Abstract
AIM: To define the benefits of three-dimensional video-assisted thoracoscopic esophagectomy (3D-VATE) over 2D-VATE for esophageal cancer.
METHODS: A total of 93 patients with esophageal cancer including 45 patients receiving 3D-VATE and 48 receiving 2D-VATE were evaluated. Data related to patient and cancer characteristics, operating time, intraoperative bleeding, morbidity and mortality, postoperative inflammatory markers, Numerical Rating Scale for postoperative pain, Constant-Murley rating system for shoulder recovery and oxygenation index (OI) were collected. All medical records were retrieved from a prospectively maintained oncological database at our institution. A retrospective study was performed to compare the short-term surgical outcomes between the two groups.
RESULTS: No significant differences were found between the two groups in either morbidity or mortality (P = 0.328). An enhanced surgical recovery was noted in the 3D group as indicated by shortened thoracoscopic operation time (3D vs 2D: 68 ± 13.79 min vs 83 ± 13 min, P < 0.01), minor intraoperative blood loss (3D vs 2D: 68.2 ± 10.7 mL vs 89.8 ± 10.4 mL, P < 0.01), earlier chest tube removal (3D vs 2D: 2.67 ± 1.01 vs 3.75 ± 1.15 d, P < 0.01), shorter length of hospital stay (3D vs 2D: 9.07 ± 2.00 vs 10.85 ± 3.40 d, P < 0.01), lower in-hospital expenses (3D vs 2D: 74968.4 ± 9637.8 vs 86211.1 ± 8519.7 RMB, P < 0.01), lower pain intensity (P < 0.01) and faster recovery of the left shoulder function (P < 0.01). Better preservation of the pulmonary function was also found in the 3D group as the decline of the OI post operation was significantly lower than that of the 2D group (P < 0.01). Changes of postoperative inflammatory markers, including procalcitonin [postoperative days (PODs) 4 and 7: P < 0.01], peripheral granulocytes (PODs 1, 4 and 7: P < 0.01) and hypersensitive C-reactive protein (POD 4: P < 0.01) in 3D-VATE patients were less than those in the 2D group. Moreover, utilization of the 3D technique extended the dissection of the thoracic lymph nodes (P < 0.01), with better exposure of nodes in the left recurrent laryngeal nerve (P = 0.031).
CONCLUSION: 3D-VATE could be a more viable technique over 2D-VATE in terms of short-term outcomes for patients with esophageal cancer.
Keywords: Esophageal cancer, Three-dimensional video-assisted thoracoscopic esophagectomy, Two-dimensional video-assisted thoracoscopic esophagectomy, Surgical outcomes
Core tip: Minimally invasive esophagectomy has been the predominant option for esophageal cancers. However, conventional two-dimensional video-assisted thoracoscopic esophagectomy (2D-VATE) is limited in its operating fields and disturbed eye-hand coordination, which may hamper necessary lymph node dissection and increase chances of surgical-related trauma. The introduction of 3D-VATE with 24-fold magnified view is designed to overcome such disadvantages. However, the benefits of 3D-VATE over 2D-VATE have not been fully studied in terms of surgical outcomes. This work, to our knowledge, is for the first time to report the definitive advantages of 3D-VATE in short-term outcomes.
INTRODUCTION
Surgery remains the treatment of choice for esophageal cancer[1]. However, conventional open esophagectomy has been associated with high rates of morbidity and mortality[2-4]. Since the first report by Cuschieri et al[5] in 1992, minimally invasive esophagectomy (MIE) has become a viable alternative. Accumulating evidence has shown that the application of MIE is associated with a substantial decrease in blood loss, fewer complications and short hospital stays[6,7]. However, MIE with two-dimensional (2D) visualization is known for its limitations, such as a restricted operating field and disturbed eye-hand coordination. These limitations may hamper necessary lymph node dissection and increase the chances of surgically related trauma[8]. The Da Vinci robotic system was developed with a three-dimensional camera, which offers a ten-fold magnified view of the operating field. This could be of great value in lymph node dissection and mediastinal dissection of the esophagus as it gives the actual depth perception to the surgeons[9]. However, the robotic system requires specific instruments that took a much longer time to prepare and a prolonged learning curve for surgeons to adopt the technique[10]. Above all, the costs and technical challenges substantially limited its application, especially in developing countries[11].
The introduction of the three-dimensional video-assisted thoracoscopic esophagectomy (3D-VATE) was designed to overcome some of the disadvantages of 2D-VATE. It offers 24-fold three-dimensional imaging, which is comparable to that of the robotic system in restoring the actual depth perception to surgeons. The learning curve of 3D-VATE is potentially shorter than that of 2D-VATE/robotic-assisted esophagectomy[12-14]. Moreover, the expenses of 3D-VATE is much lower compared to robotic-assisted esophagectomy. The cost-efficiency of 3D-VATE allows wide use in esophagectomies, especially in developing countries such as China. Because the majority of esophageal cancer patients come from rural areas with relatively low social-economic conditions, the use of 3D-VATE could be a more viable alternative for these individuals.
However, the belief that 3D-VATE outweighs 2D-VATE has not been fully explored in scope and magnitude. In this study, we included a total of 93 patients from the Eastern parts of China to compare the postoperative outcomes in patients undergoing 3D- and 2D-VATE for esophageal cancers.
