Abstract
The neuromuscular junction (NMJ) is engineered to be a highly reliable synapse to carry the control of the motor commands of the nervous system over the muscles. Its development, organization, and synaptic properties are highly structured and regulated to support such reliability and efficacy. Yet, the NMJ is also highly plastic, able to react to injury and adapt to changes. This balance between structural stability and synaptic efficacy on one hand and structural plasticity and repair on another hand is made possible by the intricate regulation of perisynaptic Schwann cells, glial cells at this synapse. They regulate both the efficacy and structural plasticity of the NMJ in a dynamic, bidirectional manner owing to their ability to decode synaptic transmission and by their interactions via trophic-related factors.
The neuromuscular junction (NMJ) allows the motor commands of the nervous system to control the muscles. Perisynaptic Schwann cells confer structural stability and efficiency, as well as adaptability and plasticity, to the NMJ.
The vertebrate neuromuscular junction (NMJ), arguably the best characterized synapse in the peripheral nervous system (PNS), is composed of three closely associated cellular components: the presynaptic nerve terminal, the postsynaptic specialization, and nonmyelinating Schwann cells. These synapse-associated glial cells are called perisynaptic Schwann cells (PSCs), or terminal Schwann cells (see reviews by Todd and Robitaille 2006; Feng and Ko 2007; Griffin and Thompson 2008; Sugiura and Lin 2011). Multiple roles of PSCs have gained great appreciation since the 1990s and, along with the novel roles of astrocytes in central synapses, have led to the concept of the “tripartite” synapse (Araque et al. 1999, 2014; Volterra et al. 2002; Auld and Robitaille 2003; Kettenmann and Ransom 2013).
Thus, to fully understand synaptic formation and function, it is critical to also consider the active and essential roles of synapse-associated glial cells. We will discuss evidence supporting the existence of a synapse–glia–synapse regulatory loop that helps maintain and restore synaptic efficacy at the NMJ. We will also explore the multiple functions that PSCs exert, functions that are adapted to a given situation at the NMJ (e.g., synapse formation, stability, and reinnervation). This will highlight the great adaptability and plasticity of the morphological and functional properties of PSCs.
In this review, we will focus on the multiple roles PSCs play in synaptic formation, maintenance, remodeling, and regeneration, as well as synaptic function and plasticity. Based on the evidence presented, we propose a model in which PSCs, through specific receptor activation, play a prominent role in a continuum of synaptic efficacy, stability, and plasticity at the NMJ. These synaptic-regulated functions allow PSCs to orchestrate the stability and plasticity of the NMJ and, hence, are important for maintaining and adapting synaptic efficacy.
THE TRIPARTITE ORGANIZATION OF THE VERTEBRATE NEUROMUSCULAR JUNCTION
At the vertebrate NMJ, the motor nerve endings are capped by nonmyelinating Schwann cells, in contrast to the motor axons, which are wrapped around by myelinating Schwann cells (Corfas et al. 2004). The existence of PSCs was first suggested by Louis-Antoine Ranvier (1878), who reported clusters of “arborization nuclei,” which were distinct from muscle fiber nuclei, and were later identified as nuclei of “teloglia” or terminal Schwann cells at the NMJ (Couteaux 1938, 1960; Tello 1944; Boeke 1949; Ko et al. 2007; Griffin and Thompson 2008). The identity and intimate contacts of Schwann cells with the nerve terminals was further confirmed with transmission, scanning and freeze-fracture electron microscopies (Heuser et al. 1976; Desaki and Uehara 1981; Ko 1981).
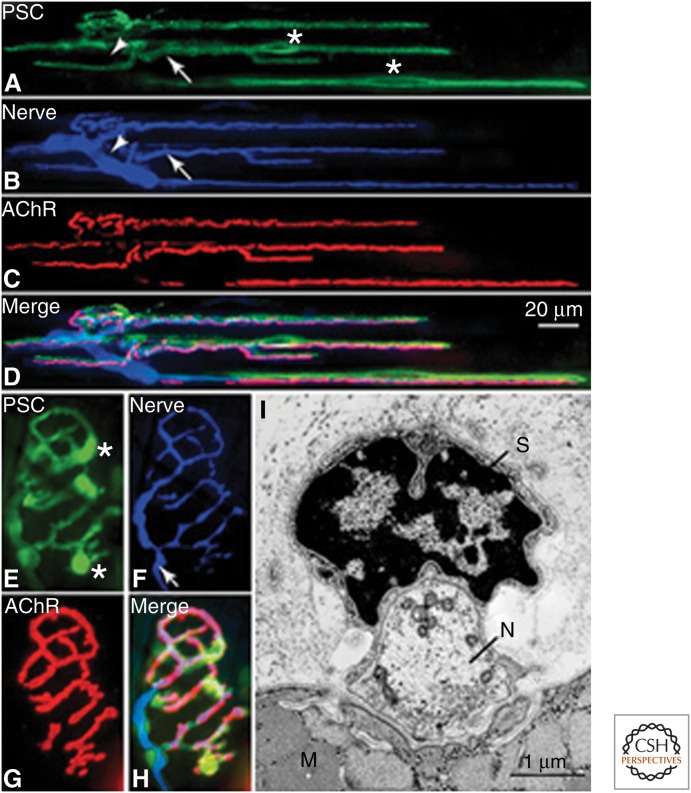
With the advance of immunofluorescence microscopy and the availability of fluorescent probes for PSCs, the tripartite nature of the vertebrate NMJ is further appreciated (Fig. 1). For amphibian muscles, two vital probes for PSCs, peanut agglutinin (PNA) (Ko 1987) and the monoclonal antibody (mAb) 2A12 (Astrow et al. 1998), have been particularly useful to reveal the tripartite organization of the NMJ and the dynamic relationship between PSCs and nerve terminals (see below). Figure 1A–D shows an example of a frog NMJ multiple labeled with mAb 2A12 for PSC somata (asterisks) and processes, with antineurofilament antibody for axons and antisynapsin I antibody for nerve terminals, and with α-bungarotoxin (α-BTX) for AChRs on muscle fibers. The merged fluorescent image (Fig. 1D) further reveals the tripartite arrangement, which can also be shown in the electron micrograph of a cross-section of the frog NMJ (Fig. 1I). Unfortunately, neither PNA nor mAb2A12 labels mammalian NMJs.
Figure 1.
The tripartite organization at the NMJ. (A–D) A frog NMJ fluorescently labeled with a monoclonal antibody (2A12) for PSCs (A, green), antineurofilament and antisynapsin I antibodies for nerve fibers and nerve terminals (B, blue), and α-bungarotoxin (α-BTX) for acetylcholine receptors (AChRs) (C, red). The merged picture (D) further shows the tripartite arrangement of the frog NMJ. PSC somata (asterisk in A) and processes (arrow in A) can be labeled with 2A12 antibody, which does not label Schwann cells along the axon (arrowheads in A and B). Scale bar in D also applies to A–C. (E–H) NMJ in a transgenic mouse that expresses green fluorescent protein (GFP) in Schwann cells (E, green) and cyan fluorescence protein (CFP) in nerve terminal (F, blue), and labeled with α-BTX for AChRs (G, red). Similar to frog NMJ shown in D, the merged picture (H) further illustrates these three closely associated elements at the mammalian NMJ. PSC somata (asterisk in E) and processes, including those associated with the preterminal axon (arrow in F), all express GFP. (I) Electron micrograph of a frog NMJ in cross section further confirms the tripartite arrangement with the PSC (S), capping the nerve terminal (N), which are in apposition with postjunctional folds on the muscle fiber (M). Scale bar, 1 µm. Glial cells maintain synaptic structure and function and promote development of the NMJ in vivo. (Panels A–H from Ko and Thompson 2003; reproduced, with permission, from the authors in conjunction with Springer Science and Business Media. Panel I from Reddy et al. 2003; reprinted, with permission, from the authors and Elsevier © 2003.)
For mammalian muscles, an antibody to the Ca2+ binding protein S100 (Reynolds and Woolf 1992) has been most commonly used for probing mammalian PSCs. Another very useful approach to label mammalian PSCs is the use of transgenic mice that express variants of the green fluorescent protein (GFP) family in axons and Schwann cells to view the dynamic behavior of axons and PSCs in living animals (Kang et al. 2003; Zuo et al. 2004; Li and Thompson 2011). Figure 1 shows an NMJ labeled with α-BTX for AChRs in a mouse that expresses GFP under the control of the S100β promoter in PSC somata (asterisks) and processes (Fig. 1E–H), and CFP in nerve terminal and the preterminal axon (arrow in Fig. 1F). The tripartite organization of the mouse NMJ is further shown in the merged image (Fig. 1H). There are other probes that can also label mammalian PSCs, for example, LNX-1 (an E3 ubiquitin ligase) (Young et al. 2005), NaV1.6 (Musarella et al. 2006), TrkC (Hess et al. 2007) in intact muscles, and antibodies to p75 neurotrophin receptor (Hassan et al. 1994), GAP-43 (Woolf et al. 1992), nestin (Kang et al. 2007), or transcription factor zinc-finger proliferation 1 (Ellerton et al. 2008) in denervated muscles.
