Abstract
Two decades after the discovery that neural stem cells (NSCs) populate some regions of the mammalian central nervous system (CNS), deep knowledge has been accumulated on their capacity to generate new neurons in the adult brain. This constitutive adult neurogenesis occurs throughout life primarily within remnants of the embryonic germinal layers known as “neurogenic sites.” Nevertheless, some processes of neurogliogenesis also occur in the CNS parenchyma commonly considered as “nonneurogenic.” This “noncanonical” cell genesis has been the object of many claims, some of which turned out to be not true. Indeed, it is often an “incomplete” process as to its final outcome, heterogeneous by several measures, including regional location, progenitor identity, and fate of the progeny. These aspects also strictly depend on the animal species, suggesting that persistent neurogenic processes have uniquely adapted to the brain anatomy of different mammals. Whereas some examples of noncanonical neurogenesis are strictly parenchymal, others also show stem cell niche-like features and a strong link with the ventricular cavities. This work will review results obtained in a research field that expanded from classic neurogenesis studies involving a variety of areas of the CNS outside of the subventricular zone (SVZ) and subgranular zone (SGZ). It will be highlighted how knowledge concerning noncanonical neurogenic areas is still incomplete owing to its regional and species-specific heterogeneity, and to objective difficulties still hampering its full identification and characterization.
Neuro-glio-genic processes have recently been shown to occur in so-called nonneurogenic regions. These processes are heterogeneous; their origins, outcomes, and physiological functions vary regionally and among species.
The central nervous system (CNS) of adult mammals is assembled during developmental neurogenesis, and its architectural specificity is maintained through a vast cohort of membrane-bound and extracellular matrix molecules (Gumbiner 1996; Bonfanti 2006). Although CNS structure is sculpted by experience-dependent synaptic plasticity at different postnatal developmental stages (critical periods) (see Sale et al. 2009) and, to a lesser extent, during adulthood (Holtmaat and Svoboda 2009), the neural networks are rather stabilized in the “mature” nervous tissue (Spolidoro et al. 2009). The differentiated cellular elements forming adult neural circuitries remain substantially unchanged in terms of their number and types, because cell renewal/addition in the CNS is very low. This situation is intuitive because connectional, neurochemical, and functional specificities are fundamental features of the mature CNS in highly complex brains, allowing specific cell types to be connected and to act in a relatively invariant way (Frotscher 1992).
Since the discovery of neural stem cells (NSCs) (Reynolds and Weiss 1992), we realized that the aforementioned rules of CNS stability have a main exception in two brain regions: the forebrain subventricular zone (SVZ) (Lois and Alvarez-Buylla 1994) and the hippocampal subgranular zone (SGZ) (Gage 2000). These “adult neurogenic sites” are remnants of the embryonic germinal layers (although indirectly for the SGZ, which forms ectopically from the embryonic germinative matrix), which retain stem/progenitor cells within a special microenvironment, a “niche,” allowing and regulating NSC activity (Kriegstein and Alvarez-Buylla 2009). In addition, the areas of destination (olfactory bulb and dentate gyrus) reached by neuroblasts generated within these neurogenic sites harbor specific, not fully identified yet, environmental signals allowing the integration of young, newborn neurons. These two “canonical” sites of adult neurogenesis have been found in all animal species studied so far, including humans (reviewed in Lindsey and Tropepe 2006; Bonfanti and Ponti 2008; Kempermann 2012; Grandel and Brand 2013). Although in several classes of vertebrates including fish, amphibians, and reptiles, adult neurogenesis is widespread in many areas of the CNS (Zupanc 2006; Chapouton et al. 2007; Grandel and Brand 2013), in mammals, the vast majority of the brain and spinal cord regions out of the germinal-layer-derived neurogenic sites are commonly referred to as “nonneurogenic parenchyma” (Sohur et al. 2006; Bonfanti and Peretto 2011; Bonfanti and Nacher 2012). However, this viewpoint has changed during the last few years. New examples of cell genesis, involving both neurogenesis and gliogenesis, have been shown to occur in the so-called nonneurogenic regions of the mammalian CNS (Table 1). Local, parenchymal progenitors that retain some proliferative capacity have been detected in most regions of the mature CNS (Horner et al. 2000; Dayer et al. 2005; Kokoeva et al. 2005; Luzzati et al. 2006; Ponti et al. 2008; reviewed in Butt et al. 2005; Nishiyama et al. 2009; Migaud et al. 2010; Bonfanti and Peretto 2011), suggesting that structural plasticity involving de novo neural cell genesis could be more widespread than previously thought. Apart from their temporal persistence (some of them represent examples of delayed developmental neurogenesis, which persist postnatally; see below), neurogliogenic processes vary as to their regional localization, origin, and final outcome. In this review, “noncanonical” neurogenic processes occurring in adult mammals will be reviewed by underlining their heterogeneity across the species and their differences in intensity and outcome with respect to canonical neurogenic sites.
Table 1.
Main sites of noncanonical neurogenesis in the mammalian brain
| Rats | Mice | Rabbits | Monkeys | |
|---|---|---|---|---|
| Neocortex | Gould et al. 2001 Dayer et al. 2005a Tamura et al. 2007 |
Shapiro et al. 2009 | Gould et al. 1999, 2001 Bernier et al. 2002 |
|
| Nakatomi et al. 2002a Pencea et al. 2001 Ohira et al. 2010a |
Magavi et al. 2000a Chen et al. 2004a |
Vessal and Darian-Smith 2010a | ||
| Corpus callosum | Pencea et al. 2001 | |||
| Piriform cortexb | Pekcec et al. 2006 | Shapiro et al. 2007 | Bernier et al. 2002 | |
| Olfactory tubercle | Shapiro et al. 2009 | Bedard et al. 2002b | ||
| Striatum | Dayer et al. 2005a | Shapiro et al. 2009 | Luzzati et al. 2006a | Bedard et al. 2002a; 2006a |
| Arvidsson et al. 2002a Pencea et al. 2001 Liu et al. 2009a |
Goldowitz and Hamre 1998a Cho et al. 2007a |
|||
| Septum | Pencea et al. 2001 | |||
| Amygdala | Shapiro et al. 2009 | Luzzati et al. 2006a | Bernier et al. 2002 | |
| Hippocampus (Ammon’s horn) | Rietze et al. 2000 | |||
| Nakatomi et al. 2002a | ||||
| Thalamus | Pencea et al. 2001 | |||
| Hypothalamus | Xu et al. 2005 | Kokoeva et al. 2007 | ||
| Xu et al. 2005a Pencea et al. 2001 Matsuzaki et al. 2009 Perez-Martin et al. 2010 |
Kokoeva et al. 2005a Pierce and Xu 2010 |
|||
| Substantia nigra | Zhao et al. 2003 Zhao and Janson Lang 2009 |
|||
| Zhao et al. 2003 | ||||
| Cerebellum | Ponti et al. 2008a | |||
| Brain stem | Bauer et al. 2005 | |||
| Bauer et al. 2005 |
Unshaded rows, spontaneous (constitutive) neurogenesis; shaded rows, experimentally induced neurogenesis (growth factor infusion, lesion, etc.). No functional integration has been shown to occur in any of the studies reported here.
aNeuronal differentiation of newborn cells has been well documented; in all other cases, neurogenesis has been shown only until the cell-specification step, and/or assessed with less accurate analyses (reslicing not performed, neuronal differentiation not clearly shown, very few cells shown in figures, insufficient or absent quantification).
bNeurogenesis reported in this region has been denied by subsequent reports. Only a set of studies are reported; gliogenesis is not considered (data modified from Bonfanti and Peretto 2011).
