Abstract
This study examined the occurrence of Anaplasma spp. and hemoplasma infection in leopard cats, Prionailurus bengalensis euptilurus, in Korea. Twenty-nine biological samples were tested by molecular analysis. Two (6.9%) and eight (27.6%) tested specimens were positive for Anaplasma bovis and hemoplasma infection, respectively. Based on our results, Anaplasma/Ehrlichia spp. and hemoplasma are regularly infecting leopard cat populations of Korea. Considering their endangered status, regular monitoring of infection by arthropod-borne pathogens known to cause clinical symptoms in feline hosts such as Anaplasma/Ehrlichia spp. and hemoplasma would be crucial as part of ongoing conservation efforts.
Keywords: Anaplasma bovis, arthropod-borne pathogens, endangered species, hemoplasma, leopard cats
The leopard cat (Prionailurus bengalensis euptilurus) is one of the few top predator species in Korean wildlife that has been designated as an endangered wildlife species by the Ministry of the Environment, Korea. A recent study reported that many wildlife species in Korea (including raccoon dogs, rodents, water deer and leopard cats) carry tick species known to function as vectors of various tick-borne pathogens [7]. Among tick-borne pathogens, Anaplasma (A.) phagocytophilum, Ehrlichia (E.) canis and hemoplasma are critical agents that can cause clinical symptoms in feline hosts such as thrombocytopenia and severe anemia [14]. In addition, A. phagocytophilum and E. canis are well known for causing human granulocytic anaplasmosis (HGA) and canine ehrlichiosis, respectively [4,9]. Occurrence of Anaplasma spp. and Ehrlichia spp. infection has previously been reported in several Korean wildlife hosts, including water deer, weasels and several species of rodent [8,9]. However, it is not known if these pathogens are infecting leopard cats in Korea. It is essential to understand the prevalence of pathogens that may influence the health status of endangered species as part of ongoing conservation efforts.
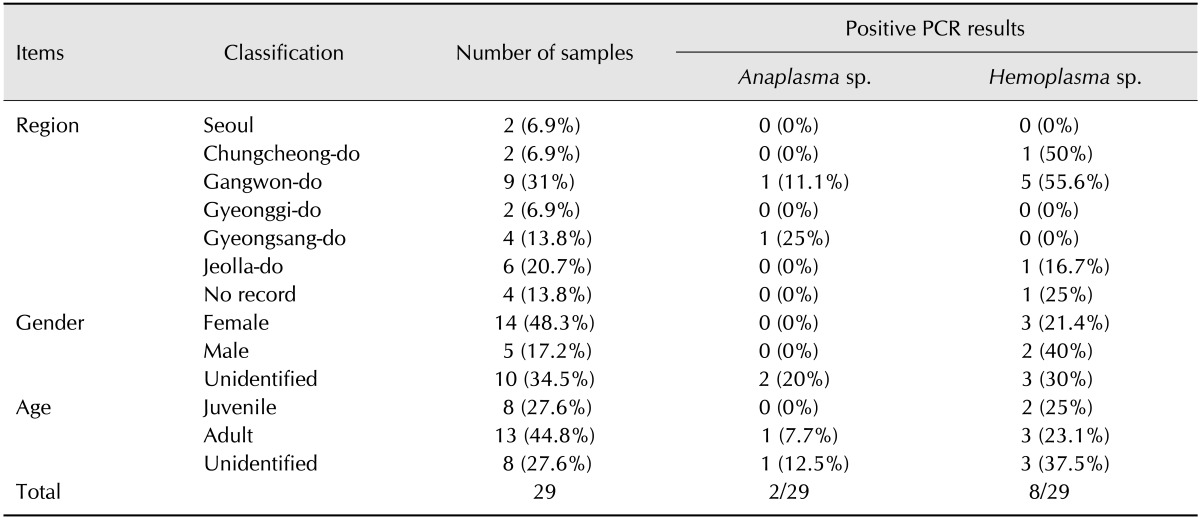
In this study, we used samples of leopard cats provided by the Conservation Genome Resource Bank for Korean Wildlife (CGRB), which maintain archives of biological samples of wildlife collected for research purposes. Sixteen frozen blood samples and 13 frozen spleen tissues samples were collected in 2003 through 2011 from various regions of Korea. Detailed information describing the samples used in this study is provided in Table 1.
Table 1. Leopard cat samples and polymerase chain reaction (PCR) results.