MATERIALS AND METHODS
Patients
Prior written informed consent was obtained from all participants and the study was approved by the Clinical Ethics Committee of the First People’s Hospital, Shanghai Jiaotong University. From April 2013 to October 2014, a total of 93 patients undergoing minimally invasive esophagectomy were selected out of the database including 45 patients with 3D-VATE and 48 with 2D-VATE. Eight patients from the 3D group were excluded, including four patients with benign esophageal diseases and two with esophageal cancer who received minimally invasive Ivor Lewis esophagectomy.
The overall operating time was the time from skin incision to the final skin closure. The thoracoscopic operating time refers to the resection time. Intraoperative bleeding was collected by anesthetists. Pulmonary complications included pneumonia, respiratory failure and adult respiratory distress syndrome (ARDS). A pneumonia diagnosis was based on radiographic evidence with a body temperature > 38.1 °C. Diagnosis of ARDS was made according to the American-European consensus conference on ARDS[15]. Systemic acute-phase responses to surgical stress were indicated by inflammatory markers on postoperative days (PODs) 1, 4 and 7, including serum hypersensitive C-reactive protein (hsCRP), white blood cells (WBCs), granulocytes (GRs) and procalcitonin (PCT).
The postoperative pain intensity was reported using numerical rating scale for pain (NRS; 0 = no pain, 10 = maximal imaginable pain)[16]. Postoperative shoulder recovery was described using the Constant-Murley rating system[17]. Changes in the oxygenation index (OI) on PODs 1, 2, 3, 5 and 7 compared to preoperative baselines were used as the indicator for pulmonary function recovery. Detailed operative procedures of 3D-VATE and 2D-VATE are described below. Tumors were staged according to the classification system of the American Joint Committee on Cancer and Union for International Cancer Control.
Surgical procedures
All patients received a combination of epidural and general anesthesia before operation. The patients were turned to the left lateral decubitus position and four trocars were placed. Operative procedure of thoracoscopic part was in a manner similar to MIE[18-20]. The thoracic duct, mediastinal pleura, and lymph nodes at para-esophageal, subcarinal, and peribronchial stations were dissected to remain en bloc with the esophagus. After completion of the total mediastinal lymph node dissection, a 28-Fr chest tube was placed, and the collapsed right lung was inflated. The patient then was turned to the supine position.
Gastric mobilization and upper abdominal lymph node dissection were performed using laparoscopy as previously reported[21]. At the end of the abdominal phase, left cervicotomy was performed, and the cervical esophagus was mobilized. The esophagogastric specimen was pulled out through the neck incision under laparoscopic observation. Esophagogastric anastomosis was performed with a side-to-side stapled anastomosis.
For 3D group, the monitor shows two separate images, one for the left eye and one for the right eye, which are presented to the corresponding eye by special filter glasses. Both images are composed of partial spectra of the three primary colors, i.e., red, green and blue. These partial spectra used for the left image are different from those of the right image and are generated with the aid of interference filters. By these means the images are coded and can be assigned to the corresponding eye via the mentioned glasses[22]. For the 2D group, the monitor presents identical images in 2D mode apart from the stereoscopic effect. We used a 30° camera in both the 2D and 3D groups.
Perioperative management
All patients were transferred to the intensive care unit (ICU) with extubation after surgery. Oxygen administration was discontinued when the O2 saturation level was > 95% of room air. Patient-controlled epidural analgesia was continued up to POD 5 in both groups. Enteral nutrition was started on POD 4. Oral intake was started after the removal of the nasogastric tube on POD 6. Blood cell counts and laboratory data were taken on PODs 1, 4 and 7. Patients were discharged when chest tube was removed.
Statistical analysis
Data are presented as mean ± SD. Comparisons were made between the two groups using Student’s t-test for continuous measures and a χ2 test for categorical variables. Significance was set as a P value < 0.05. All analyses were performed using the SPSS version 19.0 (SPSS Inc., IL, United States).
RESULTS
Patient demographics
A total of 93 patients underwent MIE at our institution with 45 3D-VATE cases and 48 2D-VATE cases. The average age was 63.8 years and 65.1 years for the 3D and 2D groups, respectively. Comorbidities such as cardiac, renal and pulmonary functions were comparable between the 3D and 2D groups (Table 1). In the 3D group, 8 patients received preoperative chemotherapy and 12 patients received radiotherapy. However, this was significantly different from the 2D group.
Table 1.
Patient demographics n (%)
| 3D-VATE n = 45 | 2D-VATE n = 48 | P value | |
| Age (yr) | 63.8 ± 10.2 | 65.1 ± 9.8 | 0.55 |
| Gender | 0.66 | ||
| Male | 29 (64.4) | 34 (70.8) | |
| Female | 16 (35.6) | 14 (29.2) | |
| Functional status | 0.25 | ||
| Independence | 44 (97.8) | 47 (97.9) | |
| Partial dependence | 1 (2.2) | 0 (0) | |
| Complete dependence | 0 (0) | 1 (2.1) | |
| Weight loss1 | 13 (28.9) | 11 (22.9) | 0.64 |
| Smoking2 | 7 (15.6) | 9 (18.8) | 0.91 |
| Use of AAS | 0 (0) | 1 (2.1) | 1 |
| LEVF% | 68.7 ± 1.5 | 68.6 ± 1.5 | 0.73 |
| FS% | 39.3 ± 2.3 | 39.5 ± 2.0 | 0.7 |
| Hypertension | 10 (22.2) | 19 (39.6) | 0.078 |
| Renal failure | 0 (0) | 2 (4.2) | 0.5 |
| Dialysis | 0 (0) | 1 (2.1) | 1 |
| Ascites | 1 (2.2) | 1 (2.1) | 0.96 |
| Esophageal varices | 0 (0) | 1 (2.1) | 1 |
| Preoperative chemotherapy | 8 (17.8) | 9 (18.8) | 1 |
| Preoperative radiation | 12 (26.7) | 13 (27.1) | 1 |
| Prior operation | 0 (0) | 1 (2.1) | 1 |
Weight loss > 10% over 6 mo;
Smoking over the past year. Prior operation within 30 d. AAS: Anabolic-androgenic steroids; LEVF: Left ventricular ejection fraction; FS: Fraction shortening; VATE: Video-assisted thoracoscopic esophagectomy; 3D: Three-dimensional; 2D: Two-dimensional.
