between PR and PD. The severity of SRT-related acute and late toxicities was evaluated at the time of recheck appointments and score based on the published scoring schemes from the Veterinary Radiation Therapy Oncology Group (VRTOG).46 Progression-free interval (PFI) was defined as the time from the start of SRT to the clinically noticeable tumour recurrence or patient death, whichever comes first. ST was defined as the time from the start of the SRT to the patient death. Patients who died of disease-unrelated to the tumour/treatment were censored from PFI and ST analyses. Kaplan–Meier analysis was performed to estimate median PFI and median ST.
Histophathological grading
Grading system used was modified from a published system for human H&N tumours.47 This system consisted of histological grading of malignancy of tumour cell population (degree of keratinization, nuclear polymorphism and number of mitosis) and tumour–host relationship (pattern of invasion, stage of invasion and lympho-plasmacytic infiltration). Modifications were determined by the primary author and a boarded veterinary pathologist (E. J. E.) (Table 2). This system was applied to the feline oral SCC of this study by the primary author with all grading overseen by this pathologist (E. J. E.).
Table 2.
A modified histopathological grading scheme for feline oral squamous cell carcinoma
| Points |
||||
|---|---|---|---|---|
| Morphologic parameter |
1 | 2 | 3 | 4 |
| Histologic grading of malignancy of tumour cell population | ||||
| Degree of keratinization |
Highly keratinized (>50% of the cell) |
Moderately keratinized (20–50% of the cells) |
Minimal keratinization (5–20% of the cells) |
No keratinization (0–5% of the cells) |
| Nuclear polymorphism |
Little nuclear polymorphism (>75% mature cells) |
Moderately abundant nuclear polymorphism (50–75% mature cells) |
Abundant nuclear polymorphism (20–50% mature cells) |
Extreme nuclear polymorphism (0–25% mature cells) |
| Number of mitosis/HPF (400×) |
0<MI≤1.5 | 1.5<MI≤3.0 | 3.0<MI≤5 | MI>5 |
|
| ||||
| Histologic grading of malignancy of tumour–host relationship | ||||
| Pattern of invasion | Pushing, well-delineated infiltrating borders |
Infiltrating, solid cords, bands and/or strands |
Small groups or cords of infiltrating cells (n>15) |
Marked and widespread cellular dissociation in small groups of cells (n<15) and/or in single cells |
| Stage of invasion (depth) |
Carcinoma in situ
and/or questionable invasion |
Distinct invasion, but involving lamina propria only |
Invasion below lamina propria adjacent to muscles, salivary gland tissues and periosteum |
Extensive and deep invasion replacing most of the stromal tissue and infiltrating jaw bone |
| Lympho- plasmacytic infiltration (at 20×) |
Throughout every field | Some within every field |
Only in some fields | None |
Mitotic index
MI was evaluated as described in our previously published study.19 Briefly, MI was determined by counting mitotic cells in 10 random HPFs (400×magnification) of H&E stained slides. The slides were read twice by a single author (H. Y.) in a blind manner and the total numbers of cells in mitosis were divided by the numbers of HPFs counted to obtain an average MI (MIave). The maximum number of mitotic cells in these HPFs for each patient was also recorded as MImax and used as one of the variables. These results were confirmed by a boarded veterinary pathologist (E. J. E.).
Immunohistochemistry for Ki67, Von-Willebrand factor (vWf, for MVD), and EGFR
The immunohistochemistry (IHC) protocol for Ki67, vWf and EGFR are described in our previously published study.19 Briefly, after antigen retrieval [citrate buffer (Dako target retrieval solution, Dako, Carpinteria, CA, USA) for Ki67 and vWf, and with protease (Dako cytomation proteolytic enzyme, Dako) for EGFR for 1 min at 125 °C] and blocking (Background sniper, Biocare medical, Concord, CA, USA) for non-specific binding (10 min at room temperature), primary antibody [mouse anti-human Ki67 monoclonal (MIB-1, Dako) 1:50 dilution; rabbit anti-human vWf (A0082, Dako) polyclonal 1:300 dilution; mouse anti-human EGFR monoclonal (ab-10, Thermo Fisher Scientific, Fremont, CA, USA)1:50 dilution] was applied and incubated overnight at 4 °C. After blocking endogenous peroxidase (3% hydrogen peroxide for 10 min), the slides were incubated with a universal secondary antibody (Dako Envision + Dual link, Dako) for 20 min at room temperature. A diaminobenzidine substrate kit (DAB substrate kit for peroxidase, Vector Laboratories, Burlingame, CA, USA) was utilized to detect immunoreactive complexes. The slides were counterstained with Mayer’s haematoxylin and permanently mounted. Appropriate positive control slides were used for each batch and for all antibodies (feline lymph nodes for Ki67 and feline urinary bladder for vWf and EGFR). Negative controls were stained exactly the same as the tumour slides except for omission of the primary antibody.
Grading of IHC stains
The evaluation of Ki67 and EGFR was completed as our previously reported study.19 Briefly, two readers (H. Y. and J. B. C.) graded all slides independently based on criteria described below. Grading was performed in a blind manner. Discrepancies were reviewed together at a multi-head microscope and consensus was reached. Grading was confirmed by a boarded veterinary pathologist (E. J. E.). If no consensus was obtained, the reading was repeated.
Scoring of the protein expression of Ki67 and EGFR
Ki67 and EGFR staining was evaluated as previously reported by our group.19 Briefly, the percent of positively stained tumour cells (0, 0%; 1, 1–5%; 2, 6–20%; 3, 21–50%; 4, ≥51%) (Ki67%) and the average intensity of positively stained tumour cells (0, negative; 1, weak; 2, moderate; 3, strong and 4, very strong) (Ki67int) were recorded and these were multiplied to obtain a total score of 0–16 (Ki67total) for Ki67. For the EGFR, the percent of positively stained tumour cells (0, 0%; 1, <10%; 2, 11–30%; 3, 31–60% and 4, ≥61%) (EGFR%) and the average intensity of positively stained tumour cells (0, negative; 1, weak; 2, moderate and 3, strong) (EGFRint) were scored and these were multiplied to obtain a total score (EGFRtotal).
Microvascular density analysis
MVD was evaluated as described in our previous study.19 Briefly, blood vessels positive for vWf was quantified using a microscope equipped with a CCD camera (Carl Zeiss Axioplan 2 imaging scope, Carl Zeiss, Thornwood, NY, USA) and image analysis software (Axio Vision 4.3 system software, Carl Zeiss). After scanning the entire field under low power field (40×magnification), the highest microvascular density area (hot spot) close to tumour cells was determined subjectively.48 Then, at 200×magnification, two distinct hot spots were picked from each slide and captured. MVD was expressed as the ratio of positively stained pixels of representative endothelium over the total amount of image pixels and the results from these two slides were averaged (Auto%Ave). Higher percentile among these two images was also recorded as Auto%Max. We also counted the number of microvessels manually as a previous study and averaged them to obtain ManualAve and the maximum count among the two hot-spot images was also recorded as ManualMax.48
pO2 measurement
pO2 in the tumour was measured immediately before and 24 h after SRT. pO2 was also measured in an accessible normal-looking area of mucus membrane outside of the treatment field to serve as an internal control. Tissue pO2 was measured using a fiber-optic pO2 measurement system (OxyLite system, Oxford Optronics, Abingdon, UK) with a ‘large area’ probe with an 8 mm window for pO2 measurement. After inducing general anaesthesia, patients inhaled admixed oxygen which was set to 28% of pO2 and were maintained for at least 15 min to equalize the oxygen concentration in the body. This produces a pO2 within the normal range for patients at altitude.49 The site and insertion angle of the probe were decided based upon the planning CT. The site for the normal tissue pO2 was decided for each case, depending on the patient position and tumour location. Sites were then gently cleaned with 4% chlorhexidine and saline. A small skin/mucosal incision was made with a #11 scalpel blade. The sheath of a 24 G intravenous catheter was used to pass the oxygen probe into the tumour and the normal tissues. Probe positioning was visually confirmed. Once the probes were placed in the tissues, pO2 measurement was started. All data was recorded using software provided by the manufacturer (Chart5, Oxford Optronics). Typically, it took approximately 5–10 min until the measurement reading stabilized. Once the measurement was completed, probes were pulled out and the incision sites were cleaned appropriately. All probes were calibrated immediately before each measurement following manufacturer’s instructions. After the measurement, the pO2 data was reviewed using the provided software. Final data was obtained by averaging approximately 10 s of reading after reached stable status. Two-tailed, paired t-test was performed to evaluate changes in pre- and post-SRT pO2 in tumour and normal tissue.