Total DNA was extracted from spleen or blood samples using a DNeasy Blood & Tissue Kit (Qiagen, Germany). Conventional PCR was conducted to amplify the 16S rRNA gene of hemoplasma and Anaplasma/Ehrlichia spp. as previously described [2,4]. The primer set used for hemoplasma detection produces a 595 base pair (bp) amplicon from Mycoplasma (M.) haemofelis and a 618 bp amplicon from M. haemominutum, whereas the size of the amplicon from Anaplasma/Ehrlichia spp. PCR was approximately 431 bp [4]. Amplified products were separated by electrophoresis on a 2% agarose gel, after which positive PCR amplicons were sequenced directly using an ABI prism 3730 DNA sequence analyzer (PE Applied Biosystems, USA) at the National Instrumentation Center for Environmental Management (NICEM) sequencing facility (Seoul, Korea). The homology of the 16S rRNA genes of the positive samples were examined using the Basic Local Alignment Search Tool (BLAST) network service. Additionally, sequences from this study were compared with those available in the GenBank database (National Center for Biotechnology Information, USA) using the ClustalW algorithm followed by analysis with the MEGA 5 program [13]. Phylogenetic trees were constructed using the neighbor-joining method and the data set was resampled 1,000 times to generate bootstrap values.
Overall, two of the 29 samples tested (6.9%) were positive for Anaplasma/Ehlichia spp., while eight (27.6%) were positive for hemoplasma infection. The two samples positive for Anaplasma/Ehlichia spp. originated from Gangwon-do and Gyeongsang-do. The gender of both infected individuals was unidentified, but the sample from Gyeongsang-do was suspected to be from an adult individual. The nucleotide sequence of amplified DNA fragments for Anaplasma/Ehrlichia spp. from both samples showed 100% homology with A. bovis sequences isolated from Japanese wild and domestic animals such as the Hokkaido bear (GenBank accession no. JN811557), domestic dog (HM131218) and raccoons (GU937020) (Fig. 1). The bootstrap values between the A. bovis sequences from GenBank and the two sequences obtained from our study were consistently low, reflecting the high homology among sequences. Sequences of Anaplasma/Ehlichia spp. from this study were deposited in GenBank under accession number KP843893 and KP843894. Amplicons for hemoplasma infection were sequenced for species-level determination.
Fig. 1. Phylogenetic tree of the partial 16S rRNA gene sequences of Anaplasma (A.) bovis isolated from this study (bold font) and other related Anaplasma spp. sequences from the GenBank database. Sequences used for comparison were as follows: A. bovis sequences from a leopard cat (Prionailurus bengalensis euptilurus; AB983439), raccoon (Procyon lotor; GU937020), Hokkaido bear (Ursus arctos lasiotus; JN811557), domestic dog (Canis lupus familiaris; HM131218) and a red deer (Cervus elaphus; KJ659043) in Japan, as well as from a goat (Capra aegagrus hircus; HQ913646) in China and two sequences from Haemaphysalis longicornis in Korea (KC311347, EU181143). In addition, an A. platys sequence from a domestic dog (Canis lupus familiaris; EF139459) in Thailand, as well as A. phagocytophilum sequences from a Korean water deer (Hydropotes inermis argyropus; GU556625) and a domestic dog (EU409557) in Korea and A. centrale and A. marginale from cattle in Japan (AF283007, FJ226454, respectively) were included in the tree. The numbers on the tree indicate bootstrap values for the branch points.
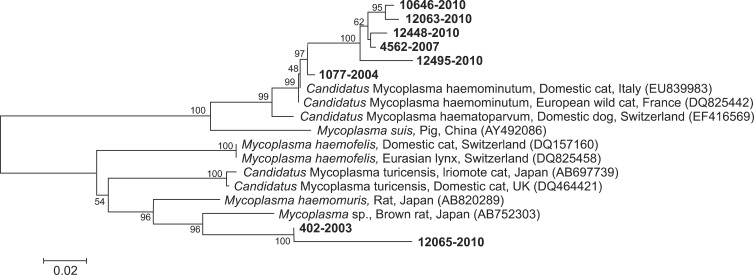
Among eight positive samples for Hemoplasma spp., six showed approximately 99 to 100% similarity to M. haemominutum sequences deposited in GenBank. Sequences with the highest similarity include those isolated from a domestic cat in Italy (EU839983), a European wild cat in France (DQ825442) and a domestic dog in Switzerland (EF416569). Although we attempted to add sequences of M. haemofelis and M. haemominutum from Korea available in GenBank (accession no. EF198144, EF198147), they were too short to be included in the tree (Fig. 2). Four of the six positive animals were from Gangwon-do (three female and an individual of unknown sex), while the other two were from Chungcheong-do (one male) and Jeolla-do region (one individual of unknown sex). Additionally, two of the individuals were identified as adults, while one individual was suspected to be a juvenile. Information about the age of other three animals was unavailable. Six sequences were deposited in GenBank under accession no. KP843885-KP843890. Meanwhile, the sequences of the other two positive for Hemoplasma spp. samples showed 94 to 95% genetic similarity to M. haemomuris reported in brown rats (Rattus norvegicus) from Japan (AB752303, AB918692) (Fig. 2). One positive sample was an adult male collected in Gangwon-do, whereas the other sample was a female juvenile from an unidentified origin. Two M. haemomuris-like sequences from this study were also deposited in GenBank under accession no. KP843891 and KP843892.
