SUMMARY
Vertebrate reproduction requires a myriad of precisely orchestrated events—in particular, the maternal production of oocytes, the paternal production of sperm, successful fertilization, and initiation of early embryonic cell divisions. These processes are governed by a host of signaling pathways. Protein kinase and phosphatase signaling pathways involving Mos, CDK1, RSK, and PP2A regulate meiosis during maturation of the oocyte. Steroid signals—specifically testosterone—regulate spermatogenesis, as does signaling by G-protein-coupled hormone receptors. Finally, calcium signaling is essential for both sperm motility and fertilization. Altogether, this signaling symphony ensures the production of viable offspring, offering a chance of genetic immortality.
From hormone-initiated and kinase/phosphatase-controlled gamete maturation, to calcium-induced capacitation and fertilization, a host of signaling pathways ensures that reproduction occurs only under optimal conditions.
1. Introduction
Mammalian reproduction depends on the proper development and maturation of both the female egg and the male sperm. These gametes fuse through a complex series of events, known as fertilization, that ensure the highest quality of offspring. Both gamete development and fertilization depend on numerous connected signaling pathways, a flaw in any of which can lead to infertility or birth defects.
The egg and sperm are haploid germ cells that, upon fertilization, reconstitute a diploid cell—the embryo. Production of haploid gametes from diploid precursors requires a modified cell cycle known as meiosis. Before meiosis, the full complement of parental chromosomes is first duplicated in S phase, to produce so-called sister chromatids (i.e., four copies of each chromosome per cell) and paternal and maternal chromosomes pair up. The homologous chromosomes from each chromosome pair are then separated in the first meiotic M phase (meiosis I, also known as MI). Subsequently, without further replication, the cells reenter M phase (meiosis II, also known as MII) to divide the sister chromatids equally into four haploid daughter cells. For male gametes, four mature sperm are generated, whereas in the female, a single final gamete (the egg) is produced together with three polar bodies. Movement through these stages of meiosis is carefully controlled by kinases, phosphatases, ubiquitin-dependent degradation of key regulators, and calcium flux.
Before acquiring the capacity to fertilize eggs, a sperm must reside in the female reproductive tract and undergo physiological changes that render it fertilization competent (Bedford 1970). The acquisition of fertilization competence and the biochemical, membrane, and enzymatic changes that underlie it are collectively known as capacitation (Austin 1951; Chang 1951). As with gamete development and maturation, capacitation and fertilization depend on careful regulation through signaling pathways. These include pathways involving gonadotropins, G-protein-coupled receptors (GPCRs), kinases, and calcium signaling (Salicioni et al. 2007).
2. Oocyte maturation
Oocyte maturation has been most extensively studied in the frog Xenopus laevis, because its very large oocytes allow both physical manipulation of the cell (microinjection of proteins, RNAs, and antisense oligonucleotides) and observation of the progression through meiosis with the naked eye. Although some notable differences have been observed, genetic studies in mammals (primarily mouse) have revealed similar overall regulation of meiotic progression (Fig. 1).
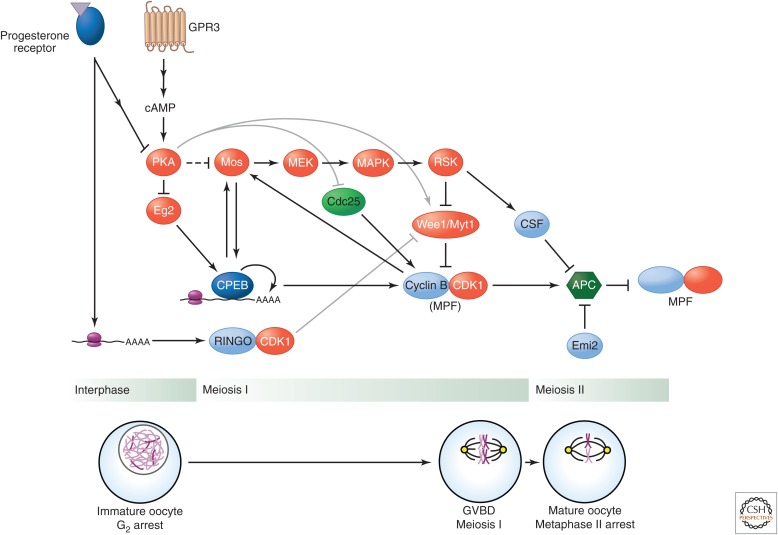
Figure 1.
Regulation of oocyte maturation. Immature oocytes are held in G2 arrest through the activity of PKA, which is stimulated by GPR3-dependent production of cAMP. Progesterone signaling leads to a loss of PKA activity, leading to disinhibition of the CDK1 activator Cdc25 and stimulation of the CDK1 inhibitor Wee1. Additionally, progesterone stimulates translation of both Mos and cyclin B proteins (the former also stimulating translation of the latter) through induction of Eg2, which phosphorylates and activates CPEB to unmask the messages and promote their polyadenylation. In parallel, progesterone stimulates translation of RINGO, which can activate CDK1 independently of cyclin and make it phosphorylate and inhibit the CDK1 inhibitor Myt1. All of these events result in activation of MPF, which drives the oocyte into MI. Maturation continues via the Mos-MEK-ERK pathway as shown. Blockade of APC (anaphase-promoting complex) activity by CSF and Emi2 holds the mature oocyte at a second arrest until the time of fertilization.
Oocytes and sperm both begin life as primordial germ cells (PGCs) that migrate to the nascent gonads (ovaries in females, testes in males) in early embryonic development. Under the influence of a variety of cytokines and growth factors, PGCs that will become oocytes continue dividing mitotically within cell clusters. In oogenesis, the premeiotic S phase is followed by a prolonged arrest in prophase I of meiosis until sexual maturity. During this phase, the oocyte is maintained in a G2-phase-arrested state through G-protein-coupled signaling (see below). When mitosis ceases, these oocytes each become surrounded by somatic granulosa and theca cells, which form the primordial follicles that serve as repositories of dormant oocytes for later ovulation. Oocytes nestled within the follicles grow and stockpile nutrients until they become competent to undergo maturation; upon receipt of appropriate hormonal signals, one follicle from the larger pool will mature fully during each menstrual cycle in the mammal. Stimulated by pituitary hormones (gonadotropins) and as a consequence of maturation-inducing steroid hormones (e.g., progesterone) synthesized by the ovarian follicle cells, the oocyte exits prophase arrest, and progresses through MI, transitioning promptly to MII without any intervening DNA replication. At MII, the oocyte arrests again awaiting fertilization.
The end product of oocyte maturation is a haploid egg capable of being fertilized. A strong MII arrest helps to prevent parthenogenesis, which is the aberrant entry of the haploid egg into the mitotic cell cycle in the absence of fertilization. In an effort to define the factors responsible for MII arrest, Masui and colleagues injected extract prepared from a mature M-phase-arrested frog egg into blastomeres formed after the first embryonic cell division (Masui and Markert 1971); injected cells remained arrested in M phase, whereas the uninjected cells continued to divide. These experiments helped to identify both maturation promoting factor (MPF), which drives entry into both MI and MII during oocyte development, and the cytostatic factor (CSF), which maintains MII arrest. We now know that MPF is equivalent to the complex of cyclin B and cyclin-dependent kinase 1 (cyclin-B–CDK1) that drives entry into mitosis in the somatic cell cycle (Dunphy et al. 1988; Labbe et al. 1989; Rhind and Russell 2012). CSF was shown to be the kinase Mos, the cellular counterpart of the viral oncoprotein v-Mos, which is expressed primarily in germ cells (Propst et al. 1987; Sagata et al. 1989). Proper maturation from MI entry through MII arrest depends on tightly controlled temporal regulation of both cyclin-B–CDK1 and Mos activity.
2.1. Meiosis I
During MI, oocytes are maintained in the G2-arrested state by high levels of cytosolic cAMP. Constitutive signaling by the GPCR GPR3 probably stimulates adenylyl cyclase to keep cAMP levels high. Indeed, overexpression of GPR3 in frog oocytes makes them resistant to the maturation effects of progesterone, and in mice lacking GPR3, oocytes mature in the absence of additional stimuli (Freudzon et al. 2005; Hinckley et al. 2005; Mehlmann 2005; Deng et al. 2008). Although sphingosine 1-phosphate and sphingosylphosphorylcholine have been proposed as GPR3 ligands, unliganded GPR3 appears to be able to stimulate adenylyl cyclase and thus the role of GPR3 in maintenance of G2 arrest is not entirely clear (Eggerickx et al. 1995; Uhlenbrock et al. 2002; Hinckley et al. 2005). Progesterone stimulation seems to antagonize the GPR3 signal, triggering a decrease in cAMP levels that is at least partly mediated by stimulation of phosphodiesterases that degrade cAMP (primarily PDE3 in the oocytes) (Tsafriri et al. 1996). This leads to a diminution of protein kinase A (PKA) activity. If PDE3 is artificially inhibited or cAMP synthesis is artificially stimulated, progesterone-induced maturation can be blocked. Conversely, injection of oocytes with the PKA inhibitor PKI can promote resumption of meiosis even without progesterone stimulation (Stanford et al. 2003).
As in the mitotic cell cycle, cyclin-B–CDK1 activity is controlled by phosphorylation of CDK1 on Y15 by Wee1-family kinases, which is opposed by the Cdc25 phosphatase (Watanabe et al. 1995; Berry and Gould 1996). The Wee1 relative Myt1 contributes to suppressing CDK1 phosphorylation, and the Cdc25c isoform mediates its subsequent dephosphorylation. In mice, this process is mediated by oocyte-specific isoforms: WEE1B and CDC25B. Loss of WEE1B irreversibly arrests oocytes in prophase (Han et al. 2005). In the G2-arrested oocyte, PKA directly phosphorylates Cdc25 on S287 (Xenopus numbering), promoting the binding of the small acidic protein 14-3-3 (Duckworth et al. 2002). 14-3-3 interferes with the ability of Cdc25 to interact with and dephosphorylate cyclin-B–CDK1 and prevents its translocation into the nucleus, where it would promote rapid cyclin-B–CDK1 activation (Kumagai and Dunphy 1999; Lopez-Girona et al. 1999; Yang et al. 1999). Thus, the drop in PKA activity required for maturation promotes Cdc25 activation. PKA also phosphorylates and activates Wee1/Myt1 (Stanford and Ruderman 2005); so the drop in PKA also promotes CDK1 activation by alleviating its suppression by these kinases.
The formation and activity of MPF is also regulated by translation of cyclin B, whose mRNA is translationally dormant before the induction of oocyte maturation owing to its very short poly-A tail. At the time of oocyte maturation, cis-acting sequences within the 3′ UTR of the cyclin B mRNA promote cytoplasmic polyadenylation, elongating the tail more than 100 nucleotides. These cytoplasmic polyadenylation elements (CPEs) within the 3′ UTR of the mRNA are bound by CPE-binding protein (CPEB) (Hake and Richter, 1994). Through a process that is not entirely clear, the drop in PKA activity that heralds the onset of oocyte maturation also induces activation of a kinase, Eg2, which phosphorylates CPEB, activating it to both unmask the mRNA and recruit a poly(A) polymerase to elongate the poly(A) tail (Andresson and Ruderman 1998; Frank-Vaillant et al. 2000; Hodgman et al. 2001).