Evaluating markers as a prognostic factor for ST and PFI
Patients were divided into two groups; above or below the median result of each marker (‘high’ and ‘low’ groups, respectively). A Log-Rank test was performed to evaluate for a prognostic significance for ST and PFI. Univariate Cox-proportional hazard analysis was also performed. Patient factors included for those analyses were sex, age at the time of diagnosis of oral SCC, body weight at the time of SRT, clinical stage, volume of GTV and tumour location. Variables with those P-values < 0.05 in either Log-Rank or univariate Cox-proportional hazard analysis were included in multivariate Cox-proportional hazard analysis.
Difference of IHC markers, histopathological grading, pre-SRT tumour pO2 and volume of GTV between clinical stages or tumour locations
Patients were sorted into either clinical stages II or III/IV. Patients were also grouped into either mandibular/maxillary or tongue/laryngeal group. Between these groups, statistical analyses were performed to evaluate any difference in IHC scores, pre-SRT tumour pO2 and volume of GTV. Student’s t-test was performed for the pre-SRT tumour pO2 and the volume of GTV. Mann–Whitney rank sum test was performed for the MI and the MVDs. Fisher exact test was performed for the Ki67, the EGFR and the histopathological grading.
Statistical analysis
All statistical analysis was performed using commercially available software (SigmaStat version 3.5 and SigmaPlot version 12, Systat Software, San Jose, CA, USA). A P-value < 0.05 was considered statistically significant.
Results
Patient information, ST and PFI
Fifteen domestic short hair, four domestic long hair and one Siamese were included in the study. Tumour locations included mandible (11), lingual/laryngeal (6) and maxilla (3). There were 12 neutered males, 7 neutered females and 1 intact male. Mean patient age and body weight at diagnosis were 12.5 years old and 4.4 kg, respectively. There were nine each patients in clinical stages II and III and two patients were in stage IV. All histopathological grading criteria were (Table 2) subjectively evenly distributed except the stage of invasion since most cases had invaded into the adjacent muscles (data not shown). Information regarding sex, age and body weight of the patients, tumour location, clinical stage, volume of GTV, prescribed dose, maximum, mean, and 95% doses of PTV, ST, PFI and reason of death are listed in Tables 3 and 4. One of the two patients who received 10 Gy×3 fractions (#3) received an additional fraction of 20 Gy when the tumour recurred 104 days after the first fraction of SRT. One of the 17 patients (#19) who received 20 Gy×1 fraction underwent multiple cytoreductive surgeries when the tumour recurred 108 days after the start of SRT. For these two patients, PFI was determined at time of first recurrence. Among the 20 patients, 12 had PET/CT examination and eight had CT examination for SRT planning. There were two patients with metastatic lymph nodes. One of them had a large SCC at left side of the neck and the mandibular and retropharyngeal lymph nodes were considered contiguous to the original tumour mass. The other patient had a metastatic superficial cervical lymph node. This patient received 16.2–16.6 Gy to its mandibular, retropharyngeal and cervical lymph nodes.
Table 3.
Summary of patients’ profile
| Patient# | Sex | Age (years) | BW (kg) | Tumour location | Clinical stage | GTV volume (cm3) |
|---|---|---|---|---|---|---|
| 1 | FS | 12.7 | 5.1 | R mandible | II: T2b, N0, M0 | 4.9 |
| 2 | MI | 14.2 | 3.8 | R mandible | III: T3b, N0, M0 | 12.8 |
| 3 | FS | 6.2 | 3.3 | R maxilla | II: T2b, N0, M0 | 5.9 |
| 4 | FS | 13.3 | 4.5 | R mandible | II: T2b, N0, M0 | 11.8 |
| 5 | MC | 12.2 | 5.6 | R mandible | III: T3b, N0, M0 | 38.9 |
| 6 | MC | 13.3 | 7.3 | L mandible | III: T3b, N0, M0 | 22.9 |
| 7 | MC | 9.8 | 6.1 | L mandible | III: T3b, N0, M0 | 18.1 |
| 8 | FS | 12.8 | 2.5 | R mandible | II: T2b, N0, M0 | 2.6 |
| 9 | MC | 12.5 | 4.3 | sublingual | II: T2a, N0, M0 | 5.3 |
| 10 | MC | 17.7 | 2.8 | R mandible | III: T3b, N0, M0 | 13.2 |
| 11 | MC | 10.5 | 4.1 | Laryngeal | IV: T3a, N3, M0 | 29.3 |
| 12 | MC | 13 | 5.2 | Sublingual | III: T3a, N0, M0 | 13.5 |
| 13 | MC | 16.7 | 4 | L maxilla | II: T2b, N0, M0 | 13 |
| 14 | MC | 8.1 | 4.8 | Laryngeal/lingual | II: T2a, N0, M0 | 5.1 |
| 15 | FS | 13.5 | 5 | R mandible | III: T3b, N0, M0 | 18.8 |
| 16 | MC | 11.1 | 2.8 | R mandible | IV: T3b, N1b, M0 | 21.3 |
| 17 | FS | 14.3 | 4.5 | Sublingual | II: T2a, N0, M0 | 6.8 |
| 18 | MC | 15 | 3.2 | R mandible | III: T3b, N0, M0 | 24.7 |
| 19 | MC | 10.7 | 4.5 | Sublingual | II: T2a, N0, M0 | 1.3 |
| 20 | FS | 11.5 | 4.1 | R maxilla | III: T3b, N0, M0 | 31.7 |
BW, Body weight; GTV, Gross tumour volume; MC, Male castrated; MI, Male intact; FS, Female spayed; L, Left; R, Right.
Table 4.
