Abstract
As part of our ongoing influenza surveillance program in South China, 19 field strains of H9N2 subtype avian influenza viruses (AIVs) were isolated from dead or diseased chicken flocks in Guangdong province, South China, between 2012 and 2013. Hemagglutinin (HA) genes of these strains were sequenced and analyzed and phylogenic analysis showed that 12 of the 19 isolates belonged to the lineage h9.4.2.5, while the other seven belonged to h9.4.2.6. Specifically, we found that all of the viruses isolated in 2013 belonged to lineage h9.4.2.5. The lineage h9.4.2.5 viruses contained a PSRSSR↓GLF motif at HA cleavage site, while the lineage h9.4.2.6 viruses contained a PARSSR↓GLF at the same position. Most of the isolates in lineage h9.4.2.5 lost one potential glycosylation site at residues 200-202, and had an additional one at residues 295-297 in HA1. Notably, 19 isolates had an amino acid exchange (Q226L) in the receptor binding site, which indicated that the viruses had potential affinity of binding to human like receptor. The present study shows the importance of continuing surveillance of new H9N2 strains to better prepare for the next epidemic or pandemic outbreak of H9N2 AIV infections in chicken flocks.
Keywords: H9N2 subtype, avian influenza virus, phylogenetic analysis, South China
Introduction
Wild aquatic birds are the most important natural host of influenza A viruses (IAVs). To date, IAVs have been classified into 18 HA subtypes (H1-H18) and 10 neuraminidase (NA) subtypes (N1-N10) according to antigenic differences in the viral surface glycoproteins [2,9,32,33]. H9N2 subtype avian influenza virus (AIV) has circulated worldwide since it was first isolated from turkeys in Wisconsin in 1966 [15]. In mainland China, H9N2 subtype AIV was first isolated from a chicken flock in Guangdong in 1994 [13]. Subsequently, this subtype virus spread extensively and prevailed in other areas of China [4,5,11,35]. Currently, it is one of the predominant AIV circulating in the poultry population in China.
H9N2 AIV infections usually cause mild respiratory symptoms and egg production decline; however, when occurring concurrently with other pathogens such as Escherichia coli it can lead to severe infection and moderate mortality in chicken populations [3,18,24]. The wide circulation of H9N2 viruses has caused tremendous economic loss in the poultry industry worldwide [35]. Some H9N2 viruses have acquired receptor binding characteristics typical of human strains (2,6-NeuAcGal) and affected humans occasionally. In addition, H9N2 AIVs may be significant donors of genetic material to emerging human pathogens. Previous studies have demonstrated that H9N2 viruses contributed the internal genes to the human H5N1, H7N9, and H10N8 viruses [10,12,20]. Therefore, the persistence of the H9N2 virus is significant for poultry industries and public health.
Vaccination is the major measure used to control infection by H9N2 AIV in China. Vaccines have been widely applied in chicken flocks since the late 1990s. However, commercial vaccines cannot provide complete protection to prevent the infection of endemic strains [29], and serious diseases caused by H9 AIVs infection in vaccinated chicken flocks have been reported continually in China [4,5]. A previous study showed that the vaccine strains and pandemic H9N2 strains belong to different lineages. The present paper describes the isolation of new emerging H9N2 subtype AIVs from chickens between 2012 and 2013 in Guangdong province, South China. Phylogenetic analysis and molecular characterization of the HA gene of the isolates were conducted to provide useful guidance for the control of H9 AIV and vaccine candidate selection.
