Abstract
The bursa of Fabricius (BF) is the acknowledged central humoral immune organ in birds. Bursal septpeptide II (BSP-II) is an immunomodulatory bioactive peptide isolated from BF. To understand the effects of BSP-II on immune induction, gene expression profiles of hybridoma cells treated with BSP-II were evaluated. Pathway analysis showed that regulated genes were involved in cytokine-cytokine receptor interactions, T cell receptor signaling pathway, and pathway in cancer. It was observed that BSP-II reduced tumor cells proliferation and stimulated p53 expression. These results indicate potential mechanisms underlying the effects of the humoral immune system on immune induction, including antitumor activities. Our study has provided a novel insight into immunotherapeutic strategies for treating human tumors.
Keywords: bursal septpeptide II, humoral immune system, pathway analysis, tumor suppressor p53
Introduction
In mammals, an organ responsible for B cell differentiation equivalent to the thymus (where T cell differentiation occurs) has not been identified [1,6]. The bursa of Fabricius (BF) is critical for antibody production in birds, and has characteristics that can help delineate the pathways of B cell development in humans or rodent models [13,16,18]. Thus, BF can serve as an invaluable model for studies on the basic mammalian immunology.
It has been reported that various biologically active peptides are present in BF, such as bursin, that selectively induce the development of avian B cells, but not T cells, from their precursors in vitro [1,5]. Bursal septpeptide I (BSP-I) and bursal pentapeptide I (BPP-I) have antiproliferative effects on tumor cells, initiate tumor suppressor p53 expression, and enhance different types of immune responses [7,9]. However, the molecular basis of bursal-derived peptides on immune induction and antitumor activity is unclear.
We recently reported that bursal-derived immunomodulatory BSP-II promotes antibody production and cellular-mediated immune responses [8]. To further elucidate the mechanism of BSP-II activity, gene profiles altered by BSP-II treatment were examined using a microarray. Pathway and gene ontology analyses revealed the potential molecular mechanisms underlying the effects of BSP-II. Furthermore, it was observed that BSP-II reduced tumor cell proliferation and stimulated p53 expression.
Materials and Methods
Cell lines
Hybridoma cells (1H5F9 strain, IgG1 κ subtype) were cultured with Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 20% (v/v) heat-inactivated fetal bovine serum (FBS; Invitrogen, USA) at 37℃ with 5% CO2. MCF-7 (3111C0001CCC000013) and HeLa (TCHu 19) tumor cells along with normal Vero (GNO10), PK15 (3115CNCB00260), MDBK (GNO7), and CEF (prepared from avian 9-day-old specific pathogen-free [SPF] embryos) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) FBS at 37℃ with 5% CO2.
Treatment of hybridoma cells
Hybridoma cells (105 cells/mL) were incubated with or without BSP-II (from 0.01 µg/mL to 5 µg/mL) for 48 h. Cell viability was measured with a 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay (Sigma, USA). Antibody concentration in the culture supernatant was measured as previously described [9] using ELISA plates coated with 10 µg/mL JEV antigen.
Microarray profiles of BSP-II-treated hybridoma cells
Hybridoma cells were treated with or without 5 µg/mL BSP-II for 4 h. Three independently generated populations of cells were used for these experiments. In brief, total RNA was harvested using TRIzol (Invitrogen) and an RNeasy kit (Qiagen, Germany) according to the manufacturers' instructions. After the isolated RNA was quantified and underwent denaturing gel electrophoresis, the samples were amplified, labeled, and hybridized to a microarray (no. 14868; Agilent Technologies, USA). The microarray data reported herein have been deposited at the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (NCBI, USA) under the accession no. GSE27340.
Semi-quantitative RT-PCR analysis
Hybridoma cells were treated with BSP-II for 4 h. Semi-quantitative RT-PCR was performed using a One-Step SYBR PrimeScript kit (Takara Bio, China) following the manufacturer's protocols with Ms4a2, Cd3d, Fgf21, Cd80, Ptprc, Nfatc4, IL2rb, Fas, and Lat.