PARENCHYMAL NEUROGENESIS
Unlike adult neurogenesis occurring in the SVZ and SGZ, which is well characterized and rather constant through different mammalian species, different “types” of neurogenic processes may occur in the adult CNS parenchyma, depending on the animal species, age, and physiological/pathological states (Bonfanti and Peretto 2011). Spontaneous (constitutive) parenchymal neurogenesis can be considered a rare phenomenon in mammals, with its regional location being dependent on the animal species (reviewed in Bonfanti and Peretto 2011; Bonfanti 2013; and summarized in Table 1). Different examples of neurogenesis occurring outside of the two germinal-layer-derived neurogenic sites have been described in rodents (Dayer et al. 2005; Kokoeva et al. 2005), rabbits (Luzzati et al. 2006; Ponti et al. 2008), and monkeys (Gould et al. 2001; Bernier et al. 2002), with remarkable differences between closely related orders (e.g., rodents and lagomorphs: cf., for example, Dayer et al. 2005; Luzzati et al. 2006; Ohira et al. 2010; Ponti et al. 2008). Some of the differences and discrepancies among studies arise from technical issues, such as analysis of a proliferative marker (e.g., BrdU), colocalization with neuronal markers, or misconception about specific markers (e.g., PSA-NCAM labeling migrating cells and not newborn neurons). Technical issues have been detailed in other reviews (e.g., Feliciano and Bordey 2013) and are only briefly mentioned below.
In adult rodents, most parenchymal neurogenesis seems to occur spontaneously at very low levels, rather being elicited/enhanced after specific physiological or pathological conditions (see below) (Dayer et al. 2005; Kokoeva et al. 2005; Luzzati et al. 2006; Ponti et al. 2008; Ohira et al. 2010; Pierce and Xu 2010). Dayer and colleagues (2005) showed the occurrence of new neurons in the deep layers of the rat cerebral cortex. By labeling newborn cells with multiple intraperitoneal injections of BrdU and using markers of both immature and mature neurons to characterize the new cells through a detailed confocal analysis at different survival times, they showed genesis of new GABAergic interneurons in both neocortex and striatum. At 4–5 wk survival time, the 0.4 ± 0.13% of the BrdU+ cells were mature NeuN+ neurons in the neocortex. Interestingly, although several newborn cells were identified close to the SVZ periventricular region, the great majority of cortical BrdU+ cells were positive for the chondroitin sulfate proteoglycan NG2 (neuron glial 2), a marker of oligodendrocyte precursors. Other studies also support the occurrence of low neurogenic events in the rat neocortex (Gould et al. 2001; Tamura et al. 2007), although no clear conclusions are provided concerning the final outcome of the newborn cells (see Bonfanti and Peretto 2011; Feliciano and Bordey 2013). Most investigators suggest that adult cortical newborn interneurons might originate from in situ progenitors, but additional work needs to examine this conclusion. Interestingly, Tamura and colleagues found that a subpopulation of NG2+/DCX+ (doublecortin) cells resides in the rat neocortex, some of which could acquire neuronal specification (Tamura et al. 2007). Neuronal progenitors have also been described in cortical layer 1 of neonatal (Breunig et al. 2012) as well as adult rats (Ohira et al. 2010), wherein they increase 1.6-fold after mild ischemia. No clear evidence of spontaneous neurogenesis has been shown in the intact mouse (as opposed to rat) cerebral cortex, thus confirming the importance of the animal species in parenchymal neurogenesis (see Table 1) and the possible differences existing in cortical structural plasticity when comparing rats and mice. On the other hand, striking results have been obtained in the lesioned mouse cortex (Magavi et al. 2000; Chen et al. 2004), but this remains controversial and awaits validation (Diaz et al. 2013).
Other examples of spontaneous parenchymal neurogenesis have been described in lagomorphs. In rabbits, newly generated neurons are spontaneously produced in two main regions of the adult CNS: the forebrain striatum and the cerebellum. In the caudate nucleus, newborn neuroblasts form longitudinally arranged, DCX- and PSA-NCAM-immunoreactive striatal chains similar to the SVZ chains (Luzzati et al. 2006). These neuroblasts are generated from clusters of proliferating cells that express the astroglial marker brain lipid-binding protein (BLBP), and about 1/6 of surviving cells differentiate into calretinin-positive striatal interneurons. Using an approach based on the carbon-14 (14C) assay to label cells and identify their age in humans, it was recently proposed that local neurogenesis does occur within the human striatum (Ernst et al. 2014). Still in rabbits, the combination of cell proliferation markers, detected at different postinjection survival times, with DCX and PSA-NCAM staining revealed a parenchymal genesis of Pax2+ interneurons in the cerebellar cortex, resulting from further proliferation of cells of neuroepithelial origin (Ponti et al. 2008). This process shows features of both delayed neurogenesis, extending until and around puberty (Ponti et al. 2006b), and persistent neurogenesis occurring, to a lesser extent, during adulthood (Ponti et al. 2008). Thus, in the striatal and cerebellar parenchyma of lagomorphs, in sharp contrast with our common knowledge concerning the CNS of other mammals, new neurons are spontaneously generated independently from remnants of germinal layers, yet their final outcome, possible integration, and role in the adult neural circuits remains obscure.
Further examples of mammalian parenchymal neurogenesis have been provided (see Table 1), yet it is not easy or intuitive to provide an ultimate list and/or classification of such processes, for several reasons. First, this is linked to a remarkable heterogeneity involving variables, such as the developmental stages at which they occur, the different levels of specification/differentiation of the newborn cells, the origin of the progenitors, the exact nature of these precursor cells, their lineage relationship to other stem cells, and the final outcome and significance of the entire process (these latter mostly unknown). A large number of reports published in this domain, although accurate and performed with multiple technical approaches, do suggest that in most cases the newly generated cells barely survive and do not fully integrate. In a recent review article (Bonfanti and Peretto 2011), five subsequent steps occurring in germinal-layer-derived (SVZ and SGZ) neurogenic processes have been dissected to critically evaluate/compare different parenchymal neurogenic events. These subsequent “levels” of neurogenesis span from cell division to possible integration of specified/differentiated cells into the CNS tissue; according to this view, the neurogenic process should be classified as complete only when all the steps are filled. As a result, all the parenchymal neurogenic processes described until now can actually be considered as incomplete. The main differences between germinal-layer-derived and parenchymal neurogenesis are listed in Table 2. It has been proposed that some parenchymal newborn neurons have a transient existence (Gould et al. 2001; Luzzati et al. 2011), and their fate and role remain unknown (Arvidsson et al. 2002; Chen et al. 2004; Liu et al. 2009; Ohira et al. 2010; Bonfanti and Peretto 2011; Luzzati et al. 2011).