Materials and Methods
Viruses
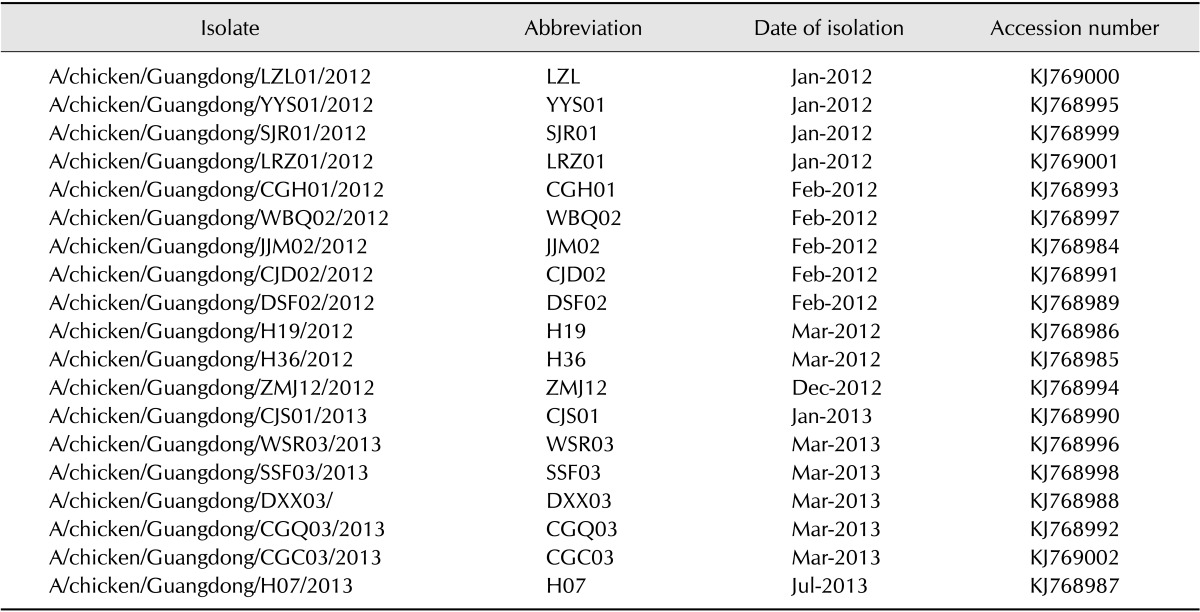
Between 2012 and 2013, 19 H9N2 subtype avian influenza viruses were isolated from vaccinated chicken flocks that had been administered A/chicken/Guangdong/SS/94 (H9N2) (SS/94) strain inactivated vaccine in Guangdong province, South China (Table 1). The sick chickens showed typical respiratory illness and moderate mortality. Tracheal swabs and clinical samples (including trachea, spleen, kidney, liver, and other tissue samples) were collected from dead or diseased chickens. Swabs and tissue suspensions prepared in phosphate-buffered saline were filtered through 0.22 µm filters and inoculated into 10-day-old specific pathogen free (SPF) chicken embryos, after which allantoic fluid containing the propagated virus was harvested for further analysis. Hemagglutination inhibition (HI) and neuraminidase inhibition (NI) tests were conducted to identify the virus subtypes using antisera specific to the reference strains of influenza viruses.
Table 1. H9N2 avian influenza viruses (AIVs) isolated from chickens in this study.
Reverse transcription polymerase chain reaction (RT-PCR), cloning and sequencing
Total RNA was extracted using TRIzol reagent (Invitrogen, USA) according to the manufacturer's instructions, then amplified by one-step RT-PCR with a PrimeScript One-Step RT-PCR Kit (Takara Bio, China) as previously described [4]. The specific primer set designed for amplification of the H9N2 HA gene was HA-F: 5'-CAAGATGGAAGTAGTATCACT-3' and HA-R: 5'-TTGCCAATTATATACAAATGT-3'. RT-PCR was conducted by subjecting the samples to 50℃ for 30 min, 94℃ for 2 min and then 30 cycles of 94℃ for 40 sec, 53℃ for 40 sec and 72℃ for 2 min, followed by final extension at 72℃ for 10 min. PCR products were purified using an Axygen AxyPrep DNA Gel Extraction Kit (Corning, USA) according to the manufacturer's instructions, after which the product was cloned into pMD-19T vector (Takara Bio) for sequencing. The purified recombinant plasmids were sequenced by Invitrogen Trading (Shanghai).
Genetic and phylogenetic analysis
Multiple sequence alignments were performed by the MUSCLE program using the MEGA5.05 software. The phylogenetic tree was constructed using the MEGA5.05 software and the neighbor-joining method and bootstrap values were calculated from 1,000 replications [7]. The potential N-glycosylation sites (PGS) of deduced amino acid sequences of HA genes were predicted using the Center for Biological Sequence Analysis online server 1.0 [14].
Antigenic analysis
Antigenic analysis was performed by HI tests to evaluate the antigenic relationships between the emerging viruses and vaccine strain SS/94. Polyclonal antibodies against eight randomly selected isolates of H9 subtype AIVs (four in h9.4.2.5 and others in h9.4.2.6) were generated using six week old SPF chickens. Each chicken was injected with 1 mL of oil emulsion-inactivated vaccine derived from the eight tested viruses and SS/94. The sera were collected on day 21 after injection. HI tests were performed as previously described [8].
Results
Homology analysis
The coding sequences of the 19 viral HA genes contained 1683 nucleotides. There were no nucleotide insertions or deletions. Homology analysis was performed to compare the nucleotide sequences of surface protein genes of the tested viruses with H9N2 representative strains, as well as with the vaccine strains China SS/94, A/chicken/Shandong/6/96 (6/96), and A/chicken/Shanghai/F/98 (F/98). The result showed that the HA gene nucleotide sequence and deduced amino acid sequence identities among the isolates ranged from 87.4 to 99.3% and 91.4 to 99.5%, respectively. The HA genes of these isolates were 90.5 to 92.8% identical to the representative virus, A/duck/Hong Kong/Y280/1997 (Y280), at the amino acid level, indicating that they belong phylogenetically to the Y280-like lineage. When compared with the three H9N2 vaccine strains, the nucleotide sequence and deduced amino acid sequence similarities among the 19 isolates ranged from 89.6% to 92.0% and 91.1% to 94.5%, respectively. These results indicated that most of the isolates are genetically distant from the vaccine strains.