Morbidity and mortality
Postoperative morbidity was similar between the groups as shown in Table 2. The morbidity of pulmonary, cardiac and renal systems showed no statistical differences. No significant differences were found between the two groups in terms of anastomotic leakage, wound infections or unplanned returns to the operating room.
Table 2.
Postoperative morbidity and mortality n (%)
| 3D-VATE (n = 45) | 3D-VATE (n = 48) | P value | |
| Morbidity | 13 (28.9) | 8 (16.7) | 0.328 |
| Mortality | 1 (2.2) | 1 (2.1) | NS |
| Pulmonary complications | NS | ||
| Pneumonia | 5 (11.1) | 7 (14.6) | 0.76 |
| Re-intubation | 4 (8.9) | 5 (10.4) | |
| Pulmonary embolism | 1 (2.2) | 1 (2.1) | |
| Ventilation > 48 h | 4 (8.9) | 4 (8.3) | |
| Wound infections | NS | ||
| Neck | 0 (0) | 0 (0) | |
| Thorax | 0 (0) | 1 (2.1) | |
| Abdomen | 0 (0) | 0 (0) | |
| Renal complications | NS | ||
| Progressive renal insufficiency | 0 (0) | 0 (0) | |
| Acute renal failure | 0 (0) | 0 (0) | |
| Urinary tract infections | 1 (2.2) | 1 (2.1) | |
| Cardiac complications | NS | ||
| Cardiac arrest | 0 (0) | 0 (0) | |
| Myocardial infarction | 0 (0) | 0 (0) | |
| Tachycardia/arrhythmias | 1 (2.2) | 4 (8.3) | 0.363 |
| Deep venous thrombosis | 0 (0) | 0 (0) | NS |
| Anastomotic leakage | 1 (2.2) | 1 (2.1) | NS |
| Unplanned return to OR | 2 (4.4) | 4 (8.3) | 0.678 |
NS: Not significant; OR: Operating room; 3D-VATE: Three-dimensional video-assisted thoracoscopic esophagectomy.
Surgical recovery indications
The overall surgical time (138 ± 14 min vs 167 ± 20 min; P < 0.001) and the thoracoscopic surgical time (68 ± 13.79 min vs 83 ± 13min, P < 0.001) were both remarkably shortened in the 3D group compared to the 2D group. Intraoperative bleeding in the 3D group was minor (68 ± 13.79 mL vs 83 ± 13 mL, P < 0.01) with earlier chest tube removal after surgery (2.67 ± 1.01 d vs 3.75 ± 1.15 d, P < 0.01), reduced length of the hospital stay (9.07 ± 2.00 d vs 10.85 ± 3.40 d, P = 0.003) and lower in-hospital expenses (RMB 74968 ± 9637 yuan vs 86211 ± 8519 yuan, P < 0.01; Table 3). Figure 1 shows the pain intensity of the patients undergoing the 3D-VATE and 2D-VATE via the numerical pain rating scale. The pain degree of the patients on PODs 3, 5 and postoperative months (POMs) 1, 2 and 3 indicates a statistically significant difference between the 3D group and the 2D group (P < 0.05) (3D vs 2D, POD 3: 3.93 ± 0.84 vs 5.48 ± 1.15, P = 0.002; POD 5: 3.96 ± 0.82 vs 5.40 ± 1.01, P = 0.01; POM 1: 4.69 ±1.15 vs 5.63 ± 1.44, P = 0.048; POM 2: 3.87 ± 0.94 vs 4.75 ± 1.2, P = 0.029; POM 3: 2.07 ± 0.863 vs 3.38 ± 1.20, P = 0.007). Figure 2 summarizes the patients’ recovery condition of the shoulder function in the two groups. The shoulder recovery condition had a statistically significant difference between the two groups on POM 1 (3D vs 2D, POM 1:87.40 ± 3.14 vs 83.50 ± 4.05, P = 0.03).
Table 3.