Table 2.
Main differences between cell genesis in adult neurogenic sites and in the parenchyma
| Neurogenic sites | Parenchyma | |
|---|---|---|
| Location | Restricted | Widespread |
| Primary progenitor cells | Neural stem cells | Neural progenitors |
| Microenvironment | Stem cell niche | Mature neuropil |
| Origin | Germinal-layer derived | No direct link with germinal layers |
| Fate (progeny) | Mainly neurons (some astrocytes and oligodendrocytes) | Mainly glial cells (some neurons) |
| Fate (process) | Complete | Incomplete |
Among the unsolved issues of parenchymal neurogenesis are the numerous reports that have not been confirmed by further studies performed by the same or other laboratories (Gould et al. 1999; Magavi et al. 2000; Nakatomi et al. 2002; Zhao et al. 2003; Rivers et al. 2008; Guo et al. 2010), along with a series of findings that have been denied in studies trying to reproduce the same results (Kornack and Rakic 2001; Frielingsdorf et al. 2004; Richardson et al. 2011). Hence, it is evident that we still do not grasp the real limits and/or opportunities of parenchymal neurogenesis and that further studies are required before finally accepting or denying the existence of some “unusual” neurogenic processes. It is important to state that this is not a criticism, rather a common trait when science explores new, unknown territories. Indeed, several unresolved aspects make parenchymal neurogenesis a difficult issue to be explored: (1) the contrast between a wide range of linages or fates displayed by parenchymal progenitors isolated in vitro (Palmer et al. 1999; Belachew et al. 2003) and far more restricted potentialities in vivo, (2) the existence of studies reporting neurogenesis in parenchymal regions yet performed by different researchers using different experimental plans and paradigms, (3) the lack of specific markers making it difficult to identify the cells of origin (i.e., progenitors), (4) the lack of information concerning the environmental factors (tissue-specific, metabolic, behavioral, etc.) involved in the induction of parenchymal progenitor cell proliferation/migration/differentiation in different pathophysiological contexts that are either mobilized from neurogenic sites or activated locally within the parenchyma (Arvidsson et al. 2002; Nakatomi et al. 2002; Thored et al. 2006; Luzzati et al. 2011), and, finally, (5) the difficulties in performing systematic analyses that homogeneously cover different animal species, brain regions, and experimental variables.
Among other difficulties in studying different types of neurogenesis is the lack of precise and unique molecular markers. For instance, NeuN is often used to claim that new neurons are produced in certain brain regions, yet NeuN is a splicing factor that is switched on very early once a neuronal cell has become postmitotic. Because NeuN marks neurons that are not fully mature yet but simply postmitotic, there is overlap between DCX and NeuN expression. PSA-NCAM and DCX have also been frequently overestimated as markers of neurogenic processes (discussed in Bonfanti and Nacher 2012).
Interestingly, the heterogeneous nature of parenchymal neurogenesis and the technical limitations of newborn neuron detection (see above) have inadvertently led to the discovery of noncanonical neurogenic mechanisms of plasticity. This is the case of the piriform cortex, which is one region in which neurogenesis was reported by different research groups and is refuted by others (see, for example, Bernier et al. 2002; Pekcec et al. 2006; Shapiro et al. 2007; Gomez-Climent et al. 2008). The piriform cortex is known to harbor a population of neurons immunoreactive for PSA-NCAM and DCX, which are two markers highly expressed in newborn neurons, but are also present in non-newly generated cells (Bonfanti 2006; Gomez-Climent et al. 2008). Deeper investigations have shown that the piriform cortex contains a population of immature, non-newly generated neurons (Gomez-Climent et al. 2008; Klempin et al. 2011). These cells, by remaining in an immature state for an undetermined amount of time, can represent a “reservoir” of structurally plastic neurons that could possibly be recruited into the preexisting neural circuits although not generated ex novo (Bonfanti and Nacher 2012). Hence, multiple forms of structural plasticity, involving noncanonical neurogenesis, can overlap within the so-called nonneurogenic tissue, increasing the complexity of the whole picture of CNS remodeling.
ADULT NEUROGENESIS IN THE HYPOTHALAMUS: PARENCHYMAL OR GERMINAL LAYER DERIVED?
One of the noncanonical sites of neurogenesis listed in Table 1 that is now well accepted is the hypothalamus, which includes a germinal-layer-derived zone. The hypothalamus is a small brain region that surrounds the third ventricle, is part of the limbic system, and contains distinct nuclei. It serves as a central homeostatic regulator of numerous physiological and behavioral functions, such as feeding, metabolism, body temperature, thirst, fatigue, aggression, sleep, circadian rhythms, and sexual behavior. To achieve these functions, the hypothalamus receives many externally generated signals, is interconnected with other brain regions, and links the nervous system to the endocrine system via the pituitary gland (hypophysis) by secreting specific hormones. In addition, the identification of adult neurogenesis raises the question about the contribution of newborn neurons to hypothalamic functions (Fig. 1) (for reviews, see Migaud et al. 2010; Yuan and Arias-Carrion 2011; Lee and Blackshaw 2012; Sousa-Ferreira et al. 2013).
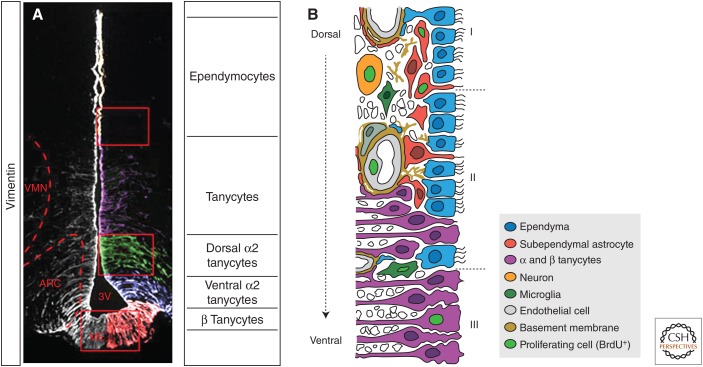
Figure 1.
Diagram of the hypothalamic neurogenic zone. (A) Coronal section (c.s.) through third ventricle (3V), immunolabeled with vimentin (left, white; right, pseudocolored), which labels tanycytes. Ependymocytes do not express vimentin. The pseudocoloring distinguishes tanycyte subtypes: purple, α1; green, dorsal α2; blue, ventral α2; red, β. (Panel A from Robins et al. (2013); reprinted, with permission, from Nature Publishing Group © 2013.) (B) Schematic drawing summarizing the structure of the rat third ventricle wall, and the type of cells capable of proliferation after insulin-like growth factor (IGF)-I stimulation. The dorsal section (I) is lined by a multiciliated ependyma (blue) over a subependymal astroglial layer (red). The ventral portion (III) is lined by an epithelium of tanycytes (magenta). The overlapping region (II) is characterized by the presence of ependyma, subependymal astrocytes, and tanycytes, among other cell types and neuropil fibers. Subependymal capillaries are endowed with a basal lamina that develops complex labyrinths of basement membranes (brown). Some subependymal astrocytes protrude among the ependymal cells by means of a process that contacts the cerebrospinal fluid and express a solitary cilium. Two microglial cells (dark green), a pericyte (greenish gray), and a neuron (orange) are also represented. Neuropil fibers are not colored. Nuclei of the cells that are able to divide after IGF-I stimulation (tanycytes, microglia, endothelial cells, neurons, and subependymal astrocytes) are colored in light green representing BrdU-positive labeling. (From Perez-Martin et al. 2010; adapted, with permission, from John Wiley and Sons © 2010.)