Phylogenetic analysis
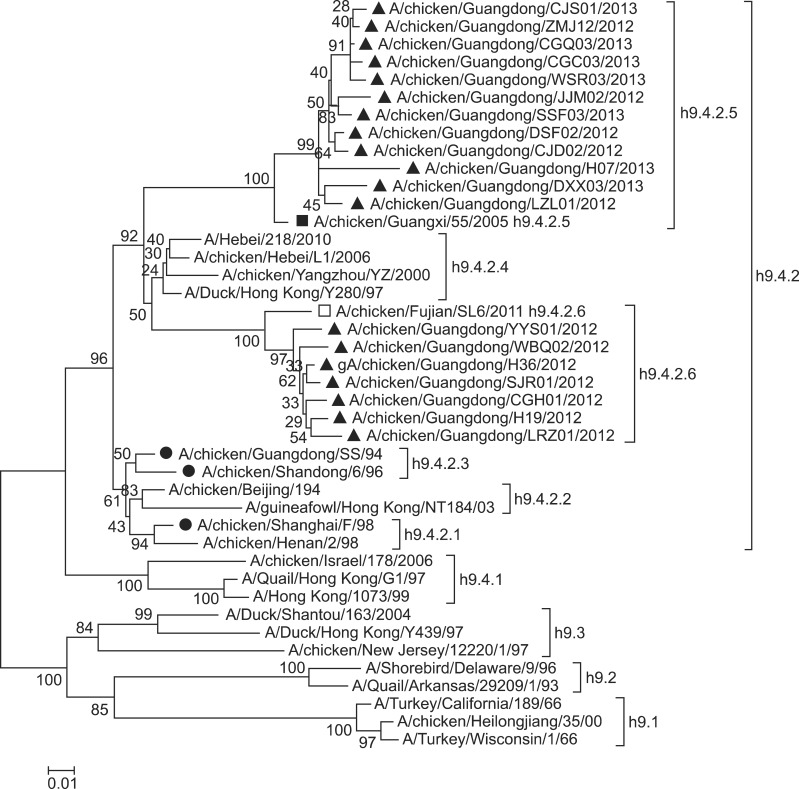
Phylogenetic analysis based on the HA genes showed that the 19 H9N2 strains isolated in the present study belonged to lineage h9.4.2 represented by Y280 or BJ194 (Fig. 1), which has been the predominant strain in China in recent years. In the tertiary lineages, the19 isolates were clustered into two lineages. Seven isolates fell into lineage h9.4.2.6, which was isolated from January to March, 2012. The other 12 isolates, which were isolated from January, 2012 to July, 2013, belonged to lineage h9.4.2.5. Notably, by the end of 2012 few of lineage h9.4.2.6 viruses were detected in flocks, and the lineage h9.4.2.5 viruses became predominant in Guangdong province. Vaccine strains SS/94 and 6/96 were clustered in lineage h9.4.2.3, and strain F/98 belonged to h9.4.2.1. All of these strains had a very different genetic distance from the newly emerging h9.4.2.5 viruses (Fig. 1).
Fig. 1. Phylogenetic tree of H9 avian influenza viruses isolated in Guangdong from 2012 to 2013 based on the viral HA gene sequences. The isolates from Guangdong were marked with squares and the vaccine strains with circles. Solid and hollow squares indicate reference to lineage h9.4.2.5 and h9.4.2.6, respectively. The second and tertiary lineages of the viruses referred to previous nomenclature systems [16,21].
Critical site analysis of deduced amino acid sequences
The cleavage sites of precursor HA protein of the H9N2 isolates showed various motifs. The lineage h9.4.2.5 viruses had the cleavage site motif PSRSSR↓GLF, while all of the lineage h9.4.2.6 viruses (except strain CGH01) harbored PAKSSR↓GLF at the cleavage sites. The remaining lineage had the motif PARSSR↓GLF (Table 2). These two motifs differed from those of vaccine strain SS/94, which contained the PAGSSR↓GLF motif at the cleavage sites. There were no groups of basic residues at the cleavage sites.
Table 2. Receptor-binding pocket, cleavage site and antigenic site of H9 subtype AIV isolates.