It has been shown that there are ∼3–5 PSC somata in both frog and mammalian mature NMJs, and that the number of PSCs is correlated with the endplate size (Herrera et al. 1990; Love and Thompson 1998; Lubischer and Bebinger 1999; Jordan and Williams 2001). It is not clear why PSCs are nonmyelinating even though they can be labeled with antibodies to myelinating glial markers, such as protein zero (P0), myelin-associated glycoprotein (MAG), galactocerebroside, and 2′, 3′-cyclic nucleotide 3′-phosphodiesterase (Georgiou and Charlton 1999). It is also not well understood why PSCs cap, but do not enclose entirely, the motor nerve terminal. An otherwise complete enclosure of the nerve terminal would obviously severely compromise synaptic function. Frog PSCs, however, project finger-like processes, which contain L-type calcium channels (Robitaille et al. 1996), into the synaptic cleft and interdigitate with active zones—sites of transmitter release. In contrast, mammalian PSC “fingers” are usually excluded from the cleft, which may be attributed to laminin 11 (α5β2γ1) in the synaptic cleft (Patton et al. 1998). Besides the synaptic cleft and the muscle surface, the basal lamina also covers PSCs (Saito and Zacks 1969; Engel 1994). However, the extracellular matrix molecules associated with PSC basal lamina are distinct from those in the synaptic cleft and the extrasynaptic muscle surface (Ko 1987; Astrow et al. 1997; Patton et al. 1997; for review, see Patton 2003). It has been suggested that the PSC-associated extracellular matrix may play a role in guiding nerve terminal sprouts at the frog NMJ (Chen and Ko 1994; Ko and Chen 1996; see below). Interestingly, fibroblast-like cells (kranocytes) capping the NMJ have also been shown (Connor and McMahan 1987; Court et al. 2008).
ROLE OF PSCs IN SYNAPTOGENESIS
The intimate arrangement of the tripartite NMJ raises a question as to whether PSCs participate in synaptogenesis. To address this question, one needs to know first if Schwann cells are necessary during the initial navigation of axons to their target muscles (Keynes 1987). It has been shown that motor axons can reach their target muscles and even form the initial nerve–muscle contacts, albeit only transiently, in mutant mice of ErbB2 (Morris et al. 1999; Woldeyesus et al. 1999; Lin et al. 2000), ErbB3 (Riethmacher et al. 1997), and Splotch (Grim et al. 1992) mutant mice, all of which lack Schwann cells in the peripheral nerves. Furthermore, functional nerve–muscle contacts can be formed in cultures without Schwann cells (Kullberg et al. 1977; Chow and Poo 1985). These studies suggest that Schwann cells are not necessary for axonal pathfinding and the initial formation of nerve–muscle contacts.
Although Schwann cells are dispensable for the initial stages of NMJ formation, they play a critical role in promoting subsequent synaptic growth, maturation, and maintenance at developing NMJs. In frog muscles, PSCs appear shortly after the earliest discernible nerve–muscle contacts in tadpoles, and PSCs then quickly extend processes beyond nerve terminals (Herrera et al. 2000). The subsequent growth of nerve terminals appears to follow along the preceding PSC sprouts as shown with repeated in vivo observations of identified developing NMJs in tadpoles (Reddy et al. 2003). Combining repeated in vivo observations with an ablation technique that takes advantage of mAb2A12 and complement-mediated lysis to selectively ablate PSCs in vivo, Reddy et al. (2003) revealed major perturbations in NMJ structure and establishment, which further shows the critical role of PSCs in promoting synaptic growth and maintenance at developing amphibian NMJs in vivo.
PSCs also play an essential role in synaptogenesis in mammalian muscles (Griffin and Thompson 2008). Trachtenberg and Thompson (1996) showed that denervation in neonate, but not in adult, leads to rapid apoptosis of mammalian PSCs, and the apoptosis can be prevented by a glial growth factor, neuregulin 1 (NRG1). In addition, PSC morphology and NMJ structure can be altered by applications of NRG1 or Schwann cell transplants to mammalian muscles (Trachtenberg and Thompson 1997). The observation that partial denervation in neonatal but not in adult rat muscles results in apoptosis of PSCs, and absence of nerve terminal sprouting in neonatal muscles further confirms the importance of PSCs in promoting synaptic growth (Lubischer and Thompson 1999). Moreover, the lack of PSCs may also play a role in the withdrawal of nerve terminals following the initial formation of nerve–muscle contacts in ErbB2 and ErbB3 mutant mice (Riethmacher et al. 1997; Morris et al. 1999; Woldeyesus et al. 1999). Interestingly, Lee et al. (2011) have found that the PSCs are reduced in number and incompletely cover the endplate site in a mutant mouse model of spinal muscular atrophy. The PSC defects may contribute to the abnormal and delayed maturation of NMJs in this neuromuscular disease. Taken together, these studies suggest that PSCs are essential for the growth and maintenance of developing motor nerve terminals at both amphibian and mammalian muscles.
The molecular mechanisms of how PSCs participate in synaptic growth and maintenance at developing NMJs are not well understood. It has been shown that frog Schwann cells express active isoform of agrin and enhance AChR aggregation in muscle culture (Yang et al. 2001). Furthermore, Schwann cell–conditioned medium promotes synaptogenesis in Xenopus nerve–muscle cultures (Peng et al. 2003). In particular, Feng and Ko (2008) have shown, using Xenopus tissue culture, that Schwann cell–conditioned medium contains transforming growth factor (TGF)-β1. TGF-β1plays a necessary and sufficient role in promoting NMJ formation, and TGF-β ligands have been implicated in synaptic pruning in the developing visual system (Bialas and Stevens 2013) and synaptic growth in Drosophila (Fuentes-Medel et al. 2012). It has also been shown that Xenopus Schwann cell–conditioned medium can acutely enhance transmitter release in developing NMJs in culture (Cao and Ko 2007). However, the in vivo role of TGF-β or other Schwann cell–derived factors in synaptogenesis at NMJs remains to be examined.
One hallmark of the mammalian NMJ formation is the innervation of multiple nerve terminals at a single NMJ (polyneuronal innervation) and the subsequent removal of all but one of the nerve endings (synapse elimination) by the second week after birth (Sanes and Lichtman 1999). The potential role of PSCs in pruning excess nerve terminals at multiply innervated NMJs in postnatal muscles has been suggested (Griffin and Thompson 2008). For example, the retraction of nerve terminals and Schwann cell processes from the sites of synapse elimination occur at a similar time course (Culican et al. 1998). Interestingly, retracting nerve terminals shed numerous membrane-bound remnants called axosomes, which are engulfed by Schwann cells during synapse elimination (Bishop et al. 2004). Using time-lapse imaging of labeled single PSCs, Brill et al. (2011) have revealed that young PSCs intermingle dynamically in contrast to the static tile patterns seen in adult NMJs. A recent study using serial electron microscopy has further shown that PSCs participate in synapse elimination by phagocytosis of nerve terminals, although the process involves all axons and PSCs do not seem to select the winner of the competing developing nerve terminals (Smith et al. 2013). However, using simultaneous Ca2+ imaging of PSCs and synaptic recording of dually innervated mouse NMJs, Darabid et al. (2013) have shown that activity of single PSCs reflects the synaptic strength of each competing nerve terminal and the state of synaptic competition. Hence, PSCs decode synaptic transmission at a later stage of synaptic competition, allowing them to identify the strongest competing nerve terminal, which is likely to win the ongoing competition. Whether PSCs play an active and necessary role in synapse elimination remain to be further explored.
ROLE OF PSCs IN SYNAPTIC MAINTENANCE, REMODELING, AND REGENERATION AT ADULT NMJs
Maintenance
It is remarkable that the tripartite organization is maintained at the adult vertebrate NMJ despite the continual mechanical disruptions by muscle contractions throughout the animal’s life span. To address the question of whether PSCs play a role in synaptic maintenance, Reddy et al. (2003) took advantage of the selective labeling of PSCs with mAb2A12 and combined this with complement-mediated cell lysis to selectively ablate PSCs from frog NMJs in vivo. They observed no significant changes in synaptic structures and function shortly after PSC ablation (within 5 h). At mouse NMJs, Halstead et al. (2004, 2005) have shown that both NMJ morphology and synaptic transmission are also not acutely affected after selective PSC ablation with an autoantibody against disialosyl epitopes of gangliosides seen in Miller Fisher syndrome. This null effect may be caused by the ability of PSCs to both decrease and increase synaptic efficacy (see below; Robitaille 1998; Castonguay and Robitaille 2001; Todd et al. 2010), hence, resulting in no net change in the synaptic output at the NMJ. However, partial or total retraction of some nerve terminals and a ∼50% reduction in transmitter release were seen 1 wk after PSC ablation. These observations suggest that, although PSC may be dispensable for the short-term maintenance, they are essential for the long-term maintenance of frog NMJs. The long-term effect of PSC ablation on the maintenance of the mammalian NMJ remains to be investigated.
Remodeling
Although the tripartite arrangement is maintained at adult NMJs, nerve terminals at frog NMJs undergo extension and/or retraction throughout adult life (Wernig et al. 1980; Herrera et al. 1990; Chen et al. 1991). To address whether PSCs also undergo similar dynamic remodeling, repeated in vivo observations of identified frog NMJs double-labeled with a vital florescent dye for nerve terminals and PNA for PSCs have been shown (Chen et al. 1991; Chen and Ko 1994; Ko and Chen 1996). These studies have revealed that PSCs and associated extracellular matrix often lead, and may guide, the nerve terminal sprouts. The dynamic relationship between PSCs and nerve terminals has also been confirmed using direct injection of fluorescent dyes into adult frog PSCs and nerve terminals (Macleod et al. 2001; Dickens et al. 2003). These findings suggest that the dynamic behavior of PSCs may contribute to the constant remodeling of nerve terminals seen at amphibian NMJs. In contrast, mammalian NMJs are relatively stable (Lichtman et al. 1987; Wigston 1989). However, there are minor nerve terminal filopodia and lamellipodia adjacent to PSCs (Robbins and Polak 1988), which also protrude short and unstable processes beyond AChR clusters at mammalian NMJs (Zuo et al. 2004). It is still unclear why adult mammalian NMJs show fewer morphological remodeling than amphibian NMJs.