Note that in every species there is at least some preformed cyclin-B–CDK1 complex (known as pre-MPF) whose activity is suppressed by phosphorylation of CDK1 at T14 and Y15. Indeed, the initial discovery of MPF relied on the ability of the injected MPF to mobilize the pre-MPF pool through autoamplification (Masui and Markert 1971; Drury and Schorderet-Slatkine 1975; Wasserman and Masui 1975). Phosphorylation by cyclin-B–CDK1 suppresses Wee1/Myt1 and activates Cdc25, which promotes more conversion of pre-MPF to MPF. In species in which most of the CDK1 is bound to cyclin B in pre-MPF complexes, new cyclin B translation is not absolutely required for induction of oocyte maturation; however, in those species that have low amounts of pre-MPF and high levels of free CDK1, cyclin B synthesis is an obligate step in maturation (Jagiello 1969; Fulka et al. 1986; Moor and Crosby 1986; Hunter and Moor 1987; Gautier and Maller 1991; Mattioli et al. 1991).
In mammals, the signal emanating from a loss of PKA activity may be conveyed directly to CDK1 via Wee1B, as this appears to be a direct PKA target. For Myt1, there is evidence for indirect pathways of inhibition. First, soon after progesterone treatment, a non-cyclin alternative activator of CDK1 known as RINGO is translated (Ferby et al. 1999). This protein can bind to and activate CDK1, causing it to phosphorylate and suppress Myt1 (Ruiz et al. 2008). This, in turn, leads to activation of cyclin-B–CDK1 complexes. A second pathway is an oocyte-specific MAP kinase (MAPK) cascade involving the MAPKKK Mos, the MAPKK MEK, and the MAPK ERK. In frogs, the terminal effector in this pathway is RSK, which can phosphorylate and inhibit Myt1 (Palmer et al. 1998). RSK can also phosphorylate Cdc25, contributing to its activation. Accordingly, injection of activated RSK into Xenopus oocytes can induce meiotic maturation and RSK inhibition interferes with progesterone-induced maturation. In mice, alternative pathways must operate (e.g., the direct inhibition of WEE1B by PKA, as described above), because mice lacking all known isoforms of RSK do not show defects in oocyte maturation (Dumont et al. 2005).
In some species, including Xenopus, activation of the Mos-ERK pathway precedes completion of MI and breakdown of the nuclear envelope (known as germinal vesicle breakdown [GVBD] in oocytes). In others, it occurs after GVBD (because cyclin-B–CDK1 can actually activate ERK). Whether the Mos-MEK-ERK-RSK pathway is involved at MI depends on the organism, and Mos accumulation is controlled by multiple mechanisms (reviewed in Fan and Sun 2004). The 3′ end of the Mos mRNA in the immature oocyte has a short poly(A) tail whose elongation (necessary for efficient translation) is masked through binding of CPEB. In addition to the Eg2-induced phosphorylation of CPEB, which unmasks and enhances the translation of Mos (Mendez et al. 2000), the stability of Mos protein is greatly enhanced by phosphorylation at S3 as both dephosphorylation of this residue and the presence of a proline at residue 2 are required for recognition by the ubiquitin-proteasome degradation system (Nishizawa et al. 1993). This site is phosphorylated in a positive-feedback loop by ERK, which stabilizes Mos. Where Mos accumulates only after GVBD, it is phosphorylated at the same site by cyclin-B–CDK1. Together, increased translation and stabilization promote Mos accumulation during maturation. Note that, in Xenopus, redundant pathways allow MI progression even when Mos is ablated; yet, the kinetics are delayed, indicating that Mos normally enhances meiotic progression in this species. Indeed, when either cyclin B synthesis or Mos synthesis is impaired, progesterone-induced GVBD can proceed, but ablation of both abolishes this.
2.2. MI-MII Transition
Mos appears to be more widely important for MII. For example, unlike RSK-knockout mice, Mos-knockout mice are sterile and oocytes fail to mature properly (Colledge et al. 1994; Hashimoto et al. 1994). To understand the role of Mos, we must first consider cyclin B dynamics in meiosis. At the time of exit from MI, cyclin B must be degraded by the anaphase-promoting complex (APC), a multisubunit E3 ubiquitin ligase also known as the cyclosome. However, because cyclin-B–CDK1 inhibits formation of prereplicative complexes necessary for DNA replication, complete loss of cyclin-B–CDK1 kinase activity (as occurs in a somatic mitosis) would result in reinitiation of S phase. Thus, cyclin B translation is ramped up immediately after GVBD. Moreover, there must be sufficiently rapid reaccumulation of cyclin B to drive MII. Mos participates in two ways: it helps to drive cyclin B synthesis, and it helps control cyclin B degradation. This allows partial but not complete loss of cyclin B at MI exit—a decrease sufficient to exit MI but not initiate S phase—followed by unimpeded cyclin B accumulation to drive MII.
A key effector of this pathway is an inhibitor of the APC known as Emi2 (reviewed in Wu and Kornbluth 2008). Emi2 binds directly to the APC, inhibiting its ability to ubiquitylate substrates and so cause their proteasomal degradation (e.g., cyclin B). Emi2 is also regulated at the level of protein stability and is a substrate of another multisubunit E3 ubiquitin ligase, SCFβ–TrCP (Liu and Maller 2005; Rauh et al. 2005; Hansen et al. 2006). Recognition by this E3 ligase, which requires a phosphodegron in its targets, depends on phosphorylation of Emi2 at critical sites within the amino-terminal half of the protein, catalyzed by cyclin-B–CDK1. It is this feedback phosphorylation of Emi2 that helps to precisely control cyclin B levels. As cyclin B is synthesized, it binds to and activates its partner CDK1. Active cyclin-B–CDK1 complexes phosphorylate Emi2, promoting its degradation, thereby alleviating suppression of the APC to allow cyclin B degradation and MI exit. If Emi2 is artificially stabilized at this transition, it causes MI arrest by preventing cyclin B degradation (Fig. 2).
Figure 2.
Regulation of Emi2 and the APC during the MI–MII transition. Phosphorylation controls Emi2 stability during oocyte maturation. At MI, CDK1 phosphorylates four amino-terminal sites (S213, T239, T252, and T267) on Emi2; this triggers Emi2 degradation, required for MI exit. At MI anaphase, cyclin B is degraded, leading to a drop in CDK1 activity. Emi2 is stabilized by dephosphorylation triggered by Mos signaling. Emi accumulates, resulting in APC inhibition, critical for S phase block and MII entry. CDK1 activity is low in MII relative to MI. Emi2 is stable in MII, as required for CSF arrest. At fertilization, Emi2 is quickly degraded through a CaMKII-mediated pathway, allowing activation of the APC and exit from MII. At the onset of MI anaphase, APC-mediated cyclin B degradation results in decreased CDK1 activity. With the Mos-PP2A pathway predominant, dephosphorylated and stabilized Emi2 protein prevents complete ubiquitylation of cyclin B by the APC. This is essential for the inhibition of S phase between MI and MII. (From Tang et al. 2008; adapted, with permission.)
If cyclin-B–CDK1 phosphorylation of Emi2 were unopposed, then ultimately accumulation of cyclin B would eradicate Emi2, allowing complete, rather than the required partial, cyclin B degradation. However, the Mos-ERK pathway interferes at this point. Emi2 is phosphorylated directly by RSK, the kinase downstream from ERK. RSK-mediated phosphorylation of Emi2 promotes docking of the protein phosphatase PP2A on Emi2 (Wu et al. 2007b). PP2A, in turn, dephosphorylates the sites on Emi2 that are phosphorylated by cyclin-B–CDK1, thereby stabilizing Emi2 and restoring its inhibitory binding to the APC (Tang et al. 2008). This allows the accumulation of cyclin B necessary for blocking S phase and, ultimately, for entry into MII. Mos may also promote inhibition of Myt1 and consequently T14 and Y15 dephosphorylation and activation of CDK1. This could help to activate cyclin-B–CDK1 as cyclin B accumulates. As MI is completed, cyclin B synthesis is markedly enhanced; eventually, this exceeds the ability of the APC to keep pace (even without Emi2 inhibition), cyclin B is degraded, and MII ensues. Although the general role of Emi2 appears to be conserved in mammals, the pathway appears to be somewhat different from that in frogs; because RSK is dispensable, another downstream target of Mos, MEK or ERK, probably phosphorylates Emi2 and recruits PP2A.
2.3. MII Arrest
Once MII is initiated, the oocyte arrests again, this time as a mature egg awaiting fertilization. Mos and Emi2 are also central to the activity that maintains this arrest (Kanki and Donoghue 1991; Hashimoto et al. 1994; Dupre et al. 2002; Madgwick et al. 2006; Ohe et al. 2007). The critical nature of Mos is clear because removal of Mos from egg extracts by immunodepletion destroys CSF activity (Daar et al. 1991), and eggs from mice or frogs lacking Mos fail to arrest in MII (Colledge et al. 1994; Hashimoto et al. 1994; Araki et al. 1996). The target of the Mos-MAPK cascade that produces the MII arrest is again the APC. Indeed, when radiolabeled cyclin B is injected into Xenopus eggs, treatment with the MEK inhibitor UO126 promotes cyclin B degradation because the APC inhibition mediated by the ERK MAPK pathway is lifted (Gross et al. 2000). Inhibition of the APC in a CSF-arrested egg requires Emi2, which is targeted by the ERK MAPK pathway during MI and MII (Tung et al. 2005). Loss of Emi2 in either frog or mouse eggs prevents MII arrest and allows parthenogenetic divisions.
Accumulation of cyclin B must be carefully regulated to maintain MII arrest. Cyclin B is synthesized continuously. If unopposed, this would make it difficult to achieve the rapid degradation of cyclin B required for a sharp cell-cycle transition upon fertilization. Again, tight regulation of Emi2 occurs through a negative-feedback loop involving its phosphorylation by cyclin-B–CDK1 and RSK. The carboxy-terminal Emi2 phosphorylations impede association of Emi2 with the APC; although the precise manner in which Emi2 inhibits the APC is not yet clear, the physical association of Emi2 with the APC is critical for APC inhibition and the amino-terminal phosphorylations dissociate Emi2 from the APC, allowing cyclin B degradation (Wu et al. 2007a). Thus, the Mos-ERK-RSK pathway maintains the CSF arrest, restricting cyclin B levels within a narrow limit. Although the precise sites of phosphorylation do not appear to be conserved from Xenopus to mammals, the overall mode of regulation may be conserved through phosphorylation of alternative sites.
3. Sperm maturation
Spermatogenesis occurs over the course of several weeks and encompasses three successive phases (Sharpe 1994): proliferation, meiosis, and differentiation. During proliferation, spermatogonial stem cells (SSCs) differentiate into spermatogonia. These undergo several mitotic divisions, giving rise to spermatocytes. After two meiotic divisions, spermatocytes form haploid spermatids. The final transformation of spermatids into mature sperm entails a major physical and structural reorganization of the cell that is known as spermiogenesis (Fig. 3).