Summary of prescribed protocol, doses to planning target volume, survival time, progression-free interval, reason of death and treatment response
| SRT prescription |
Dose to PTV (Gy) |
Reason of death |
Treatment response |
|||||
|---|---|---|---|---|---|---|---|---|
| Patient# | Maximum | Mean | 95% | ST (days) | PFI (days) | |||
| 1 | 10 Gy×3 | 33.1 | 29.8 | 28.8 | 132 | 131 | PD | SD |
| 2 | 20 Gy×× 1 | 27.8 | 18.6 | 15 | 156 | 156 | PD | – |
| 3 | 10 Gy×3 + 20 Gy× 1 | 35.9 | 30.7 | 26.2 | 359 | 104 | PD | SD |
| 4 | 20 Gy×1 | 27.7 | 18.9 | 15.8 | 21 | 21 | Heart failure | – |
| 5 | 20 Gy×1 | 27.4 | 20.2 | 16.8 | 69 | 69 | PD | – |
| 6 | 20 Gy×1 | 31.6 | 22.4 | 17.4 | 21 | 21 | Acute Renal Failure |
– |
| 7 | 20 Gy×1 | 32.8 | 22.7 | 18.2 | 84 | 84 | PD | SD |
| 8 | 20 Gy×1 | 27.3 | 20.7 | 19.5 | 206 | 206 | PD | CR |
| 9 | 20 Gy×1 | 30.7 | 22.3 | 19 | 77 | 75 | PD | CR |
| 10 | 20 Gy×1 | 27.3 | 20.5 | 13 | 23 | 23 | PD | – |
| 11 | 20 Gy×1 | 30 | 21.8 | 15.3 | 106 | 87 | PD | SD |
| 12 | 20 Gy×1 | 30.8 | 22.4 | 19.7 | 44 | 44 | PD | SD |
| 13 | 20 Gy×1 | 31.2 | 22.6 | 19.2 | 104 | 54 | PD | SD |
| 14 | 20 Gy×1 | 28 | 19.2 | 15.8 | 84 | 59 | PD | – |
| 15 | 20 Gy×1 | 31 | 21.5 | 18.2 | 190 | 164 | PD | SD |
| 16 | 20 Gy×1 | 34.4 | 22.7 | 19.2 | 14 | 14 | PD | – |
| 17 | 20 Gy×1 | 29.2 | 21.8 | 19.4 | 140 | 140 | PD | PR |
| 18 | 20 Gy×1 | 28.7 | 21.8 | 17.7 | 70 | 57 | PD | PR |
| 19 | 20 Gy ×1+ cytoreductive surgery |
26.7 | 21.4 | 19.8 | 355 | 108 | PD | PR |
| 20 | 20 Gy×1 | 3.03 | 23.1 | 18.3 | 145 | 128 | PD | SD |
CR, complete response; PD, progressive disease; PFI, progression-free interval; PR, partial response; PTV, planned target volume; SD, stable disease; SRT, stereotactic radiation therapy; ST, survival.
Among the 20 cats, 2 cats were euthanized due to tumour/treatment-unrelated problems both at day 21 (Case#4: heart failure, case#6: acute renal failure) (Table 4). These two cats had no obvious progression of disease when they were euthanized. They were censored from ST and PFI analysis. Median ST was 106 days (range: 14–359 days) (Fig. 1A). Median PFI was 87 days (range: 14–206 days) (Fig. 1B) (range: 14–206 days).
Figure 1.
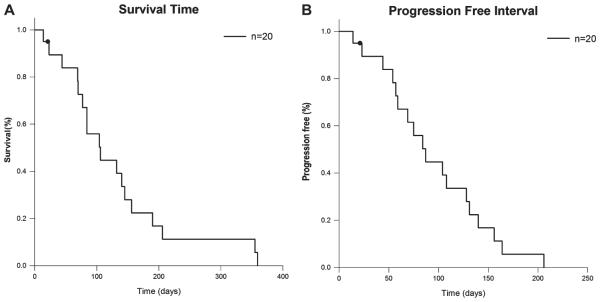
Kaplan–Meier graphs representing (A) survival time and (B) progression-free interval of feline patients with oral squamous cell carcinoma treated by stereotactic radiation therapy. Black circles indicate censored patients.
Thirteen cats underwent either CT or 18F-FDG PET/CT 30 days post-SRT in average. Among them, two cats (15.4%) showed CR, eight cats (61.5%) showed SD and three cats (23.1%) showed PR. Overall response rate was 38.5% (Table 4).
Evaluating markers as a prognostic factor for ST and PFI
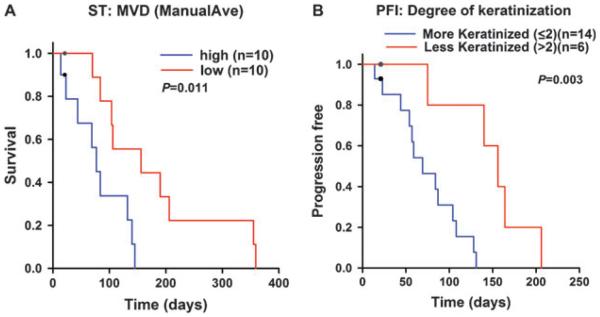
As previously reported, Ki67 showed nuclear staining.19 vWf and EGFR showed cytoplasmic and membranous staining, respectively.19 Log-Rank test revealed multiple variables that were significantly prognostic for ST and PFI. For ST, EGFRint, ManualAve, patient sex and volume of GTV showed statistically significant differences between the two groups. To evaluate prognostic importance of these variables by accounting for mutual impact, we conducted multivariate Cox-proportional hazard analysis (Table 5). This test revealed that patients with higher ManualAve (P=0.041, RR=1.06, 95% CI=1.002, 1.1) (Figs 2A,B and 3A) or male patients (P=0.025, for female: RR=0.26, 95% CI=0.08, 0.84) had significantly shorter ST than patients with lower ManualAve or female patients (Table 5). For PFI, EGFRint, degree of keratinization and patient sex are statistically significant in the Log-Rank analysis. In the multivariate Cox-proportional hazard analysis, patients with higher degree of keratinization (P=0.049, RR=0.087, 95% CI=0.0076, 0.99) (Figs 2C, D and 3B) or male patients (P=0.016, for female: RR=0.17, 95% CI=0.042, 0.72) had significantly shorter PFI (Table 6). No other histopathological grading parameters showed a statistical significance.
Table 5.
Results of survival analysis
| MVD (ManualAve) | n (median), range | Sex | n (median), range |
|---|---|---|---|
| Low | 10 (156), 70–359 | Female | 7 (145), 132–359 |
| High | 10 (77), 14–145 | Male | 13 (84), 14–156 |
| Log Rank P-value | 0.011 | Log Rank P-value | 0.029 |
| Cox P-value | 0.041 | Cox P-value | 0.025 |
| RR (95% Cl) | 1.06 (1.002, 1.1) | RR (95% Cl) | 0.26 (0.08, 0.84) |
For each group, number of patient, median and range of survival time, P-value of Log Rank test, P-value of multivariate Cox proportional hazard analysis, relative risk, and lower/upper 95% confidence interval are shown. CI: Confidence interval, MannualAve: average of manually counted microvessels, MVD: microvascular density, RR: risk ratio.
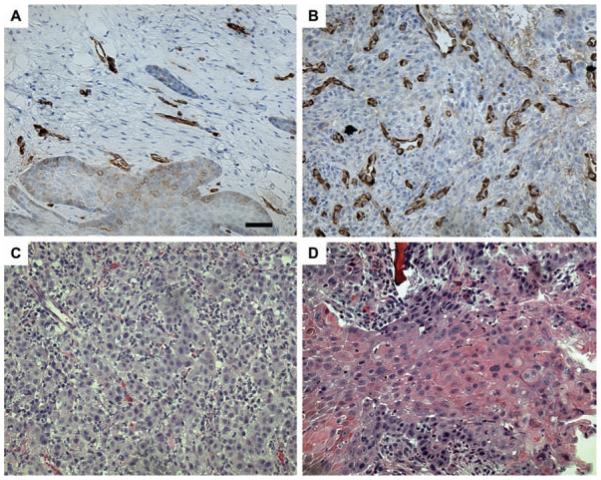
Figure 2.
Representative microscopic images of (A) low and (B) high microvascular density and (C) low and (D) high degree of keratinization. Bar=20 μm.
Figure 3.
Kaplan–Meier graphs representing outcome of feline patients with oral squamous cell carcinoma treated by stereotactic radiation therapy. (A) Survival time stratified by low (red) or high (blue) in the microvascular density (ManualAve). (B) Progression-free interval stratified by low (blue, more keratinized group) or high (red, less keratinized group) in scoring of the degree of keratinization histopathological grading system. Black circles indicate censored patients.