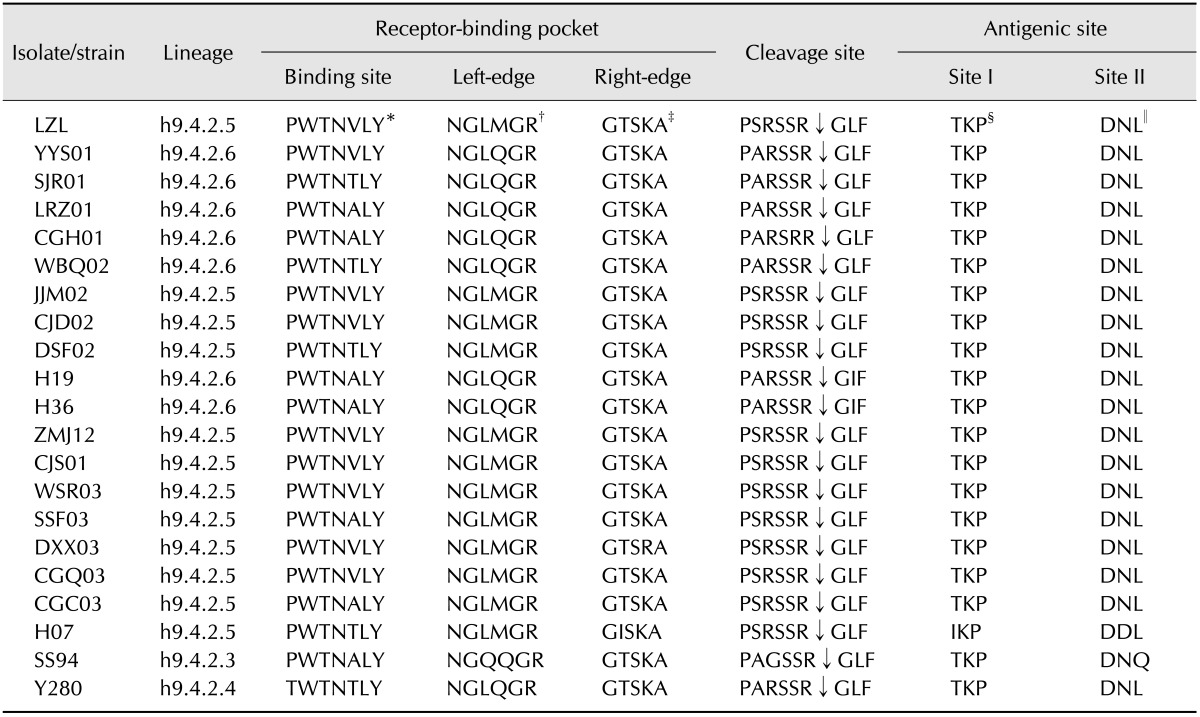
*Amino acid residues at position 98 (H3 numbering), 153, 155, 183, 190, 194, and 195, respectively. †Amino acid residues at position 224-229. ‡Amino acid residues at position 134-138. §Amino acid residues at position 135, 157, and 162, respectively. ∥Amino acid residues at position 145, 193, and 226, respectively.
The amino acid residues at receptor binding site (RBS) of the HA protein of the 19 isolates detected in this study were conserved except for those at position 190, which had a V190A (or T) substitution (Table 2). On the left-edge of the receptor-binding pocket, lineage h9.4.2.5 isolates had methionine (M) at position 227, while lineage h9.4.2.6 viruses and the vaccine strain SS/94 had glutamine (Q) at the same position. In addition, all 19 isolates had leucine (L) at position 226, which showed that these viruses preferentially bind to NeuAca2,6-Gal linkage. The amino acids in the antigenic sites of most (18/19) isolates were quite well conserved, and only two substitutions (T135I and N193D) were found in isolate H07.
Potential N-glycosylation sites (PGS) of HAs were predicted using the Center for Biological Sequence Analysis online server 1.0. The results showed that there were seven PGSs (positions 11-13, 123-125, 280-282, 287-289, and 295-297 in HA1, and positions 154-156 and 213-215 in HA2) of the lineage h9.4.2.5 viruses (Table 3). An exception was found in the LZL01 strain, which carried an additional PGS at position 200-202 in HA1 (Table 3). When compared with the lineage h9.4.2.5 viruses, the lineage h9.4.2.6 viruses lacked PGS at position 295-297 and had an additional PGS at position 200-202 in HA1, as well as seven PGSs in the HA protein. Vaccine strain SS/94 was similar to lineage h9.4.2.6 viruses that lacked PGS at position 295-297 when compared with lineage h9.4.2.5 viruses. An exception was virus SJR01, which lost one PGS at 200-202.
Table 3. Potential N-glycosylation sites analysis of HA amino acid sequences of H9 AIV isolates.