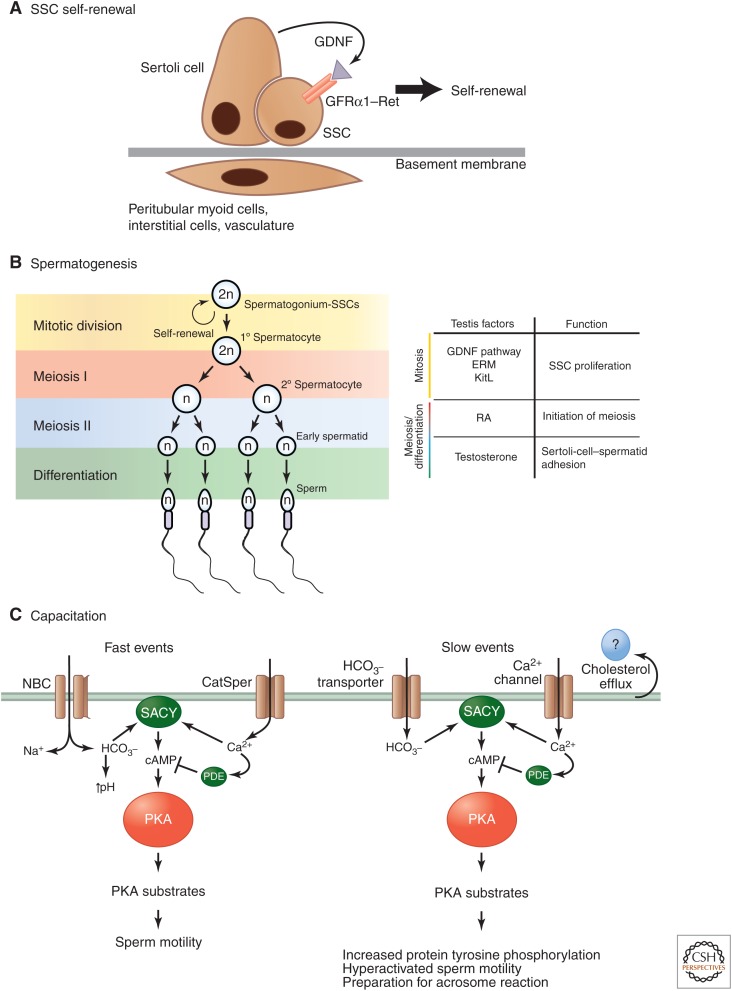
Figure 3.
Regulation of sperm maturation. (A) Spermatogenesis is a continuous process that starts after puberty. This is possible because a subset of spermatogonium, spermatogonial stem cells (SSCs), are capable of self-renewal. Glial-cell-line-derived neurotrophic factor (GDNF) and the receptor complex composed of Ret protooncoprotein and the GDNF family receptor α1 (GFRα1) are key signaling events for self-renewal. (From Hofmann 2008; adapted, with permission.) (B) Spermatocytes undergo a series of maturation steps before differentiating into sperm. These are tightly regulated by interaction with Sertoli cells and testis factors such as GDNF, ERM, KitL, and retinoic acid (RA). (C) Sperm must additionally undergo capacitation before they are capable of fertilization. This includes both fast and slow events. The fast events stimulate flagellar activity through a calcium flux that is controlled by the adenylyl cyclase SACY and mediated by the CatSper and sodium/bicarbonate (NBC) channels. Slow events increase motility and introduce changes that prepare sperm for fertilization. These include increases in both intracellular calcium levels and tyrosine phosphorylation of PKA substrates. cAMP levels are also controlled by endogenous phosphodiesterase (PDE). Unknown cholesterol acceptors present in uterine/oviduct fluids mediate cholesterol efflux during capacitation. (From Visconti 2009; adapted, with permission.)
As oocyte maturation is supported by follicular cells, so is spermatogenesis supported by nongermline cells in and around the seminiferous tubules. These include Sertoli, Leydig, and myoid cells (Hermo et al. 2010). The Sertoli cells perform a plethora of functions to sustain germ cells through all stages of development. They express receptors for growth factors and hormones and secrete many essential regulatory factors required for spermatogenesis. Lying outside the tubules in the interstitial space and in close proximity to blood vessels, Leydig cells are responsible for the regulated production of androgens in the testis. Lastly, in the peritubular tissue, along with extracellular matrix, myoid cells support the seminiferous tubules (Yoshida et al. 2007; de Rooij 2009). These cells create a microenvironment that allows SSCs to self-renew and/or differentiate, as is required for continual fertility (Nalam and Matzuk 2010).
Complete spermatogenesis requires the coordinated action of peptide and steroid hormones, which are important regulators of seminiferous tubule function (McLachlan et al. 2002). Only somatic cells in the testis express hormone receptors; therefore these cells (i.e., Sertoli cells) are the exclusive mediators of hormone activity in spermatogenesis. Of the two gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which also play important roles during oogenesis (Richards and Pangas 2010), FSH has broader involvement in spermatogenesis whereas LH functions primarily in the regulation of testosterone production by Leydig cells (Ruwanpura et al. 2010). However, both gonadotropins exert their biological effects by activating cognate GPCRs. In mice, following formation of the seminiferous cords, gonocytes, the precursors of SSCs, proliferate until day E15–16, when they become quiescent, which is coincident with changes in the expression of several cell-cycle proteins (van den Ham et al. 2003). Formation and proliferation of SSCs is observed during the first postnatal week and continues with the synchronous first wave of spermatogenesis that extends in the prepubertal testis over the first 35 days after birth (Itman et al. 2006).
3.1. Stem Cell Proliferation and Maintenance
The predominant role of FSH is regulating Sertoli cell proliferation during prepubertal development (Holdcraft and Braun 2004). Stimulation of the FSH receptor on Sertoli cells activates several downstream signaling cascades, including those involving cAMP, calcium release, ERK, PI3K, and phospholipases A2 and C. These activate the cAMP-responsive transcription factor CREB, leading to stimulation of gene expression (Ruwanpura et al. 2010). Testosterone produced in response to LH mostly functions via the nuclear androgen receptor (AR) and its subsequent stimulation of gene expression (Wang et al. 2009; Sever and Glass 2013). It may also act via alternative mechanisms, which also result in phosphorylation of CREB. These “nongenomic” pathways are thought to involve the activation of several kinases, including Src (Cheng et al. 2007). Importantly for proliferation of both Sertoli cells and SSCs, FSH stimulation increases expression and secretion of glial-cell-line-derived neurotrophic factor (GDNF) (Hu et al. 1999), a distant member of the transforming growth factor β (TGFβ) family superfamily that regulates the proliferation of uncommitted SSCs (Meng et al. 2000; Hermo et al. 2010).
GDNF promotes the activation and expression of many signaling molecules, including the transcription factors Fos and BCL6. GDNF also stimulates Ras, Akt, and Src-family kinases; Akt activation, specifically, is important for preventing apoptosis of SSCs (Sariola and Saarma 2003; Hermo et al. 2010; Oatley and Brinster 2012). These signals are initiated following its engagement of a receptor complex composed of the Ret proto-oncoprotein (McGuinness et al. 1996) and the GDNF family receptor α1 (GFRα1) protein (Meng et al. 2000). GFRA1 appears to be expressed only by a subpopulation of SSCs, possibly a marker of true “stemness.” This is consistent with the effects of its down-regulation, which causes widespread inhibition of SSC proliferation and differentiation (reviewed in Nalam and Matzuk 2010). SSCs lacking GFRα1 display reduced phosphorylation of Ret at Y1062, a known binding site for several of the downstream targets of this pathway. Additionally, loss of either member of the receptor complex leads to defective SSC proliferation and differentiation (Naughton et al. 2006). The GDNF pathway is thus essential for the maintenance of uncommitted SSCs.
SSC renewal requires the transcription factor Ets variant gene 5 (ERM) a product of the Sertoli cells. Deletion of ERM in mice results in inhibition of spermatogenesis following the first wave (Chen et al. 2005). Thus, although GDNF plays a critical role in spermatogenesis during the perinatal period, ERM does so at puberty. Expression of ERM and GDNF is increased in Sertoli cells by addition of the fibroblast growth factor (FGF) 2 and activation of the FGF2 receptor, which stimulates ERK and PI3K signaling. Therefore, FGFs may also play an important role in regulating the functions of Sertoli cells that control the establishment of the SSC pool (Simon et al. 2007).
Another factor regulating proliferation and differentiation of SSCs is the protooncoprotein Kit, a receptor tyrosine kinase expressed at high levels in differentiating spermatogonia and early spermatocytes (Sorrentino et al. 1991). In undifferentiated spermatogonia, Kit expression is suppressed by the transcription factor Plzf. Plzf is necessary for the maintenance of undifferentiated SSCs. Plzf-null mice display accumulation of Kit-positive cells and depletion of germ cells (Buaas et al. 2004; Costoya et al. 2004), and dominant white spotting (W) mutants affecting the Kit locus inhibit spermatogonia differentiation without affecting either mitosis of undifferentiated SSCs or initiation of meiosis in spermatocytes (Yoshinaga et al. 1991). Furthermore, in the postnatal testis, expression of the Kit ligand (KitL, also known as stem cell factor [SCF]), in Sertoli cells is critical for spermatogenesis (Flanagan et al. 1991), and mutations in KitL phenocopy the spermatogenic defects observed in W mutant mice (Bedell and Mahakali Zama 2004). The binding of KitL to Kit causes receptor dimerization and phosphorylation of cytoplasmic tyrosine residues. These phosphorylated residues become anchoring sites for SH2-domain containing proteins such as phospholipase Cγ (PLCγ), Src, and PI3K. Consequent induction of calcium release, phosphorylation of target proteins such as p70S6K (Feng et al. 2000), and gene expression leads to proliferation and differentiation of spermatogonia (Sette et al. 2000). Note that spermatogonia from Kit-null testes can nevertheless undergo spermatogenesis, which indicates other parallel pathways must also operate (Nalam and Matzuk 2010).
3.2. Spermatocyte Meiosis and Release
After differentiation, spermatogonia undergo a few mitotic divisions before entering meiosis, which is delayed until puberty. Retinoic acid (RA) (see Sever and Glass 2013) is a key signaling molecule in meiotic initiation (Vernet et al. 2006), and Sertoli cells are believed to be the main source of RA in the postnatal testes. Importantly, although RA is generated in Sertoli cells by the enzyme aldehyde dehydrogenase family 1, subfamily A1 (ALDH1A1), male gonads also express an enzyme responsible for RA degradation, cytochrome P450, family 26, subfamily b polypeptide 1 (CYP26B1) (Bowles et al. 2006). This limits the availability of RA and postpones meiosis in the male.
Hormonal signaling plays an important role in control of meiosis and production of functional spermatids. Suppression of FSH production and signaling leads to reduced numbers of pachytene spermatocytes (Matthiesson et al. 2006) and compromises the release of sperm, which suggests that FSH regulates adhesion between Sertoli cells and spermatids (Saito et al. 2000; Ruwanpura et al. 2010). Spermatocyte meiosis is arguably even more dependent on testosterone signaling. In mice lacking AR in Sertoli cells spermatogenesis is arrested at the late spermatocyte stage and spermiation, which is the release of the mature spermatids into the lumen of the seminiferous tubules, fails (Chang et al. 2004; De Gendt et al. 2004). Moreover, in adult mice that lack both gonadotropins owing to a defect in the GnRH gene, testosterone supplementation alone can restore full spermatogenesis (Haywood et al. 2003). The processes modulated by testosterone during spermiogenesis include adhesion between Sertoli cells and different stage spermatids. Testosterone is thought to regulate expression and/or function of proteins required for the assembly and/or disassembly of adhesion junctions, including α and β integrins, focal adhesion kinases, and Src kinases (Ruwanpura et al. 2008, 2010; Shupe et al. 2011).