Fig. 2. Phylogenetic tree of the partial 16S rRNA gene sequences of hemoplasmas isolated from this study (bold font) and related mycoplasmas from GenBank database. Sequences used for comparison were as follows: Candidatus M. haemominutum sequences from a domestic cat (Felis catus; EU839983) and an European wild cat (Felis silvestris silvestris; DQ825442), Candidatus M. haematoparvum from a domestic dog (Canis lupus familiaris; EF416569), Mycoplasma (M.) suis from a pig (Sus scrofa domesticus; AY492086), M. haemofelis from a domestic cat (DQ157160) and an Eurasian lynx (Lynx lynx; DQ825458), Candidatus M. turicensis from an Iriomote cat (Prionailurus bengalensis iriomotensis; AB697739) and a domestic cat (DQ464421), and M. haemomuris or M. haemomuris-like sequences from brown rats (Rattus norvegicus; AB820289, AB752303, respectively). The numbers on the tree indicate bootstrap values for the branch points.
In this study, we tested for infection of A. phagocytophilum and/or E. canis in leopard cats, but no positive specimens were observed. However, A. bovis was identified in two of the investigated cats. A. bovis is an Anaplasma spp. known to cause anaplasmosis in domestic and wild ruminants along with A. marginale and A. centrale [6]. In Korea, water deer infected with A. bovis and its vector tick species have previously been reported [7,9]. However, a recent study from Japan [14] detected A. bovis in Tsushima leopard cats with 15% prevalence. Although the pathogenicity and/or main vector species of Anaplasma/Ehrlichia infection in leopard cats is currently unknown, the recent discovery of A. bovis infection in leopard cats indicates the need for further study of the potential impact of Anaplasma/Ehrlichia spp. pathogens on these animals in the wild.
In previous studies, wild feline species such as the Iriomote cat and Eurasian lynx were infected with hemoplasma species from domestic cats, such as M. haemominutum, M. haemofelis and 'Candidatus M. turicensis' [5,10]. However, in the present study, the hemoplasma sequence from two positive samples showed the highest similarity (94-95%) to Myoplasma sp. or M. haemomuris, which have only identified in brown rats to date (AB820289, AB752303). Further study is needed to better characterize the genetic sequences from these two M. haemomuris-like pathogens. Nevertheless, the relatively high prevalence of hemoplasma infection (27.6%) observed in the present study and the finding that our samples cover a wide geographical and temporal range indicate that leopard cats in Korea are regularly infected with feline hemoplasma, similar to other wild feline species.
For mesocarnivores such as leopard cats, ecological competition with free-roaming domestic animals including feral dogs and cats represents an important challenge [15]. In addition, free-ranging domestic animals can serve as hosts in the area, maintaining a sufficient population size for constant circulation of pathogens and causing infection of wild animals that may share the habitat [12]. Because pathogens are shared between wild feline species and domestic cats, the potential overlap of habitat range of leopard cats and stray/feral cats in rural areas may provide higher opportunities for cross-species transmission of feline pathogens to leopard cats [1]. The prevalence of Anaplasma/Ehrlichia spp. and hemoplasma or its vector species in stray/feral cats has frequently been reported [3,11,16]. Since we are currently unaware of ecological interactions among leopard cats and domestic/stray cats in Korea, understanding pathogens of leopard cats that can be transmitted across different host species will have critical implications to leopard cat management strategies.
Acknowledgments
We express our gratitude to Seonmi Lee for her help analyzing the genetic sequences. This work was supported by the National Research Foundation of Korea grant funded by the Korean Government (NRF-2012S1A5B6034265).