Degeneration and Regeneration
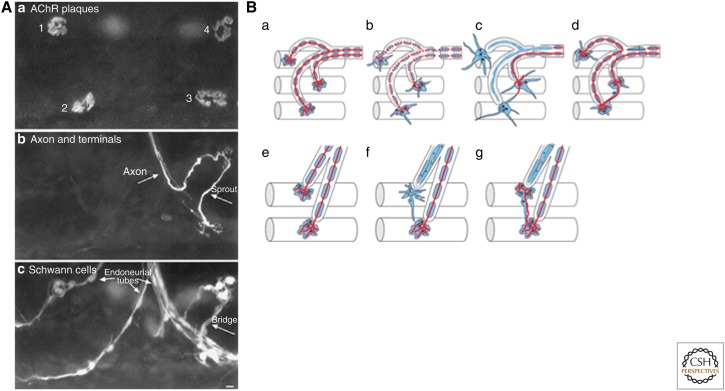
After nerve injury, nerve terminals degenerate and PSCs become phagocytic to remove debris of degenerating nerve terminals at denervated NMJs (Birks et al. 1960). It is interesting to note that Schwann cells at denervated NMJs can release acetylcholine (Dennis and Miledi 1974) although its functional significance is unknown. One seminal work that stimulated our current belief of the novel role of PSCs was the discovery of profuse sprouting of PSC processes shortly after denervation at the mammalian NMJ (Reynolds and Woolf 1992; Astrow et al. 1994; Son and Thompson 1995a,b). Furthermore, on reinnervation, nerve terminals grow along PSC “bridges” formed with PSC sprouts from adjacent denervated junction, and form the so-called “escaped fibers” to innervate the adjacent denervated endplates (Fig. 2A,Ba–d). A similar role of PSC “bridges” in guiding nerve terminal sprouts has also been shown after partial denervation at the adult NMJ (Fig. 2A,Be–g) (Son and Thompson 1995a,b; Love and Thompson 1999). The dynamic relationship between PSC and regenerating nerve terminals after nerve injury at NMJs has been examined with repeated in vivo observations of the same NMJs labeled with vital dyes (O’Malley et al. 1999; Koirala et al. 2000), or in transgenic mice that express GFP in Schwann cells and CYP in axons (Kang et al. 2003). These in vivo studies further confirm that PSC sprouts guide regenerating nerve terminals following nerve injury. It has been suggested that NRG1-ErbB signaling is involved in PSC sprouting, as exogenous application of NRG1 to neonatal muscles or expression of constitutively activated ErbB2 receptors in PSCs induces sprouting and migration of PSCs away from endplate sites (Trachtenberg and Thompson 1997; Hayworth et al. 2006; Moody et al. 2006).
Figure 2.
PSCs regulate NMJ repair and remodeling. (A) Sprouting after partial denervation. Four endplates are depicted in a rat soleus muscle (3 days after partial denervation) triple labeled with Cy5 conjugated α-BTX for AChRs (a), antibodies to neurofilament and synaptic vesicle protein (with an FITC-conjugated secondary antibody) for axons and nerve terminals (b), and antibody to S100 (with a rhodamine-conjugated secondary antibody) for PSCs and Schwann cells associated with the endoneurial tubes (c). Following partial denervation, Schwann cell processes extend profusely beyond the original endplate sites rich in AChR clusters (compare a and c). Although endplates 1 and 2 remain denervated, endplate 3 becomes innervated by a nerve sprout growing along a Schwann cell “bridge” linked to endplate 4, which is innervated (compare a and b). The role of PSCs in guiding nerve terminal sprouting is further depicted in a cartoon in Be–g. (From Love and Thompson 1999; reprinted, with permission, from the Society for Neuroscience © 1999.) (B) Schematic diagram summarizing the role of PSCs in reinnervation after nerve injury (a–d) and in sprouting after partial denervation (e–g) at mammalian NMJs. (a) Normal muscle fibers with intact NMJs (nerves in red, Schwann cells in blue). (b) PSCs sprout after nerve injury. (c) Regenerating nerve fibers grow along the endoneurial tubes and reinnervate synaptic sites (the middle muscle fiber). In addition, PSCs protrude processes further to form “bridges” connecting neighboring synaptic sites. (d) The PSC “bridges” guide regenerating nerve terminals to innervate adjacent endplates. The regenerating nerve fibers can continue to grow in a retrograde direction along other endoneurial tubes to innervate more endplates. (e) Normal muscle fibers with intact NMJs. (f) Partial denervation induces PSC sprouting. (g) Nerve terminals sprout along PSC bridges. (Panel B modified from data in Kang et al. 2003.)
The essential role of PSCs in synaptic repair has also been shown by the absence of nerve terminal sprouting following partial denervation when PSC bridge formation is blocked by direct stimulation or exercise of muscles (Love and Thompson 1999; Love et al. 2003; Tam and Gordon 2003). The importance of PSC sprouts has further been implicated in mdx mice (a model for Duchenne muscular dystrophy), in which presynaptic expression of neuronal nitric oxide synthase is decreased and formation of PSC “bridges” is impaired, suggesting that these defects may contribute to the less effective reinnervation and muscle weakness in these mutant muscles (Personius and Sawyer 2005; Marques et al. 2006). PSCs have also been shown to express the chemorepellent Semaphorin 3A in a subset of NMJs that are vulnerable in amyotrophic lateral sclerosis (ALS) (De Winter et al. 2006). Enhanced expression of a cell-surface glycoprotein, CD44, in PSCs in an ALS mouse model further suggests a potential role of PSCs in the motor neuron disease (Gorlewicz et al. 2009). Impaired PSC sprouting seen in aged muscles may also explain the poor reinnervation after nerve injury during aging (Kawabuchi et al. 2001). Besides guiding presynaptic nerve terminals, PSCs are thought to play a role in clustering postsynaptic AChRs by expressing neuronal isoforms of agrin at the frog NMJ (Yang et al. 2001). Furthermore, PSCs may play a role in the synthesis of AChRs by expressing neuregulin-2 at the mammalian NMJ (Rimer et al. 2004). Together, these results suggest that PSCs play an important role in synaptic repair following degeneration and disease at the NMJ.
PHYSIOLOGICAL ROLES OF PSCs IN SYNAPTIC FUNCTION
The roles of PSCs in the regulation of maintenance and morphological plasticity of the NMJ underline a large degree of plasticity in PSCs as they must be able to change their properties in various synaptic contexts. Furthermore, these properties imply that PSCs must be able to analyze the synaptic situation to adjust to the changing synaptic environment. To this end, PSCs decode synaptic properties of the NMJ by the detection of synaptic transmission, and attune to the fine changes that can take place. Hence, PSCs detect synaptic communication, decode the message and, in return, modulate synaptic properties in an intricate way adapted to the synaptic context.
PSCs DETECT SYNAPTIC TRANSMISSION
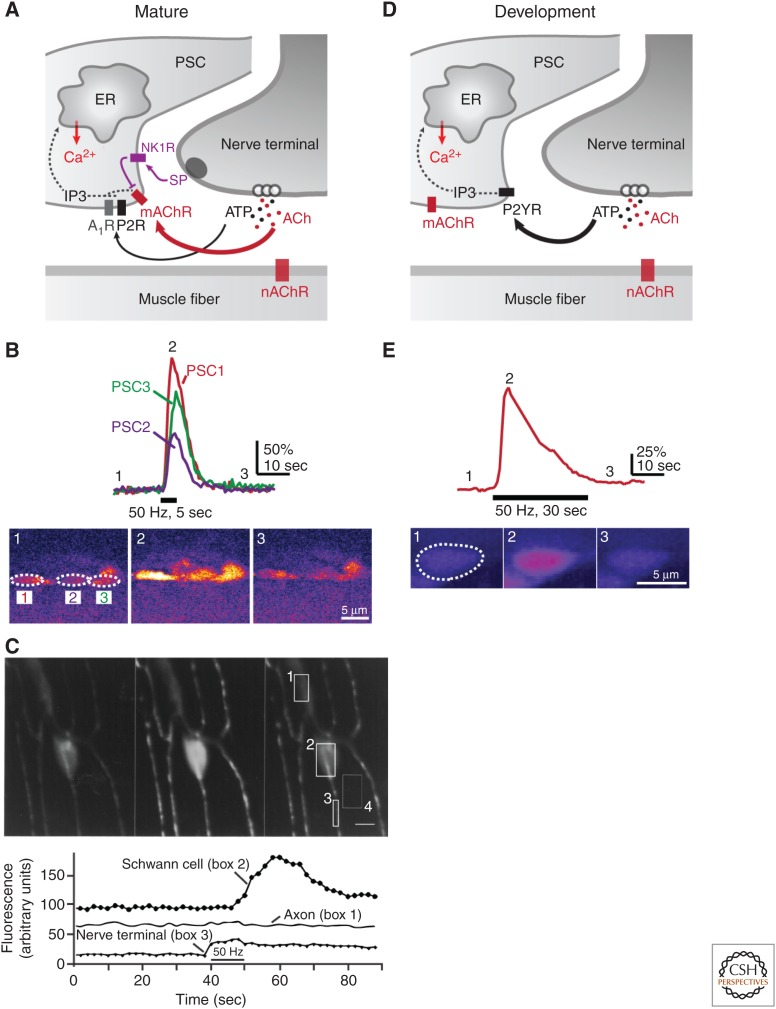
The development of fluorescent probes to detect free intracellular Ca2+ (Tsien 1981) has been a major advance for the study of the dynamic properties of glial cells and PSCs in particular. Indeed, the excitability of PSCs, like other glial cells, does not rely on electrical properties like neurons but rather on a biochemical excitability that largely relies on Ca2+-dependent mechanisms (Auld and Robitaille 2003; Araque et al. 2014). Observations at the vertebrate NMJ were among the first to show that glial cells associated with intact chemical synapses detected synaptic transmission via G protein–coupled receptors (GPCRs) that controlled internal stores of Ca2+ (Fig. 3A,C) (Jahromi et al. 1992; Reist and Smith 1992). PSCs at other vertebrate NMJs were also shown to detect the release of neurotransmitters on stimulation of the motor nerve (Fig. 3A,B) (Rochon et al. 2001; Lin and Bennett 2006; Todd et al. 2007, 2010).
Figure 3.