3.3. Sperm Capacitation and Calcium Channels
After entering the oviduct, most mammalian sperm associate with epithelial cells in the isthmus creating a sperm reservoir. Sperm are sporadically released from this reservoir and migrate to the ampulla, which is the site of fertilization (Suarez 2008b). The mechanisms that facilitate this release are unclear but they involve the loss of BSPs (originally isolated from the cow, so named bovine seminal plasma proteins) and other extrinsic proteins from the sperm; these events are seemingly precipitated by the progression of capacitation events, including hyperactivation (a change in sperm motility) (Suarez 2008a). Unfolding over several hours, sperm capacitation begins as sperm come in contact with the female reproductive tract and involves a series of sequential and simultaneous processes. Sperm acquire motility immediately after their release from the cauda epididymis (for reviews see Yanagimachi 1994; Salicioni et al. 2007). These subsequent changes involve both modification of the plasma membrane phospholipid composition and increases in intracellular calcium concentration. Early in capacitation, activation of soluble adenylyl cyclase (SACY) (Chen et al. 2000) causes an increase in cAMP levels, which activates PKA containing a unique catalytic subunit (Cα2 or Cs). These events are initiated by an increase in the intracellular concentration of bicarbonate, which activates the enzyme by promoting closure of the catalytic active site and metal recruitment (Steegborn et al. 2005). This is itself triggered by the high concentrations of bicarbonate in the seminal fluid, which enters via the Na+/HCO3− cotransporter. PKA activation coincides with phosphorylation of a number of proteins, although its targets remain mostly unknown (Signorelli et al. 2012) except for FSCB, a 270-kDa protein involved in the biogenesis of the fibrous sheath (Li et al. 2007), a cytoskeletal structure in the mammalian sperm flagellum. The ongoing alkalinization, which activates the downstream sperm-specific plasma membrane calcium channel CatSper (Ren et al. 2001), also increases intracellular calcium levels (Carlson et al. 2003). Together, this increase and the PKA-regulated phosphorylation of proteins on tyrosine residues, including A-kinase-anchoring proteins (AKAPs) (Ficarro et al. 2003), drive the activation of flagellar motility that is necessary for both sperm migration and residence in the female reproductive tract.
Additional aspects of capacitation that are more protracted are required for sperm to fertilize the egg. These occur closer to the site of the ovulated egg(s) and include a general increase in protein phosphorylation, loss of cholesterol from the plasma membrane, hyperactivation (acquisition of an asymmetrical, nonlinear motility pattern), and increased susceptibility to stimuli that promote the acrosome reaction. These slow processes are also regulated by bicarbonate; however, they additionally require the transfer of cholesterol from the plasma membrane to unknown cholesterol acceptors present in the uterine/oviduct fluids. Also, despite the dominant role of PKA in sperm capacitation, research suggests that other kinases, such as Src-family kinases and PKC, and phosphatases such as PP1α, PP1γ2, PP2A, and PP2B may play a role regulating the rapid and slow events of capacitation (reviewed in Visconti et al. 2011; Signorelli et al. 2012).
Hyperactivated sperm show increased flagellar bend amplitudes (Yanagimachi 1970), which is most often observed in the oviduct. This amplification generates enough power for the sperm to make its way through the oviductal mucus, cumulus matrix, and the zona pellucida (ZP) surrounding the egg (Chang and Suarez 2010; Hung and Suarez 2010). CATSPER channels and calcium are critical for hyperactivation; either the absence of external calcium or the presence of mutations in any of the four CATSPER subunits results in a failure to hyperactivate (Carlson et al. 2003), and CATSPER-defective sperm cannot leave the oviduct sperm reservoir and are incapable of penetrating the ZP. The mechanism of CATSPER regulation in the oviduct remains unclear. Progesterone is a possible regulator (Lishko et al. 2011; Strunker et al. 2011) produced by cumulus cells and is present in the follicular fluid (Sun et al. 2005). It may act as the long-sought chemoattractant. In this regard, hyperactivation could promote sperm chemotaxis (Chang and Suarez 2010). Indeed, progesterone can induce increases in intracellular calcium near the base of the sperm head—the site where the calcium stores are located (Fukami et al. 2001). Note that in marine species such as the sea urchin Arbacia punctulata, in which sperm migrate toward the oocyte along a chemoattractant gradient, motility is regulated by incremental increases in intracellular calcium concentration caused by the binding of the chemoattractant resact to the sperm (Bohmer et al. 2005). Progesterone could act similarly in mammals.
4. Fertilization
4.1. The Acrosome Reaction in Sperm
Before fusing with the egg’s plasma membrane, sperm must undergo the acrosome reaction (Yanagimachi and Mahi 1976). The acrosome is a Golgi-derived organelle that lies above the tip of the sperm head. Its contents are released following fusion between the outer acrosomal membrane and the plasma membrane (Kim et al. 2011), and this reaction is critical for interaction with the ZP of the egg. Only capacitated sperm are capable of undergoing the acrosome reaction. Progesterone produced by the cumulus cells surrounding the oocyte has been proposed as the possible inducer of this reaction (Osman et al. 1989; Jin et al. 2011). Upon breaching the egg’s plasma membrane, the sperm induces the initiation of embryonic development by evoking an increase in the intracellular concentration of free calcium, a signaling mechanism that regulates numerous cellular processes (Fig. 4) (Berridge et al. 2000b; Bootman 2012).
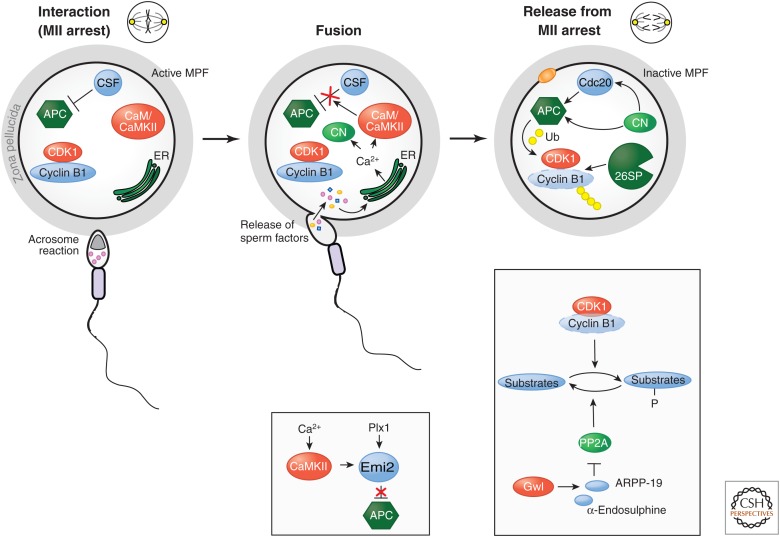
Figure 4.
Fertilization events. Both egg and sperm must undergo complex changes before fertilization can occur. Upon interaction with the zona pelucida, sperm undergo a calcium-flux-driven reorganization of SNARE fusion proteins, called the acrosome reaction. Fusion with the egg membrane releases factors from the sperm into the cytoplasm. These factors stimulate calcium release from the egg ER and subsequent activation of CaMKII and calcineurin (CN). The consequences of CaMKII activation include phosphorylation of Emi2, which promotes its Plx1 and SCFβ-TrCP-dependent degradation and consequent reactivation of the APC. CN activation promotes dephosphorylation of Cdc20 and also the Cdc27 subunit of the APC. At the same time, dephosphorylation of M phase phosphoproteins is promoted by inactivation of the Greatwall kinase (Gwl), thereby alleviating inhibition of PP2A, allowing it to dephosphorylate M phase CDK1 substrates.
The signaling mechanisms leading to the acrosome reaction are well characterized (Arnoult et al. 1996; Evans and Florman 2002). Activation of PLCδ4 by a calcium influx and the consequent generation of inositol 1,4,5-trisphosphate (IP3) (Distelhorst and Bootman 2011) causes a second influx of calcium (Fukami et al. 2001). This promotes exocytosis by stimulating the reorganization of a SNARE protein in the sperm membranes (Mayorga et al. 2007). This reorganization is facilitated by the NSF and α-SNAP proteins and the calcium sensor synaptotagmin. Release of proteolytic enzymes and hyaluronidase from the acrosome aids the penetration of the cumulus cells and ZP by the sperm (Kim et al. 2008)
How the sperm interacts with ZP has been a point of some dispute. Early studies implicated several sperm-surface enzymes as critical for the interaction of the sperm with the egg coat (Lu and Shur 1997; Ikawa et al. 2010), but more recent work supports the involvement of a disintegrin and metalloproteinase family member, ADAM3 (Shamsadin et al. 1999). On the egg side, recent studies point to the amino-terminal domain of ZP2, which is cleaved after fertilization by the egg cortical granule component ovastacin, as the ZP ligand (reviewed in Avella et al. 2013). After binding, sperm must penetrate the ZP, probably using sperm-associated serine proteases such as acrosin and testisin (also known as PRSS21 or TESP5) (Baba et al. 1994; Yamashita et al. 2008).
4.2. Fusion and Egg Activation Leading to Fertilization
Soon after negotiating the ZP, the sperm fuses with the egg plasma membrane (Evans and Florman 2002). Early studies implicated the proteases ADAM1b and ADAM2, together known as fertilin, but gene knockout studies showed that fertilin is not required for fusion (Inoue et al. 2007). Subsequent work identified IZUMO, a member of the immunoglobulin superfamily, as the protein that appears to mediate fusion (Inoue et al. 2005; Sosnik et al. 2009). In the egg, the tetraspanin protein CD9 has been shown to be critical for fusion (Le Naour et al. 2000; Miyado et al. 2000). Whether CD9 and IZUMO directly interact is not clear.
Fusion of the gametes produces a calcium signal in the egg. A variety of calcium signals are required for egg activation; these reflect both the plasticity of the signaling machinery as well as the distinct requirements for egg activation in different species. Species typically either display a single increase in calcium concentration (e.g., in sea urchins, starfish, frogs, and fish) or show calcium oscillations (e.g., in nemertian worms, ascidians, and mammals) (Stricker 1999; Stricker and Whitaker 1999; Miyazaki and Ito 2006). The mechanism(s) that mediate calcium influx remains poorly characterized in mammalian eggs. Cells use several calcium influx mechanisms, including receptor-operated channels (ROCs) and voltage-operated calcium channels (VOCCs) (Berridge et al. 2000a; Tosti and Boni 2004; Smyth et al. 2006). Calcium influx may be attained, at least in part, by store-operated calcium entry (SOCE), a mechanism regulated by ER calcium levels and driven by store-operated calcium channels (SOCs) (Park et al. 2009). Stromal interaction molecule (STIM1) in the ER acts as a calcium sensor (Liou et al. 2005; Roos et al. 2005) and causes opening of Orai1, a channel partner protein in the plasma membrane to replenish stores (Feske et al. 2006; Vig et al. 2006).
The IP3 receptor (IP3R) on the ER is the main intracellular calcium-release channel in many mammalian cell types (reviewed in Berridge et al. 2000b; Bootman et al. 2001; Bootman 2012). Although mammalian oocytes, eggs, and the surrounding cells express all three IP3R isoforms (reviewed in Fissore et al. 1999a; Berridge et al. 2000b; Díaz-Muñoz et al. 2008), oocytes and eggs overwhelmingly express the type I IP3R isoform (Kume et al. 1997; Fissore et al. 1999b; Jellerette et al. 2000; Tokmakov et al. 2002), which requires binding by both calcium and IP3 for activation and is stimulated at low calcium levels and inhibited at high calcium levels (Iino 1990a,b; Finch et al. 1991). This makes it especially suited to support long-lasting oscillations. The importance of IP3R1 in fertilization is supported by studies showing injection of an anti-IP3R1 antibody blocks sperm-initiated calcium oscillations (Miyazaki et al. 1992) and egg activation in mice (Xu et al. 1994) and works in the same manner in other vertebrates (Parys et al. 1994; Thomas et al. 1998; Yoshida et al. 1998; Runft et al. 1999; Goud et al. 2002; Iwasaki et al. 2002).