Treatment of cell lines with BSP-II
MCF-7 and HeLa cells were added to 96-well flat-bottomed microtiter plates (2 × 105 cells/mL), and incubated with or without BSP-II (from 5 µg/mL to 100 µg/mL). In addition, MDBK, PK15, Vero, and CEF cells were treated with or without BSP-II at concentrations ranging from 0.4 µg/mL to 100 µg/mL. After 48 h, cell proliferation was measured using an MTT-based method.
Cell transfection and in vitro luciferase assay
Vero cells were transfected with a p53-luciferase (p53-Luc) reporter plasmid encoding 14 tandem repeats of the p53 consensus binding sites (Stratagene, USA) according to the manufacturer's instructions. After 24 h, the transfected cells were treated with or without BSP-II (from 0.2 µg/mL to 20 µg/mL) for approximately 24 h. Luciferase activity was measured according to the manufacturer's protocol. Vero cells treated with doxorubicin (Dox; Sigma), which has been reported to induce p53 expression [10], were used as the positive control. Additionally, Vero cells were transfected for 16 h to 24 h, treated with 20 µM α-pifithrin for 2 h, and then stimulated with or without BSP-II. Luciferase activity was then assayed 22 h later.
Western blot analysis
Vero cells were treated with or without BSP-II for 24 h. Positive control cells were treated with Dox. Cell proteins were collected and subjected to Western blotting as previously described [14] with anti-p53 monoclonal antibody (DO-1; Santa Cruz Biotechnology, USA), anti-Bax polyclonal antibody (N-20; Santa Cruz Biotechnology), and anti-β-actin monoclonal antibody (AC-15; Sigma).
Statistical analysis
Data were recorded as the mean ± standard deviation (SD). Biochemical and physiological parameters were analyzed using an ANOVA followed by Dunnett's test with SPSS software (IBM, USA) to evaluate differences between groups.
Results
Impact of BSP-II treatment on hybridoma cells
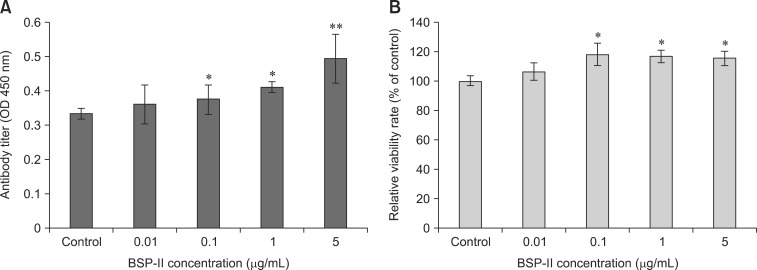
In the current study, it was observed that BSP-II increased antibody production by hybridoma cells in a dose-dependent manner (panel A in Fig. 1). Furthermore, BSP-II significantly induced proliferation of the hybridoma cells (p < 0.05; panel B in Fig. 1).
Fig. 1. The impact of bursal septpeptide II (BSP-II) on hybridoma cells. (A) After incubation with the indicated concentrations of BSP-II for 48 h, concentrations of antibody in secreted into the cell supernatant were measured with ELISA method. (B) Cell viability was also measured with MTT assay. Bars represent the mean ± standard deviation (SD) of three independent experiments. *p < 0.05 and **p < 0.01 compared to the control.
Microarray profiles of BSP-II-treated hybridoma cells
The threshold used to screen for up- or downregulated genes was a fold change ≥ 1.5. Using microarray analysis, 707 upregulated genes and 572 downregulated genes were identified after BSP-II treatment. Additionally, the pathway analysis revealed that five pathways were regulated following BSP-II administration (Table 1).
Table 1. Differentially regulated genes affected by BSP-II treatment.