There are several convincing studies showing constitutive neurogenesis in the adult hypothalamus of mammals, including rodents, rats, mice, voles (Pencea et al. 2001; Kokoeva et al. 2005; Xu et al. 2005; Matsuzaki et al. 2009; Perez-Martin et al. 2010; Pierce and Xu 2010; Lee et al. 2012; Li et al. 2012; Werner et al. 2012), and sheep (Migaud et al. 2011) using BrdU as well as genetic lineage tracing. BrdU was injected intraperitoneally (repeated injections except in Migaud et al. 2011) or infused intracerebroventricularly (Kokoeva et al. 2005; Li et al. 2012); 6 to 53 d postinjection immunostaining for neuronal markers (e.g., DCX and NeuN) as well as glial markers (e.g., glial fibrillary acidic protein [GFAP] for astrocytes) were performed. BrdU+NeuN+ cells were routinely identified albeit at various percentages of the total BrdU+ cell population ranging from <10% to 37%. Adult hypothalamic neurogenesis is significantly influenced by external stimuli, such as diet (for a review, see Yon et al. 2013) and social environment (Fowler et al. 2002), which could explain variabilities or discrepancies in the number of newborn neurons between studies. The number of newborn neurons is much lower than in the SVZ and SGZ. The hypothalamus is, thus, predominantly gliogenic and displays a low rate of neurogenesis under unstimulated conditions. One study did not identify any BrdU+ cells in the hypothalamus of sheep despite detecting BrdU+ cells in the SGZ and using higher intraperitoneal (i.p.) doses of BrdU (Hawken et al. 2009). The reason for this discrepancy remains unclear. One of the above studies also labeled tanycytes, which are one of the cell types touching the third ventricle with GFP-recombinant adenoviral injection (Xu et al. 2005). Using this complementary approach, they identified GFP+ NeuN+ cells that displayed synaptic structure by electron microscopy. Some of the above and additional studies used genetic fate mapping from different populations of tanycytes along the third ventricles (see below for details) and identified newborn neurons under control conditions (Lee et al. 2012; Li et al. 2012; Haan et al. 2013; Robins et al. 2013). The rate of constitutive neurogenesis was nevertheless low (only a few cells per section) under understimulated conditions.
The following features of adult hypothalamic neurogenesis provide some clues into its functional significance. Newborn neurons acquire identities and functional phenotypes relevant for energy-balance regulation. A subset of newborn neurons express the anorexigenic marker proopiomelanocortin (POMC) and the orexigenic markers, neuropeptide-Y (Kokoeva et al. 2005; Li et al. 2012; Haan et al. 2013) and agouti-related protein (AgRP) (Pierce and Xu 2010), and respond to fasting and leptin-induced signaling (Kokoeva et al. 2005; Pierce and Xu 2010; Haan et al. 2013). Leptin, produced by white adipose tissue, plays a fundamental role in maintaining neuroendocrine and body weight homeostasis. Some newborn neurons also express orexin A, which influences wakefulness (Xu et al. 2005). In addition, neurospheres from the adult hypothalamus generated functional neurons expressing the markers listed above (Sousa-Ferreira et al. 2011).
Adult neurogenesis is influenced by growth factors (e.g., brain-derived neurotrophic factor [BDNF], ciliary neurotrophic factor [CNTF], insulin-like growth factor [IGF]-1, fibroblast growth factor [FGF]) (for review, see Sousa-Ferreira et al. 2013), by genetically triggered death of AgRP neurons (Pierce and Xu 2010), and by external stimuli relevant to homeostatic body functions regulated by the hypothalamus, such as diet (Haan et al. 2013), social environment (Fowler et al. 2002), heat (Matsuzaki et al. 2009), and physiological adaptation owing to seasonal changes (Migaud et al. 2011). More direct functional relevance of hypothalamic neurogenesis comes from studies decreasing or ablating cell proliferation and, thus, neurogenesis and examining the outcome of energy balance and feeding. Ablation of neurogenesis with radiation altered the weight and metabolic activity of adult mice (Lee et al. 2012). Similarly, depletion of NSCs through IKK-β/NF-κB activation led to impaired neuronal differentiation and ultimately the development of obesity and prediabetes (Li et al. 2012). Cytosine arabinoside (AracC) treatment to eliminate cell proliferation prevented the proliferative and anorexigenic effects of CNTF (Kokoeva et al. 2005) and decreased food intake and body adiposity in mice lacking newborn AgRP neurons (Pierce and Xu 2010). Collectively, these studies strongly suggest that adult hypothalamic neurogenesis is important for the feeding regulation and energy balance.
The presence of newborn neurons in the adult hypothalamus implies the presence of NSCs or neural progenitor cells (NPCs). Indeed, a study in 1996 and additional ones later on reported that cells surrounding the third ventricle could generate neurospheres (Weiss et al. 1996; Xu et al. 2005; Li et al. 2012; Robins et al. 2013) and monolayer cell culture generating neurons and glia (Markakis et al. 2004). Based on 24-h post-BrdU experiments, proliferative cells have been identified in both the parenchyma and along the third ventricle (for a review, see Migaud et al. 2010). Although no studies have further examined cells acting as NPCs from the parenchyma, accumulating evidence suggests that cells along the third ventricle, in particular tanycytes, act as NSCs. Studies focused on this ventricular region because its cytoarchitecture resembles that of the SVZ with the presence of ependymal cells and a specialized glia, tanycytes, with a radial glia-like morphology. The tanycytes resemble SVZ B1 type cells, which are considered to be NSCs, and display a basal process that contacts the ventricle, a single primary cilium, and an apical process that projects into the parenchyma and capillaries (Perez-Martin et al. 2010). The proliferative zone along the third ventricle wall in the hypothalamus can be divided into two parts: the medial part containing proliferative tanycytes and subependymal astrocytes, and the ventral part (median eminence) containing proliferative tanycytes. Medial and ventral tanycytes are distinct populations called α and β tanycytes, respectively, based on their location and antigenic properties. Both populations express NSC markers, such as nestin and Sox2, but only α tanycytes express GFAP and GLAST as shown in SVZ B1 type cells and β tanycytes, and a subset of α tanycytes express FGF-10 (Li et al. 2012; Robins et al. 2013).