The signals that initiate the calcium increase at fertilization have proven elusive (Whitaker 2006; Parrington et al. 2007). Nonetheless, in some species, especially those with a single calcium transient at fertilization, Src-family kinases and PLCγ have been shown to lead to production of IP3 during fertilization (Giusti et al. 1999; Sato et al. 2000). The receptor responsible for recruiting and activating the kinases remains to be identified (Mahbub Hasan et al. 2005). Similarly, it is unclear how sperm induce calcium oscillations in mammals. Calcium responses can be initiated in eggs from various species, including mammals by the same agonists that cause calcium release in somatic cells (Katayama et al. 1993; Miyazaki and Ito 2006). However, these fail to replicate the pattern of calcium oscillations associated with fertilization; other mechanism(s) might, therefore, be at play. Studies of sea urchin, ascidian eggs, and later, mammalian eggs showed that injection of sperm extracts or whole sperm can replicate fertilization-like responses in these species (Stice and Robl 1990; Swann 1990; Tesarik and Testart 1994; Nakano et al. 1997; Stricker 1997; Swann and Lai 1997; Wu et al. 1997; Kurokawa and Fissore 2003; Malcuit et al. 2006; Swann et al. 2006) strengthening the notion of a sperm cytosolic factor (SF). The mechanism may involve the release of SF into the ooplasm after fusion of the gametes. Importantly, the SF is not IP3 or calcium but an uncharacterized protein moiety (Swann 1990; Wu et al. 1997; Kyozuka et al. 1998; Harada et al. 2007). Once initiated, calcium oscillations trigger all events of egg activation (Schultz and Kopf 1995). The presence of oscillations ensures the persistent degradation of Emi2 and the stepwise progression of activation, as events such as cortical granule exocytosis exit require fewer calcium increases than exit from MII or recruitment of maternal RNAs (Ducibella et al. 2002).
Cytosolic preparations from mammalian sperm possess high PLC activity (Parrington et al. 1999; Jones et al. 2000; Rice et al. 2000), and this is highly sensitive to calcium and could therefore be (Rice et al. 2000) the oscillation initiator. Indeed, a novel sperm-specific PLCζ (Saunders et al. 2002) has been identified (Fujimoto et al. 2004; Kouchi et al. 2004) and can evoke appropriate oscillations in mouse (Saunders et al. 2002), rat (Ito et al. 2008), human (Rogers et al. 2004), bovine (Malcuit et al. 2005; Ross et al. 2008), porcine (Yoneda et al. 2006), and equine (Bedford-Guaus et al. 2008) eggs. The findings that aberrant PLCζ expression is associated with infertility, and sperm from patients with repeated fertilization failure after intracytoplasmic sperm injection (ICSI), which also fail to initiate calcium oscillations (Yoon et al. 2008; Heytens et al. 2009), supports the idea that PLCζ released from the sperm triggers oscillations in fertilized eggs. Nevertheless, questions remain regarding its expression during spermatogenesis and storage, the means by which release into the ooplasm occurs, and how activation occurs upon egg entry.
5. From egg to zygote
Once fertilization has occurred, interphase nuclei form separately around the paternal (sperm) and maternal (Eggerickx et al. 1995) genomes in structures known as pronuclei. The paternal genome undergoes a series of transformations that result in both exchange of spermatic chromatin packaging proteins (protamines) for maternal histones and demethylation of many repressed paternal genes. Upon completion of DNA replication, the pronuclear envelopes break down and the maternal and paternal chromosomes comingle on a common mitotic spindle in the zygote. In this transition to the zygote, maternal mRNA transcripts are largely degraded; many are replaced by zygote-specific transcripts both to take over the function of maternal housekeeping messages and to allow expression of new proteins that will build the early embryo.
At fertilization, the egg exits its MII arrest, in part as a consequence of events set in motion by the calcium response, which inactivates CSF through degradation of Mos and reactivates the APC, leading to cyclin degradation. Reactivation of the APC appears to be mediated in part by the calcium-dependent phosphatase calcineurin (also known as PP2B). Inhibition of calcineurin by cyclosporine A halts destruction of cyclin B. Not only has a core component of the APC, Cdc27, been shown to serve as a calcineurin substrate, but the APC-activating subunit Cdc20 is also a substrate of calcineurin. To exit meiosis, a host of CDK1–cyclin-B substrates must be dephosphorylated. This is accomplished largely by PP2A (as described above). Premature inactivation of CDK1–cyclin-B substrates would prohibit entry into or maintenance of MII. Inappropriate PP2A activation is therefore prevented through binding of a small peptide called Arpp19 and α endosulfine. Phosphorylation of Arpp19 by the conserved mitotic kinase Greatwall, which is activated by CDK1 at M phase entry, allows it to inhibit the PP2A isoform B55δ. At M phase exit, Greatwall is inactivated, which alleviates inhibition of PP2A and consequently allows the requisite dephosphorylation of M phase substrates. Additionally, rapid destruction of Emi2 alleviates inhibition of the APC, leading to loss of CDK1 activity (through degradation of cyclin B). In Xenopus eggs, this is triggered by calcium via a calcium-calmodulin-dependent kinase (CaMKII). CaMKII phosphorylates Emi2 protein on T195. This creates a docking site on Emi2 for another kinase, Plx1 in Xenopus, which then phosphorylates Emi2 within a sequence (the phosphodegron) required for recognition by the SCFβ-TrCP ubiquitin ligase. This series of events leads to ubiquitylation and degradation of Emi2, liberation of the APC, and degradation of substrates required for M phase exit. Note the Plx1-phosphorylation site does not appear to be conserved in mammals, and it is not known whether the ability of CaMKII to trigger Emi2 degradation in mammalian eggs is also triggered by recruitment Plk1 (the mammalian Plk1 ortholog). The culmination of these events allows the fertilized egg to exit arrest and proceed with cell division.
6. Concluding remarks
In gametes of both sexes, development, maturation, and fertilization require the careful coordination of complex processes. From hormone-initiated and kinase/phosphatase-controlled maturation, to calcium-induced capacitation and fertilization, regulatory mechanisms ensure that reproduction occurs only under conditions in which they are best poised for success. For males, the signals that regulate spermatogenesis are at first contained within the testis, but following spermiation and ejaculation, proper sperm function depends on factors outside of the male reproductive tract: for external fertilizers, factors in the outside environment; and for internal fertilizers, the milieu of the female reproductive tract. Taking into account these potentially harsh environments, male reproduction relies on the production of large quantities of sperm, with progenitor cells retaining mitotic capacity into adulthood. For female internal fertilizers, the production of eggs is more contained, restricted to the follicle until ovulation and to the female ducts until fertilization and beyond, relying on the production of few gametes; in contrast, externally fertilizing females, like their male counterparts, produce large numbers of gametes. Even for these organisms, though, oocytes and eggs are self-contained developmental units capable of sustaining the early stages of development whose progenitors have generally entered meiosis and have lost the capacity to regenerate.
In every species, regardless of the site of fertilization, cascades of protein modifications regulate cell-cycle transitions to guarantee that oocytes can be fertilized only at an appropriate time. Hormonal regulation and calcium signaling promote capacitation of sperm only in the correct environment for fertilization. Furthermore, multiple overlapping pathways provide the checks and balances that are necessary to prevent defective reproduction.
Although great progress has been made over the last 50 years in detailing the molecular events underlying most aspects of vertebrate fertilization, there are still aspects of gamete development and fertilization whose precise regulation by cell signaling events remain to be determined. Elucidation of these events is likely to have important implications for the continued development of reproductive technologies and for maximizing the health of gametes, and thus of progeny.
Footnotes
Editors: Lewis Cantley, Tony Hunter, Richard Sever, and Jeremy Thorner
Additional Perspectives on Signal Transduction available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Andresson T, Ruderman JV. 1998. The kinase Eg2 is a component of the Xenopus oocyte progesterone-activated signaling pathway. EMBO J 17: 5627–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Naito K, Haraguchi S, Suzuki R, Yokoyama M, Inoue M, Aizawa S, Toyoda Y, Sato E. 1996. Meiotic abnormalities of c-mos knockout mouse oocytes: Activation after first meiosis or entrance into third meiotic metaphase. Biol Reprod 55: 1315–1324. [DOI] [PubMed] [Google Scholar]
- Arnoult C, Cardullo RA, Lemos JR, Florman HM. 1996. Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida. Proc Natl Acad Sci 93: 13004–13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CR. 1951. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B 4: 581–596. [DOI] [PubMed] [Google Scholar]
- Avella MA, Xiong B, Dean J. 2013. The molecular basis of gamete recognition in mice and humans. Mol Hum Reprod 19: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Azuma S, Kashiwabara S, Toyoda Y. 1994. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem 269: 31845–31849. [PubMed] [Google Scholar]
- Bedell MA, Mahakali Zama A. 2004. Genetic analysis of Kit ligand functions during mouse spermatogenesis. J Androl 25: 188–199. [DOI] [PubMed] [Google Scholar]
- Bedford JM. 1970. Sperm capacitation and fertilization in mammals. Biol Reprod 2: 128–158. [PubMed] [Google Scholar]
- Bedford-Guaus SJ, Yoon SY, Fissore RA, Choi YH, Hinrichs K. 2008. Microinjection of mouse phospholipase Cζ complementary RNA into mare oocytes induces long-lasting intracellular calcium oscillations and embryonic development. Reprod Fertil Dev 20: 875–883. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. 2000a. Signal transduction. The calcium entry pas de deux. Science 287: 1604–1605. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. 2000b. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21. [DOI] [PubMed] [Google Scholar]
- Berry LD, Gould KL. 1996. Regulation of Cdc2 activity by phosphorylation at T14/Y15. Prog Cell Cycle Res 2: 99–105. [DOI] [PubMed] [Google Scholar]
- Bohmer M, Van Q, Weyand I, Hagen V, Beyermann M, Matsumoto M, Hoshi M, Hildebrand E, Kaupp UB. 2005. Ca2+ spikes in the flagellum control chemotactic behavior of sperm. EMBO J 24: 2741–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bootman MD. 2012. Calcium signaling. Cold Spring Harb Perspect Biol 4: a011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, et al. 2001. Calcium signalling—An overview. Sem Cell Dev Biol 12: 3–10. [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, et al. 2006. Retinoid signaling determines germ cell fate in mice. Science 312: 596–600. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. 2004. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 36: 647–652. [DOI] [PubMed] [Google Scholar]
- Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, Hille B, Garbers DL, Babcock DF. 2003. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci 100: 14864–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC. 1951. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168: 697–698. [DOI] [PubMed] [Google Scholar]