PSCs detect synaptic transmission. (A) Diagram depicting the receptors and their actions by which PSCs detect synaptic transmission at mature NMJ and the main regulatory mechanisms. (B) (Top) Changes in fluorescence of a Ca2+ indicator in PSCs of a mature mouse NMJ before, during, and after motor nerve stimulation. (Bottom) False color confocal images of the PSCs loaded with a Ca2+ indicator and from which the traces have been measured. (C) Images of an amphibian neuromuscular preparation showing the changes in fluorescence observed in the axonal compartment (1), the soma of a PSC (2), and the presynaptic terminal area (3) before, during, and after motor nerve stimulation (bar). (D) Diagram depicting the receptors and their actions by which PSCs detect synaptic transmission at developing NMJ. (E) (Top) Changes in fluorescence of a Ca2+ indicator in a PSC of an immature (P7) mouse NMJ before, during, and after motor nerve stimulation. (Bottom) False color confocal images of the PSCs loaded with a Ca2+ indicator and from which the traces have been measured. (Panel C from Reist and Smith 1992; reprinted, with permission, from the National Academy of Sciences.)
PSCs at mature amphibian and mouse NMJs possess muscarinic and purinergic receptors that regulate the release of Ca2+ from internal stores (Fig. 3A) (Robitaille 1995; Robitaille et al. 1997; Castonguay and Robitaille 2001; Rochon et al. 2001). At adult NMJs, detection of synaptic transmission by PSCs is mediated by muscarinic receptors (M1, M3, or M5) (Wright et al. 2009) and by purinergic receptors, in particular adenosine A1 receptors (Rochon et al. 2001). Although the characterization of the muscarinic receptor system follows a clear nomenclature, the properties of the purinergic receptor systems still elude a clear classification (Robitaille et al. 1997; Rochon et al. 2001; Rousse et al. 2010).
Consistent with their dynamic involvement in the regulation of the formation and maintenance of the NMJ, PSCs at immature NMJs (at postnatal day 7) also detect the activity of nerve terminals involved in synaptic competition at the mouse NMJ (Fig. 3D,E) (Darabid et al. 2013). Interestingly, the detection of synaptic activity is solely dependent on purinergic receptors, although muscarinic receptors are present and functional. This appears to be dependent upon the localization of the purinergic receptors close to active zones, whereas muscarinic receptors appear more evenly distributed over the PSCs (Darabid et al. 2013).
Interestingly, the biochemical excitability of PSCs, which allows them to detect neurotransmitter release, can be regulated. Indeed, Bourque and Robitaille (1998) showed that the peptide substance P released during sustained and intense synaptic activity at the mature amphibian NMJ caused a reduction in the sensitivity of the muscarinic detection, leading to a reduction in the size of the nerve-evoked Ca2+ responses in PSCs (Fig. 3A). Another molecule, nitric oxide (NO), acts in an autocrine manner. Descarries et al. (1998) observed that the synthesizing enzymes for NO are present in PSCs and that NO reduced the efficacy of ATP to elicit Ca2+ elevation in PSCs of mature amphibian NMJ.
Three major conclusions can be reached when comparing the properties of PSCs at different NMJs. First, the basic mechanisms are common throughout the different types of NMJ studied. Indeed, the detection of synaptic transmission by PSCs at adult NMJs is always carried by muscarinic and/or purinergic receptors (Robitaille 1995; Robitaille et al. 1997; Rochon et al. 2001; Colomar and Robitaille 2004; Darabid et al. 2013), indicating that fundamental mechanisms are preserved throughout a large sample of NMJs and developmental stages. Second, and somewhat contradictory, PSC properties are also tuned with the properties of the NMJ they are associated with. For instance, PSCs of weaker (e.g., soleus muscle) and stronger (levator auris longus [LAL] muscle) NMJs respond differently to nerve-evoked release of neurotransmitters where the weaker synapses systematically evoked smaller Ca2+ elevation in PSCs. These differences are largely the result of the different intrinsic properties of the PSCs at the different synapses (Rousse et al. 2010). Third, the excitability of PSCs can be dynamically modulated either by presynaptic signaling or in an autocrine manner, indicating that the properties of the PSCs and the possible resulting modulation can be adapted (Bélair et al. 2010). Hence, similar to the neuronal elements at the synapse, there are basic, fundamental mechanisms that drive PSCs excitability and responsiveness to synaptic activity, but these properties are in tune with the properties of the synapse they are associated with. This is a fundamental property because it implies that PSCs are adapted to a given synaptic environment and, hence, can participate to the regulation of NMJ properties in a precise and adapted manner.
PSCs DECODE SYNAPTIC PROPERTIES AND ACTIVITY
The different properties of PSCs according to the different synaptic context further suggested that the Ca2+-dependent biochemical excitability of PSCs allowed them to decode synaptic activity. Furthermore, owing to the impacts on cell activity of cytoplasmic changes of Ca2+ and the importance of the amplitude and kinetics of such changes, one could argue that such changes represent a code that reflects the level and type of synaptic activity.
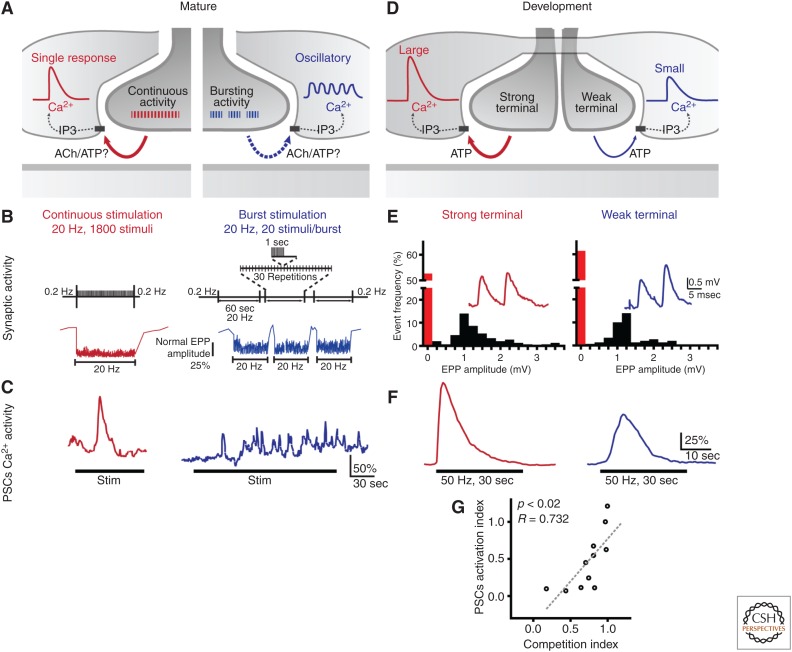
There are two recent observations that support this possibility. First, Todd et al. (2010) reported that PSCs at the mouse soleus NMJs detected two different patterns of synaptic activity with different kinetics and amplitude of Ca2+ responses (Fig. 4A–C). One pattern was a continuous stimulation (20 Hz, 90 sec) while the other generated a series of burst stimulation (same total duration and number of stimuli). The pattern of continuous stimulation generated a large phasic Ca2+ elevation, similar to those previously reported (Fig. 4B,C) (Jahromi et al. 1992; Robitaille 1995; Rochon et al. 2001; Todd et al. 2007, 2010). However, the bursting stimulation generated a sustained Ca2+ elevation on which irregular and small Ca2+ elevations were observed (Fig. 4B,C). This revealed that the different properties of synaptic signaling was decoded by PSCs and reflected in the differences in the timing, duration, and pattern of Ca2+ elevation.
Figure 4.
PSCs decode synaptic information. (A) Diagram depicting the Ca2+ responses in PSCs and the mechanisms involved when motor nerve activity is induced using two different patterns of stimulation (continuous or bursting activity) at mouse mature NMJs. (B) The bursting pattern consisting of 30 repetitions of 20 pulses at 20 Hz repeated every 2 sec and a continuous pattern of stimulation at 20 Hz for 90 sec. (C) Typical Ca2+ responses elicited by the bursting and the continuous motor nerve stimulation illustrated in B. Note the difference in the kinetics of the Ca2+ responses revealing the ability of PSCs to decode the pattern of synaptic activity. (D) Diagram depicting the Ca2+ responses in PSCs elicited by independent activity of competing nerve terminals (weak and strong) at NMJs during synapse formation. (E) Quantal analysis based on the failure rates of two competing inputs at an immature NMJ. Note the larger percentage of failures of the weak nerve terminal. (F) Independent Ca2+ responses in the PSC that covers the same two terminals (weak and strong) in E. Note the difference in the amplitude of the two responses, the stronger terminal eliciting a larger Ca2+ response. (G) A PSC activation index as a function of the synaptic strength index showing a continuum in the amplitude of Ca2+ responses as a function of the relative strength of competing nerve terminals. These results indicate that a single PSC can decipher the strength of nerve terminals competing for the territory at a same NMJ. (Panels B and C from data in Todd et al. 2010; and panels E–G from data in Darabid et al. 2013.)
PSCs ability to decode the nature of the synaptic properties was also unraveled during the course of synaptic competition that occurs postnatal at the NMJ (Fig. 4D–G). As indicated above, Darabid et al. (2013) studied PSCs ability to detect transmitter release evoked selectively by two nerve terminals competing for the same postsynaptic site. Ca2+ elevations were quite variable and were dependent on the synaptic strength (amount of transmitter release) of each nerve terminal (Fig. 4E,F). Indeed, the stronger nerve terminal (releasing more neurotransmitter) systematically induced larger Ca2+ responses than the weak nerve terminal (Fig. 4F) (Darabid et al. 2013). Importantly, PSC Ca2+ responses were unaltered in the presence of the K+ channel blocker (tetraethylamonium [TEA]), which increased transmitter release without affecting directly PSCs excitability (Rousse et al. 2010; Darabid et al. 2013). This indicates that differences in Ca2+ kinetics elicited by the two nerve terminals were also determined by intrinsic properties of PSCs. These results indicate that PSCs not only detect the two terminals, but also decode the ongoing competition.
As a whole, these observations indicate that PSCs, through dynamic Ca2+ regulation, decode synaptic communication in a given situation. This is particularly important when considering the PSCs as synaptic partners because their properties should be adapted to a given synaptic environment.