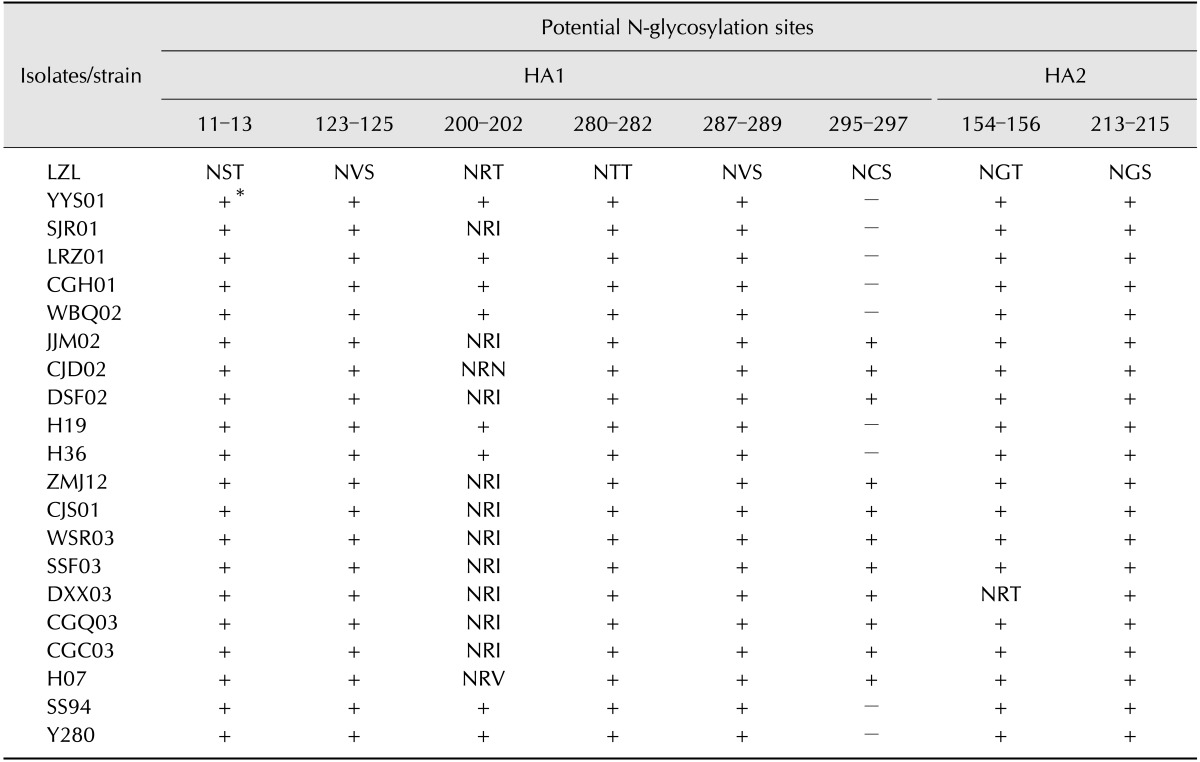
∥Positive (+) indicates the same as A/chicken/Guangdong/LZL01/2012. ∥Negative (-) indicates the absence of N-glycosylation sites.
Antigenic analysis
To evaluate the antigenic relationship between the emerging viruses and current commercial vaccine strain, eight viruses and vaccine strain SS/94 were investigated with antiserum by cross HI tests. As shown in Table 4, antisera against h9.4.2.5 viruses CJS01 and JJM02 reacted well with the emerging viruses (HI tier ≥ 1280), and the four antisera against the h9.4.2.6 viruses reacted well with the emerging viruses (HI tier ≥ 1280), but none of the antisera against emerging viruses reacted well with the SS/94. Moreover, the SS/94 antisera showed low HI titer (≤ 320) against the emerging H9N2 viruses. These results suggest that the SS/94 strain was antigenic different when compared to the H9N2 viruses circulating in Guangdong Province.
Table 4. Homologous and heterogeneous hemagglutination inhibition titers of some H9 isolates.
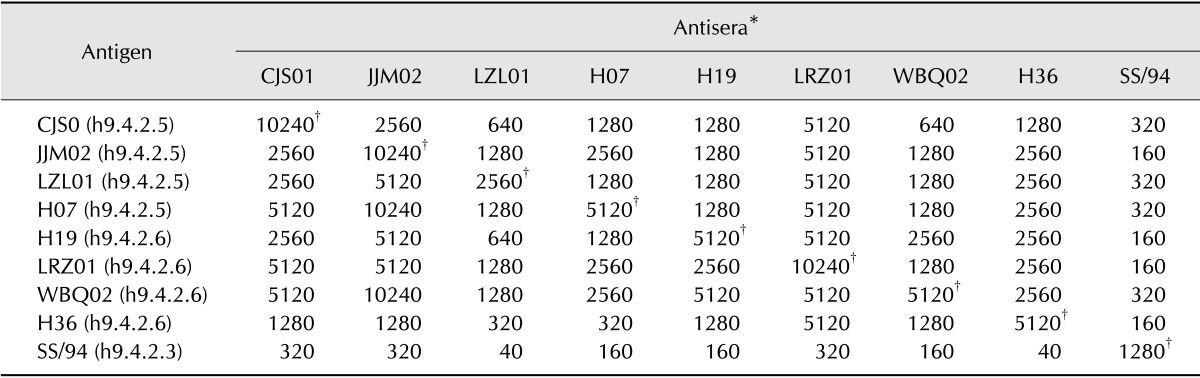
*Antisera were diluted tenfold. †Homologous titers are shown.