Table 6.
Results of progression-free interval analysis
| Degree of keratinization | n (median), range | Sex | n (median), range |
|---|---|---|---|
| Low | 14 (69), 14–131 | Female | 7 (131), 104–206 |
| High | 6 (156), 75–206 | Male | 13 (69), 14–156 |
| Log Rank P-value | 0.003 | Log Rank P-value | 0.005 |
| Cox P-value | 0.049 | Cox P-value | 0.016 |
| RR (95% CI) | 0.087 (0.0076, 0.99) | RR (95% CI) | 0.17 (0.042, 0.72) |
For each group, number of patient, median and range of progression-free interval, P-value of Log Rank test, P-value of multivariate Cox proportional hazard analysis, relative risk and lower/upper 95% confidence interval are shown. CI, confidence interval; RR, risk ratio.
Patients #1 and #3 received 10 Gy×3 fractions whereas other 18 patients received 20 Gy×1 fx. Also, patient #19 underwent multiple cytoreductive surgeries when the tumour recurred. Therefore, we conducted the same ST analysis but this time, without these three cases. When patient #1, 3, 19 were excluded from the ST analysis, patients with higher ManualAve (P=0.014, RR=1.1, 95% CI=1.02, 1.3) or male patients (P=0.007, for female: RR=0.042, 95% CI=0.0039, 0.42) still had significantly shorter ST in the multivariate Cox-proportional hazard analysis. Because patients #1 and #3 underwent different SRT protocol from other 18 patients, PFI analysis without these 2 patients were also performed. In this analysis, male patients (P=0.038, for female: RR=0.13, 95% CI=0.019, 0.89) still had significantly shorter PFI in the multivariate Cox-proportional hazard analysis.
pO2 measurement
Pre- and post-SRT pO2 in tumour and normal tissue were summarized in Fig. 4. pO2 measurement was not performed in one patient (SCC #10) due to the patient’s condition while under anaesthesia. Four of the 19 patients had only the pre-SRT pO2 measurement (SCC #2,4,16: poor general condition, SCC #8: equipment failure). Paired t-test revealed that post-SRT tumour pO2 was significantly lower than pre-SRT tumour pO2 (n=15, mean=19.2 versus 11.02 mm Hg, P=0.047) (Fig. 4). Paired t-test also revealed that pO2 in pre-SRT tumour (n=18) and post-SRT tumour (n=15) are significantly lower than those in pre-SRT normal tissue and post-SRT normal tissue, respectively (both P<0.001, 21.5 versus 75.8 mm Hg and 11.02 versus 68.8 mm Hg, respectively) (Fig. 4). pO2 in the normal tissues was normal in both pre- and post-SRT.
Figure 4.
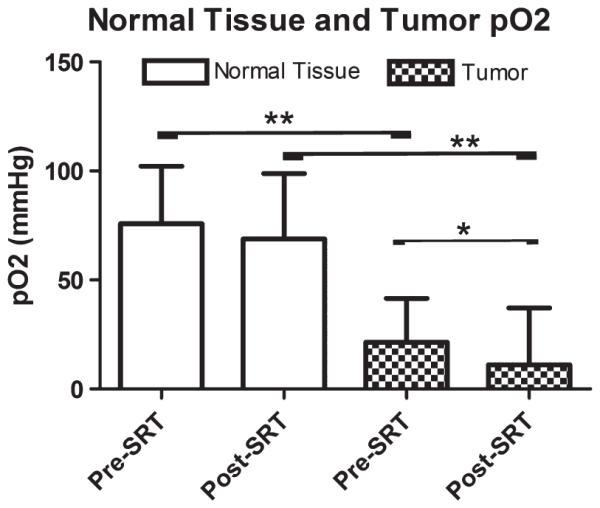
Bar graphs representing changes of oxygen tension in the tumour (checked pattern) and nearby normal tissues (blank) measured in feline patients with oral squamous cell carcinoma, measured immediately before SRT and 24 h post-SRT. Asterisk represents P=0.047 and double asterisk represents P<0.001.
Difference of IHC markers, pre-SRT pO2 and volume of GTV between patients with different clinical stages or tumour locations
Statistically significant difference was not found between clinical stages II and III/IV. Statistical analyses revealed that patients with Tongue/Laryngeal tumours had significantly higher ManualAve and ManualMax than patients with Mandibular/Maxillary tumours (both P≤0.001).
SRT-related toxicities
Toxicities and treatment-related complications are summarized in Table 7. No patients showed acute toxicity in skin or mucous membrane with VRTOG scores 2 or higher. Patient #13 showed refractory glaucoma in the left eye approximately 2 months after SRT. CT examination which was performed at this time revealed local tumour progression but the exact cause of glaucoma of the left eye could not be determined. Other patients showed no late effects or score 1 late effect in their skin/hair or eyes. Fractured/displaced mandible was observed in 6 of 11 mandibular cases, fibrosis in 3 of 6 lingual/laryngeal cases and oro-nasal fistula in 1 of 3 maxillary cases.
Table 7.
Treatment-related toxicities and complications
| Acute effects |
Late effects |
|||||
|---|---|---|---|---|---|---|
| Patient# | Skin | Mucous membrane |
Eyes | Skin/hair | Eyes | Treatment-related complications |
| 1 | 0 | 0 | 0 | 1 | 0 | Fractured mandible |
| 2 | 0 | 0 | 0 | 0 | 0 | Fractured mandible |
| 3 | 0 | 0 | 0 | 1 | 0 | |
| 4 | 0 | 0 | 0 | – | – | Fractured mandible |
| 5 | 1 | 0 | 0 | – | – | Fractured mandible |
| 6 | 0 | 0 | 0 | – | – | Fractured mandible |
| 7 | 0 | 0 | 0 | – | – | |
| 8 | 0 | 0 | 0 | 0 | 0 | |
| 9 | 0 | 0 | 0 | – | – | |
| 10 | 1 | 0 | 0 | – | – | |
| 11 | 0 | 0 | 0 | 0 | 0 | |
| 12 | 0 | 0 | 0 | – | – | Fibrosis |
| 13 | 0 | 0 | 1 | 1 | 2 | |
| 14 | 0 | 1 | 0 | 0 | 0 | |
| 15 | 1 | 0 | 0 | 1 | 0 | Fractured mandible |
| 16 | 1 | 0 | 1 | – | – | |
| 17 | 1 | 0 | 0 | 0 | 0 | Fibrosis |
| 18 | 1 | 1 | 0 | 0 | 0 | |
| 19 | 0 | 0 | 0 | 0 | 0 | Fibrosis |
| 20 | 1 | 0 | 0 | 1 | 0 | Oro-nasal fistula |
Discussion
Feline oral SCC is a locally aggressive cancer that responds to local treatment initially but almost always recurs locally.43 Although our knowledge about its aetiology/biology has been deepened by previous studies,19,50,51 knowledge about tumour biology that possibly leads to better local tumour control is still scarce.7,9,52–55 This study was to evaluate the biological variables that possibly act as a prognostic marker in feline patients with oral SCC treated by the novel type of radiation therapy, SRT.