- Chang H, Suarez SS. 2010. Rethinking the relationship between hyperactivation and chemotaxis in mammalian sperm. Biol Reprod 83: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. 2004. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci 101: 6876–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. 2000. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628. [DOI] [PubMed] [Google Scholar]
- Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, et al. 2005. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature 436: 1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Watkins SC, Walker WH. 2007. Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in sertoli cells. Endocrinology 148: 2066–2074. [DOI] [PubMed] [Google Scholar]
- Colledge WH, Carlton MB, Udy GB, Evans MJ. 1994. Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature 370: 65–68. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. 2004. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet 36: 653–659. [DOI] [PubMed] [Google Scholar]
- Daar I, Paules RS, Vande Woude GF. 1991. A characterization of cytostatic factor activity from Xenopus eggs and c-mos-transformed cells. J Cell Biol 114: 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, et al. 2004. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci 101: 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Lang S, Wylie C, Hammes SR. 2008. The Xenopus laevis isoform of G protein-coupled receptor 3 (GPR3) is a constitutively active cell surface receptor that participates in maintaining meiotic arrest in X. laevis oocytes. Mol Endocrinol 22: 1853–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij DG. 2009. The spermatogonial stem cell niche. Microsc Res Tech 72: 580–585. [DOI] [PubMed] [Google Scholar]
- Díaz-Muñoz M, de la Rosa Santander P, Juárez-Espinosa AB, Arellano RO, Morales-Tlalpan V. 2008. Granulosa cells express three inositol 1,4,5-trisphosphate receptor isoforms: Cytoplasmic and nuclear Ca2+ mobilization. Reprod Biol Endocrinol 6: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelhorst CW, Bootman MD. 2011. Bcl-2 interaction with the inositol 1,4,5-trisphosphate receptor: Role in Ca2+ signaling and disease. Cell Calcium 50: 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury KC, Schorderet-Slatkine S. 1975. Effects of cycloheximide on the “autocatalytic” nature of the maturation promoting factor (MPF) in oocytes of Xenopus laevis. Cell 4: 269–274. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP. 2002. Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev Biol 250: 280–291. [PubMed] [Google Scholar]
- Duckworth BC, Weaver JS, Ruderman JV. 2002. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc Natl Acad Sci 99: 16794–16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J, Umbhauer M, Rassinier P, Hanauer A, Verlhac MH. 2005. p90RSK is not involved in cytostatic factor arrest in mouse oocytes. J Cell Biol 169: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy WG, Brizuela L, Beach D, Newport J. 1988. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell 54: 423–431. [DOI] [PubMed] [Google Scholar]
- Dupre A, Jessus C, Ozon R, Haccard O. 2002. Mos is not required for the initiation of meiotic maturation in Xenopus oocytes. EMBO J 21: 4026–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggerickx D, Denef JF, Labbe O, Hayashi Y, Refetoff S, Vassart G, Parmentier M, Libert F. 1995. Molecular cloning of an orphan G-protein-coupled receptor that constitutively activates adenylate cyclase. Biochem J 309: 837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JP, Florman HM. 2002. The state of the union: The cell biology of fertilization. Nat Cell Biol 4: S57–S63. [DOI] [PubMed] [Google Scholar]
- Fan HY, Sun QY. 2004. Involvement of mitogen-activated protein kinase cascade during oocyte maturation and fertilization in mammals. Biol Reprod 70: 535–547. [DOI] [PubMed] [Google Scholar]
- Feng LX, Ravindranath N, Dym M. 2000. Stem cell factor/c-kit up-regulates cyclin D3 and promotes cell cycle progression via the phosphoinositide 3-kinase/p70 S6 kinase pathway in spermatogonia. J Biol Chem 275: 25572–25576. [DOI] [PubMed] [Google Scholar]
- Ferby I, Blazquez M, Palmer A, Eritja R, Nebreda AR. 1999. A novel p34cdc2-binding and activating protein that is necessary and sufficient to trigger G2/M progression in Xenopus oocytes. Genes Dev 13: 2177–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. 2006. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185. [DOI] [PubMed] [Google Scholar]
- Ficarro S, Chertihin O, Westbrook VA, White F, Jayes F, Kalab P, Marto JA, Shabanowitz J, Herr JC, Hunt DF, et al. 2003. Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J Biol Chem 278: 11579–11589. [DOI] [PubMed] [Google Scholar]
- Finch EA, Turner TJ, Goldin SM. 1991. Subsecond kinetics of inositol 1,4,5-trisphosphate-induced calcium release reveal rapid potentiation and subsequent inactivation by calcium. Ann NY Acad Sci 635: 400–403. [DOI] [PubMed] [Google Scholar]
- Fissore RA, Long CR, Duncan RP, Robl JM. 1999a. Initiation and organization of events during the first cell cycle in mammals: Applications in cloning. Cloning 1: 89–100. [DOI] [PubMed] [Google Scholar]
- Fissore RA, Longo FJ, Anderson E, Parys JB, Ducibella T. 1999b. Differential distribution of inositol trisphosphate receptor isoforms in mouse oocytes. Biol Reprod 60: 49–57. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Chan DC, Leder P. 1991. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell 64: 1025–1035. [DOI] [PubMed] [Google Scholar]
- Frank-Vaillant M, Haccard O, Thibier C, Ozon R, Arlot-Bonnemains Y, Prigent C, Jessus C. 2000. Progesterone regulates the accumulation and the activation of Eg2 kinase in Xenopus oocytes. J Cell Sci 113: 1127–1138. [DOI] [PubMed] [Google Scholar]
- Freudzon L, Norris RP, Hand AR, Tanaka S, Saeki Y, Jones TL, Rasenick MM, Berlot CH, Mehlmann LM, Jaffe LA. 2005. Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J Cell Biol 171: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S, Yoshida N, Fukui T, Amanai M, Isobe T, Itagaki C, Izumi T, Perry AC. 2004. Mammalian phospholipase Cζ induces oocyte activation from the sperm perinuclear matrix. Dev Biol 274: 370–383. [DOI] [PubMed] [Google Scholar]
- Fukami K, Nakao K, Inoue T, Kataoka Y, Kurokawa M, Fissore RA, Nakamura K, Katsuki M, Mikoshiba K, Yoshida N, et al. 2001. Requirement of phospholipase Cδ4 for the zona pellucida-induced acrosome reaction. Science 292: 920–923. [DOI] [PubMed] [Google Scholar]
- Fulka J Jr, Motlik J, Fulka J, Crozet N. 1986. Activity of maturation promoting factor in mammalian oocytes after its dilution by single and multiple fusions. Dev Biol 118: 176–181. [DOI] [PubMed] [Google Scholar]
- Gautier J, Maller JL. 1991. Cyclin B in Xenopus oocytes: Implications for the mechanism of pre-MPF activation. EMBO J 10: 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti AF, Carroll DJ, Abassi YA, Foltz KR. 1999. Evidence that a starfish egg Src family tyrosine kinase associates with PLC-γ1 SH2 domains at fertilization. Dev Biol 208: 189–199. [DOI] [PubMed] [Google Scholar]
- Goud PT, Goud AP, Leybaert L, Van Oostveldt P, Mikoshiba K, Diamond MP, Dhont M. 2002. Inositol 1,4,5-trisphosphate receptor function in human oocytes: Calcium responses and oocyte activation-related phenomena induced by photolytic release of InsP3 are blocked by a specific antibody to the type I receptor. Mol Hum Reprod 8: 912–918. [DOI] [PubMed] [Google Scholar]
- Gross SD, Schwab MS, Taieb FE, Lewellyn AL, Qian YW, Maller JL. 2000. The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90RSK. Curr Biol 10: 430–438. [DOI] [PubMed] [Google Scholar]
- Hake LE, Richter JD. 1994. CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell 79: 617–627. [DOI] [PubMed] [Google Scholar]
- Han SJ, Chen R, Paronetto MP, Conti M. 2005. WEE1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol 15: 1670–1676. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Tung JJ, Jackson PK. 2006. CaMKII and polo-like kinase 1 sequentially phosphorylate the cytostatic factor Emi2/XErp1 to trigger its destruction and meiotic exit. Proc Natl Acad Sci 103: 608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y, Matsumoto T, Hirahara S, Nakashima A, Ueno S, Oda S, Miyazaki S, Iwao Y. 2007. Characterization of a sperm factor for egg activation at fertilization of the newt Cynops pyrrhogaster. Dev Biol 306: 797–808. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa Y, et al. 1994. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature 370: 68–71. [DOI] [PubMed] [Google Scholar]
- Haywood M, Spaliviero J, Jimemez M, King NJ, Handelsman DJ, Allan CM. 2003. Sertoli and germ cell development in hypogonadal (hpg) mice expressing transgenic follicle-stimulating hormone alone or in combination with testosterone. Endocrinology 144: 509–517. [DOI] [PubMed] [Google Scholar]
- Hermo L, Pelletier RM, Cyr DG, Smith CE. 2010. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: Background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech 73: 241–278. [DOI] [PubMed] [Google Scholar]
- Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, Fissore RA, Hamer R, Deane CM, Ruas M, et al. 2009. Reduced amounts and abnormal forms of phospholipase Cζ (PLCζ) in spermatozoa from infertile men. Hum Reprod 24: 2417–2428. [DOI] [PubMed] [Google Scholar]
- Hinckley M, Vaccari S, Horner K, Chen R, Conti M. 2005. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol 287: 249–261. [DOI] [PubMed] [Google Scholar]
- Hodgman R, Tay J, Mendez R, Richter JD. 2001. CPEB phosphorylation and cytoplasmic polyadenylation are catalyzed by the kinase IAK1/Eg2 in maturing mouse oocytes. Development 128: 2815–2822. [DOI] [PubMed] [Google Scholar]
- Hofmann MC. 2008. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol 288: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdcraft RW, Braun RE. 2004. Hormonal regulation of spermatogenesis. Int J Androl 27: 335–342. [DOI] [PubMed] [Google Scholar]
- Hu J, Shima H, Nakagawa H. 1999. Glial cell line-derived neurotropic factor stimulates sertoli cell proliferation in the early postnatal period of rat testis development. Endocrinology 140: 3416–3421. [DOI] [PubMed] [Google Scholar]
- Hung PH, Suarez SS. 2010. Regulation of sperm storage and movement in the ruminant oviduct. Soc Reprod Fertil Suppl 67: 257–266. [DOI] [PubMed] [Google Scholar]
- Hunter AG, Moor RM. 1987. Stage-dependent effects of inhibiting ribonucleic acids and protein synthesis on meiotic maturation of bovine oocytes in vitro. J Dairy Sci 70: 1646–1651. [DOI] [PubMed] [Google Scholar]
- Iino M. 1990a. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol 95: 1103–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. 1990b. Calcium release mechanisms in smooth muscle. Jpn J Pharmacol 54: 345–354. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Inoue N, Benham AM, Okabe M. 2010. Fertilization: A sperm’s journey to and interaction with the oocyte. J Clin Invest 120: 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N, Ikawa M, Isotani A, Okabe M. 2005. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 434: 234–238. [DOI] [PubMed] [Google Scholar]
- Inoue N, Yamaguchi R, Ikawa M, Okabe M. 2007. Sperm-egg interaction and gene manipulated animals. Soc Reprod Fertil Suppl 65: 363–371. [PubMed] [Google Scholar]
- Itman C, Mendis S, Barakat B, Loveland KL. 2006. All in the family: TGF-β family action in testis development. Reproduction 132: 233–246. [DOI] [PubMed] [Google Scholar]
- Ito M, Shikano T, Oda S, Horiguchi T, Tanimoto S, Awaji T, Mitani H, Miyazaki S. 2008. Difference in Ca2+ oscillation-inducing activity and nuclear translocation ability of PLCZ1, an egg-activating sperm factor candidate, between mouse, rat, human, and medaka fish. Biol Reprod 78: 1081–1090. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Chiba K, Uchiyama T, Yoshikawa F, Suzuki F, Ikeda M, Furuichi T, Mikoshiba K. 2002. Molecular characterization of the starfish inositol 1,4,5-trisphosphate receptor and its role during oocyte maturation and fertilization. J Biol Chem 277: 2763–2772. [DOI] [PubMed] [Google Scholar]