Discussion
H9N2 subtype virus can infect various poultry species for long periods of time without showing symptoms. When this subtype co-infects with other subtypes, such as H5N1 and H7N9, new subtypes of viruses might emerge [36]. Vaccination is considered to be a cost-effective measure for avian influenza control compared to stamping-out policy [19]. In China, inactivated H9N2 vaccines have been use since the 1990s. However, in the present study, 19 H9N2 strains were isolated from vaccinated chicken flocks in Guangdong during 2012-2013, which revealed that the vaccine did not provide complete protection against infection.
Previous studies have reported that the prevalent strain in China belonged to clade h9.4.2 [4,5,16,17,21]. Jiang et al. [17] studied the phylogenic relationships of the HA gene of H9 AIV that circulated in China from 2008 to 2011, and showed that lineage h9.4.2.5 and emerging lineage h9.4.2.6 were dominant. The emerging lineage h9.4.2.6 emerged first in southern China in 2010 and subsequently extended to northern China [5,17]. Phylogenic analysis of this study showed that lineage h9.4.2.5 and h9.4.2.6 were the predominant clades in Guangdong province from 2012 to 2013. Notably, all viruses isolated in 2013 belonged to lineage h9.4.2.5, while no h9.4.2.6 virus was detected, indicating that H9N2 viruses circulated persistently in domestic chickens and were genetically diverse. Large numbers of chicken are transported to Guangdong province from other areas, which may explain the genetic diversity of H9 AIV in Guangdong.
Glycosylation of viral envelope proteins, which is responsible for viral immune evasion and persistence, is used by several enveloped viruses to escape, block, or minimize the virus-neutralizing antibody response [1]. Generally, H9N2 AIVs vary in the number of PGSs on HA; however, no single specific glycosylation site has been found to correspond to adaptation to land-based poultry [25]. The lineage h9.4.2.5 of the H9 subtype AIVs isolated from 2010 to 2012 usually contain eight PGSs in the HA [5]. In this study, most isolates (11/12) of lineage h9.4.2.5 lost one PGS at residues 200-202 in HA1, causing by the substitution of threonine (T) to another residue. The loss of PGS in HA has been reported to increase the affinity to human-like receptors and virulence in mice in the H5N1 and H1N1 virus, respectively [26,28]. Accordingly, further study to determine whether the loss of PGS appeared in the glucoprotein of the new emerging H9N2 virus enhanced the pathogenicity is warranted.
The receptor specificity of HA is considered to be a factor of host range restriction of influenza viruses [31]. Human-like influenza with L226 (H3 numbering) at HA protein preferentially binds to the α-2,6 sialyl glycan of the receptor, whereas avian-like influenza with Q (glutamine) 226 prefer to bind to α-2,3 sialyl glycan [27,34]. Viruses with mutation at position 226 in HA RBS have the potential to adapt to new hosts [23]. Substitution of Q226L and G228S at HA in H5N1 has been shown to change the viral receptor binding specificity from binding avian-like receptor to human-like receptor [6,22]. In the present study, we found that all isolates in Guangdong province had 226 L from 2012 to 2013, indicating that they had the ability to cross species to infect humans. No G228S was found in this study. Amino acid at position 341 (H3 numbering) in combination with short stalk NA was reported to increase HA cleavage efficiency [30]. All isolates of lineage h9.4.2.5 in this study contained serine (S), whereas lineage h9.4.2.6 contained alanine at this position.
Vaccination is the predominant strategy to prevent and control H9N2 virus. Vaccine strain SS/94 was used to control H9N2 virus in Guangdong province. Antigenic analysis showed that the emerging viruses circulating in Guangdong were antigenically distinct from SS/94. Accordingly, H9N2 vaccine candidates from current circulating strains that reacted well with the emerging strains should be used to update the H9N2 vaccine in the Guangdong area.
In the present study, we found that H9N2 AIVs circulate continually in chicken flocks in Guangdong province. The results also showed that the lineage h9.4.2.5 viruses losing one PGS at position 200-202 would become the prevalent strains in this area. In addition, genetic and phylogenetic analysis results indicated that the prevalent strains and vaccine strain belonged to different clades and are only distantly related. Furthermore, all of the isolates contain human-like receptor and might have the potential to cross species to infect humans. Accordingly, it is recommended that continual surveillance of the circulation of H9N2 in Guangdong and Update vaccine be implemented to prevent and control H9N2 AIV.
Acknowledgments
This work was supported by the Natural Science Foundation of Guangdong Province (grant no. S2013030013313) and the Technology Planning Project of Guangdong Province of China (grant nos. 2012B020306002 and 2012B091100078).