Recent genetic fate mapping studies clearly identified tanycytes as NSCs (Xu et al. 2005; Lee et al. 2012; Li et al. 2012; Haan et al. 2013; Robins et al. 2013). However, some confusion persists in terms of the function of the two populations of tanycytes, which by themselves remain a very elusive population of cells that resemble astrocytes. Using GLAST-CreERT2 mice, α tanycytes generated neurospheres up to 10 passages and gave rise to both α and β tanycytes, neurons, and glia in vivo (Robins et al. 2013). Neurogenesis rates were low under unstimulated conditions, suggesting that α tanycytes are quiescent NSCs. Other studies using nestin-CreERT2 (Lee et al. 2012) or FGF10-CreERT2 (Haan et al. 2013) mice found that β tanycytes also generate neurons and astrocytes. However, these later studies were performed in young adults (up to postnatal day 60). It thus remains to be determined whether there are two distinct populations of NSCs or whether α tanycytes generate β tanycytes, which retain self-renewal properties. To complicate the matter, another elegant study suggested that subventricular astrocytes can proliferate and behave as NPCs on stimulation with IGF-1 (Perez-Martin et al. 2010). These cells acquired a striking morphology resembling that of SVZ B1 type cells. It remains to be examined whether these cells could be a dormant population of NSCs.
For the hypothalamus to be considered as a true neurogenic site, it is important to identify a classical neurogenic niche containing quiescent NSCs, niche cells, as well as a supportive microenvironment. Such findings could provide clearer evidence that this process is long lasting in adults and not a case of delayed development as reported for other regions (see next paragraph). As mentioned above, cells with NSC properties exist in the third ventricular niche. Niche cells may include the ependymal cells and perhaps astrocytes, and endothelial cells as shown in the SVZ, although their individual function on NSC behavior and neurogenesis needs to be clarified. The third ventricle wall contains a vascular plexus contacted by tanycytes and extracellular matrix elements that can trap growth factors provided either by surrounding cells, the blood, or the cerebrospinal fluid. In conclusion, all of the above studies define the third ventricle zone as a bona fide neurogenic and gliogenic zone in the adult hypothalamus. Clearly, many provocative questions remain regarding hypothalamic neurogenesis. For example, does neurogenesis exist in the human hypothalamus? What are the percentages of the different neuronal types generated under specific conditions of external stimulation? Do tanycytes give rise to similar neuronal populations in the hypothalamus of diverse mammalian species? Do tanycytes and neurogenesis play a role in pathophysiological conditions, such as obesity? Finally, it appears that tanycytes play an important function in hypothalamic plasticity, but the mechanisms by which tanycytes serve as a sensor of an organismal physiological state remains to be identified.
POSTNATAL EXTENSION OF EMBRYONIC NEUROGENESIS: POSSIBLE OVERLAPPINGS WITH PARENCHYMAL NEUROGENESIS
Although neurogenesis starts early during mammalian development, many neurons are generated postnatally following a wide range of temporal windows in different regions and in different animal species. The majority of these neurons are granule cells, namely, small-sized, relatively morphologically uniform neurons, yet displaying remarkable differences in their function and neurotransmitter content (Kuhn and Blomgren 2011). This general behavior of delayed neurogenesis involves topographical and temporal variations within the same brain region. Because we know that neurogenic processes continue throughout life in areas such as the olfactory bulb and the dentate gyrus, a distinction should be made between protracted or delayed neurogenesis, as a transitory extension of developmental neurogenesis for some periods after birth (e.g., cerebellar granule cells), and persistent neurogenesis, namely, a constitutive neurogenic process that can decrease in intensity with age, but does not come to an end (see Bonfanti and Peretto 2011). Protracted neurogenesis should be viewed as a morphogenic process accomplished after birth, and the mouse olfactory bulb is a typical example of overlapping between protracted and persistent neurogenesis. Postnatal morphogenesis involving glomerular formation is delayed until the first week after birth (Bailey et al. 1999), then adult neurogenesis from the SVZ grants interneuron turnover throughout life. In rodents, it was estimated that 41% of granule cells in the main olfactory bulb are generated during the first postnatal week (which is the late embryonic period in humans), 23% during the second postnatal week, and only 14% thereafter (Hinds 1968; Bayer 1983). Recently, Sanai et al. (2011) showed that the rostral migratory stream (RMS) coming from the SVZ in humans is dramatically reduced postnatally, virtually disappearing around the 18th month of life. The fact that SVZ neurogenesis directed to the olfactory bulb is active throughout life in rodents but mainly restricted to postnatal periods in humans is also a prototypical example of variability in mammalian protracted/persistent neurogenesis.
Protracted neurogenesis also occurs in parenchymal regions not linked to germinal-layer-derived stem cell niches. For instance, some striatal projection neurons are generated postnatally in rats (Wright et al. 2013) and guinea pigs (Luzzati et al. 2014). In mammals, the most striking prototype of protracted neurogenesis is the cerebellum. Although the genesis of most cerebellar cell types occurs very early from the periventricular neuroepithelium lining the fourth ventricle, interneurons complete their centrifugal migration through the white matter and their specification postnatally (Maricich and Herrup 1999). Yet, this is not just a migratory event because cell proliferation of the progenitors still occurs in prospective white matter (Leto et al. 2009), thus assuming features of a protracted, parenchymal cell genesis. In addition, the postnatal mammalian cerebellum undergoes a genesis of granule cells through a transitory, secondary germinative layer localized on its surface (the external germinal layer [EGL]). The EGL can be considered as a germinal layer, because it is formed by tangential subpial displacement of cell precursors from the germinal trigone of the fourth ventricle that persists during the first 2 postnatal weeks. Some neural precursors in the EGL also express GFAP as reported in the adult SVZ (Silbereis et al. 2010). This transitory germinal zone, after radial, centripetal migration of granule cell precursors, progressively reduces its thickness, then disappears at specific ages in different species (from 3 wk in mice to 11 mo in humans, which is very early compared with the onset of puberty [Ponti et al. 2008, 2010]).
Recent studies revealed that protracted cerebellar neurogenesis extends around and beyond puberty in the New Zealand white rabbit (Ponti et al. 2006b), then persisting, to a lesser extent, during adulthood in the absence of germinal layers (Ponti et al. 2008, 2010). Thus, unlike other mammals, in lagomorphs an overlapping of protracted (germinal-layer derived) and adult (parenchymal) cerebellar neurogenesis do occur. In the hypothalamus, it remains unclear how long neurogenesis persists postnatally and whether it would disappear in young humans.
Finally, some examples of protracted neurogenesis can be confused with parenchymal neurogenesis. This is the case of secondary migratory pathways from the SVZ germinal layer to different regions of the surrounding tissue. In postnatal mice, some SVZ-derived progenitors also migrate in a ventral migratory mass across the nucleus accumbens into the basal forebrain giving rise to granule neurons in the islands of Calleja and along a ventrocaudal migratory stream originating at the elbow of the RMS, then reaching the olfactory tubercle (De Marchis et al. 2004). Postnatally generated interneurons (small axonless neurons) originating in the neonatal SVZ have been shown to reach the cortex, being incorporated in its neural circuits (Le Magueresse et al. 2011). These late developmental processes also show heterogeneity among mammals. In young rabbits, up to puberty (6–12 mo), SVZ-derived parenchymal chains of neuroblasts leave the neurogenic layer migrating through the corpus callosum to reach the frontal cortex (parenchymal chains) (Luzzati et al. 2003; Ponti et al. 2006a), and a similar stream has been described in the ventromedial prefrontal cortex of postnatal humans (Sanai et al. 2011).