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Choi TY, Kwon HS, Woo DG, Park CH. Habitat selection and management of the leopard cat (Prionailurus bengalensis) in a rural area of Korea. Korean J Environ Ecol. 2012;26:322–332. [Google Scholar]
- 2.Criado-Fornelio A, Martinez-Marcos A, Buling-Saraña A, Barba-Carretero JC. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: a molecular study. Vet Microbiol. 2003;93:307–317. doi: 10.1016/s0378-1135(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 3.Duarte A, Marques V, Correia JHD, Neto I, Bráz BS, Rodrigues C, Martins T, Rosado R, Ferreira JP, Santos-Reis M, Tavares L. Molecular detection of haemotropic Mycoplasma species in urban and rural cats from Portugal. J Feline Med Surg. 2015;17:516–522. doi: 10.1177/1098612X14550172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eddlestone SM, Diniz PPVP, Neer TM, Gaunt SD, Corstvet R, Cho D, Hosgood G, Hegarty B, Breitschwerdt EB. Doxycycline clearance of experimentally induced chronic Ehrlichia canis infection in dogs. J Vet Intern Med. 2007;21:1237–1242. doi: 10.1892/07-061.1. [DOI] [PubMed] [Google Scholar]
- 5.Hirata M, Tateno M, Sakuma M, Nakanishi N, Izawa M, Asari Y, Okamura M, Shimokawa Miyama T, Setoguchi A, Endo Y. An epidemiological survey of hemoplasma infection in Iriomote cats (Prionailurus bengalensis iriomotensis) J Vet Med Sci. 2012;74:1531–1537. doi: 10.1292/jvms.12-0094. [DOI] [PubMed] [Google Scholar]
- 6.Jilintai, Seino N, Hayakawa D, Suzuki M, Hata H, Kondo S, Matsumoto K, Yakoyama N, Inokuma H. Molecular survey for Anaplasma bovis and Anaplasma phagocytophilum infection in cattle in a pastureland where sika deer appear in Hokkaido, Japan. Jpn J Infect Dis. 2009;62:73–75. [PubMed] [Google Scholar]
- 7.Kim HC, Hang SH, Chong ST, Klein TA, Choi CY, Nam HY, Chae HY, Lee H, Ko S, Kang JG, Chae JS. Ticks collected from selected mammalian hosts surveyed in the Republic of Korea during 2008-2009. Korean J Parasitol. 2011;49:331–335. doi: 10.3347/kjp.2011.49.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim CM, Yi YH, Yu DH, Lee MJ, Cho MR, Desai AR, Shringi S, Klein TA, Kim HC, Song JW, Baek LJ, Chong ST, O'Guinn ML, Lee JS, Lee IY, Park JH, Foley J, Chae JS. Tick-borne rickettsial pathogens in ticks and small mammals in Korea. Appl Environ Microbiol. 2006;72:5766–5776. doi: 10.1128/AEM.00431-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang JG, Ko S, Kim YJ, Yang HJ, Lee H, Shin NS, Choi KS, Chae JS. New genetic variants of Anaplasma phagocytophilum and Anaplasma bovis from Korean water deer (Hydropotes inermis argyropus) Vector Borne Zoonotic Dis. 2011;11:929–938. doi: 10.1089/vbz.2010.0214. [DOI] [PubMed] [Google Scholar]
- 10.Meli ML, Cattori V, Martínez F, López G, Vargas A, Simón MA, Zorrilla I, Muñoz A, Palomares F, López-Bao JV, Pastor J, Tandon R, Willi B, Hofmann-Lehmann R, Lutz H. Feline leukemia virus and other pathogens as important threat to the survival of the critically endangered Iberian lynx (Lynx pardinus) PLoS One. 2009;4:e4744. doi: 10.1371/journal.pone.0004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spada E, Proverbio D, Galluzzo P, Della Pepa A, Bagnagatti De Giorgi G, Perego R, Ferro E. Prevalence of haemoplasma infections in stray cats in northern Italy. ISRN Microbiol. 2014;2014:298352. doi: 10.1155/2014/298352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzán G, Ceballos G. The role of feral mammals on wildlife infectious disease prevalence in two nature reserves within Mexico City limits. J Zoo Wildl Med. 2005;36:479–484. doi: 10.1638/04-078.1. [DOI] [PubMed] [Google Scholar]
- 13.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tateno M, Nishio T, Sakuma M, Nakanishi N, Izawa M, Asari Y, Okamura M, Maruyama S, Miyama TS, Setoguchi A, Endo Y. Molecular epidemiologic survey of Bartonella, Ehrlichia, and Anaplasma infections in Japanese Iriomote and Tsushima leopard cats. J Wildl Dis. 2013;49:646–652. doi: 10.7589/2012-07-194. [DOI] [PubMed] [Google Scholar]
- 15.Vanak AT, Gompper ME. Interference competition at the landscape level: the effect of free-ranging dogs on a native mesocarnivore. J Appl Ecol. 2010;47:1225–1232. [Google Scholar]
- 16.Yu DH, Kim HW, Desai AR, Han IA, Li YH, Lee MJ, Kim IS, Chae JS, Park J. Molecular detection of feline hemoplasmas in feral cats in Korea. J Vet Med Sci. 2007;69:1299–1301. doi: 10.1292/jvms.69.1299. [DOI] [PubMed] [Google Scholar]