Surgical recovery indicators
| 3D-VATE (%) n = 45 | 2D-VATE (%) n = 48 | P value | |
| Thoracoscopic operating time1 (min) | 68 ± 13.79 | 83 ± 13 | < 0.01 |
| Operating time2 (min) | 138 ± 14 | 167 ± 20 | < 0.01 |
| Bleeding (mL) | 68.2 ± 10.7 | 89.8 ± 10.4 | < 0.01 |
| Chest drains (mL) | 306.6 ± 56.2 | 366.4 ± 62.6 | < 0.01 |
| Chest tube duration (d) | 2.67 ± 1.01 | 3.75 ± 1.15 | < 0.01 |
| Length of stay (d) | 9.07 ± 2.00 | 10.85 ± 3.40 | 0.003 |
| Expenses (RMB) | 74968.4 ± 9637.8 | 86211.1 ± 8519.7 | < 0.01 |
Thoracoscopic operating time refers to the resection time;
Operating time is the time from skin incision to the final skin closure. VATE: Video-assisted thoracoscopic esophagectomy; 3D: Three-dimensional; 2D: Two-dimensional.
Figure 1.
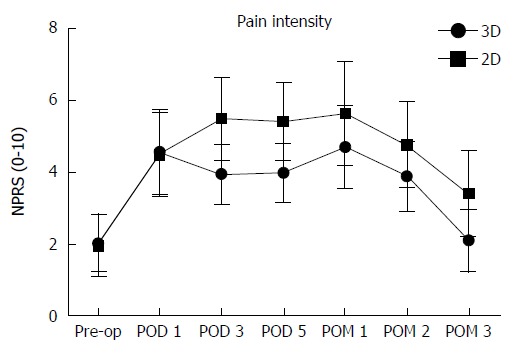
Pain intensity. The pain intensity of the patients undergoing the three-dimensional video-assisted thoracoscopic esophagectomy (3D-VATE) and two-dimensional (2D)-VATE was showed via the numerical pain rating scale (NPRS). The pain degree of the patients on postoperative PODs 3, 5 and POMs 1, 2 and 3 indicated a statistically significant difference between the 3D group and the 2D group (P < 0.05). 3D vs 2D, POD 3: 3.93 ± 0.84 vs 5.48 ± 1.15, P = 0.002; POD 5: 3.96 ± 0.82 vs 5.40 ± 1.01, P = 0.01; POM 1: 4.69 ± 1.15 vs 5.63 ± 1.44, P = 0.048; POM 2: 3.87 ± 0.94 vs 4.75 ± 1.2, P = 0.029; POM 3: 2.07 ± 0.863 vs 3.38 ± 1.20, P = 0.007. pre-op: Preoperation; POD: Postoperative day; POM: Postoperative month.
Figure 2.
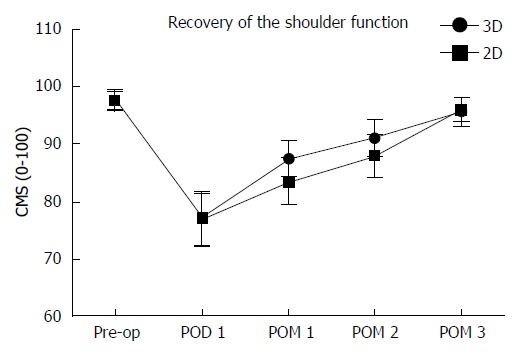
Recovery of the shoulder function. The shoulder recovery condition had a statistically significant difference between the two groups on POM 1 (3D vs 2D, POM 1:87.40 ± 3.14 vs 83.50 ± 4.05, P = 0.03). CMS: Constant-Murley scale; pre-op: Pre-operation; POD: Postoperative day; POM: Postoperative month; 3D: Three-dimensional; 2D: Two-dimensional.
Pulmonary function recovery
Decline of the OI on PODs 1, 2, 3, 5 and 7 was significantly smaller in the 3D group compared to that in the 2D group (Table 4, Figure 3), indicating a better preservation of the pulmonary function in the 3D group (POD 1: 71.01 ± 17.92 mmHg vs 86.25 ± 15.91 mmHg; POD 2: 66.71 ± 17.58 mmHg vs 132.22 ± 25.04 mmHg; POD 3: 113.69 ± 20.25 mmHg vs 126.14 ± 22.96 mmHg; POD 5: 76.79 ± 23.52 mmHg vs 117.25 ± 34.88 mmHg; POD 7: 87.26 ± 19.88 mmHg vs 107.83 ± 27.11 mmHg, P < 0.01).
Table 4.
Preoperative baselines of pulmonary function
| 3D-VATE (%) (n = 45) | 2D-VATE (%) (n = 48) | P value | |
| Smoking1 | 7 (15.6) | 9 (18.8) | 0.91 |
| FEV1 (L) | 2.73 ± 0.36 | 2.77 ± 0.36 | 0.65 |
| FEV1% | 85.1 ± 5.8 | 86.6 ± 6.5 | 0.26 |
| Dlco% | 91.9 ± 3.88 | 92.8 ± 3.78 | 0.23 |
| PaO2 (mmHg) | 407.8 ± 19.7 | 402.1 ± 18.7 | 0.16 |
| BMI | 22.5 ± 2.0 | 22.2 ± 2.0 | 0.44 |
| ASA class | 2.58 ± 0.54 | 2.58 ± 0.53 | 0.96 |
Smoking (in the past year). FEV1: Forced expiratory volume in one second; Dlco: Diffusing capacity of the lung for carbon monoxide; PaO2: Oxygen tension; BMI: Body mass index; ASA class: American Society of Anesthesiologists physical status classification system; 3D: Three-dimensional; 2D: Two-dimensional.
Figure 3.