We found that patients with higher MVD had shorter ST compared to patients with lower MVD. This finding is in contrast to a previous human study in which treatment outcome of patients with H&N SCC was worse in patients with lower MVD.23 In that study, patients underwent fractionated radiation therapy instead of SRT. Although not proven in human patients, it has been presumed that the tumouricidal effect of SRT, which typically delivers higher dose per fraction, consists of radiation damage directly to the cancer cells and indirect damage secondary to tumour vasculature damage, whereas the effect of fractionated XRT is mainly from the direct killing of the cancer cells.24 It has also been shown that the MVD decreases immediately after a large single dose of radiation (10–15 Gy), remains reduced for varying periods and occasionally re-grows to the original level.24 In a study with human glioblastoma, tumour started re-growing when blood perfusion recovered 3 weeks after an initial reduction in MVD and perfusion and an increase of hypoxic fraction caused by a single large dose of radiation (15 Gy).56 Although we did not evaluate MVD serially and the correlation between MVD and tissue hypoxia is still unclear, patients with higher pre-SRT MVD may have had more surviving tumour vasculature and a lower hypoxic fraction compared to patients with lower pre-SRT MVD, resulting in continuous proliferation of cancer cells. This may have led to significantly earlier local failure in patients with higher pre-SRT MVD in this study. This result suggests that additional treatments targeting tumour vasculature such as metronomic chemotherapy may be beneficial although this additional treatment may also increase the chance of normal tissue late toxicities by damaging normal endothelial cells unless the treatment is targeted to the tumour vasculature. Further study of serial evaluation of the change in MVD before and post-SRT is needed.57
Statistical analyses revealed that patients with less keratinized SCC had longer PFI compared to patients with more keratinized SCC. In human H&N SCC, one study reported that lower degree of keratinization correlates to better treatment outcome.58 Other studies also showed that human patients with more differentiated SCC had significantly poorer local tumour control than patients with less differentiated SCC.59,60 The patients in these three studies underwent conventional fractionated XRT. Keratinized SCC is generally considered to be more differentiated. Less differentiated tumours are generally considered to be more aggressive. Although these results seem counterintuitive, our result regarding the degree of keratinization is in accordance with the findings in human SCC studies. Because this variable is easily evaluated using H&E stained tumour specimens, it might be worth continuing to evaluate the prognostic significance to verify its usefulness in a larger patient population.
We found that the male patients had significantly shorter ST and PFI compared to female patients. As of our knowledge, this has never been reported and a plausible reason for the difference between males and females still needs to be investigated in a larger patient population.
Patients with low scores of EGFR intensity had significantly shorter ST and PFI than those with high scores in the Log-Rank analyses although this was not significant in the multivariate Cox-proportional hazard analysis. Further investigation is needed to exclude the chance of type II error caused by the small sample population in this study.
In this study, median ST and median DFI were 106 and 87 days, respectively. Although inter-study comparison is difficult, these outcomes in the current study do not appear to be superior to previous studies.3,5,8 However, advantages of this study include fewer anaesthesia events, less cosmetic and functional changes compared to more radical surgery, and lower probability of side effects compared to other type of palliative radiation therapy.1 The major disadvantage of SRT is its cost. Because the current study failed to prove an advantage in long-term local control over other treatment modalities and SRT showed about 40% of overall response rate with rapid improvement of clinical symptoms in many cases, it should be considered as a palliative treatment as is in human H&N SCC.26–28
Tumour vasculature is not the same as normal tissue vasculature.61 Tumour vasculature is characterized by its tortuous, dilated, dead-ended and leaky structure.30,61 It has been shown that the tumour vasculature is less dense and less effective to deliver oxygen and nutrients to the surrounding cells.61 Moreover, blood flow in the tumour is slow and bi-directional.61 Although no study has evaluated the blood flow dynamics and vascular structure in feline oral SCC, it might be reasonable to assume that these well-known characteristics of tumour vasculature can be applied to this feline cancer. In our study, we performed direct measurement of tissue pO2 in the oral SCC and nearby normal tissues using a fiber-optic system. In human H&N SCC, tumour pO2 as a prognostic value has been extensively studied with controversial results.2,34,41,62. In the current study, no correlations were found between pre-SRT tumour pO2 and ST or PFI. There are a couple of possible explanations for this result. First, the treatment protocol in the current study SRT was different from these previous studies that used fractionated protocols. Second, the oxygen measurement probe used in this study was different from the one used in previous reports, in which polarographic oxygen probes were used.34,62 In our study, we used the fiber-optic probe. Although histogram analysis is probably the most ideal technique that is currently available to evaluate tissue oxygen status, we obtained a single-point reading of pO2 with a wide window probe. This probe has an 8 mm of reading window and therefore, the pO2 readout is the average of the pO2 around the window. Given the fact that the tissue pO2 changes dramatically in less than 100 μm,29 hypoxic areas may have been masked by non-hypoxic areas with this averaging. However, the fiber-optic probe has some advantages over the polarographic probes and those include higher sensitivity in the lower range (pO2 < 10 mm Hg) of pO2 and no oxygen consumption by the probe.63 Even with those advantages, fiber-optic probes lack spatial information of pO2 and a novel pO2 measurement modality which allows us quick and accurate measurement in multiple points and is as practical as the fiber-optic system will be needed to deepen our knowledge about tumour pO2.
In this study, pre-SRT mean pO2 in the tumour was significantly lower than that in the normal tissues. A statistically significant difference between the tumour and normal tissues was also seen at 24 h after SRT. These findings are in accordance with the general consensus that tumour is more hypoxic than normal tissues.62,64 However, as is shown in Figure 4, we observed a large inter-patient variation of pO2 in normal tissues. This variation has been reported in human literature too.62,64,65 These previous studies have also shown that there is a large intra-patient/intra-organ heterogeneity of pO2, by using the histogram technique. Although we performed only a ‘single-point measurement’ in the normal subcutaneous tissues, the inter-patient heterogeneity we observed may suggest that the oxygenation status in feline normal tissues is similar to that in human normal tissues. Previous reports have also shown that there is some inter-patient heterogeneity in tumour pO2.2,34,41,62 Although different measurement techniques were used among the previous studies as well as between these studies and our current studies, the heterogeneity in tumour pO2 we observed may suggest that it might be worth further investigation for prognostication. Tumour oxygenation status is an important tumour-microenvironmental factor that affects the response of tumour to radiation therapy especially photon therapy.29 For photon XRT, oxygen enhancement ratio (OER), an indicator of how much higher dose is required under hypoxic condition to obtain the same radiobiological effects seen under oxygenated condition, has been reported to be around 2.5 at lower dose range (~3 Gy) and become higher up to 3.5 as the dose increases (5–25 Gy) in Chinese hamster cells.29 Although we showed that the pO2 in the tumour is lower than that in the surrounding normal tissues, an obvious question would be the magnitude of hypoxia at which level we see a significant reduction in radiobiological effects. A study that was reported in 1953 has shown that about 3 mm Hg of pO2 (0.5%) is required to obtain 50% radiosensitivity of fully oxygenated tumours and under 30 mm Hg of pO2, cells are 100% radiosensitive.66 This data, however, was obtained by using mouse tumour cells and some plant cells and therefore, it may not be appropriate to extrapolate these numbers directly to feline oral SCC and other types of malignancies. Clinically, there are studies that evaluated significance of pre-treatment tumour pO2 as a prognostic marker for human H&N SCC patients treated with fractionated XRT.2,33,34,41,62,67 One of these studies evaluated pO2 in 397 patients with H&N SCC.33 In this study, patients whose fraction of pO2 ≤2.5 mm Hg was less than 19% of total measurement had significantly better overall survival than those whose fraction of pO2 <2.5 mm Hg was more than 19%. Moreover, the fraction of pO2 ≤2.5 mm Hg was the most statistically significant prognostic marker for patient survival.33 Patients in this study underwent curative intent fractionated XRT with or without chemotherapy/surgery. Four other studies set the cut-off value pO2 between 2.5 and 10 mm Hg and found significantly worse prognosis in patients with higher hypoxic fraction.2,34,41,67 Therefore, it may be reasonable to assume that clinically significant cut-off value for the pO2 histogram in human patients with H&N SCC treated with fractionated XRT is between 2.5 and 10 mm Hg. In the current study, however, we treated patients with feline oral SCC with SRT, not fractionated protocol. As mentioned below, it seems like that there is a substantial difference in the events occurring in the tumour cells and their microenvironment in response to XRT between SRT and fractionated regimen. Furthermore, because the method we performed averages the pO2 around the probe, it does not provide us a histogram. And finally, our study has smaller patient population compared to these human H&N SCC studies. Because of these differences/limitations in this study, our future direction to understand the clinical impact of tumour oxygenation includes a more detailed pO2 measurement such as histogram evaluation in a larger patient population.