In conclusion, the lack of consensus, especially in the addition of new neurons in the cerebral cortex, likely stems from the low rate of cortical neurogenesis and the fact that it may be limited to neonatal/postnatal periods. Although postnatal neurogenesis could result from SVZ cell displacement, it cannot be excluded that local, parenchymal progenitors also might contribute if appropriately activated (Feliciano and Bordey 2013).
PROGENITORS INVOLVED IN NONCANONICAL NEUROGENIC PROCESSES
Progenitors giving rise to noncanonical neurogenesis are still obscure as to their origin and cellular identity. In some cases, they show features of glial elements (see, for example, Luzzati et al. 2006), yet most studies of parenchymal neurogenesis reported the identity of newborn cells, but did not identify the cell of origin (Magavi et al. 2000; Ponti et al. 2008; Ohira et al. 2010). What is intriguing, in particular for the long-term perspective of brain repair, is the fact that most parenchymal progenitors can be activated from a relatively quiescent state by different homeostatic, experimental, and pathological contexts (Sohur et al. 2006).
To further complicate the picture is the existence of glial progenitors retaining proliferative capacity in wide areas of the mature CNS (reviewed in Dawson et al. 2000; Butt et al. 2005; Nishiyama et al. 2009; Trotter et al. 2010). The largest class of these cells express NG2 (Horner et al. 2000) and are often referred to simply as NG2 cells. They are also called synantocytes (Butt et al. 2005) or polydendrocytes (Nishiyama et al. 2009), and are morphologically, antigenically, and functionally distinct from mature astrocytes, oligodendrocytes, and microglia. NG stands for neuron-glia, and NG2+ cells can express neuronal markers during differentiation (see Crociara et al. 2013) leading to confusion and perhaps erroneous data interpretation in the literature. Despite their proliferative capacity and potentialities in vitro, NG2+ cells do not contribute to neurogenesis in vivo (reviewed in Boda and Buffo 2010; Trotter et al. 2010; Richardson et al. 2011). Nevertheless, some of these cells are oligodendrocyte progenitor cells that generate oligodendrocytes in the mature CNS (Horner et al. 2000; Dubois-Dalcq et al. 2008; Crociara et al. 2013) and may have multifaceted functions (e.g., neuromodulatory and neuroprotective functions, homeostatic regulations of synaptic functions) that remain to be further investigated (Boda and Buffo 2014).
In conclusion, adult neurogenesis in mammals is restricted to the SVZ, SGZ, and the hypothalamic ventricular zone and subserves homeostatic roles. In contrast, neurogliogenesis in the CNS parenchyma remains substantially obscure as to its origin(s), outcome(s), and physiological function(s) (Bonfanti 2013). Yet, various examples of “reactive” neurogenesis are known to occur (Arvidsson et al. 2002; Thored et al. 2006; Ohira et al. 2010; Pierce and Xu 2010; Luzzati et al. 2011), suggesting that certain pathological states can stimulate either migration of progenitors from the neurogenic sites or activation of local neural progenitors. What remains poorly investigated is whether the adult brain parenchyma belonging to spontaneously nonneurogenic areas could be endowed with quiescent progenitors, which can be stimulated to awake under specific environmental conditions, independently from the contribution of germinal layers.
Footnotes
Editors: Fred H. Gage, Gerd Kempermann, and Hongjun Song
Additional Perspectives on Neurogenesis available at www.cshperspectives.org
REFERENCES
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. 2002. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8: 963–970. [DOI] [PubMed] [Google Scholar]
- Bailey MS, Puche AC, Shipley MT. 1999. Development of the olfactory bulb: Evidence for glia-neuron interactions in glomerular formation. J Comp Neurol 415: 423–448. [PubMed] [Google Scholar]
- Bauer S, Hay M, Amilhon B, Jean A, Moyse E. 2005. In vivo neurogenesis in the dorsal vagal complex of the adult rat brainstem. Neuroscience 130: 75–90. [DOI] [PubMed] [Google Scholar]
- Bayer SA. 1983. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res 50: 329–340. [DOI] [PubMed] [Google Scholar]
- Bedard A, Cossette M, Levesque M, Parent A. 2002a. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci Lett 328: 213–216. [DOI] [PubMed] [Google Scholar]
- Bedard A, Levesque M, Bernier PJ, Parent A. 2002b. The rostral migratory stream in adult squirrel monkeys: Contribution of new neurons to the olfactory tubercle and involvement of the antiapoptotic protein Bcl-2. Eur J Neurosci 16: 1917–1924. [DOI] [PubMed] [Google Scholar]
- Bedard A, Gravel C, Parent A. 2006. Chemical characterization of newly generated neurons in the striatum of adult primates. Exp Brain Res 170: 501–512. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. 2003. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol 161: 169–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. 2002. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci 99: 11464–11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda E, Buffo A. 2010. Glial cells in non-germinal territories: Insights into their stem/progenitor properties in the intact and injured nervous tissue. Arch Ital Biol 148: 119–136. [PubMed] [Google Scholar]
- Boda E, Buffo A. 2014. Beyond cell replacement: Unresolved roles of NG2-expressing progenitors. Front Neurosci 8: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L. 2006. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol 80: 129–164. [DOI] [PubMed] [Google Scholar]
- Bonfanti L. 2013. The (real) neurogenic/gliogenic potential of the postnatal and adult brain parenchyma. ISRN Neurosci 2013: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L, Nacher J. 2012. New scenarios for neuronal structural plasticity in non-neurogenic brain parenchyma: The case of cortical layer II immature neurons. Prog Neurobiol 98: 1–15. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Peretto P. 2011. Adult neurogenesis in mammals—A theme with many variations. Eur J Neurosci 34: 930–950. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Ponti G. 2008. Adult mammalian neurogenesis and the New Zealand white rabbit. Vet J 175: 310–331. [DOI] [PubMed] [Google Scholar]
- Breunig JJ, Gate D, Levy R, Rodriguez J Jr, Kim GB, Danielpour M, Svendsen CN, Town T. 2012. Rapid genetic targeting of pial surface neural progenitors and immature neurons by neonatal electroporation. Neural Dev 7: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. 2005. Synantocytes: The fifth element. J Anat 207: 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapouton P, Jagasia R, Bally-Cuif L. 2007. Adult neurogenesis in non-mammalian vertebrates. BioEssays 29: 745–757. [DOI] [PubMed] [Google Scholar]
- Chen J, Magavi SS, Macklis JD. 2004. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci 101: 16357–16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SR, Benraiss A, Chmielnicki E, Samdani A, Economides A, Goldman SA. 2007. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J Clin Invest 117: 2889–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crociara P, Parolisi R, Conte D, Fumagalli M, Bonfanti L. 2013. Cellular and molecular characterization of multipolar Map5-expressing cells: A subset of newly generated, stage-specific parenchymal cells in the mammalian central nervous system. PLoS ONE 8: e63258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Levine JM, Reynolds R. 2000. NG2-expressing cells in the central nervous system: Are they oligodendroglial progenitors? J Neurosci Res 61: 471–479. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. 2005. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol 168: 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchis S, Fasolo A, Puche AC. 2004. Subventricular zone-derived neuronal progenitors migrate into the subcortical forebrain of postnatal mice. J Comp Neurol 476: 290–300. [DOI] [PubMed] [Google Scholar]