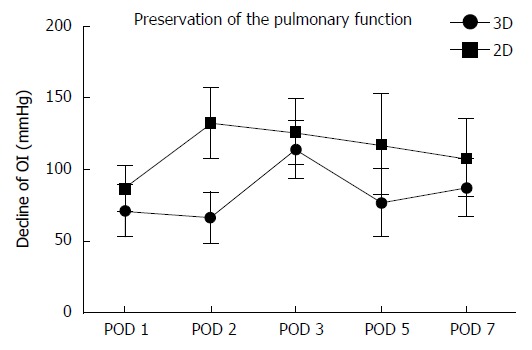
Preservation of the pulmonary function. Decline of the OI on PODs 1, 2, 3, 5 and 7 was significantly smaller in the 3D group compared to that in the 2D group (POD 1: 71.01 ± 17.92 mmHg vs 86.25 ± 15.91 mmHg; POD 2: 66.71 ± 17.58 mmHg vs 132.22 ± 25.04 mmHg; POD 3: 113.69 ± 20.25 mmHg vs 126.14 ± 22.96 mmHg; POD 5: 76.79 ± 23.52 mmHg vs 117.25 ± 34.88 mmHg; POD 7: 87.26 ± 19.88 mmHg vs 107.83 ± 27.11 mmHg, P < 0.01). OI: Oxygenation index; POD: Postoperative day; 3D: Three-dimensional; 2D: Two-dimensional.
Inflammatory markers
Systemic responses to surgical stress were studied to evaluate the surgical invasiveness. Increases in inflammatory markers, including hsCRP, WBCs, granulocytes and PCT, were significantly lower on POD 4 in the 3D group (3D vs 2D, hsCRP: 0.32 ± 0.14 μg/L vs 0.71 ± 0.14 μg/L, P < 0.01). In addition, the rise of GR on PODs 1 and 4 were significantly lower in the 3D group (POD 1: 4.88 ± 1.18 μg/L vs 6.13 ± 1.42 μg/L; POD 4; 3.59 ± 0.85 μg/L vs 6.25 ± 1.21 μg/L, P < 0.01). The rates for other inflammatory factors were equivalent between the two groups (Table 5).
Table 5.
Inflammatory markers
| 3D-VATE n = 45 | 2D-VATE n = 48 | P value | |
| Preoperative baselines | |||
| WBC (109/L) | 5.54 ± 0.88 | 5.39 ± 0.89 | 0.45 |
| GR (109/L) | 3.88 ± 0.55 | 3.77 ± 0.52 | 0.28 |
| hsCRP (mg/L) | 1.76 ± 0.43 | 1.82 ± 0.43 | 0.51 |
| PCT (μg/L) | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.97 |
| Postoperative outcomes | |||
| ΔWBC | |||
| POD1 (109/L) | 5.64 ± 1.60 | 6.75 ± 1.37 | 0.001 |
| POD4 (109/L) | 3.87 ± 1.05 | 6.65 ± 1.01 | < 0.01 |
| POD7 (109/L) | 2.48 ± 0.50 | 2.54 ± 0.66 | 0.667 |
| ΔGR | |||
| POD1 (109/L) | 4.88 ± 1.18 | 6.13 ± 1.42 | < 0.01 |
| POD4 (109/L) | 3.59 ± 0.85 | 6.25 ± 1.21 | < 0.01 |
| POD7 (109/L) | 2.24 ± 0.63 | 2.68 ± 0.67 | 0.001 |
| ΔCRP | |||
| POD1 (mg/L) | 42.5 ± 15.3 | 40.1 ± 16.9 | 0.544 |
| POD4 (mg/L) | 102.5 ± 61.3 | 137.9 ± 64.1 | < 0.01 |
| POD7 (mg/L) | 88.4 ± 47.1 | 91.5 ± 41.3 | 0.225 |
| ΔPCT | |||
| POD1 (μg/L) | 0.71 ± 0.17 | 0.68 ± 0.20 | 0.414 |
| POD4 (μg/L) | 0.32 ± 0.14 | 0.71 ± 0.14 | < 0.01 |
| POD7 (μg/L) | 0.46 ± 0.23 | 1.01 ± 0.26 | < 0.01 |
WBCs: White blood cells; GR: Granulocytes; hsCRP: Hypersensitive C-reactive protein; PCT: Procalcitonin; Δ: Data compared to baselines; VATE: Video-assisted thoracoscopic esophagectomy; 3D: Three-dimensional; 2D: Two-dimensional.
Lymph node dissection and exposure
Total lymph node dissection, including nodes from the abdomen, thorax and neck, was not significantly different between the two groups. However, the 3D technique greatly extended dissection of the thoracic lymph nodes (P = 0.008) with better exposure of nodes in the regions of the left recurrent laryngeal nerve (P < 0.01) and the aortic arch (P = 0.005; Table 6).
Table 6.