In contrast to the well-accepted concept of re-oxygenation after a small fraction of radiation, we did not observe an increase of tumour pO2.29 Instead, we observed a significant reduction of tumour pO2 after SRT. Previous studies with xenograft models suggested that when higher dose per fraction is used, radiation damage to the endothelial cells becomes critical and leads these cells to initiate apoptotic cascades as early as 3 h post-irradiation 68,69 In the mouse xenograft model, the threshold dose for endothelial cell death has been suggested to be around 10 Gy.68 In a study with human H&N SCC mouse xenograft, a single dose of 10 Gy caused a significant reduction of hypoxic fraction with a minimum value reached at 7 h post-irradiation followed by a steady increase of the hypoxic fraction until 11 days post-irradiation.70 The hypoxic fraction at that time (11 days post-irradiation) was significantly higher than that at between 2 and 96 h post-irradiation.70 Although the endothelial cell damage, at least partially, may be the cause of the significant reduction of tumour pO2 after SRT observed in this study, spatial and serial information of pO2 and MVD is essential to understand the in vivo effect of SRT on the tumour endothelial cells and tumour microenvironment more in detail.
In our previous study, we reported that the MVD in lingual SCC tended to be higher than that in mandibular/maxillary SCC.19 In this study, we found the same difference between tongue/laryngeal group and mandibular/maxillary group, but this time the difference was statistically significant (P<0.001). The impact of this significant difference to the patient outcome is unclear because the tumour location (mandibular/maxillary versus tongue/laryngeal) was not a prognostic factor in our current study. To clarify this discrepancy between our current and previous studies, we think that it is important to increase the patient population and continue evaluating the difference in MVD between lingual/laryngeal SCC and mandibular/maxillary SCC.
In this study, no patients showed acute or late toxicities with score of 3 in the skin/mucous membrane, however, ST was not long enough to adequately study late effects (Table 7). No patients showed acute toxicities or if they did, the score was 1 in skin, or mucous membrane. These acute toxicities were self-limiting and no treatment was indicated. There was a patient with possible radiation-induced glaucoma in the left eye recognized 8 weeks post-SRT (patient #13). This patient had left maxillary SCC and received a single fraction of 20 Gy. The left eye was in the treatment field. Although the multi-leaf collimator was used to minimize the dose to the left eye, approximately 30% of the eye, mostly the ventral aspect, received 10 Gy. Post-mortem examination of the eye was not performed due to owner’s request. A CT examination at the time of diagnosis of the glaucoma revealed significant progression of SCC but could not differentiate whether the glaucoma was caused by tumour progression or toxicity of SRT. Because there are only three maxillary SCC cats whose ST is more than 2 months, it is difficult to decide the precise incidence rate of SRT-related glaucoma at this point. In the future, patients with maxillary SCC should be closely watched post-SRT for any signs of intra-ocular inflammation or glaucoma. In the other patients with mandibular, laryngeal or lingual SCC, there was no sign of acute/late toxicity in their eyes, demonstrating the advantage of a sharp drop-off of radiation dose outside the treatment target for intensity modulation and SRT. We also observed fracture of mandible, lingual fibrosis and oro-nasal fistula formation typically 2–3 months post-SRT. Those complications were seen in 50% of cats in this study. Although clear distinction of the cause of those complications is impossible (radiation-induced toxicity versus tumour invasion or tumour-induced inflammation), it may be safe to assume that the complication rate following SRT is relatively high in cats with locally advanced oral SCC. Unless effective reconstruction techniques or tissue engineering is developed, it is likely that these complications will continue to be a problem in patients with any type of destructive oral malignancies.
In conclusion, although the tumour rapid response to SRT with alleviation of clinical symptoms and fewer anaesthesia episode are advantages of SRT, the associated cost, the higher chance of late complications and relatively short period of local tumour control limit the use of this modality as a sole treatment, especially the protocol of a single fraction of 20 Gy, to a palliative purpose only. MVD and degree of keratinization appear to be prognostic indicators for patients undergoing SRT and anti-angiogenic treatment may help improving local tumour control. Changes in tumour pO2 following SRT in feline oral SCC suggests tumour vasculature in this tumour responds in a similar fashion as is reported in human H&N SCC but serial monitoring might allow more detailed insight about its dynamics. This may also help identify possible treatment targets that amplify the effect of SRT. Acute toxicity was minimal, although late toxicities/treatment-related toxicities impacted patient quality of life.
Footnotes
This study was presented orally at the Annual Conference of the Veterinary Cancer Society, Las Vegas, NV, October 2012.
References
- 1.Bregazzi VS, LaRue SM, Powers BE, Fettman MJ, Ogilvie GK, Withrow SJ. Response of feline oral squamous cell carcinoma to palliative radiation therapy. Veterinary Radiology and Ultrasound. 2001;42:77–79. doi: 10.1111/j.1740-8261.2001.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 2.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. International Journal of Radiation Oncology, Biology, and Physics. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 3.Fidel J, Lyons J, Tripp C, Houston R, Wheeler B, Ruiz A. Treatment of oral squamous cell carcinoma with accelerated radiation therapy and concomitant carboplatin in cats. Journal of Veterinary Internal Medicine. 2011;25:504–510. doi: 10.1111/j.1939-1676.2011.0721.x. [DOI] [PubMed] [Google Scholar]
- 4.Fidel JL, Sellon RK, Houston RK, Wheeler BA. A nine-day accelerated radiation protocol for feline squamous cell carcinoma. Veterinary Radiology and Ultrasound. 2007;48:482–485. doi: 10.1111/j.1740-8261.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 5.Hutson CA, Willauer CC, Walder EJ, Stone JL, Klein MK. Treatment of mandibular squamous cell carcinoma in cats by use of mandibulectomy and radiotherapy: seven cases (1987–1989) Journal of the American Veterinary Medical Association. 1992;201:777–781. [PubMed] [Google Scholar]
- 6.Jones PD, de Lorimier LP, Kitchell BE, Losonsky JM. Gemcitabine as a radiosensitizer for nonresectable feline oral squamous cell carcinoma. Journal of the American Animal Hospital Association. 2003;39:463–467. doi: 10.5326/0390463. [DOI] [PubMed] [Google Scholar]
- 7.Martin CK, Werbeck JL, Thudi NK, Lanigan LG, Wolfe TD, Toribio RE, Rosol TJ. Zoledronic acid reduces bone loss and tumor growth in an orthotopic xenograft model of osteolytic oral squamous cell carcinoma. Cancer Research. 2010;70:8607–8616. doi: 10.1158/0008-5472.CAN-10-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Northrup NC, Selting KA, Rassnick KM, Kristal O, O’Brien MG, Dank G, Dhaliwal RS, Jagannatha S, Cornell KK, Gieger TL. Outcomes of cats with oral tumors treated with mandibulectomy: 42 cases. Journal of the American Animal Hospital Association. 2006;42:350–360. doi: 10.5326/0420350. [DOI] [PubMed] [Google Scholar]