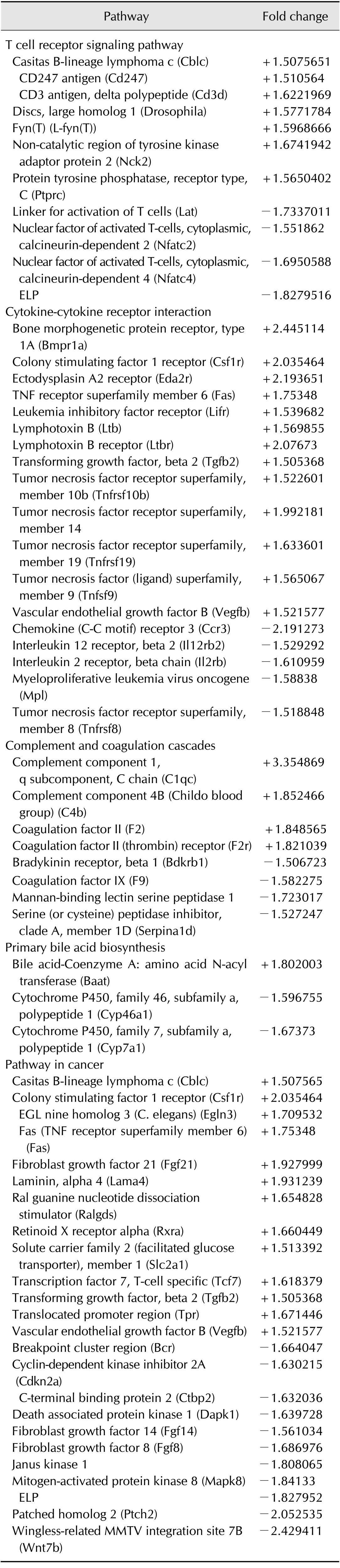
The threshold used to screen for regulated genes was fold change ≥ 1.5. "+/-" represent the appearance of up- (+) or downregulated (-) probe sets.
Pathway analysis of gene expression profiles in BSP-II-treated hybridoma cells-T cell receptor signaling pathway
In this study, various genes whose products are involved in T cell receptor signaling pathway were downregulated in BSP-II-treated hybridoma cells (Table 1), including the linker for activation of T cells (Lat); nuclear factor of activated T cells, calcineurin-dependent, cytoplasmic 2 (Nfatc2) and 4 (Nfatc4); and Plcg1. The expression of Cblc, Cd247, CD3 antigen, Cd3d, Fyn, Nck2, and Ptprc was upregulated by BSP-II administration in the hybridoma cells (Table 1).
Cytokine-cytokine receptor interaction
Various members of the tumor necrosis factor receptor superfamily activated by BSP-II are involved in cytokine-cytokine receptor interaction signaling (Table 1). Expression of TNF receptor superfamily member 6 (Fas), tumor necrosis factor superfamily member 9 (Tnfsf9) which is ligand mediated TNF superfamily, Tnfrsf10b, Tnfrsf19, and member 14 (the herpesvirus entry mediator) was upregulated by BSP-II stimulation. In contrast, Tnfrsf8 was downregulated. Certain interleukin receptors, such as Il12rb2 and Il2rb, were also downregulated by BSP-II. These data indicated that BSP-II, which has been previously reported to function as an immunomodulatory factor in the humoral immune system [8], has diverse effects on cytokine immune responses.
Complement and coagulation cascades
Four complement genes were activated, including complement component 1, q subcomponent, C chain (C1qc); complement component 4B (C4b), coagulation factor II (F2), and coagulation factor II (thrombin) receptor (F2r). Moreover, the expression of bradykinin receptor, beta 1 (Bdkrb1); coagulation factor IX (F9), mannan-binding lectin serine peptidase 1 (Masp1); and serine (or cysteine) peptidase inhibitor, clade A, member 1D (Serpina1d) was inhibited in hybridoma cells by BSP-II treatment (Table 1).