PSCs MODULATE SYNAPTIC ACTIVITY AND PLASTICITY
The ability of PSCs to be in tune with the properties of the synapse and decode the pattern of synaptic activity at adult NMJs and ongoing synaptic competition at developing NMJs are strong indicators that PSCs should be able, in return, to talk back to the pre- and postsynaptic elements and modulate the properties of the synaptic communication.
The first observation of synaptic activity modulation by PSCs was made by Robitaille (1998), using the amphibian NMJ. He showed that injection of molecules that increased G-protein activity specifically in PSCs reduced the amount of transmitter release (Fig. 5A). More importantly, he showed that blocking G-protein activation prevented a large portion of synaptic depression, a short-term synaptic plasticity that occurs at this synapse (Fig. 5B,C). Hence, this was one of the first examples of direct evidence that glial cells at an intact vertebrate synapse were controlling transmitter release and modulating synaptic plasticity. This piece of evidence was a key observation from which the concept of the “tripartite synapse” originated (Araque et al. 1999; Auld and Robitaille 2003). This provided a direct demonstration of the dynamic, bidirectional neuron–glia interactions that occur at the NMJ and further emphasizes that PSCs are active and competent synaptic partners at this synapse.
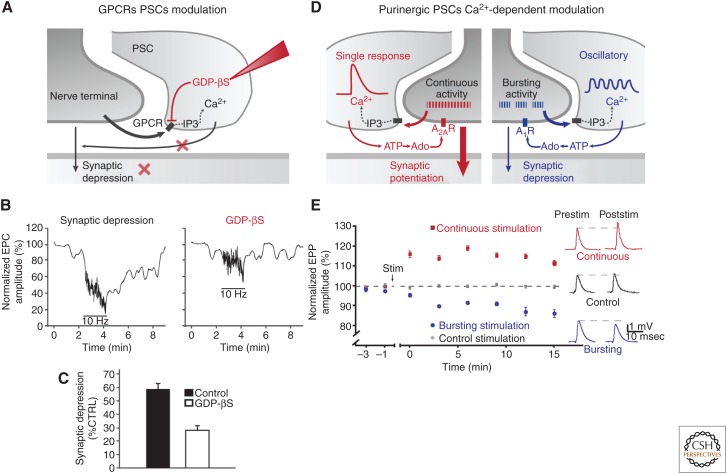
Figure 5.
PSCs modulate synaptic transmission and plasticity. (A) Diagram of the glial mechanisms involved in synaptic regulation at the mature amphibian NMJ on manipulation of GTP-binding proteins. (B) (Left) Relative endplate potential amplitude at the frog NMJ before (0.2 Hz), during (10 Hz, 90 sec), and after (0.2 Hz) high-frequency motor nerve stimulation. Note the occurrence of synaptic depression during the high-frequency stimulation. (Right) Same protocol performed on the same NMJ as in the left panel, but following the injection of GDP-βS in a PSC to block G-protein activity. (C) Histogram illustrating the average depression in control and after injection of GDP-βS in PSCs. Note that synaptic depression was significantly reduced following the selective G-protein blockade in PSCs. (D) Diagram of the glial mechanisms involved in synaptic regulation at the mature mouse NMJ as a result of the differential activation of PSCs by the two different patterns of stimulation. (E) Changes in EPP amplitude at mouse NMJ evoked by the two patterns of stimulation illustrated in Figure 4B. The continuous stimulation induced a long-lasting potentiation that was caused by the phasic and rapid Ca2+ elevation in PSC (inset), whereas the bursting pattern of stimulation induced a long-lasting depression that was caused by the small and sustained changes in Ca2+ in PSCs. Both forms of plasticity were altered when selectively blocking Ca2+ elevation in PSCs. These results indicate that, based on their decoding of synaptic activity, PSCs regulate synaptic efficacy and plasticity. Stim, stimulation. (Panels B and C from data in Robitaille 1998; and panel E from data in Todd et al. 2010.)
Synaptic plasticity at any synapses is often a balance of reduction (depression) and increase (potentiation) of synaptic efficacy. Hence, it was hypothesized that, if glial cells are indeed competent partners, they would also have the ability to increase synaptic efficacy. This was observed by Castonguay and Robitaille (2001), who showed that selectively chelating Ca2+ in PSCs of amphibian NMJs resulted in an increase in synaptic depression, suggesting that a potentiation event was perturbed.
These results indicate that PSCs have the ability to both decrease and increase synaptic efficacy, hence, fine tuning the net output at the NMJ regulating muscle functions. However, these results did not indicate that PSCs used this ability to simultaneously regulate synaptic efficacy in a given synaptic context. This was unraveled when studying the decoding ability of PSCs. As indicated above, Todd et al. (2010) observed that different patterns of synaptic activity elicited Ca2+ responses with different kinetics and amplitude (Figs. 4 and 5D). Concomitantly, these different patterns of motor nerve stimulation induced different forms of synaptic plasticity, such that the continuous stimulation produced a long-lasting potentiation, whereas the bursting pattern generated a long-lasting depression (Fig. 5D,E). Using selective blockade of Ca2+ elevation in PSCs through photo-activation of caged Ca2+, Todd et al. (2010) showed that the different Ca2+ signaling in PSCs were responsible for the different synaptic plasticity. This differential modulation was caused by the activation of different types of adenosine receptors (A1 receptors causing depression, A2A receptors causing the potentiation) on hydrolysis of ATP following its release by PSCs (Fig. 5D). Hence, not only do PSCs decode the ongoing synaptic transmission but, as a result, they also react differentially to produce an adapted modulation.
This modulation further illustrates that PSCs, much like other glial cells, release neuromodulatory substances identified as gliotransmitters (Araque et al. 2014). In addition to the involvement of ATP and adenosine in the differential modulation of synaptic transmission, observations from amphibian, lizard, and mouse NMJs indicated that PSCs may also produce and release other potential neuromodulatory substances, such as glutamate, prostaglandins, and nitric oxide (Descarries et al. 1998; Pinard et al. 2003; Pinard and Robitaille 2008; Lindgren et al. 2013). However, it is unclear whether PSCs combine any of these gliotransmitters and whether the same PSC can release them in a differential manner.
PLASTICITY OF PSC PROPERTIES
The results discussed above highlight the fine and efficient regulation of transmitter release by PSCs. In addition, acute modulations in the excitability of PSCs have been discussed, providing evidence that these cells are intrinsically plastic, capable of adapting to a changing synaptic environment. More importantly, it raises the question as to whether PSCs could undergo long term changes in their properties, allowing them to adjust to the changes in the synaptic properties themselves. Two sets of recent evidence supports this possibility.
First, Bélair et al. (2005, 2010) showed that the presynaptic properties of the amphibian NMJ undergo significant adaptation following two different in vivo approaches to alter long-term properties of the presynaptic release of neurotransmitters. Interestingly, PSC properties also underwent a long-term plasticity of their properties. These changes were not directly correlated with the level of transmitter release and instead involved an alteration of the muscarinic- and purinergic-dependent activation of PSCs (Bélair et al. 2010). Hence, this resulted in the alteration of the PSCs decoding ability and possibly of the outcome of their modulation of synaptic transmission. Importantly, these observations reveal that, similar to neurons, glial cells also undergo plastic changes in their properties. Furthermore, it remains to be determined whether the changes in PSCs properties contributed to the changes in the synaptic properties.
The second evidence of long-term plasticity of PSC properties originates from the study of their properties during synapse development. Indeed, it was shown that PSCs detect transmitter release mainly via purinergic receptors during synaptic competition (Darabid et al. 2013) even though muscarinic receptors are present and functional. This is quite different from the situation at mature NMJ in which PSCs detection of synaptic activity heavily relies on the activation of muscarinic receptors. This switch of the type of signaling mechanisms during the maturation of the synapse reflects the adaptation of the function of these cells from the context of synapse formation to a stable and synaptically reliable one.
Hence, not only do PSCs interact dynamically and in a bidirectional manner with the pre- and postsynaptic elements of the NMJ, but also these interactions are highly plastic indicating that PSCs regulation of the NMJ properties can also be adaptive.
PSCs INTEGRATE SYNAPTIC ACTIVITY TO ESTABLISH SYNAPTIC PROPERTIES
The two main roles of PSCs at the NMJ (i.e., morphological stability/plasticity and synaptic regulation) appear as two independent functions. However, a number of observations indicate that in fact both functions are tightly linked and are essential for the balance between synaptic efficacy and stability and synaptic plasticity and repair. Indeed, the same receptor systems that PSCs use to detect and decode synaptic transmission are also used to regulate a number of genes involved in their reaction on injury (Georgiou et al. 1994, 1999). In fact, using the amphibian NMJ model, these investigators have shown that interruption of synaptic communication is sufficient to trigger an injury-like response in PSCs. This was mediated specifically by the muscarinic receptors, not the purinergic ones, and appears not to depend directly on Ca2+, but rather on CREB-like regulation pathways. Interestingly, Wright et al. (2009) observed that the blockade of muscarinic receptors in vivo induced injury-related changes in PSCs, in particular an abundant level of PSC process sprouting that is normally observed after axonal injury (Son and Thompson 1995b). This suggests that the muscarinic receptor system is particularly important in regulating the PSCs in a mode of maintenance and regulation of synaptic efficacy. Consistent with the data at mature NMJs, it is remarkable that in condition when important changes occur at the NMJ such at developing NMJs during synaptic competition, only purinergic receptors (not the muscarinic ones) are actively recruited by synaptic transmission. Hence, it appears that the contribution of muscarinic receptors is much reduced in situations in which major morphological and functional rearrangement of the NMJs are required (synapse formation or after injury). However, it is unclear whether these changes in receptor activation are caused by the level of receptor expression, the type of receptors, and/or the cellular mechanisms they control. Furthermore, the regulation of PSCs excitability by trophic factors, such as neurotrophin-3 (NT-3), brain-derived neurotophic factor (BDNF), or nerve growth factor (NGF) (Todd et al. 2007), that are also involved in NMJ formation and stabilization also points to the possibility that PSCs two main functions are very interdependent.