- Diaz F, McKeehan N, Kang W, Hebert JM. 2013. Apoptosis of glutamatergic neurons fails to trigger a neurogenic response in the adult neocortex. J Neurosci 33: 6278–6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Williams A, Stadelmann C, Stankoff B, Zalc B, Lubetzki C. 2008. From fish to man: Understanding endogenous remyelination in central nervous system demyelinating diseases. Brain 131: 1686–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisén J. 2014. Neurogenesis in the striatum of the adult human brain. Cell 156: 1072–1083. [DOI] [PubMed] [Google Scholar]
- Feliciano DM, Bordey A. 2013. Newborn cortical neurons: Only for neonates? Trends Neurosci 36: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. 2002. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol 51: 115–128. [DOI] [PubMed] [Google Scholar]
- Frielingsdorf H, Schwarz K, Brundin P, Mohapel P. 2004. No evidence for new dopaminergic neurons in the adult mammalian substantia nigra. Proc Natl Acad Sci 101: 10177–10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M. 1992. Specificity of interneuronal connections. Ann Anat 174: 377–382. [DOI] [PubMed] [Google Scholar]
- Gage FH. 2000. Mammalian neural stem cells. Science 287: 1433–1438. [DOI] [PubMed] [Google Scholar]
- Goldowitz D, Hamre K. 1998. The cells and molecules that make a cerebellum. Trends Neurosci 21: 375–382. [DOI] [PubMed] [Google Scholar]
- Gomez-Climent MA, Castillo-Gomez E, Varea E, Guirado R, Blasco-Ibanez JM, Crespo C, Martinez-Guijarro FJ, Nacher J. 2008. A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb Cortex 18: 2229–2240. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG. 1999. Neurogenesis in the neocortex of adult primates. Science 286: 548–552. [DOI] [PubMed] [Google Scholar]
- Gould E, Vail N, Wagers M, Gross CG. 2001. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci 98: 10910–10917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandel H, Brand M. 2013. Comparative aspects of adult neural stem cell activity in vertebrates. Dev Genes Evol 223: 131–147. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. 1996. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 84: 345–357. [DOI] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L, Vaccarino F, Pleasure D. 2010. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci 30: 12036–12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, El AE, Bellusci S, Hajihosseini MK. 2013. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci 33: 6170–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawken PA, Jorre TJ, Rodger J, Esmaili T, Blache D, Martin GB. 2009. Rapid induction of cell proliferation in the adult female ungulate brain (Ovis aries) associated with activation of the reproductive axis by exposure to unfamiliar males. Biol Reprod 80: 1146–1151. [DOI] [PubMed] [Google Scholar]
- Hinds JW. 1968. Autoradiographic study of histogenesis in the mouse olfactory bulb. I. Time of origin of neurons and neuroglia. J Comp Neurol 134: 287–304. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. 2009. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10: 647–658. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. 2000. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci 20: 2218–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. 2012. New neurons for “survival of the fittest.” Nat Rev Neurosci 13: 727–736. [DOI] [PubMed] [Google Scholar]
- Klempin F, Kronenberg G, Cheung G, Kettenmann H, Kempermann G. 2011. Properties of doublecortin (DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS ONE 6: e25760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. 2005. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science 310: 679–683. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. 2007. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol 505: 209–220. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. 2001. Cell proliferation without neurogenesis in adult primate neocortex. Science 294: 2127–2130. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. 2009. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32: 149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Blomgren K. 2011. Developmental dysregulation of adult neurogenesis. Eur J Neurosci 33: 1115–1122. [DOI] [PubMed] [Google Scholar]
- Lee DA, Blackshaw S. 2012. Functional implications of hypothalamic neurogenesis in the adult mammalian brain. Int J Dev Neurosci 30: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, et al. 2012. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci 15: 700–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse C, Alfonso J, Khodosevich K, Arroyo Martin AA, Bark C, Monyer H. 2011. “Small axonless neurons”: Postnatally generated neocortical interneurons with delayed functional maturation. J Neurosci 31: 16731–16747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto K, Bartolini A, Yanagawa Y, Obata K, Magrassi L, Schilling K, Rossi F. 2009. Laminar fate and phenotype specification of cerebellar GABAergic interneurons. J Neurosci 29: 7079–7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tang Y, Cai D. 2012. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol 14: 999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey BW, Tropepe V. 2006. A comparative framework for understanding the biological principles of adult neurogenesis. Prog Neurobiol 80: 281–307. [DOI] [PubMed] [Google Scholar]
- Liu F, You Y, Li X, Ma T, Nie Y, Wei B, Li T, Lin H, Yang Z. 2009. Brain injury does not alter the intrinsic differentiation potential of adult neuroblasts. J Neurosci 29: 5075–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. 1994. Long-distance neuronal migration in the adult mammalian brain. Science 264: 1145–1148. [DOI] [PubMed] [Google Scholar]
- Luzzati F, Peretto P, Aimar P, Ponti G, Fasolo A, Bonfanti L. 2003. Glia-independent chains of neuroblasts through the subcortical parenchyma of the adult rabbit brain. Proc Natl Acad Sci 100: 13036–13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati F, De Marchis S, Fasolo A, Peretto P. 2006. Neurogenesis in the caudate nucleus of the adult rabbit. J Neurosci 26: 609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati F, De Marchis S, Parlato R, Gribaudo S, Schutz G, Fasolo A, Peretto P. 2011. New striatal neurons in a mouse model of progressive striatal degeneration are generated in both the subventricular zone and the striatal parenchyma. PLoS ONE 6: e25088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati F, Nato G, Oboti L, Vigna E, Rolando C, Armentano M, Bonfanti L, Fasolo A, Peretto P. 2014. Quiescent neuronal progenitors are activated in the juvenile guinea pig lateral striatum and give rise to transient neurons. Development 141: 4065–4075. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. 2000. Induction of neurogenesis in the neocortex of adult mice. Nature 405: 951–955. [DOI] [PubMed] [Google Scholar]
- Maricich SM, Herrup K. 1999. Pax-2 expression defines a subset of GABAergic interneurons and their precursors in the developing murine cerebellum. J Neurobiol 41: 281–294. [DOI] [PubMed] [Google Scholar]
- Markakis EA, Palmer TD, Randolph-Moore L, Rakic P, Gage FH. 2004. Novel neuronal phenotypes from neural progenitor cells. J Neurosci 24: 2886–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki K, Katakura M, Hara T, Li G, Hashimoto M, Shido O. 2009. Proliferation of neuronal progenitor cells and neuronal differentiation in the hypothalamus are enhanced in heat-acclimated rats. Pflugers Arch 458: 661–673. [DOI] [PubMed] [Google Scholar]
- Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I, Pillon D. 2010. Emerging new sites for adult neurogenesis in the mammalian brain: A comparative study between the hypothalamus and the classical neurogenic zones. Eur J Neurosci 32: 2042–2052. [DOI] [PubMed] [Google Scholar]
- Migaud M, Batailler M, Pillon D, Franceschini I, Malpaux B. 2011. Seasonal changes in cell proliferation in the adult sheep brain and pars tuberalis. J Biol Rhythms 26: 486–496. [DOI] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. 2002. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110: 429–441. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. 2009. Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity. Nat Rev Neurosci 10: 9–22. [DOI] [PubMed] [Google Scholar]
- Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, et al. 2010. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci 13: 173–179. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. 1999. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci 19: 8487–8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekcec A, Loscher W, Potschka H. 2006. Neurogenesis in the adult rat piriform cortex. Neuroreport 17: 571–574. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. 2001. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci 21: 6706–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martin M, Cifuentes M, Grondona JM, Lopez-Avalos MD, Gomez-Pinedo U, Garcia-Verdugo JM, Fernandez-Llebrez P. 2010. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur J Neurosci 31: 1533–1548. [DOI] [PubMed] [Google Scholar]
- Pierce AA, Xu AW. 2010. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci 30: 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G, Aimar P, Bonfanti L. 2006a. Cellular composition and cytoarchitecture of the rabbit subventricular zone and its extensions in the forebrain. J Comp Neurol 498: 491–507. [DOI] [PubMed] [Google Scholar]
- Ponti G, Peretto P, Bonfanti L. 2006b. A subpial, transitory germinal zone forms chains of neuronal precursors in the rabbit cerebellum. Dev Biol 294: 168–180. [DOI] [PubMed] [Google Scholar]
- Ponti G, Peretto P, Bonfanti L. 2008. Genesis of neuronal and glial progenitors in the cerebellar cortex of peripuberal and adult rabbits. PLoS ONE 3: e2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G, Crociara P, Armentano M, Bonfanti L. 2010. Adult neurogenesis without germinal layers: The “atypical” cerebellum of rabbits. Arch Ital Biol 148: 147–158. [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. 1992. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255: 1707–1710. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Young KM, Tripathi RB, McKenzie I. 2011. NG2-glia as multipotent neural stem cells: Fact or fantasy? Neuron 70: 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietze R, Poulin P, Weiss S. 2000. Mitotically active cells that generate neurons and astrocytes are present in multiple regions of the adult mouse hippocampus. J Comp Neurol 424: 397–408. [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. 2008. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci 11: 1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, Goetz M, Ninkovic J, Briancon N, Maratos-Flier E, Flier JS, et al. 2013. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun 4: 2049. [DOI] [PubMed] [Google Scholar]
- Sale A, Berardi N, Maffei L. 2009. Enrich the environment to empower the brain. Trends Neurosci 32: 233–239. [DOI] [PubMed] [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, et al. 2011. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LA, Ng KL, Kinyamu R, Whitaker-Azmitia P, Geisert EE, Blurton-Jones M, Zhou QY, Ribak CE. 2007. Origin, migration and fate of newly generated neurons in the adult rodent piriform cortex. Brain Struct Funct 212: 133–148. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Ng K, Zhou QY, Ribak CE. 2009. Subventricular zone-derived, newly generated neurons populate several olfactory and limbic forebrain regions. Epilepsy Behav 1: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereis J, Heintz T, Taylor MM, Ganat Y, Ment LR, Bordey A, Vaccarino F. 2010. Astroglial cells in the external granular layer are precursors of cerebellar granule neurons in neonates. Mol Cell Neurosci 44: 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohur US, Emsley JG, Mitchell BD, Macklis JD. 2006. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos Trans R Soc Lond B Biol Sci 361: 1477–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Ferreira L, Alvaro AR, Aveleira C, Santana M, Brandao I, Kugler S, de Almeida LP, Cavadas C. 2011. Proliferative hypothalamic neurospheres express NPY, AGRP, POMC, CART and Orexin-A and differentiate to functional neurons. PLoS ONE 6: e19745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Ferreira L, Almeida LP, Cavadas C. 2013. Role of hypothalamic neurogenesis in feeding regulation. Trends Endocrinol Metab 26: 80–88. [DOI] [PubMed] [Google Scholar]
- Spolidoro M, Sale A, Berardi N, Maffei L. 2009. Plasticity in the adult brain: Lessons from the visual system. Exp Brain Res 192: 335–341. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. 2007. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci 25: 3489–3498. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. 2006. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 24: 739–747. [DOI] [PubMed] [Google Scholar]
- Trotter J, Karram K, Nishiyama A. 2010. NG2 cells: Properties, progeny and origin. Brain Res Rev 63: 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessal M, Darian-Smith C. 2010. Adult neurogenesis occurs in primate sensorimotor cortex following cervical dorsal rhizotomy. J Neurosci 30: 8613–8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. 1996. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci 16: 7599–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner L, Muller-Fielitz H, Ritzal M, Werner T, Rossner M, Schwaninger M. 2012. Involvement of doublecortin-expressing cells in the arcuate nucleus in body weight regulation. Endocrinology 153: 2655–2664. [DOI] [PubMed] [Google Scholar]
- Wright J, Stanic D, Thompson LH. 2013. Generation of striatal projection neurons extends into the neonatal period in the rat brain. J Physiol 591: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C. 2005. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol 192: 251–264. [DOI] [PubMed] [Google Scholar]
- Yon MA, Mauger SL, Pickavance LC. 2013. Relationships between dietary macronutrients and adult neurogenesis in the regulation of energy metabolism. Br J Nutr 109: 1573–1589. [DOI] [PubMed] [Google Scholar]
- Yuan TF, Arias-Carrion O. 2011. Adult neurogenesis in the hypothalamus: Evidence, functions, and implications. CNS Neurol Disord Drug Targets 10: 433–439. [DOI] [PubMed] [Google Scholar]
- Zhao M, Janson Lang AM. 2009. Bromodeoxyuridine infused into the cerebral ventricle of adult mice labels nigral neurons under physiological conditions—A method to detect newborn nerve cells in regions with a low rate of neurogenesis. J Neurosci Methods 184: 327–331. [DOI] [PubMed] [Google Scholar]
- Zhao M, Momma S, Delfani K, Carlen M, Cassidy RM, Johansson CB, Brismar H, Shupliakov O, Frisen J, Janson AM. 2003. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci 100: 7925–7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupanc GK. 2006. Neurogenesis and neuronal regeneration in the adult fish brain. J Comp Physiol A 192: 649–670. [DOI] [PubMed] [Google Scholar]