Histopathological results and lymph node dissection/exposure n (%)
| 3D-VATE n = 45 | 2D-VATE n = 48 | P value | |
| Tumor location | NS | ||
| Upper third of the esophagus | 8 (17.8) | 9 (18.8) | |
| Middle third of the esophagus | 29 (64.4) | 30 (62.5) | |
| Lower third of the esophagus | 8 (17.8) | 9 (18.8) | |
| Histological type | NS | ||
| Adenocarcinoma | 0 (0) | 1 (2.1) | |
| Squamous cell carcinoma | 45 (100) | 47 (97.9) | |
| TNM stage | NS | ||
| I | 12 (26.7) | 9 (18.8) | |
| II | 16 (35.5) | 13 (27.1) | |
| III | 17 (37.8) | 26 (54.2) | |
| Lymph nodes dissection | |||
| Total1 LNN | 24.8 ± 5.2 | 21.4 ± 6.3 | NS |
| Thoracic LNN | 13.13 ± 3.43 | 8.96 ± 4.05 | < 0.01 |
| Thoracic LN group | 2.56 ± 1.12 | 2.00 ± 0.85 | 0.008 |
| Laryngeal recurrent nerve LNN (L) | 2.67 ± 1.15 | 1.17 ± 0.83 | < 0.01 |
| Laryngeal recurrent nerve LNN (R) | 2.27 ± 1.74 | 2.33 ± 1.39 | NS |
| Esophageal LNN | 3.64 ± 2.05 | 3.58 ± 1.16 | NS |
| Subcarinal LNN | 3.89 ± 2.59 | 2.73 ± 1.08 | 0.005 |
| Lymph nodes exposure | |||
| Recurrent laryngeal nerve (L) (Y) | 41 (91.1) | 35 (72.9) | 0.031 |
| Recurrent laryngeal nerve (R) (Y) | 43 (95.6) | 43 (89.6) | 0.44 |
Inclusive for lymph nodes harvested from the abdomen, chest and neck. TNM: Tumor node metastasis; LNN: Lymph node number; L: Left; R: Right; NS: Not significant; VATE: Video-assisted thoracoscopic esophagectomy; 3D: Three-dimensional; 2D: Two-dimensional.
DISCUSSION
Shortened length of hospital stay, reduced impairment of pulmonary function, minor invasiveness and more extensive lymphadenectomy were found among patients undergoing 3D-VATE compared to 2D-VATE in our study.
The shorter hospital stay may indicate an accelerated recovery from surgery in the 3D group. Contributive factors included reduced intraoperative time, blood loss and postoperative pain when compared to the 2D group. Oh et al[10] reviewed 43 cases undergoing robotic-assisted thoracoscopic surgery in pulmonary lobectomy and found that 3D visualization could facilitate a faster resection compared to open cases. Boone et al[9] reported that 47 patients with resectable esophageal cancer undergoing robot-assisted thoracoscopic esophagectomy achieved a significant decrease in resection time due to the magnified view of the 3D-HD camera. The 3D camera has to be moved more frequently during the operation, as small amounts of bleeding can interfere significantly with visualization, which may prolong overall procedure duration. However, decreased total blood loss allows the surgeons to have more freedom and greater efficiency in difficult tasks[9,10,13]. In our study, the resection time continued to improve in the 3D group as experience accumulated despite these complexities. Furthermore, postoperative pain intensity is another important indicator for postoperative recovery. The present study found a significantly lower pain score and an earlier recovery of shoulder function in the 3D group compared to the 2D group. With a more proper mediastinal dissection in the aid of 3D imaging, surgical injuries were reduced such as repeated compression and stretching of nerves and muscles.
In the present study, we found that the decline of the OI over preoperative baselines was much lower in the 3D group compared to the 2D group, suggesting reduced surgical-related pulmonary impairment. Because anesthesia management was not different between the two groups, decreased postoperative pain could be an important contributor to better preservation of postoperative pulmonary function in the 3D group. This finding is consistent with previous studies as postoperative pain intensity has been identified as an independent predictive factor for pulmonary function recovery[23]. Moreover, reduced duration of lung deflation due to shortened resection time in the 3D group is another factor accounting for fewer changes in the OI for the 3D-VATE.
Systemic responses to postoperative stress are another indicator for surgical invasiveness[23]. Inflammatory factors, including C-reactive protein (CRP), peripheral leukocytes, granulocytes counts and PCT, are commonly used for acute phase responses to tissue injury[24,25]. In this study, we compared the changes in these variables before and after surgery in the two groups. Both groups showed significant increases in these inflammatory markers compared to preoperative baselines, with the 3D group to a lesser extent. These findings suggested minor postoperative stress in the 3D group. With a 24-fold magnified view of the operation field, the 3D high-definition VATE offers a more meticulous and precise dissection in a confined surgical field. This results in minor surgical invasiveness and consequent postoperative stress responses as indicated in our findings.
The lymph node status is a major prognostic factor for esophageal carcinomas[26,27]. A more extensive lymphadenectomy is positively correlated to better survival in patients with esophageal cancer. Even in patients who initially presented with a locally curable disease, 20% were found to eventually have node-positive disease that required esophagectomy[28]. However, studies showed that lymph node dissection was inadequate for patients undergoing MIE and open esophagectomy[28-30]. In our study, we found that utilization of a 3D high-definition camera allows better exposure of thoracic lymph nodes and thus more extensive resection. Dissection of lymph nodes in regions of the left recurrent laryngeal nerve and aortic arch is not practical for open and 2D esophagectomy due to limited information on spatial depth, which can be derived only from secondary spatial depth cues and experience. The 3D technique is designed to overcome this defect. It returns the actual depth perception to the surgeons, which facilitates the improvement of surgical performance[13,30,31].