We propose a model to integrate the different functions and properties of PSCs according to the different functional states of the NMJ. We propose that activation of PSCs by GPCRs determines the balance between synaptic efficacy/maintenance and remodeling/repair (Fig. 6). At adult NMJ, normal synaptic activity would be detected by a set of muscarinic and purinergic receptors that would regulate the feedback modulation to synaptic functions (modulation, left loop). However, the same receptors also impose a regulation of the expression of a number of genes that allow PSCs to ensure the maintenance and efficacy of the NMJ (maintenance, right loop). On injury or diseases, the balance between muscarinic and purinergic receptor activation would be impaired, altering the gene regulation, thus allowing a change in PSCs phenotype that would allow them to enter into a repair mode (repair, far right loop). Ultimately, this would allow the NMJ to be repaired and synaptic communication reestablished. At this point, PSCs would regain their normal functions (left and right loops).
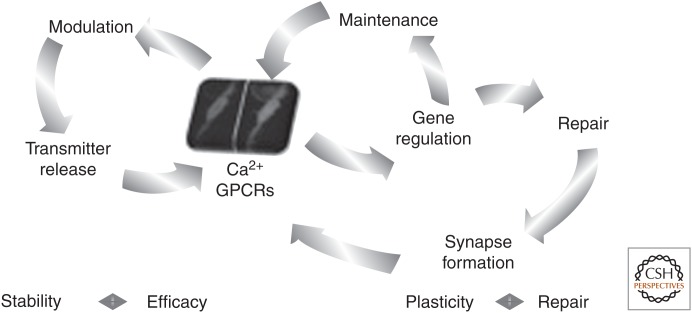
Figure 6.
Model of PSCs balanced regulation of NMJ stability and plasticity. PSCs (illustrated as a responding cell) detect synaptic activation through activation of G protein–coupled receptors leading to the activation of Ca2+-dependent events that lead to the modulation of synaptic transmission and plasticity (modulation, left loop). The same receptor activation also leads to the regulation of the expression of a number of genes that promote PSCs activity to sustain maintenance and stability of the NMJ (maintenance, right loop). However, on dysregulation of transmitter release or following injury, the signaling in PSCs is perturbed, leading to a change in the gene regulation and a switch of PSC phenotype from maintenance to repair (repair, far right loop). This repair mode includes removing of remnants of injured nerve terminals and PSC bridging processes to facilitate nerve terminal sprouting toward denervated endplates. Hence, PSCs can integrate both the efficacy and the plasticity of the NMJ to establish the appropriate response according to the state of the NMJ. GPCRs, G protein–coupled receptors.
CONCLUDING REMARKS
PSCs have two main critical functions at the NMJ. First, they control synapse stability. During development, they guide growing nerve terminals and are essential for synaptic growth and maintenance at developing NMJs. Chronic absence of PSCs results in retraction of nerve terminals and reduction in synaptic function, suggesting a long-term maintenance role of PSCs. Similar to developing NMJs, PSCs in adult muscles guide nerve terminal growth during synaptic sprouting and repair after nerve injury. Second, they control synapse plasticity. Owing to their dynamic detection of synaptic transmission, PSCs can control both the efficacy and the maintenance of the NMJ. This surveillance allows them to alter their properties to allow for synapse repair on injury or other weakening of the synapse. The future challenges would be to unravel the molecular mechanisms of synapse–glia interactions and their likely involvement in neurodegenerative diseases.
ACKNOWLEDGMENTS
We thank Dr. Zhihua Feng, Dr. Clare Reynell, and Houssam Darabid for their critical comments. We also thank Houssam Darabid for his help in the preparation of the figures and Danielle Arbour for the data of Figure 3B. This work is supported by Grants from the National Institutes of Health (NIH) to C.-P.K., and the Canadian Institutes for Health Research to R.R. (MOP-14137 and MOP-111070), a Leader Opportunity Fund from the Canadian Foundation of Innovation, and an Infrastructure Grant from Fonts Recherche Quebec-Santé (FRQ-S) to the Groupe de Recherche sur le Système Nerveux Central.
Footnotes
Editors: Ben A. Barres, Marc R. Freeman, and Beth Stevens
Additional Perspectives on Glia available at www.cshperspectives.org
REFERENCES
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. 1999. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci 22: 208–215. [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SHR, Robitaille R, Volterra A. 2014. Gliotransmitters travel in time and space. Neuron 81: 728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrow SH, Son YJ, Thompson WJ. 1994. Differential neural regulation of a neuromuscular junction-associated antigen in muscle fibers and Schwann cells. J Neurobiol 25: 937–952. [DOI] [PubMed] [Google Scholar]
- Astrow SH, Tyner TR, Nguyen MT, Ko CP. 1997. A Schwann cell matrix component of neuromuscular junctions and peripheral nerves. J Neurocytol 26: 63–75. [DOI] [PubMed] [Google Scholar]
- Astrow SH, Qiang H, Ko CP. 1998. Perisynaptic Schwann cells at neuromuscular junctions revealed by a novel monoclonal antibody. J Neurocytol 27: 667–681. [DOI] [PubMed] [Google Scholar]
- Auld DS, Robitaille R. 2003. Perisynaptic Schwann cells at the neuromuscular junction: Nerve- and activity-dependent contributions to synaptic efficacy, plasticity, and reinnervation. Neuroscientist 9: 144–157. [DOI] [PubMed] [Google Scholar]
- Bélair E-L, Vallée J, Robitaille R. 2005. Long-term in vivo modulation of synaptic efficacy at the neuromuscular junction of Rana pipiens frogs. J Physiol 569.1: 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélair E-L, Vallée J, Robitaille R. 2010. Bidirectional plasticity of glial cells induced by chronic treatments in vivo. J Physiol 588.7: 1039–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialas AR, Stevens B. 2013. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci 16: 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Birks R, Katz B, Miledi R. 1960. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol 150: 145–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. 2004. Axon branch removal at developing synapses by axosome shedding. Neuron 44: 651–661. [DOI] [PubMed] [Google Scholar]
- Boeke J. 1949. The sympathetic endformation, its synaptology, the interstitial cells, the periterminal network, and its bearing on the neurone theory. Discussion and critique. Acta Anatomica 8: 18–61. [DOI] [PubMed] [Google Scholar]
- Bourque MJ, Robitaille R. 1998. Endogenous peptidergic modulation of perisynaptic Schwann cells at the frog neuromuscular junction. J Physiol 512: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill MS, Lichtman JW, Thompson W, Zuo Y, Misgeld T. 2011. Spatial constraints dictate glial territories at murine neuromuscular junctions. J Cell Biol 195: 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Ko CP. 2007. Schwann cell-derived factors modulate synaptic activities at developing neuromuscular synapses. J Neurosci 27: 6712–6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castonguay A, Robitaille R. 2001. Differential regulation of transmitter release by presynaptic and glial Ca2+ internal stores at the neuromuscular synapse. J Neurosci 21: 1911–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Ko CP. 1994. Extension of synaptic extracellular matrix during nerve terminal sprouting in living frog neuromuscular junctions. J Neurosci 14: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Folsom DB, Ko CP. 1991. The remodeling of synaptic extracellular matrix and its dynamic relationship with nerve terminals at living frog neuromuscular junctions. J Neurosci 11: 2920–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow I, Poo MM. 1985. Release of acetylcholine from embryonic neurons upon contact with muscle cell. J Neurosci 5: 1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomar A, Robitaille R. 2004. Glial modulation of synaptic transmission at the neuromuscular junction. Glia 47: 284–289. [DOI] [PubMed] [Google Scholar]
- Connor EA, McMahan UJ. 1987. Cell accumulation in the junctional region of denervated muscle. J Cell Biol 104: 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G, Velardez MO, Ko CP, Ratner N, Peles E. 2004. Mechanisms and roles of axon–Schwann cell interactions. J Neurosci 24: 9250–9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court FA, Gillingwater TH, Melrose S, Sherman DL, Greenshields KN, Morton AJ, Harris JB, Willison HJ, Ribchester RR. 2008. Identity, developmental restriction and reactivity of extralaminar cells capping mammalian neuromuscular junctions. J Cell Sci 121: 3901–3911. [DOI] [PubMed] [Google Scholar]
- Couteaux R. 1938. Sur l’origine de la sole des plaques motrices. CR Soc Biol 127: 218–221. [Google Scholar]
- Couteaux R. 1960. Motor end-plate structure. The Structure and function of muscle (ed. Bourne GH), pp. 337–380. Academic, New York. [Google Scholar]
- Culican SM, Nelson CC, Lichtman JW. 1998. Axon withdrawal during synapse elimination at the neuromuscular junction is accompanied by disassembly of the postsynaptic specialization and withdrawal of Schwann cell processes. J Neurosci 18: 4953–4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabid H, Arbour D, Robitaille R. 2013. Glial cells decipher synaptic competition at the mammalian neuromuscular junction. J Neurosci 33: 1297–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis MJ, Miledi R. 1974. Electrically induced release of acetylcholine from denervated Schwann cells. J Physiol 237: 431–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaki J, Uehara Y. 1981. The overall morphology of neuromuscular junctions as revealed by scanning electron microscopy. J Neurocytol 10: 101–110. [DOI] [PubMed] [Google Scholar]
- Descarries LM, Cai S, Robitaille R. 1998. Localization and characterization of nitric oxide synthase at the frog neuromuscular junction. J Neurocytol 27: 829–40. [DOI] [PubMed] [Google Scholar]