Pathway in cancer
Among the genes regulated by BSP-II according to the microarray analysis, the most dramatically affected were ones involved in pathway in cancer. Prominent among this group of activated genes were Csf1r, fibroblast growth factor 21 (Fgf21); and laminin, alpha 4 (Lama4). On the other hand, the expression of Janus kinase 1 (Jak1), mitogen-activated protein kinase 8 (Mapk8), patched homolog 2 (Ptch2), and wingless-related mouse mammary tumor virus (MMTV) integration site 7B (Wnt7b) was repressed after BSP-II exposure (Table 1). Furthermore, Cblc and transcription factor 7, T-cell specific (Tcf7) were upregulated by BSP-II treatment, indicating a link between the avian humoral immune system and tumor development. The expression of cyclin-dependent kinase inhibitor 2A (Cdkn2a) and death associated protein kinase 1 (Dapk1) was downregulated. Additionally, there were various fibroblast growth factors, including down-regulated fibroblast growth factor 14 (Fgf14) and Fgf8, and up-regulated Fgf21 and Vegfb. Ral guanine nucleotide dissociation stimulator (Ralgds) expression was induced after BSP-II treatment.
High-dose BSP-II inhibition of tumor cell proliferation
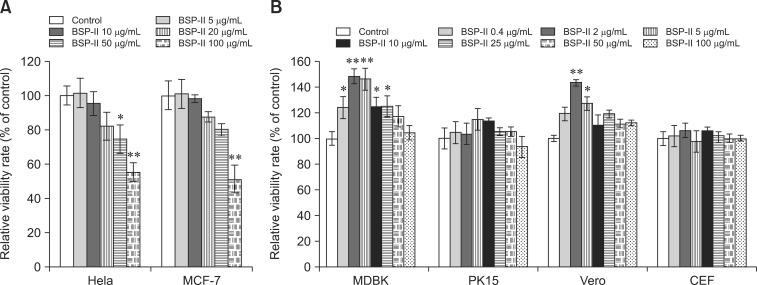
In this study, MCF-7 and HeLa cells were used to investigate the effect of BSP-II at concentrations ranging from 5 µg/mL to 100 µg/mL on tumor cell proliferation. Results of the study showed that BSP-II reduced MCF-7 proliferation at concentrations of 10, 20, 50, and 100 µg/mL in a dose-dependent manner (panel A in Fig. 2). HeLa cell proliferation was decreased by 4.75%, 18.1%, 25.5%, and 44.7% with 10, 20, 50, and 100 µg/mL BSP-II, respectively (panel A in Fig. 2).
Fig. 2. The antiproliferative effect of BSP-II on tumor cells. After 48 h of treatment with BSP-II, viability of tumor (A) and normal (B) cells was evaluated with MTT assay, respectively. Bars represent the mean ± SD of three independent experiments. *p < 0.05 and **p < 0.01 compared to the control.
Furthermore, the effects of BSP-II on the proliferation of four normal cell lines (MDBK, PK15, Vero, and CEF) were evaluated. The results showed that BSP-II increased MDBK and Vero cell viability in a dose-dependent manner (panel B in Fig. 2). However, no significant differences were observed in the viability of BSP-II-treated CEF and PK15 cells compared to the controls (panel B in Fig. 2).
BSP-II enhancement of p53 activity and protein expression
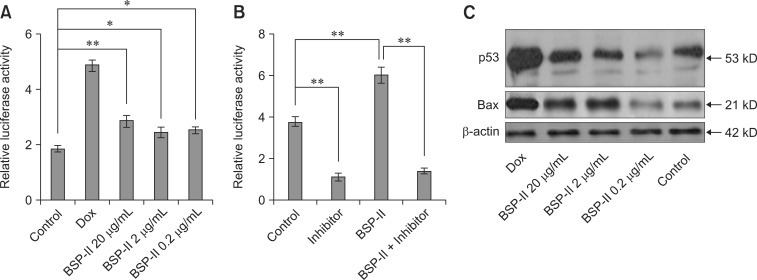
To further understand the antitumor role of BSP-II, the activities of p53 were measured during BSP-II treatment. The results showed that luciferase activity was significantly increased following BSP-II exposure compared to the untreated control (panel A in Fig. 3). However, the mRNA expression of p53 in BSP-II-treated Vero cells was not increased (not shown). To inhibit p53-luciferase activity, transfected Vero cells were treated with a specific p53 inhibitor, 20 µM α-pifithrin, for 2 h before BSP-II administration. The results showed that α-pifithrin significantly inhibited p53-luciferase activity with or without BSP-II (panel B in Fig. 3), suggesting that α-pifithrin might suppress BSP-II-induced p53 luciferase activity.