All data of the present study were retrieved from a prospectively maintained oncological database at our institution with standardized systematic collection of medical records. This database helped to reduce observation bias to a great extent as analysis of patient profiles showed no significant difference between the two groups. However, there are several limitations to our study. First, both surgeon-specific and team-related factors could lead to information bias. It was not until January 2013 that we introduced 3D-VATE to the treatment of esophageal cancer. To ensure sufficient training in 3D-VATE esophagectomy, we slowly accumulated nearly 30 patients with clinical stage I esophageal cancer before surgeons of our thoracic department gradually passed their learning curve by April 2013. Second, selection of patients undergoing 3D-VATE was mainly on a first-come and first-served basis in our study. However, confounding factors such as patient willingness, educational backgrounds and economic conditions contributed to selection biases, which were unfortunately not available for further analysis in the retrospective study. Indications of MIE for esophageal cancers remain controversial. In this study, the inclusion criterion for MIE was non-discriminative of early or advanced disease stage[31]. Further randomized controlled studies are needed to determine the benefits of 3D-VATE in long-term outcomes
In conclusion, compared with 2D-VATE successful utilization of 3D-VATE for esophageal carcinomas was associated with an accelerated recovery, the preservation of pulmonary function, reduced surgical stress and more extensive lymphadenectomy. In conclusion, 3D-VATE could be a more advantageous technique over 2D-VATE. Further investigations are needed to confirm this conclusion.
COMMENTS
Background
The optimal surgical approach for esophageal cancer remains controversial. Three-dimensional video-assisted thoracoscopic esophagectomy (3D-VATE) is believed to offer unique advantages when compared to conventional 2D thoracoscopic technique. However, the benefits of 3D-VATE over 2D-VATE have not been fully studied.
Research frontiers
The 3D-VATE is a minimally invasive technique with less surgical stress and faster recovery compared to open approach. It offers a 24-fold three dimensional imaging, which facilitates the restoring of the actual depth perception to surgeons and improvement of surgical performance.
Innovations and breakthroughs
The use of 3D-VATE for esophageal carcinomas was associated with an accelerated recovery, the preservation of pulmonary function, reduced surgical stress and more extensive lymphadenectomy. In conclusion, 3D-VATE could be a more advantageous technique over 2D-VATE.
Applications
The cost-efficiency of 3D-VATE allows the wide use in esophagectomies, especially in developing countries such as China. Since the majority of esophageal cancer patients come from rural areas with relatively low social-economic conditions, the 3D-VATE could be a more viable alternative for these individuals.
Terminology
The 3D-VATE is a minimally invasive technique that uses three-dimensional video, which offers a 24-fold three dimensional imaging that helps to restore the actual depth perception to surgeons.
Peer-review
The paper is well written and well designed. It presents a novel idea. The manuscript is excellent and shows the great improvement in the surgical treatment of esophageal cancer.
Footnotes
Institutional review board statement: This study was approved by the Clinical Ethics Committee of Shanghai General Hospital, School of Medicine, Shanghai Jiaotong University.
Informed consent statement: All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement: No conflict of interest is declared.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 21, 2015
First decision: June 19, 2015
Article in press: August 31, 2015
P- Reviewer: Caboclo JLF, Dobrucali AM, Ghanaat M S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Barreto JC, Posner MC. Transhiatal versus transthoracic esophagectomy for esophageal cancer. World J Gastroenterol. 2010;16:3804–3810. doi: 10.3748/wjg.v16.i30.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagpal K, Ahmed K, Vats A, Yakoub D, James D, Ashrafian H, Darzi A, Moorthy K, Athanasiou T. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc. 2010;24:1621–1629. doi: 10.1007/s00464-009-0822-7. [DOI] [PubMed] [Google Scholar]
- 3.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155–3162. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 5.Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb. 1992;37:7–11. [PubMed] [Google Scholar]
- 6.Wright CD, Kucharczuk JC, O’Brien SM, Grab JD, Allen MS. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg. 2009;137:587–95; discussion 596. doi: 10.1016/j.jtcvs.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 7.Wei B, D’Amico TA. Thoracoscopic versus robotic approaches: advantages and disadvantages. Thorac Surg Clin. 2014;24:177–188, vi. doi: 10.1016/j.thorsurg.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Camarillo DB, Krummel TM, Salisbury JK. Robotic technology in surgery: past, present, and future. Am J Surg. 2004;188:2S–15S. doi: 10.1016/j.amjsurg.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 9.Boone J, Borel Rinkes IH, van Hillegersberg R. Robot-assisted thoracolaparoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc. 2007;21:2342–2343. doi: 10.1007/s00464-007-9604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh DS, Cho I, Karamian B, DeMeester SR, Hagen JA. Early adoption of robotic pulmonary lobectomy: feasibility and initial outcomes. Am Surg. 2013;79:1075–1080. [PubMed] [Google Scholar]