- De Winter F1, Vo T, Stam FJ, Wisman LA, Bär PR, Niclou SP, van Muiswinkel FL, Verhaagen J. 2006. The expression of the chemorepellent Semaphorin 3A is selectively induced in terminal Schwann cells of a subset of neuromuscular synapses that display limited anatomical plasticity and enhanced vulnerability in motor neuron disease. Mol Cell Neurosci 32: 102–117. [DOI] [PubMed] [Google Scholar]
- Dickens P, Hill P, Bennett MR. 2003. Schwann cell dynamics with respect to newly formed motor-nerve terminal branches on mature (Bufo marinus) muscle fibers. J Neurocytol 32: 381–392. [DOI] [PubMed] [Google Scholar]
- Ellerton EL, Thompson WJ, Rimer M. 2008. Induction of zinc-finger proliferation 1 expression in non-myelinating Schwann cells after denervation. Neuroscience 153: 975–985. [DOI] [PubMed] [Google Scholar]
- Engel AG. 1994. The neuromuscular junction. In Myology (ed. Engel AG, Franzini-Armstrong C). McGraw-Hill, New York. [Google Scholar]
- Feng Z, Ko CP. 2007. Neuronal glia interactions at the vertebrate neuromuscular junction. Curr Opin Pharmacol 7: 316–324. [DOI] [PubMed] [Google Scholar]
- Feng Z, Ko CP. 2008. Schwann cells promote synaptogenesis at the neuromuscular junction via transforming growth factor-β1. J Neurosci 28: 9599–9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Medel Y, Ashley J, Barria R, Maloney R, Freeman M, Budnik V. 2012. Integration of a retrograde signal during synapse formation by glia-secreted TGF-β ligand. Curr Biol 22: 1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou J, Charlton MP. 1999. Non-myelin-forming perisynaptic Schwann cells express protein zero and myelin-associated glycoprotein. Glia 27: 101–109. [DOI] [PubMed] [Google Scholar]
- Georgiou J, Robitaille R, Trimble WS, Charlton MP. 1994. Synaptic regulation of glial protein expression in vivo. Neuron 12: 443–455. [DOI] [PubMed] [Google Scholar]
- Georgiou J, Robitaille R, Charlton MP. 1999. Muscarinic control of cytoskeleton in perisynaptic glia. J Neurosci 19: 3836–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlewicz A, Wlodarczyk J, Wilczek E, Gawlak M, Cabaj A, Majczynski H, Nestorowicz K, Herbik MA, Grieb P, Slawinska U, et al. 2009. CD44 is expressed in non-myelinating Schwann cells of the adult rat, and may play a role in neurodegeneration-induced glial plasticity at the neuromuscular junction. Neurobiol Dis 34: 245–258. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Thompson WJ. 2008. Biology and pathology of nonmyelinating Schwann cells. Glia 56: 1518–1531. [DOI] [PubMed] [Google Scholar]
- Grim M, Halata Z, Franz T. 1992. Schwann cells are not required for guidance of motor nerves in the hindlimb in Splotch mutant mouse embryos. Anat Embryol (Berl) 186: 311–318. [DOI] [PubMed] [Google Scholar]
- Halstead SK, O’Hanlon GM, Humphreys PD, Morrison DB, Morgan BP, Todd AJ, Plomp JJ, Willison HJ. 2004. Anti-disialoside antibodies kill perisynaptic Schwann cells and damage motor nerve terminals via membrane attack complex in a murine model of neuropathy. Brain 127: 2109–2123. [DOI] [PubMed] [Google Scholar]
- Halstead SK, Morrison I, O’Hanlon GM, Humphreys PD, Goodfellow JA, Plomp JJ, Willison HJ. 2005. Anti-disialosyl antibodies mediate selective neuronal or Schwann cell injury at mouse neuromuscular junctions. Glia 52: 177–189. [DOI] [PubMed] [Google Scholar]
- Hassan SM, Jennekens FG, Veldman H, Oestreicher BA. 1994. GAP-43 and p75NGFR immunoreactivity in presynaptic cells following neuromuscular blockade by botulinum toxin in rat. J Neurocytol 23: 354–363. [DOI] [PubMed] [Google Scholar]
- Hayworth CR, Moody SE, Chodosh LA, Krieg P, Rimer M, Thompson WJ. 2006. Induction of neuregulin signaling in mouse Schwann cells in vivo mimics responses to denervation. J Neurosci 26: 6873–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera AA, Banner LR, Nagaya N. 1990. Repeated, in vivo observation of frog neuromuscular junctions: Remodelling involves concurrent growth and retraction. J Neurocytol 19: 85–99. [DOI] [PubMed] [Google Scholar]
- Herrera AA, Qiang H, Ko CP. 2000. The role of perisynaptic Schwann cells in development of neuromuscular junctions in the frog (Xenopus laevis). J Neurobiol 45: 237–254. [DOI] [PubMed] [Google Scholar]
- Hess DM, Scott MO, Potluri S, Pitts EV, Cisterni C, Balice-Gordon RJ. 2007. Localization of TrkC to Schwann cells and effects of neurotrophin-3 signaling at neuromuscular synapses. J Comp Neurol 501: 465–482. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS, Landis DM. 1976. Preservation of synaptic structure by rapid freezing. Cold Spring Harb Symp Quant Biol 40: 17–24. [DOI] [PubMed] [Google Scholar]
- Jahromi BS, Robitaille R, Charlton MP. 1992. Transmitter release increases intracellular calcium in perisynaptic Schwann cells in situ. Neuron 8: 1069–1077. [DOI] [PubMed] [Google Scholar]
- Jordan CL, Williams TJ. 2001. Testosterone regulates terminal Schwann cell number and junctional size during developmental synapse elimination. Dev Neurosci 23: 441–451. [DOI] [PubMed] [Google Scholar]
- Kang H, Tian L, Thompson W. 2003. Terminal Schwann cells guide the reinnervation of muscle after nerve injury. J Neurocytol 32: 975–985. [DOI] [PubMed] [Google Scholar]
- Kang H, Tian L, Son YJ, Zuo Y, Procaccino D, Love F, Hayworth C, Trachtenberg J, Mikesh M, Sutton L, et al. 2007. Regulation of the intermediate filament protein nestin at rodent neuromuscular junctions by innervation and activity. J Neurosci 27: 5948–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabuchi M, Zhou CJ, Wang S, Nakamura K, Liu WT, Hirata K. 2001. The spatiotemporal relationship among Schwann cells, axons and postsynaptic acetylcholine receptor regions during muscle reinnervation in aged rats. Anat Rec 264: 183–202. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Ransom BR. 2013. Neuroglia. Oxford University Press, New York. [Google Scholar]
- Keynes RJ. 1987. Schwann cells during neural development and regeneration: Leaders or followers? Trends Neurosci 10: 137–139. [Google Scholar]
- Ko CP. 1981. Electrophysiological and freeze-fracture studies of changes following denervation at frog neuromuscular junctions. J Physiol 321: 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CP. 1987. A lectin, peanut agglutinin, as a probe for the extracellular matrix in living neuromuscular junctions. J Neurocytol 16: 567–576. [DOI] [PubMed] [Google Scholar]
- Ko CP, Chen L. 1996. Synaptic remodeling revealed by repeated in vivo observations and electron microscopy of identified frog neuromuscular junctions. J Neurosci 16: 1780–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CP, Thompson W. 2003. Special issue—The neuromuscular junction. J Neurocytol 32: 423–1037. [PubMed] [Google Scholar]
- Ko CP, Sugiura Y, Feng Z. 2007. The biology of perisynaptic (terminal) Schwann cells. In Biology of Schwann cells (ed. Armati PJ), pp. 72–99. Cambridge University Press, New York. [Google Scholar]
- Koirala S, Qiang H, Ko CP. 2000. Reciprocal interactions between perisynaptic Schwann cells and regenerating nerve terminals at the frog neuromuscular junction. J Neurobiol 44: 343–360. [DOI] [PubMed] [Google Scholar]
- Kullberg RW, Lentz TL, Cohen MW. 1977. Development of the myotomal neuromuscular junction in Xenopus laevis: An electrophysiological and fine-structural study. Dev Biol 60: 101–129. [DOI] [PubMed] [Google Scholar]
- Lee YI, Mikesh M, Smith I, Rimer M, Thompson W. 2011. Muscles in a mouse model of spinal muscular atrophy show profound defects in neuromuscular development even in the absence of failure in neuromuscular transmission or loss of motor neurons. Dev Biol 356: 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Thompson WJ. 2011. Nerve terminal growth remodels neuromuscular synapses in mice following regeneration of the postsynaptic muscle fiber. J Neurosci 31: 13191–13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman JW, Magrassi L, Purves D. 1987. Visualization of neuromuscular junctions over periods of several months in living mice. J Neurosci 7: 1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YQ, Bennett MR. 2006. Schwann cells in rat vascular autonomic nerves activated via purinergic receptors. Neuroreport 17: 531–535. [DOI] [PubMed] [Google Scholar]
- Lin W, Sanchez HB, Deerinck T, Morris JK, Ellisman M, Lee KF. 2000. Aberrant development of motor axons and neuromuscular synapses in erbB2-deficient mice. Proc Natl Acad Sci 97: 1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren CA, Newman ZL, Morford JJ, Ryan SB, Battani KA, Su Z. 2013. Cyclooxygenase-2, prostaglandin E2 glycerol ester and nitric oxide are involved in muscarine-induced presynaptic enhancement at the vertebrate neuromuscular junction. J Physiol 591: 4749–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love FM, Thompson WJ. 1998. Schwann cells proliferate at rat neuromuscular junctions during development and regeneration. J Neurosci 18: 9376–9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love FM, Thompson WJ. 1999. Glial cells promote muscle reinnervation by responding to activity-dependent postsynaptic signals. J Neurosci 19: 10390–10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love FM, Son YJ, Thompson WJ. 2003. Activity alters muscle reinnervation and terminal sprouting by reducing the number of Schwann cell pathways that grow to link synaptic sites. J Neurobiol 54: 566–576. [DOI] [PubMed] [Google Scholar]
- Lubischer JL, Bebinger DM. 1999. Regulation of terminal Schwann cell number at the adult neuromuscular junction. J Neurosci 19: RC46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubischer JL, Thompson WJ. 1999. Neonatal partial denervation results in nodal but not terminal sprouting and a decrease in efficacy of remaining neuromuscular junctions in rat soleus muscle. J Neurosci 19: 8931–8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod GT, Dickens PA, Bennett MR. 2001. Formation and function of synapses with respect to Schwann cells at the end of motor nerve terminal branches on mature amphibian (Bufo marinus) muscle. J Neurosci 21: 2380–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques MJ1, Pereira EC, Minatel E, Neto HS. 2006. Nerve-terminal and Schwann-cell response after nerve injury in the absence of nitric oxide. Muscle Nerve 34: 225–231. [DOI] [PubMed] [Google Scholar]