Fig. 3. p53 transcription and protein expression after BSP-II treatment. Vero cells were transfected with p53 Luc and pRL-TK plasmid, and then cultured with or without BSP-II for 24 h. p53-luciferase activity levels were then measured (A). The transfected Vero cells were also pre-incubated with α-pifithrin for 2 h, incubated with or without BSP-II (2 µg/mL) for 22 h, and the level of p53-luciferase activity was measured (B). Non-transfected Vero cells were cultured with or without BSP-II for 24 h, and Western blotting analysis was performed to detect p53 and Bax protein expressions (C). Transfected or non-transfected Vero cells were treated with Dox as a positive control. Bars represent the mean ± SD of three independent experiments. *p < 0.05 and **p < 0.01 compared to the control. Dox: doxorubicin.
To determine whether the upregulation of p53 expression was induced by BSP-II, p53 protein expression was examined following treatment with BSP-II at different concentrations. An increased level of p53 protein was detected with 20 µg/mL BSP-II (panel C in Fig. 3). However, 0.2 µg/mL BSP-II reduced p53 protein levels by 22%. Expression of the pro-apoptosis molecule Bax was also examined after BSP-II treatment. Western blotting analysis revealed that the level of Bax protein was increased in Vero cells incubated with 20 µg/mL BSP-II (panel C in Fig. 3). However, the Bax protein expression was decreased by 19.5% in the presence of 0.2 µg/mL of BSP-II compared to the control cells.
Discussion
BF is the acknowledged central humoral immune organ in birds. It has been reported that bursal-derived BSP-II has immunological regulatory effects on antibody production, cytokines levels, and T lymphocyte subtype composition [8]. However, the mechanism of BSP-II effects on the immune response is unclear. Therefore, hybridoma cells were used as an immunocyte model in this study to determine the potential molecular basis of BSP-II activity using microarray analysis. The pathway analysis demonstrated that BSP-II regulated the expression of various genes in hybridoma cells, including ones involved in cytokine-cytokine receptor interaction, T cell receptor signaling pathway, complement and coagulation cascades, pathway in cancer, and primary bile acid biosynthesis. These findings demonstrated that BSP-II could induce the activation of different cellular signal pathways, resulting in various immune consequences.
BSP-II is reported to promote the modification of T cell subtypes [8]. In the current study, various genes affected by BSP-II were involved in T cell receptor signaling pathways. Optimal T cell activation requires Ca2+-calcineurin-nuclear factor of activated T cells (NFAT) signaling along with Fyn expression and tyrosine phosphorylation [22]. NFAT expression was downregulated after BSP-II treatment, which might cause a suppression of Ca2+-induced responsiveness in T cells. Lat is a transmembrane adaptor protein that plays an essential role in TCR-mediated signaling and thymocyte development [19]. Interestingly, BSP-II enhanced the expression of Cblc, Fyn, Ptprc, and Nck2 in hybridoma cells. Our current findings suggested that BSP-II might alter T cell responsiveness by necessarily mutual activities of calmodulin and tyrosine phosphatase/kinase-related factors. Therefore, data from our investigation indicated the potential molecular basis of BSP-II-mediated effects on TCR signaling and T cell activation.
It has been reported that BSP-II induces the productions of Th1 type (IFN-γ) and Th2 type (IL-4) cytokines [8], indicating a potential impact of BSP-II on cytokine-related signal activation. In the current study, it was observed that 18 differentially expressed genes after BSP-II treatment were involved in cytokine-cytokine receptor interactions, including various members of the TNF receptor superfamily that participate the regulation of immune and inflammatory responses [3]. T helper (Th) cells are at the center of an important communication loop in which IL-2/IL-2r and bioactive IL-12 play a central role [23]. Regulation of the Interleukin (IL)-12rb2 subunit expression might play a role in developing T helper 1 (Th1) and Th2 cells [21]. It was observed that Il12rb2 was downregulated after BSP-II treatment. These findings indicate potential molecular signals by which BSP-II induced the production of different types of cytokines.