- 9.Wypij JM, Fan TM, Fredrickson RL, Barger AM, de Lorimier LP, Charney SC. In vivo and in vitro efficacy of zoledronate for treating oral squamous cell carcinoma in cats. Journal of Veterinary Internal Medicine. 2008;22:158–163. doi: 10.1111/j.1939-1676.2007.0010.x. [DOI] [PubMed] [Google Scholar]
- 10.Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scandinavian Journal of Dental Research. 1987;95:229–249. doi: 10.1111/j.1600-0722.1987.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakano T, Oka K. Differential values of Ki-67 index and mitotic index of proliferating cell population. An assessment of cell cycle and prognosis in radiation therapy for cervical cancer. Cancer. 1993;72:2401–2408. doi: 10.1002/1097-0142(19931015)72:8<2401::aid-cncr2820720818>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Nakano T, Oka K, Ishikawa A, Morita S. Immunohistochemical prediction of radiation response and local control in radiation therapy for cervical cancer. Cancer Detection and Prevention. 1998;22:120–128. doi: 10.1046/j.1525-1500.1998.00771.x. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Oka K, Ohno T, Kato S, Tsujii H, Nakano T. Prognostic impact of mitotic index of proliferating cell populations in cervical cancer patients treated with carbon ion beam. Cancer. 2009;115:1875–1882. doi: 10.1002/cncr.24189. [DOI] [PubMed] [Google Scholar]
- 14.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. Journal of Cellular Physiology. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Melzer K, Guscetti F, Rohrer Bley C, Sumova A, Roos M, Kaser-Hotz B. Ki67 reactivity in nasal and periocular squamous cell carcinomas in cats treated with electron beam radiation therapy. Journal of Veterinary Internal Medicine. 2006;20:676–681. doi: 10.1892/0891-6640(2006)20[676:krinap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Arteaga CL. Epidermal growth factor receptor dependence in human tumors: more than just expression? The Oncologist. 2002;7(Suppl. 4):31–39. doi: 10.1634/theoncologist.7-suppl_4-31. [DOI] [PubMed] [Google Scholar]
- 17.Herbst RS. Review of epidermal growth factor receptor biology. International Journal of Radiation Oncology, Biology, and Physics. 2004;59(Suppl. 2):21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 18.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Research. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 19.Yoshikawa H, Ehrhart E, Charles JB, Thamm DH, Larue SM. Immunohistochemical characterization of feline oral squamous cell carcinoma. American Journal of Veterinary Research. 2012;73:1801–1806. doi: 10.2460/ajvr.73.11.1801. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez AA, Krigman HR, Whitaker RS, Dodge RK, Rodriguez GC. The prognostic significance of angiogenesis in epithelial ovarian carcinoma. Clinical Cancer Research. 1999;5:587–591. [PubMed] [Google Scholar]
- 21.Erovic BM, Neuchrist C, Berger U, El-Rabadi K, Burian M. Quantitation of microvessel density in squamous cell carcinoma of the head and neck by computer-aided image analysis. Wiener Klinische Wochenschrift. 2005;117(1–2):53–57. doi: 10.1007/s00508-004-0298-3. [DOI] [PubMed] [Google Scholar]
- 22.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. Journal of the National Cancer Institute. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 23.Kaanders JHAM, Wijffels KIEM, Marres HAM, Ljungkvist ASE, Pop LAM, van den Hoogen FJA, de Wilde PCM, Bussink J, Raleigh JA, van der Kogel AJ. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Research. 2002;62:7066–7074. [PubMed] [Google Scholar]
- 24.Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS) Radiation Research. 2012;177:311–327. doi: 10.1667/rr2773.1. [DOI] [PubMed] [Google Scholar]
- 25.Song CW, Park HJ, Griffin RJ, Levitt SH. Radiobiology of stereotactic radiosurgery and stereotactic body radiation therapy. In: Levitt SH, Purdy J, Perez C, Vijayakumar S, editors. Technical Basis of Radiation Therapy. 5 Springer-Verlag; New York: 2012. pp. 51–61. [Google Scholar]
- 26.Kawaguchi K, Yamada H, Horie A, Sato K. Radiosurgical treatment of maxillary squamous cell carcinoma. International Journal of Oral and Maxillofacial Surgery. 2009;38:1205–1207. doi: 10.1016/j.ijom.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Kodani N, Yamazaki H, Tsubokura T, Shiomi H, Kobayashi K, Nishimura T, Aibe N, Ikeno H, Nishimura T. Stereotactic body radiation therapy for head and neck tumor: disease control and morbidity outcomes. Journal of Radiation Research. 2011;52:24–31. doi: 10.1269/jrr.10086. [DOI] [PubMed] [Google Scholar]
- 28.Siddiqui F, Patel M, Khan M, McLean S, Dragovic J, Jin JY, Movsas B, Ryu S. Stereotactic body radiation therapy for primary, recurrent, and metastatic tumors in the head-and-neck region. International Journal of Radiation Oncology, Biology, and Physics. 2009;74:1047–1053. doi: 10.1016/j.ijrobp.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Hall E, Giaccia A. Radiobiology for the Radiologist. 6 Lippincott Williams & Wilkins; Philadelphia: 2006. Oxygen effect and reoxygenation; pp. 85–105. [Google Scholar]
- 30.Weinberg R. The Biology of Cancer. Taylor & Francis Group; Garland Science, New York: 2007. Dialogue replaces monologue: heterotypic interactions and the biology of angiogenesis; pp. 527–586. [Google Scholar]
- 31.Hockel M, Schlenger K, Hockel S, Vaupel P. Hypoxic cervical cancers with low apoptotic index are highly aggressive. Cancer Research. 1999;59:4525–4528. [PubMed] [Google Scholar]
- 32.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 33.Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, Becker A, Adam M, Molls M, Dunst J, Terris DJ, Overgaard J. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiotherapy and Oncology. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiotherapy and Oncology. 1996;41:31–39. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 35.Fyles A, Milosevic M, Hedley D, Pintilie M, Levin W, Manchul L, Hill RP. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. Journal of Clinical Oncology. 2002;20:680–687. doi: 10.1200/JCO.2002.20.3.680. [DOI] [PubMed] [Google Scholar]
- 36.Knocke TH, Weitmann HD, Feldmann HJ, Selzer E, Potter R. Intratumoral pO2-measurements as predictive assay in the treatment of carcinoma of the uterine cervix. Radiotherapy and Oncology. 1999;53:99–104. doi: 10.1016/s0167-8140(99)00139-5. [DOI] [PubMed] [Google Scholar]
- 37.Milosevic M, Fyles A, Hedley D, Hill R. The human tumor microenvironment: invasive (needle) measurement of oxygen and interstitial fluid pressure. Seminars in Radiation Oncology. 2004;14:249–258. doi: 10.1016/j.semradonc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Lyng H, Sundfor K, Trope C, Rofstad EK. Disease control of uterine cervical cancer: relationships to tumor oxygen tension, vascular density, cell density, and frequency of mitosis and apoptosis measured before treatment and during radiotherapy. Clinical Cancer Research. 2000;6:1104–1112. [PubMed] [Google Scholar]
- 39.Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiotherapy and Oncology. 2000;57:39–43. doi: 10.1016/s0167-8140(00)00223-1. [DOI] [PubMed] [Google Scholar]