- Jagiello GM. 1969. Meiosis and inhibition of ovulation in mouse eggs treated with actinomycin D. J Cell Biol 42: 571–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellerette T, He CL, Wu H, Parys JB, Fissore RA. 2000. Down-regulation of the inositol 1,4,5-trisphosphate receptor in mouse eggs following fertilization or parthenogenetic activation. Dev Biol 223: 238–250. [DOI] [PubMed] [Google Scholar]
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. 2011. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci 108: 4892–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Matsuda M, Parrington J, Katan M, Swann K. 2000. Different Ca2+-releasing abilities of sperm extracts compared with tissue extracts and phospholipase C isoforms in sea urchin egg homogenate and mouse eggs. Biochem J 346: 743–749. [PMC free article] [PubMed] [Google Scholar]
- Kanki JP, Donoghue DJ. 1991. Progression from meiosis I to meiosis II in Xenopus oocytes requires de novo translation of the mosxe protooncogene. Proc Natl Acad Sci 88: 5794–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Miyazaki S, Oshimi Y, Oshimi K. 1993. Ca2+ response in single human T cells induced by stimulation of CD4 or CD8 and interference with CD3 stimulation. J Immunol Meth 166: 145–153. [DOI] [PubMed] [Google Scholar]
- Kim E, Yamashita M, Kimura M, Honda A, Kashiwabara SI, Baba T. 2008. Sperm penetration through cumulus mass and zona pellucida. Int J Dev Biol 52: 677–682. [DOI] [PubMed] [Google Scholar]
- Kim KS, Foster JA, Kvasnicka KW, Gerton GL. 2011. Transitional states of acrosomal exocytosis and proteolytic processing of the acrosomal matrix in guinea pig sperm. Mol Reprod Dev 78: 930–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S. 2004. Recombinant phospholipase Cζ has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem 279: 10408–10412. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. 1999. Binding of 14-3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev 13: 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume S, Yamamoto A, Inoue T, Muto A, Okano H, Mikoshiba K. 1997. Developmental expression of the inositol 1,4,5-trisphosphate receptor and structural changes in the endoplasmic reticulum during oogenesis and meiotic maturation of Xenopus laevis. Dev Biol 182: 228–239. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Fissore RA. 2003. ICSI-generated mouse zygotes exhibit altered calcium oscillations, inositol 1,4,5-trisphosphate receptor-1 down-regulation, and embryo development. Mol Hum Reprod 9: 523–533. [DOI] [PubMed] [Google Scholar]
- Kyozuka K, Deguchi R, Mohri T, Miyazaki S. 1998. Injection of sperm extract mimics spatiotemporal dynamics of Ca2+ responses and progression of meiosis at fertilization of ascidian oocytes. Development 125: 4099–4105. [DOI] [PubMed] [Google Scholar]
- Labbe JC, Picard A, Peaucellier G, Cavadore JC, Nurse P, Doree M. 1989. Purification of MPF from starfish: Identification as the H1 histone kinase p34cdc2 and a possible mechanism for its periodic activation. Cell 57: 253–263. [DOI] [PubMed] [Google Scholar]
- Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. 2000. Severely reduced female fertility in CD9-deficient mice. Science 287: 319–321. [DOI] [PubMed] [Google Scholar]
- Li YF, He W, Jha KN, Klotz K, Kim YH, Mandal A, Pulido S, Digilio L, Flickinger CJ, Herr JC. 2007. FSCB, a novel protein kinase A-phosphorylated calcium-binding protein, is a CABYR-binding partner involved in late steps of fibrous sheath biogenesis. J Biol Chem 282: 34104–34119. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. 2005. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. 2011. Progesterone activates the principal Ca2+ channel of human sperm. Nature 471: 387–391. [DOI] [PubMed] [Google Scholar]
- Liu J, Maller JL. 2005. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr Biol 15: 1458–1468. [DOI] [PubMed] [Google Scholar]
- Lopez-Girona A, Furnari B, Mondesert O, Russell P. 1999. Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature 397: 172–175. [DOI] [PubMed] [Google Scholar]
- Lu Q, Shur BD. 1997. Sperm from β1,4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reactions and penetrate the zona pellucida poorly. Development 124: 4121–4131. [DOI] [PubMed] [Google Scholar]
- Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT. 2006. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol 174: 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahbub Hasan AK, Sato K, Sakakibara K, Ou Z, Iwasaki T, Ueda Y, Fukami Y. 2005. Uroplakin III, a novel Src substrate in Xenopus egg rafts, is a target for sperm protease essential for fertilization. Dev Biol 286: 483–492. [DOI] [PubMed] [Google Scholar]
- Malcuit C, Knott JG, He C, Wainwright T, Parys JB, Robl JM, Fissore RA. 2005. Fertilization and inositol 1,4,5-trisphosphate (IP3)-induced calcium release in type-1 inositol 1,4,5-trisphosphate receptor down-regulated bovine eggs. Biol Reprod 73: 2–13. [DOI] [PubMed] [Google Scholar]
- Malcuit C, Maserati M, Takahashi Y, Page R, Fissore RA. 2006. Intracytoplasmic sperm injection in the bovine induces abnormal [Ca2+]i responses and oocyte activation. Reprod Fertil Dev 18: 39–51. [DOI] [PubMed] [Google Scholar]
- Masui Y, Markert CL. 1971. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool 177: 129–145. [DOI] [PubMed] [Google Scholar]
- Matthiesson KL, McLachlan RI, O’Donnell L, Frydenberg M, Robertson DM, Stanton PG, Meachem SJ. 2006. The relative roles of follicle-stimulating hormone and luteinizing hormone in maintaining spermatogonial maturation and spermiation in normal men. J Clin Endocrinol Metab 91: 3962–3969. [DOI] [PubMed] [Google Scholar]
- Mattioli M, Galeati G, Bacci ML, Barboni B. 1991. Changes in maturation-promoting activity in the cytoplasm of pig oocytes throughout maturation. Mol Reprod Dev 30: 119–125. [DOI] [PubMed] [Google Scholar]
- Mayorga LS, Tomes CN, Belmonte SA. 2007. Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life 59: 286–292. [DOI] [PubMed] [Google Scholar]
- McGuinness OM, Moreton RB, Johnson MH, Berridge MJ. 1996. A direct measurement of increased divalent cation influx in fertilised mouse oocytes. Development 122: 2199–2206. [DOI] [PubMed] [Google Scholar]
- McLachlan RI, O’Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, Robertson DM. 2002. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res 57: 149–179. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM. 2005. Oocyte-specific expression of Gpr3 is required for the maintenance of meiotic arrest in mouse oocytes. Dev Biol 288: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez R, Hake LE, Andresson T, Littlepage LE, Ruderman JV, Richter JD. 2000. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature 404: 302–307. [DOI] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, et al. 2000. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287: 1489–1493. [DOI] [PubMed] [Google Scholar]
- Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, Ryu F, Suzuki K, Kosai K, Inoue K, Ogura A, et al. 2000. Requirement of CD9 on the egg plasma membrane for fertilization. Science 287: 321–324. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Ito M. 2006. Calcium signals for egg activation in mammals. J Pharmacol Sci 100: 545–552. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. 1992. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science 257: 251–255. [DOI] [PubMed] [Google Scholar]
- Moor RM, Crosby IM. 1986. Protein requirements for germinal vesicle breakdown in ovine oocytes. J Embryol Exp Morphol 94: 207–220. [PubMed] [Google Scholar]
- Nakano Y, Shirakawa H, Mitsuhashi N, Kuwabara Y, Miyazaki S. 1997. Spatiotemporal dynamics of intracellular calcium in the mouse egg injected with a spermatozoon. Mol Hum Reprod 3: 1087–1093. [DOI] [PubMed] [Google Scholar]
- Nalam RL, Matzuk MM. 2010. Local signalling environments and human male infertility: What we can learn from mouse models. Expert Rev Mol Med 12: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. 2006. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod 74: 314–321. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Furuno N, Okazaki K, Tanaka H, Ogawa Y, Sagata N. 1993. Degradation of Mos by the N-terminal proline (Pro2)-dependent ubiquitin pathway on fertilization of Xenopus eggs: Possible significance of natural selection for Pro2 in Mos. EMBO J 12: 4021–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. 2012. The germline stem cell niche unit in mammalian testes. Physiol Rev 92: 577–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohe M, Inoue D, Kanemori Y, Sagata N. 2007. Erp1/Emi2 is essential for the meiosis I to meiosis II transition in Xenopus oocytes. Dev Biol 303: 157–164. [DOI] [PubMed] [Google Scholar]
- Osman RA, Andria ML, Jones AD, Meizel S. 1989. Steroid induced exocytosis: The human sperm acrosome reaction. Biochem Biophys Res Commun 160: 828–833. [DOI] [PubMed] [Google Scholar]
- Palmer A, Gavin AC, Nebreda AR. 1998. A link between MAP kinase and p34cdc2/cyclin B during oocyte maturation: p90RSK phosphorylates and inactivates the p34cdc2 inhibitory kinase Myt1. EMBO J 17: 5037–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. 2009. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136: 876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrington J, Jones KT, Lai A, Swann K. 1999. The soluble sperm factor that causes Ca2+ release from sea-urchin (Lytechinus pictus) egg homogenates also triggers Ca2+ oscillations after injection into mouse eggs. Biochem J 341: 1–4. [PMC free article] [PubMed] [Google Scholar]
- Parrington J, Davis LC, Galione A, Wessel G. 2007. Flipping the switch: How a sperm activates the egg at fertilization. Dev Dyn 236: 2027–2038. [DOI] [PubMed] [Google Scholar]
- Parys JB, McPherson SM, Mathews L, Campbell KP, Longo FJ. 1994. Presence of inositol 1,4,5-trisphosphate receptor, calreticulin, and calsequestrin in eggs of sea urchins and Xenopus laevis. Dev Biol 161: 466–476. [DOI] [PubMed] [Google Scholar]
- Propst F, Rosenberg MP, Iyer A, Kaul K, Vande Woude GF. 1987. c-mos proto-oncogene RNA transcripts in mouse tissues: Structural features, developmental regulation, and localization in specific cell types. Mol Cell Biol 7: 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU. 2005. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature 437: 1048–1052. [DOI] [PubMed] [Google Scholar]
- Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. 2001. A sperm ion channel required for sperm motility and male fertility. Nature 413: 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Rhind N, Russell P. 2012. Signaling pathways that regulate cell division. Cold Spring Harb Perspect Biol 4: a005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A, Parrington J, Jones KT, Swann K. 2000. Mammalian sperm contain a Ca2+-sensitive phospholipase C activity that can generate InsP3 from PIP2 associated with intracellular organelles. Dev Biol 228: 125–135. [DOI] [PubMed] [Google Scholar]
- Richards JS, Pangas SA. 2010. The ovary: Basic biology and clinical implications. J Clin Invest 120: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NT, Hobson E, Pickering S, Lai FA, Braude P, Swann K. 2004. Phospholipase Czeta causes Ca2+ oscillations and parthenogenetic activation of human oocytes. Reproduction 128: 697–702. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. 2005. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PJ, Beyhan Z, Iager AE, Yoon SY, Malcuit C, Schellander K, Fissore RA, Cibelli JB. 2008. Parthenogenetic activation of bovine oocytes using bovine and murine phospholipase Cζ. BMC Dev Biol 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz EJ, Hunt T, Nebreda AR. 2008. Meiotic inactivation of Xenopus Myt1 by CDK/XRINGO, but not CDK/cyclin, via site-specific phosphorylation. Mol Cell 32: 210–220. [DOI] [PubMed] [Google Scholar]
- Runft LL, Watras J, Jaffe LA. 1999. Calcium release at fertilization of Xenopus eggs requires type I IP3 receptors, but not SH2 domain-mediated activation of PLCγ or Gq-mediated activation of PLCβ. Dev Biol 214: 399–411. [DOI] [PubMed] [Google Scholar]