The activation of signaling cascades essential for the immune response is partially achieved through the coordinated activities of complement components and coagulation factors including C1qc, C4b, F2, F2r, and F9. The complement system is an essential component of innate immunity that participates in the pathogenesis of inflammatory diseases and host defense. Therefore, regulation of the immune response through alterations in complement and coagulation signaling caused by BSP-II provides novel insight into factors involved in the avian humoral immune system.
Low-dose BSP-II has been reported to stimulate the proliferation of HeLa and SP2/0 cells [8]. In the current study, it was observed that high doses of BSP-II (from 10 µg/mL to 100 µg/mL) inhibited HeLa and MCF-7 cell proliferation. BSP-II differently regulates the proliferation of cancer and normal cells, suggesting that this factor has potentially selective antiproliferation effects on tumor cells at experimental concentrations. The expression patterns of genes involved in cancer-associated pathways were observed with the microarray analysis. The competitive interaction between tumors and the immune system is very complex [20]. BF is the organ responsible for B cell differentiation in birds [6]. Therefore, data showing the effect of bursal-derived BSP-II on cancer-related pathways in hybridoma cells might give important insight into the relationship between the humoral immune system and antitumor immunity.
One of the most important tumor suppressor proteins, p53, plays a crucial role in the induction of apoptosis following DNA damage [4]. To further understand the antitumor role of BSP-II, the activities of p53 were assessed during BSP-II treatment. Data showed that BSP-II activated p53-luciferase activity, and enhanced p53 and Bax expression. Bax (Bcl-2-associated X protein) is a pro-apoptotic protein [11,15]. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c [17]. The ratio of Bcl-2 to Bax determines cell fate following pro-apoptotic stimulation [12]. Although BSP-II could enhance Bax expression, the effects of BSP-II on Bcl-2 and caspase proteins are unclear, and will be studied in the future. Upregulated levels of DAPK, a Ca2+/calmodulin-regulated serine/threonine kinase that positively regulates intracellular signaling pathways important for various types of apoptotic cell death [2], affected cancer-related pathways after BSP-II treatment. These results suggested that the bursal-derived factor might participate in the tumor development.
In conclusion, our data have provided novel insights into the role of BSP-II. This factor appears to influence potential mechanisms of the humoral immune system and antitumor activities. Findings from this investigation about the molecular cascade that underlies the effects of the humoral immune system may help the development of immunotherapeutic strategies for treating tumors in humans.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation (no. 31302067), Jiangsu Natural Science Foundation (no. BK20130682), Youth Science and Technology Innovation Fund of Nanjing Agricultural University (no. KJ2013023), the National Agriculture Special Research Project for Non-Profit Trades, Ministry of Agriculture, China (no. 200803020), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We are grateful to Dr. Ming Yao (The Shanghai Cancer Institute, China) for kindly providing MCF-7 cells. We also thank KangChen Bio-tech (China) for performing the microarray analysis.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Audhya T, Kroon D, Heavner G, Viamontes G, Goldstein G. Tripeptide Structure of bursin, a selective B-cell-differentiating hormone of the bursa of fabricius. Science. 1986;231:997–999. doi: 10.1126/science.3484838. [DOI] [PubMed] [Google Scholar]
- 2.Bialik S, Kimchi A. The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem. 2006;75:189–200. doi: 10.1146/annurev.biochem.75.103004.142615. [DOI] [PubMed] [Google Scholar]