- 40.Stadler P, Becker A, Feldmann HJ, Hansgen G, Dunst J, Wurschmidt F, Molls M. Influence of the hypoxic subvolume on the survival of patients with head and neck cancer. International Journal of Radiation Oncology, Biology, and Physics. 1999;44:749–754. doi: 10.1016/s0360-3016(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 41.Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiotherapy and Oncology. 1999;53:113–117. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 42.Dunst J, Hansgen G, Lautenschlager C, Fuchsel G, Becker A. Oxygenation of cervical cancers during radiotherapy and radiotherapy + cis-retinoic acid/interferon. International Journal of Radiation Oncology, Biology, and Physics. 1999;43:367–373. doi: 10.1016/s0360-3016(98)00361-7. [DOI] [PubMed] [Google Scholar]
- 43.Liptak JM, Withrow SJ. Cancer of the gastrointestinal tract. In: Withrow SJ, Vail DM, editors. Small Animal Clinical Oncology. 2007. pp. 455–475. [Google Scholar]
- 44.Yoshikawa H, Harmon JF, Custis JT, Larue SM. Repeatability of a planning target volume expansion protocol for radiation therapy of regional lymph nodes in canine and feline patients with head tumors. Veterinary Radiology and Ultrasound. 2012;53:667–672. doi: 10.1111/j.1740-8261.2012.01972.x. [DOI] [PubMed] [Google Scholar]
- 45.Yoshikawa H, Randall EK, Kraft SL, Larue SM. Comparison between 2-(18)F-Fluoro-2-Deoxy-D-Glucose positron emission tomography and contrast-enhanced computed tomography for measuring gross tumor volume in cats with oral squamous cell carcinoma. Veterinary Radiology and Ultrasound. 2013;54:307–313. doi: 10.1111/vru.12016. [DOI] [PubMed] [Google Scholar]
- 46.Ladue T, Klein MK. Toxicity criteria of the veterinary radiation therapy oncology group. Veterinary Radiology and Ultrasound. 2001;42:475–476. doi: 10.1111/j.1740-8261.2001.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 47.Okada Y, Mataga I, Katagiri M, Ishii K. An analysis of cervical lymph nodes metastasis in oral squamous cell carcinoma. Relationship between grade of histopathological malignancy and lymph nodes metastasis. International Journal of Oral and Maxillofacial Surgery. 2003;32:284–288. doi: 10.1054/ijom.2002.0303. [DOI] [PubMed] [Google Scholar]
- 48.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Research and Treatment. 1995;36:169–180. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 49.Thrall DE, Larue SM, Pruitt AF, Case B, Dewhirst MW. Changes in tumour oxygenation during fractionated hyperthermia and radiation therapy in spontaneous canine sarcomas. International Journal of Hyperthermia. 2006;22:365–373. doi: 10.1080/02656730600836386. [DOI] [PubMed] [Google Scholar]
- 50.Snyder LA, Bertone ER, Jakowski RM, Dooner MS, Jennings-Ritchie J, Moore AS. p53 expression and environmental tobacco smoke exposure in feline oral squamous cell carcinoma. Veterinary Pathology. 2004;41:209–214. doi: 10.1354/vp.41-3-209. [DOI] [PubMed] [Google Scholar]
- 51.Bertone ER, Snyder LA, Moore AS. Environmental and lifestyle risk factors for oral squamous cell carcinoma in domestic cats. Journal of Veterinary Internal Medicine. 2003;17:557–562. [PubMed] [Google Scholar]
- 52.Marconato L, Buchholz J, Keller M, Bettini G, Valenti P, Kaser-Hotz B. Multimodal therapeutic approach and interdisciplinary challenge for the treatment of unresectable head and neck squamous cell carcinoma in six cats: a pilot study. Veterinary and Comparative Oncology. 2012;11:101–112. doi: 10.1111/j.1476-5829.2011.00304.x. [DOI] [PubMed] [Google Scholar]
- 53.Hayes A, Scase T, Miller J, Murphy S, Sparkes A, Adams V. COX-1 and COX-2 expression in feline oral squamous cell carcinoma. Journal of Comparative Pathology. 2006;135(2–3):93–99. doi: 10.1016/j.jcpa.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Bergkvist GT, Argyle DJ, Pang LY, Muirhead R, Yool DA. Studies on the inhibition of feline EGFR in squamous cell carcinoma: enhancement of radiosensitivity and rescue of resistance to small molecule inhibitors. Cancer Biology and Therapy. 2011;11:927–937. doi: 10.4161/cbt.11.11.15525. [DOI] [PubMed] [Google Scholar]
- 55.Looper JS, Malarkey DE, Ruslander D, Proulx D, Thrall DE. Epidermal growth factor receptor expression in feline oral squamous cell carcinomas. Veterinary and Comparative Oncology. 2006;4:33–40. doi: 10.1111/j.1476-5810.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 56.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. The Journal of Clinical Investigation. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nature Reviews. Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 58.Reddy SP, Raslan WF, Gooneratne S, Kathuria S, Marks JE. Prognostic significance of keratinization in nasopharyngeal carcinoma. American Journal of Otolaryngology. 1995;16:103–108. doi: 10.1016/0196-0709(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 59.Johansen LV, Grau C, Overgaard J. Squamous cell carcinoma of the nasopharynx–an analysis of treatment results in 149 consecutive patients. Acta Oncologica. 2001;40:801–809. doi: 10.1080/02841860152703418. [DOI] [PubMed] [Google Scholar]
- 60.Taguchi T, Tsukuda M, Mikami Y, Matsuda H, Tanigaki Y, Horiuchi C, Nishimura G, Nagao J. Treatment results and prognostic factors for advanced squamous cell carcinoma of the head and neck treated with concurrent chemoradiotherapy. Auris, Nasus, Larynx. 2009;36:199–204. doi: 10.1016/j.anl.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 61.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nature Reviews. Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 62.Eschwege F, Bourhis J, Girinski T, Lartigau E, Guichard M, Deble D, Kepta L, Wilson GD, Luboinski B. Predictive assays of radiation response in patients with head and neck squamous cell carcinoma: a review of the Institute Gustave Roussy experience. International Journal of Radiation Oncology, Biology, and Physics. 1997;39:849–853. doi: 10.1016/s0360-3016(97)00509-9. [DOI] [PubMed] [Google Scholar]
- 63.Griffiths JR, Robinson SP. The OxyLite: a fibre-optic oxygen sensor. British Journal of Radiology. 1999;72:627–630. doi: 10.1259/bjr.72.859.10624317. [DOI] [PubMed] [Google Scholar]
- 64.Terris DJ, Dunphy EP. Oxygen tension measurements of head and neck cancers. Archives of Otolaryngology – Head & Neck Surgery. 1994;120:283–287. doi: 10.1001/archotol.1994.01880270031006. [DOI] [PubMed] [Google Scholar]
- 65.Braun RD, Lanzen JL, Snyder SA, Dewhirst MW. Comparison of tumor and normal tissue oxygen tension measurements using OxyLite or microelectrodes in rodents. American Journal of Physiology – Heart and Circulatory Physiology. 2001;280:H2533–H2544. doi: 10.1152/ajpheart.2001.280.6.H2533. [DOI] [PubMed] [Google Scholar]
- 66.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. British Journal of Radiology. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 67.Gatenby RA, Kessler HB, Rosenblum JS, Coia LR, Moldofsky PJ, Hartz WH, Broder GJ. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. International Journal of Radiation Oncology, Biology, and Physics. 1988;14:831–838. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- 68.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 70.Bussink J, Kaanders JH, Rijken PF, Raleigh JA, Van der Kogel AJ. Changes in blood perfusion and hypoxia after irradiation of a human squamous cell carcinoma xenograft tumor line. Radiation Research. 2000;153:398–404. doi: 10.1667/0033-7587(2000)153[0398:cibpah]2.0.co;2. [DOI] [PubMed] [Google Scholar]