- 11.Ding R, Tong X, Xu S, Zhang D, Gao X, Teng H, Qu J, Wang S. [A comparative study of Da Vinci robot system with video-assisted thoracoscopy in the surgical treatment of mediastinal lesions] Zhongguo Fei Ai Za Zhi. 2014;17:557–562. doi: 10.3779/j.issn.1009-3419.2014.07.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park A, Lee G, Seagull FJ, Meenaghan N, Dexter D. Patients benefit while surgeons suffer: an impending epidemic. J Am Coll Surg. 2010;210:306–313. doi: 10.1016/j.jamcollsurg.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Storz P, Buess GF, Kunert W, Kirschniak A. 3D HD versus 2D HD: surgical task efficiency in standardised phantom tasks. Surg Endosc. 2012;26:1454–1460. doi: 10.1007/s00464-011-2055-9. [DOI] [PubMed] [Google Scholar]
- 14.Veronesi G. Robotic thoracic surgery: technical considerations and learning curve for pulmonary resection. Thorac Surg Clin. 2014;24:135–41, v. doi: 10.1016/j.thorsurg.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 15.van der Sluis PC, Verhage RJ, van der Horst S, van der Wal WM, Ruurda JP, van Hillegersberg R. A new clinical scoring system to define pneumonia following esophagectomy for cancer. Dig Surg. 2014;31:108–116. doi: 10.1159/000357350. [DOI] [PubMed] [Google Scholar]
- 16.McCaffrey M, Beebe A. Giving narcotics for pain. Nursing. 1989;19:161–165. [PubMed] [Google Scholar]
- 17.Romeo AA, Mazzocca A, Hang DW, Shott S, Bach BR. Shoulder scoring scales for the evaluation of rotator cuff repair. Clin Orthop Relat Res. 2004;(427):107–114. doi: 10.1097/01.blo.0000142624.05526.dd. [DOI] [PubMed] [Google Scholar]
- 18.Jamieson GG, Lamb PJ, Thompson SK. The role of lymphadenectomy in esophageal cancer. Ann Surg. 2009;250:206–209. doi: 10.1097/SLA.0b013e3181b16cd1. [DOI] [PubMed] [Google Scholar]
- 19.Chen BF, Zhu CC, Wang CG, Ma DH, Lin J, Zhang B, Kong M. [Clinical comparative study of minimally invasive esophagectomy versus open esophagectomy for esophageal carcinoma] Zhonghua Wai Ke Za Zhi. 2010;48:1206–1209. [PubMed] [Google Scholar]
- 20.Pennathur A, Awais O, Luketich JD. Technique of minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2010;89:S2159–S2162. doi: 10.1016/j.athoracsur.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 21.Kim DJ, Hyung WJ, Lee CY, Lee JG, Haam SJ, Park IK, Chung KY. Thoracoscopic esophagectomy for esophageal cancer: feasibility and safety of robotic assistance in the prone position. J Thorac Cardiovasc Surg. 2010;139:53–59.e1. doi: 10.1016/j.jtcvs.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Kunert W, Storz P, Kirschniak A. For 3D laparoscopy: a step toward advanced surgical navigation: how to get maximum benefit from 3D vision. Surg Endosc. 2013;27:696–699. doi: 10.1007/s00464-012-2468-0. [DOI] [PubMed] [Google Scholar]
- 23.Nagahiro I, Andou A, Aoe M, Sano Y, Date H, Shimizu N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg. 2001;72:362–365. doi: 10.1016/s0003-4975(01)02804-1. [DOI] [PubMed] [Google Scholar]
- 24.Grande M, Tucci GF, Adorisio O, Barini A, Rulli F, Neri A, Franchi F, Farinon AM. Systemic acute-phase response after laparoscopic and open cholecystectomy. Surg Endosc. 2002;16:313–316. doi: 10.1007/s00464-001-9042-5. [DOI] [PubMed] [Google Scholar]
- 25.Hildebrandt U, Kessler K, Plusczyk T, Pistorius G, Vollmar B, Menger MD. Comparison of surgical stress between laparoscopic and open colonic resections. Surg Endosc. 2003;17:242–246. doi: 10.1007/s00464-001-9148-9. [DOI] [PubMed] [Google Scholar]
- 26.Veeramachaneni NK, Zoole JB, Decker PA, Putnam JB, Meyers BF. Lymph node analysis in esophageal resection: American College of Surgeons Oncology Group Z0060 trial. Ann Thorac Surg. 2008;86:418–21; discussion 421. doi: 10.1016/j.athoracsur.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 27.Alline M, Bertrand MM, Colombo PE, Mourregot A, Rouanet P. [Lymph node dissection: what for? From esophagus to rectum: surgical and lymph node related prognostic factors] Bull Cancer. 2014;101:368–372. doi: 10.1684/bdc.2014.1929. [DOI] [PubMed] [Google Scholar]
- 28.Rizk NP, Ishwaran H, Rice TW, Chen LQ, Schipper PH, Kesler KA, Law S, Lerut TE, Reed CE, Salo JA, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg. 2010;251:46–50. doi: 10.1097/SLA.0b013e3181b2f6ee. [DOI] [PubMed] [Google Scholar]
- 29.Barnett SA, Rizk NP. Randomized clinical trials in esophageal carcinoma. Surg Oncol Clin N Am. 2010;19:59–80. doi: 10.1016/j.soc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Lin J, Kang M, Chen C, Lin R, Zheng W, Zhug Y, Deng F, Chen S. Thoracolaparoscopy oesophagectomy and extensive two-field lymphadenectomy for oesophageal cancer: introduction and teaching of a new technique in a high-volume centre. Eur J Cardiothorac Surg. 2013;43:115–121. doi: 10.1093/ejcts/ezs151. [DOI] [PubMed] [Google Scholar]
- 31.Dhamija A, Rosen JE, Dhamija A, Gould Rothberg BE, Kim AW, Detterbeck FC, Boffa DJ. Learning curve to lymph node resection in minimally invasive esophagectomy for cancer. Innovations (Phila) 2014;9:286–291. doi: 10.1097/IMI.0000000000000082. [DOI] [PubMed] [Google Scholar]