- 3.Blink SE, Fu YX. IgE regulates T helper cell differentiation through FcαγRIII mediated dendritic cell cytokine modulation. Cell Immunol. 2010;264:54–60. doi: 10.1016/j.cellimm.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehme KA, Blattner C. Regulation of p53: insights into a complex process. Crit Rev Biochem Mol Biol. 2009;44:367–392. doi: 10.3109/10409230903401507. [DOI] [PubMed] [Google Scholar]
- 5.Brand A, Gilmour DG, Goldstein G. Lymphocyte-differentiating hormone of bursa of Fabricius. Science. 1976;193:319–321. doi: 10.1126/science.180600. [DOI] [PubMed] [Google Scholar]
- 6.Davison F, Kaspers B, Schat KA, editors. Avian Immunology. 1st ed. New York: Academic Press; 2008. [Google Scholar]
- 7.Feng X, Liu T, Wang F, Cao R, Zhou B, Zhang Y, Mao X, Chen P, Zhang H. Isolation, antiproliferation on tumor cell and immunomodulatory activity of BSP-I, a novel bursal peptide from chicken humoral immune system. Peptides. 2011;32:1103–1109. doi: 10.1016/j.peptides.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Feng X, Su X, Wang F, Wei J, Wang F, Cao R, Zhou B, Mao X, Zheng Q, Chen P. Isolation and potential immunological characterization of TPSGLVY, a novel bursal septpeptide isolated from the bursa of Fabricius. Peptides. 2010;31:1562–1568. doi: 10.1016/j.peptides.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Feng XL, Liu QT, Cao RB, Zhou B, Wang FQ, Deng WL, Qiu YF, Zhang Y, Ishag H, Ma ZY, Zheng QS, Chen PY. A bursal pentapeptide (BPP-I), a novel bursal-derived peptide, exhibits antiproliferation of tumor cell and immunomodulator activity. Amino Acids. 2012;42:2215–2222. doi: 10.1007/s00726-011-0961-8. [DOI] [PubMed] [Google Scholar]
- 10.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 12.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 13.Pike KA, Baig E, Ratcliffe MJH. The avian B-cell receptor complex: distinct roles of Igα and IgXMLLink_XYZ in B-cell development. Immunol Rev. 2004;197:10–25. doi: 10.1111/j.0105-2896.2004.0111.x. [DOI] [PubMed] [Google Scholar]
- 14.Qiu Y, Shen Y, Li X, Liu Q, Ma Z. Polyclonal antibody to porcine p53 protein: a new tool for studying the p53 pathway in a porcine model. Biochem Biophys Res Commun. 2008;377:151–155. doi: 10.1016/j.bbrc.2008.09.117. [DOI] [PubMed] [Google Scholar]
- 15.Ranger AM, Malynn BA, Korsmeyer SJ. Mouse models of cell death. Nat Genet. 2001;28:113–118. doi: 10.1038/88815. [DOI] [PubMed] [Google Scholar]
- 16.Ratcliffe MJH. Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development. Dev Comp Immunol. 2006;30:101–118. doi: 10.1016/j.dci.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 18.Sayegh CE, Ratcliffe MJH. Perinatal deletion of B cells expressing surface Ig molecules that lack V(D)J-encoded determinants in the bursa of Fabricius is not due to intrafollicular competition. J Immunol. 2000;164:5041–5048. doi: 10.4049/jimmunol.164.10.5041. [DOI] [PubMed] [Google Scholar]
- 19.Shen S, Chuck MI, Zhu M, Fuller DM, Yang CW, Zhang W. The importance of LAT in the activation, homeostasis, and regulatory function of T cells. J Biol Chem. 2010;285:35393–35405. doi: 10.1074/jbc.M110.145052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soloski MJ. Recognition of tumor cells by the innate immune system. Curr Opin Immunol. 2001;13:154–162. doi: 10.1016/s0952-7915(00)00198-9. [DOI] [PubMed] [Google Scholar]
- 21.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan AH, Wong SC, Lam KP. Regulation of mouse inducible costimulator (ICOS) expression by Fyn-NFATc2 and ERK signaling in T cells. J Biol Chem. 2006;281:28666–28678. doi: 10.1074/jbc.M604081200. [DOI] [PubMed] [Google Scholar]
- 23.Wiesel M, Joller N, Ehlert AK, Crouse J, Spörri R, Bachmann MF, Oxenius A. Th cells act via two synergistic pathways to promote antiviral CD8+ T cell responses. J Immunol. 2010;185:5188–5197. doi: 10.4049/jimmunol.1001990. [DOI] [PubMed] [Google Scholar]