- Moody SE, Chodosh LA, Krieg P, Rimer M, Thompson WJ. 2006. Induction of neuregulin signaling in mouse Schwann cells in vivo mimics responses to denervation. J Neurosci 26: 6873–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Lin W, Hauser C, Marchuk Y, Getman D, Lee KF. 1999. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron 23: 273–283. [DOI] [PubMed] [Google Scholar]
- Musarella M, Alcaraz G, Caillol G, Boudier JL, Couraud F, Autillo-Touati A. 2006. Expression of Nav1.6 sodium channels by Schwann cells at neuromuscular junctions: Role in the motor endplate disease phenotype. Glia 53: 13–23. [DOI] [PubMed] [Google Scholar]
- O’Malley JP, Waran MT, Balice-Gordon RJ. 1999. In vivo observations of terminal Schwann cells at normal, denervated, and reinnervated mouse neuromuscular junctions. J Neurobiol 38: 270–286. [PubMed] [Google Scholar]
- Patton BL. 2003. Basal lamina and the organization of neuromuscular synapses. J Neurocytol 32: 883–903. [DOI] [PubMed] [Google Scholar]
- Patton BL, Miner JH, Chiu AY, Sanes JR. 1997. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol 139: 1507–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton BL, Chiu AY, Sanes JR. 1998. Synaptic laminin prevents glial entry into the synaptic cleft. Nature 393: 698–701. [DOI] [PubMed] [Google Scholar]
- Peng HB, Yang JF, Dai Z, Lee CW, Hung HW, Feng ZH, Ko CP. 2003. Differential effects of neurotrophins and Schwann cell-derived signals on neuronal survival/growth and synaptogenesis. J Neurosci 23: 5050–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Personius KE, Sawyer RP. 2005. Terminal Schwann cell structure is altered in diaphragm of mdx mice. Muscle Nerve 32: 656–663. [DOI] [PubMed] [Google Scholar]
- Pinard A, Robitaille R. 2008. Postsynaptic nitrinergic modulation underlies glutamate-induced synaptic depression at a vertebrate neuromuscular junction. Eur J Neurosci 28: 577–587. [DOI] [PubMed] [Google Scholar]
- Pinard A, Lévesque S, Vallée J, Robitaille R. 2003. Glutamatergic modulation of synaptic plasticity at a PNS vertebrate cholinergic synapse. Eur J Neurosci 18: 3241–3250. [DOI] [PubMed] [Google Scholar]
- Ranvier L. 1878. Leçons sur l’histologie du système nerveux. F. Savy, Paris. [DOI] [PubMed] [Google Scholar]
- Reddy LV, Koirala S, Sugiura Y, Herrera AA, Ko CP. 2003. Glial cells maintain synaptic structure and function and promote development of the neuromuscular junction in vivo. Neuron 40: 563–580. [DOI] [PubMed] [Google Scholar]
- Reist NE, Smith SJ. 1992. Neurally evoked calcium transients in terminal Schwann cells at the neuromuscular junction. Proc Natl Acad Sci 89: 7625–7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ML, Woolf CJ. 1992. Terminal Schwann cells elaborate extensive processes following denervation of the motor endplate. J Neurocytol 21: 50–66. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. 1997. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature 389: 725–730. [DOI] [PubMed] [Google Scholar]
- Rimer M, Prieto AL, Weber JL, Colasante C, Ponomareva O, Fromm L, Schwab MH, Lai C, Burden SJ. 2004. Neuregulin-2 is synthesized by motor neurons and terminal Schwann cells and activates acetylcholine receptor transcription in muscle cells expressing ErbB4. Mol Cell Neurosci 26: 271–281. [DOI] [PubMed] [Google Scholar]
- Robbins N, Polak J. 1988. Filopodia, lamellipodia and retractions at mouse neuromuscular junctions. J Neurocytol 17: 545–561. [DOI] [PubMed] [Google Scholar]
- Robitaille R. 1995. Purinergic receptors and their activation by endogenous purines at perisynaptic glial cells of the frog neuromuscular junction. J Neurosci 15: 7121–7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R. 1998. Modulation of synaptic efficacy and synaptic depression by glial cells at the frog neuromuscular junction. Neuron 21: 847–855. [DOI] [PubMed] [Google Scholar]
- Robitaille R, Bourque MJ, Vandaele S. 1996. Localization of L-type Ca2+ channels at perisynaptic glial cells of the frog neuromuscular junction. J Neurosci 16: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R, Jahromi BS, Charlton MP. 1997. Muscarinic Ca2+ responses resistant to muscarinic antagonists at perisynaptic Schwann cells of the frog neuromuscular junction. J Physiol 504: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon D, Rousse I, Robitaille R. 2001. Synapse–glia interactions at the mammalian neuromuscular junction. J Neurosci 21: 3819–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousse I, St-Amour A, Darabid H, Robitaille R. 2010. Synapse–glia interactions are governed by synaptic and intrinsic glial properties. Neuroscience 167: 621–32. [DOI] [PubMed] [Google Scholar]
- Saito A, Zacks SI. 1969. Ultrastructure of Schwann and perineural sheaths at the mouse neuromuscular junction. Anat Rec 164: 379–390. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. 1999. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci 22: 389–442. [DOI] [PubMed] [Google Scholar]
- Smith IW, Mikesh M, Lee Y, Thompson WJ. 2013. Terminal Schwann cells participate in the competition underlying neuromuscular synapse elimination. J Neurosci 33: 17724–17736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YJ, Thompson WJ. 1995a. Nerve sprouting in muscle is induced and guided by processes extended by Schwann cells. Neuron 14: 133–141. [DOI] [PubMed] [Google Scholar]
- Son YJ, Thompson WJ. 1995b. Schwann cell processes guide regeneration of peripheral axons. Neuron 14: 125–132. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Lin W. 2011. Neuron-glia interactions: The roles of Schwann cells in neuromuscular synapse formation and function. Biosci Rep 31: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SL, Gordon T. 2003. Neuromuscular activity impairs axonal sprouting in partially denervated muscles by inhibiting bridge formation of perisynaptic Schwann cells. J Neurobiol 57: 221–234. [DOI] [PubMed] [Google Scholar]
- Tello JF. 1944. Sobre una vaina que envuelve toda la ramificacion del axon en las terminaciones motrices de los musculos estriados. Trabajos Inst Cajal Invest Biol (Madrid) 36: 1–59. [Google Scholar]
- Todd KJ, Robitaille R. 2006. Neuron-glia interactions at the neuromuscular synapse. Novartis Found Symp 276: 222–229; discussion 229–237, 275–281. [PubMed] [Google Scholar]
- Todd KJ, Auld DS, Robitaille R. 2007. Differential acute neurotrophin signalling to synaptic glia at the mouse neuromuscular junction. Eur J Neurosci 25: 1287–1296. [DOI] [PubMed] [Google Scholar]
- Todd KJ, Darabid H, Robitaille R. 2010. Perisynaptic glia discriminate patterns of motor nerve activity and influence plasticity at the neuromuscular junction. J Neurosci 30: 11870–11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Thompson WJ. 1996. Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature 379: 174–177. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Thompson WJ. 1997. Nerve terminal withdrawal from rat neuromuscular junctions induced by neuregulin and Schwann cells. J Neurosci 17: 6243–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. 1981. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature 290: 527–528. [DOI] [PubMed] [Google Scholar]
- Volterra A, Magistretti PJ, Haydon PG. 2002. The tripartite synapse: Glia in synaptic transmission. Oxford University Press, New York. [Google Scholar]
- Wernig A, Pecot-Dechavassine M, Stover H. 1980. Sprouting and regression of the nerve at the frog neuromuscular junction in normal conditions and after prolonged paralysis with curare. J Neurocytol 9: 278–303. [DOI] [PubMed] [Google Scholar]
- Wigston DJ. 1989. Remodeling of neuromuscular junctions in adult mouse soleus. J Neurosci 9: 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldeyesus MT, Britsch S, Riethmacher D, Xu L, Sonnenberg-Riethmacher E, Abou-Rebyeh F, Harvey R, Caroni P, Birchmeier C. 1999. Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev 13: 2538–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Reynolds ML, Chong MS, Emson P, Irwin N, Benowitz LI. 1992. Denervation of the motor endplate results in the rapid expression by terminal Schwann cells of the growth-associated protein GAP-43. J Neurosci 12: 3999–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MC, Wright MC, Potluri S, Wang X, Dentcheva E, Gautam D, Tessler A, Wess J, Rich MM, Son YJ. 2009. Distinct muscarinic acetylcholine receptor subtypes contribute to stability and growth, but not compensatory plasticity, of neuromuscular synapses. J Neurosci 29: 14942–14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Cao G, Koirala S, Reddy LV, Ko CP. 2001. Schwann cells express active agrin and enhance aggregation of acetylcholine receptors on muscle fibers. J Neurosci 21: 9572–9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Nie J, Wang X, McGlade CJ, Rich MM, Feng G. 2005. LNX1 is a perisynaptic Schwann cell specific E3 ubiquitin ligase that interacts with ErbB2. Mol Cell Neurosci 30: 238–248. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lubischer JL, Kang H, Tian L, Mikesh M, Marks A, Scofield VL, Maika S, Newman C, Krieg P, et al. 2004. Fluorescent proteins expressed in mouse transgenic lines mark subsets of glia, neurons, macrophages, and dendritic cells for vital examination. J Neurosci 24: 10999–11009. [DOI] [PMC free article] [PubMed] [Google Scholar]