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Ansari IH, Kwon B, Osorio FA, Pattnaik AK. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J Virol. 2006;80:3994–4004. doi: 10.1128/JVI.80.8.3994-4004.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker AT, Varghese JN, Laver WG, Air GM, Colman PM. Three-dimensional structure of neuraminidase of subtype N9 from an avian influenza virus. Proteins. 1987;2:111–117. doi: 10.1002/prot.340020205. [DOI] [PubMed] [Google Scholar]
- 3.Brown IH, Banks J, Manvell RJ, Essen SC, Shell W, Slomka M, Londt B, Alexander DJ. Recent epidemiology and ecology of influenza A viruses in avian species in Europe and the Middle East. Dev Biol (Basel) 2006;124:45–50. [PubMed] [Google Scholar]
- 4.Chen F, Yan ZQ, Liu J, Ji J, Chang S, Liu D, Qin JP, Ma JY, Bi YZ, Xie QM. Phylogenetic analysis of hemagglutinin genes of 40 H9N2 subtype avian influenza viruses isolated from poultry in China from 2010 to 2011. Virus Genes. 2012;45:69–75. doi: 10.1007/s11262-012-0742-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen RA, Lai HZ, Li L, Liu YP, Pan WL, Zhang WY, Xu JH, He DS, Tang ZX. Genetic variation and phylogenetic analysis of hemagglutinin genes of H9 avian influenza viruses isolated in China during 2010-2012. Vet Microbiol. 2013;165:312–318. doi: 10.1016/j.vetmic.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Chutinimitkul S, van Riel D, Munster VJ, van den Brand JMA, Rimmelzwaan GF, Kuiken T, Osterhaus ADME, Fouchier RAM, de Wit E. In vitro assessment of attachment pattern and replication efficiency of H5N1 influenza A viruses with altered receptor specificity. J Virol. 2010;84:6825–6833. doi: 10.1128/JVI.02737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards S. OIE laboratory standards for avian influenza. Dev Biol (Basel) 2006;124:159–162. [PubMed] [Google Scholar]
- 9.Fouchier RAM, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwaan GF, Olsen B, Osterhaus ADME. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005;79:2814–2822. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Zhang Y, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 11.Ge FF, Zhou JP, Liu J, Wang J, Zhang WY, Sheng LP, Xu F, Ju HB, Sun QY, Liu PH. Genetic evolution of H9 subtype influenza viruses from live poultry markets in Shanghai, China. J Clin Microbiol. 2009;47:3294–3300. doi: 10.1128/JCM.00355-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan Y, Shortridge KF, Krauss S, Chin PS, Dyrting KC, Ellis TM, Webster RG, Peiris M. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J Virol. 2000;74:9372–9380. doi: 10.1128/jvi.74.20.9372-9380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo YJ, Krauss S, Senne DA, Mo IP, Lo KS, Xiong XP, Norwood M, Shortridge KF, Webster RG, Guan Y. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology. 2000;267:279–288. doi: 10.1006/viro.1999.0115. [DOI] [PubMed] [Google Scholar]
- 14.Gupta R, Brunak S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac Symp Biocomput. 2002:310–322. [PubMed] [Google Scholar]
- 15.Homme PJ, Easterday BC. Avian influenza virus infections. I. Characteristics of influenza A-turkey-Wisconsin-1966 virus. Avian Dis. 1970;14:66–74. [PubMed] [Google Scholar]
- 16.Ji K, Jiang WM, Liu S, Chen JM, Chen J, Hou GY, Li JP, Huang BX. Characterization of the hemagglutinin gene of subtype H9 avian influenza viruses isolated in 2007-2009 in China. J Virol Methods. 2010;163:186–189. doi: 10.1016/j.jviromet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Jiang W, Liu S, Hou G, Li J, Zhuang Q, Wang S, Zhang P, Chen J. Chinese and global distribution of H9 subtype avian influenza viruses. PLoS One. 2012;7:e52671. doi: 10.1371/journal.pone.0052671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishida N, Sakoda Y, Eto M, Sunaga Y, Kida H. Co-infection of Staphylococcus aureus or Haemophilus paragallinarum exacerbates H9N2 influenza A virus infection in chickens. Arch Virol. 2004;149:2095–2104. doi: 10.1007/s00705-004-0372-1. [DOI] [PubMed] [Google Scholar]