- Ruwanpura SM, McLachlan RI, Stanton PG, Meachem SJ. 2008. Follicle-stimulating hormone affects spermatogonial survival by regulating the intrinsic apoptotic pathway in adult rats. Biol Reprod 78: 705–713. [DOI] [PubMed] [Google Scholar]
- Ruwanpura SM, McLachlan RI, Meachem SJ. 2010. Hormonal regulation of male germ cell development. J Endocrinol 205: 117–131. [DOI] [PubMed] [Google Scholar]
- Sagata N, Watanabe N, Vande Woude GF, Ikawa Y. 1989. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature 342: 512–518. [DOI] [PubMed] [Google Scholar]
- Saito K, O’Donnell L, McLachlan RI, Robertson DM. 2000. Spermiation failure is a major contributor to early spermatogenic suppression caused by hormone withdrawal in adult rats. Endocrinology 141: 2779–2785. [DOI] [PubMed] [Google Scholar]
- Salicioni AM, Platt MD, Wertheimer EV, Arcelay E, Allaire A, Sosnik J, Visconti PE. 2007. Signalling pathways involved in sperm capacitation. Soc Reprod Fertil Suppl 65: 245–259. [PubMed] [Google Scholar]
- Sariola H, Saarma M. 2003. Novel functions and signalling pathways for GDNF. J Cell Sci 116: 3855–3862. [DOI] [PubMed] [Google Scholar]
- Sato K, Tokmakov AA, Iwasaki T, Fukami Y. 2000. Tyrosine kinase-dependent activation of phospholipase Cγ is required for calcium transient in Xenopus egg fertilization. Dev Biol 224: 453–469. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. 2002. PLCζ: A sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 129: 3533–3544. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Kopf GS. 1995. Molecular-basis of mammalian egg activation. Curr Top Dev Biol 30: 21–62. [DOI] [PubMed] [Google Scholar]
- Sette C, Dolci S, Geremia R, Rossi P. 2000. The role of stem cell factor and of alternative c-kit gene products in the establishment, maintenance and function of germ cells. Int J Dev Biol 44: 599–608. [PubMed] [Google Scholar]
- *.Sever R, Glass CK. 2013. Signaling by nuclear receptors. Cold Spring Harb Perspect Biol 5: a016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsadin R, Adham IM, Nayernia K, Heinlein UA, Oberwinkler H, Engel W. 1999. Male mice deficient for germ-cell cyritestin are infertile. Biol Reprod 61: 1445–1451. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. 1994. Regulation of spermatogenesis. Raven Press, New York. [Google Scholar]
- Shupe J, Cheng J, Puri P, Kostereva N, Walker WH. 2011. Regulation of Sertoli-germ cell adhesion and sperm release by FSH and nonclassical testosterone signaling. Mol Endocrinol 25: 238–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli J, Diaz ES, Morales P. 2012. Kinases, phosphatases and proteases during sperm capacitation. Cell Tissue Res 349: 765–782. [DOI] [PubMed] [Google Scholar]
- Simon L, Ekman GC, Tyagi G, Hess RA, Murphy KM, Cooke PS. 2007. Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, Sertoli cell proteins essential for spermatogonial stem cell maintenance. Exp Cell Res 313: 3090–3099. [DOI] [PubMed] [Google Scholar]
- Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW Jr. 2006. Emerging perspectives in store-operated Ca2+ entry: Roles of Orai, Stim and TRP. Biochim Biophys Acta 1763: 1147–1160. [DOI] [PubMed] [Google Scholar]
- Sorrentino V, Giorgi M, Geremia R, Besmer P, Rossi P. 1991. Expression of the c-kit proto-oncogene in the murine male germ cells. Oncogene 6: 149–151. [PubMed] [Google Scholar]
- Sosnik J, Miranda PV, Spiridonov NA, Yoon SY, Fissore RA, Johnson GR, Visconti PE. 2009. Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J Cell Sci 122: 2741–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JS, Ruderman JV. 2005. Changes in regulatory phosphorylation of Cdc25C Ser287 and WEE1 Ser549 during normal cell cycle progression and checkpoint arrests. Mol Biol Cell 16: 5749–5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JS, Lieberman SL, Wong VL, Ruderman JV. 2003. Regulation of the G2/M transition in oocytes of Xenopus tropicalis. Dev Biol 260: 438–448. [DOI] [PubMed] [Google Scholar]
- Steegborn C, Litvin TN, Levin LR, Buck J, Wu H. 2005. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat Struct Mol Biol 12: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice SL, Robl JM. 1990. Activation of mammalian oocytes by a factor obtained from rabbit sperm. Mol Reprod Dev 25: 272–280. [DOI] [PubMed] [Google Scholar]
- Stricker SA. 1997. Intracellular injections of a soluble sperm factor trigger calcium oscillations and meiotic maturation in unfertilized oocytes of a marine worm. Dev Biol 186: 185–201. [DOI] [PubMed] [Google Scholar]
- Stricker SA. 1999. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol 211: 157–176. [DOI] [PubMed] [Google Scholar]
- Stricker SA, Whitaker M. 1999. Confocal laser scanning microscopy of calcium dynamics in living cells. Microsc Res Tech 46: 356–369. [DOI] [PubMed] [Google Scholar]
- Strunker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. 2011. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471: 382–386. [DOI] [PubMed] [Google Scholar]
- Suarez SS. 2008a. Control of hyperactivation in sperm. Hum Reprod Update 14: 647–657. [DOI] [PubMed] [Google Scholar]
- Suarez SS. 2008b. Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol 52: 455–462. [DOI] [PubMed] [Google Scholar]
- Sun F, Bahat A, Gakamsky A, Girsh E, Katz N, Giojalas LC, Tur-Kaspa I, Eisenbach M. 2005. Human sperm chemotaxis: Both the oocyte and its surrounding cumulus cells secrete sperm chemoattractants. Hum Reprod 20: 761–767. [DOI] [PubMed] [Google Scholar]
- Swann K. 1990. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development 110: 1295–1302. [DOI] [PubMed] [Google Scholar]
- Swann K, Lai FA. 1997. A novel signalling mechanism for generating Ca2+ oscillations at fertilization in mammals. BioEssays 19: 371–378. [DOI] [PubMed] [Google Scholar]
- Swann K, Saunders CM, Rogers NT, Lai FA. 2006. PLCζ: A sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Sem Cell Dev Biol 17: 264–273. [DOI] [PubMed] [Google Scholar]
- Tang W, Wu JQ, Guo Y, Hansen DV, Perry JA, Freel CD, Nutt L, Jackson PK, Kornbluth S. 2008. Cdc2 and Mos regulate Emi2 stability to promote the meiosis I-meiosis II transition. Mol Biol Cell 19: 3536–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesarik J, Testart J. 1994. Treatment of sperm-injected human oocytes with Ca2+ ionophore supports the development of Ca2+ oscillations. Biol Reprod 51: 385–391. [DOI] [PubMed] [Google Scholar]
- Thomas TW, Eckberg WR, Dube F, Galione A. 1998. Mechanisms of calcium release and sequestration in eggs of Chaetopterus pergamentaceus. Cell Calcium 24: 285–292. [DOI] [PubMed] [Google Scholar]
- Tokmakov AA, Sato KI, Iwasaki T, Fukami Y. 2002. Src kinase induces calcium release in Xenopus egg extracts via PLCγ and IP3-dependent mechanism. Cell Calcium 32: 11–20. [DOI] [PubMed] [Google Scholar]
- Tosti E, Boni R. 2004. Electrical events during gamete maturation and fertilization in animals and humans. Hum Reprod Update 10: 53–65. [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Chun SY, Zhang R, Hsueh AJ, Conti M. 1996. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: Studies using selective phosphodiesterase inhibitors. Dev Biol 178: 393–402. [DOI] [PubMed] [Google Scholar]
- Tung JJ, Hansen DV, Ban KH, Loktev AV, Summers MK, Adler JR 3rd, Jackson PK. 2005. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc Natl Acad Sci 102: 4318–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbrock K, Gassenhuber H, Kostenis E. 2002. Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell Signal 14: 941–953. [DOI] [PubMed] [Google Scholar]
- van den Ham R, van Dissel-Emiliani FM, van Pelt AM. 2003. Expression of the scaffolding subunit A of protein phosphatase 2A during rat testicular development. Biol Reprod 68: 1369–1375. [DOI] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M. 2006. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology 147: 96–110. [DOI] [PubMed] [Google Scholar]
- Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, et al. 2006. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol 16: 2073–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE. 2009. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci 106: 667–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Krapf D, de la Vega-Beltran JL, Acevedo JJ, Darszon A. 2011. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl 13: 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Tzeng CR, Chang C. 2009. Androgen receptor roles in spermatogenesis and fertility: Lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev 30: 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman WJ, Masui Y. 1975. Effects of cyclohexamide on a cytoplasmic factor initiating meiotic naturation in Xenopus oocytes. Exp Cell Res 91: 381–388. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Broome M, Hunter T. 1995. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J 14: 1878–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M. 2006. Calcium at fertilization and in early development. Physiol Rev 86: 25–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Kornbluth S. 2008. Across the meiotic divide—CSF activity in the post-Emi2/XErp1 era. J Cell Sci 121: 3509–3514. [DOI] [PubMed] [Google Scholar]
- Wu H, He CL, Fissore RA. 1997. Injection of a porcine sperm factor triggers calcium oscillations in mouse oocytes and bovine eggs. Mol Reprod Dev 46: 176–189. [DOI] [PubMed] [Google Scholar]
- Wu JQ, Hansen DV, Guo Y, Wang MZ, Tang W, Freel CD, Tung JJ, Jackson PK, Kornbluth S. 2007a. Control of Emi2 activity and stability through Mos-mediated recruitment of PP2A. Proc Natl Acad Sci 104: 16564–16569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Guo Y, Yamada A, Perry JA, Wang MZ, Araki M, Freel CD, Tung JJ, Tang W, Margolis SS, et al. 2007b. A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest. Curr Biol 17: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Kopf GS, Schultz RM. 1994. Involvement of inositol 1,4,5-trisphosphate-mediated Ca2+ release in early and late events of mouse egg activation. Development 120: 1851–1859. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Honda A, Ogura A, Kashiwabara S, Fukami K, Baba T. 2008. Reduced fertility of mouse epididymal sperm lacking Prss21/Tesp5 is rescued by sperm exposure to uterine microenvironment. Genes Cells 13: 1001–1013. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. 1970. The movement of golden hamster spermatozoa before and after capacitation. J Reprod Fertil 23: 193–196. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. 1994. Mammalian fertilization. In The physiology of reproduction, 2nd ed. (ed. Knobil E, Neill JD), Vol. 1, pp. 189–317. Raven, New York. [Google Scholar]
- Yanagimachi R, Mahi CA. 1976. The sperm acrosome reaction and fertilization in the guinea-pig: A study in vivo. J Reprod Fertil 46: 49–54. [DOI] [PubMed] [Google Scholar]
- Yang J, Winkler K, Yoshida M, Kornbluth S. 1999. Maintenance of G2 arrest in the Xenopus oocyte: A role for 14-3-3-mediated inhibition of Cdc25 nuclear import. EMBO J 18: 2174–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A, Kashima M, Yoshida S, Terada K, Nakagawa S, Sakamoto A, Hayakawa K, Suzuki K, Ueda J, Watanabe T. 2006. Molecular cloning, testicular postnatal expression, and oocyte-activating potential of porcine phospholipase Cζ. Reproduction 132: 393–401. [DOI] [PubMed] [Google Scholar]
- Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE, et al. 2008. Human sperm devoid of PLC, zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J Clin Invest 118: 3671–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Sensui N, Inoue T, Morisawa M, Mikoshiba K. 1998. Role of two series of Ca2+ oscillations in activation of ascidian eggs. Dev Biol 203: 122–133. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. 2007. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 317: 1722–1726. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T, Nishikawa S. 1991. Role of c-kit in mouse spermatogenesis: Identification of spermatogonia as a specific site of c-kit expression and function. Development 113: 689–699. [DOI] [PubMed] [Google Scholar]