- 19.Lee CW, Senne DA, Suarez DL. Effect of vaccine use in the evolution of Mexican lineage H5N2 avian influenza virus. J Virol. 2004;78:8372–8381. doi: 10.1128/JVI.78.15.8372-8381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin YP, Shaw M, Gregory V, Cameron K, Lim W, Klimov A, Subbarao K, Guan Y, Krauss S, Shortridge K, Webster R, Cox N, Hay A. Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc Natl Acad Sci U S A. 2000;97:9654–9658. doi: 10.1073/pnas.160270697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Liu X, Cheng J, Peng D, Jia L, Huang Y. Phylogenetic analysis of the hemagglutinin genes of twenty-six avian influenza viruses of subtype H9N2 isolated from chickens in China during 1996-2001. Avian Dis. 2003;47:116–127. doi: 10.1637/0005-2086(2003)047[0116:PAOTHG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Maines TR, Chen LM, Van Hoeven N, Tumpey TM, Blixt O, Belser JA, Gustin KM, Pearce MB, Pappas C, Stevens J, Cox NJ, Paulson JC, Raman R, Sasisekharan R, Katz JM, Donis RO. Effect of receptor binding domain mutations on receptor binding and transmissibility of avian influenza H5N1 viruses. Virology. 2011;413:139–147. doi: 10.1016/j.virol.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matrosovich M, Tuzikov A, Bovin N, Gambaryan A, Klimov A, Castrucci MR, Donatelli I, Kawaoka Y. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J Virol. 2000;74:8502–8512. doi: 10.1128/jvi.74.18.8502-8512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nili H, Asasi K. Natural cases and an experimental study of H9N2 avian influenza in commercial broiler chickens of Iran. Avian Pathol. 2002;31:247–252. doi: 10.1080/03079450220136567. [DOI] [PubMed] [Google Scholar]
- 25.Perez DR, Lim W, Seiler JP, Yi G, Peiris M, Shortridge KF, Webster RG. Role of quail in the interspecies transmission of H9 influenza A viruses: molecular changes on HA that correspond to adaptation from ducks to chickens. J Virol. 2003;77:3148–3156. doi: 10.1128/JVI.77.5.3148-3156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reading PC, Pickett DL, Tate MD, Whitney PG, Job ER, Brooks AG. Loss of a single N-linked glycan from the hemagglutinin of influenza virus is associated with resistance to collectins and increased virulence in mice. Respir Res. 2009;10:117. doi: 10.1186/1465-9921-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers GN, Paulson JC, Daniels RS, Skehel JJ, Wilson IA, Wiley DC. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983;304:76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- 28.Sun X, Jayaraman A, Maniprasad P, Raman R, Houser KV, Pappas C, Zeng H, Sasisekharan R, Katz JM, Tumpey TM. N-linked glycosylation of the hemagglutinin protein influences virulence and antigenicity of the 1918 pandemic and seasonal H1N1 influenza A viruses. J Virol. 2013;87:8756–8766. doi: 10.1128/JVI.00593-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y, Pu J, Fan L, Sun H, Wang J, Zhang Y, Liu L, Liu J. Evaluation of the protective efficacy of a commercial vaccine against different antigenic groups of H9N2 influenza viruses in chickens. Vet Microbiol. 2012;156:193–199. doi: 10.1016/j.vetmic.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Tan Y, Wei K, Sun H, Shi Y, Pu J, Yang H, Gao GF, Yin Y, Feng W, Perez DR, Liu J. Amino acid 316 of hemagglutinin and the neuraminidase stalk length influence virulence of H9N2 influenza virus in chickens and mice. J Virol. 2013;87:2963–2968. doi: 10.1128/JVI.02688-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki Y. Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biol Pharm Bull. 2005;28:399–408. doi: 10.1248/bpb.28.399. [DOI] [PubMed] [Google Scholar]
- 32.Tong S, Li Y, Rivailler P, Conrardy C, Castillo DAA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, Turmelle AS, Moran D, Rogers S, Shi M, Tao Y, Weil MR, Tang K, Rowe LA, Sammons S, Xu X, Frace M, Lindblade KA, Cox NJ, Anderson LJ, Rupprecht CE, Donis RO. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A. 2012;109:4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Johnson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013;9:e1003657. doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weis W, Brown JH, Cusack S, Paulson JC, Skehel JJ, Wiley DC. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988;333:426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- 35.Xu KM, Smith GJ, Bahl J, Duan L, Tai H, Vijaykrishna D, Wang J, Zhang JX, Li KS, Fan XH, Webster RG, Chen H, Peiris JS, Guan Y. The genesis and evolution of H9N2 influenza viruses in poultry from southern China, 2000 to 2005. J Virol. 2007;81:10389–10401. doi: 10.1128/JVI.00979-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao G, Gu X, Lu X, Pan J, Duan Z, Zhao K, Gu M, Liu Q, He L, Chen J, Ge S, Wang Y, Chen S, Wang X, Peng D, Wan H, Liu X. Novel reassortant highly pathogenic H5N2 avian influenza viruses in poultry in China. PLoS One. 2012;7:e46183. doi: 10.1371/journal.pone.0046183. [DOI] [PMC free article] [PubMed] [Google Scholar]