Abstract
Host-directed therapies are a relatively new and promising approach to treatment of tuberculosis. Modulation of specific host immune pathways, including those that impact inflammation and immunopathology, can limit mycobacterial infection and pathology, both in cell culture and in animal models. This review explores a range of host pathways and drugs, some already approved for clinical use that have the potential to provide new adjunctive therapies for tuberculosis. Drugs targeting host processes may largely avoid the development of bacterial antibiotic resistance, a major public health concern for tuberculosis. However, these drugs may also have generally increased risk for side effects on the host. Understanding the specific mechanisms by which these drugs act and the relationship of these mechanisms to Mycobacterium tuberculosis pathogenesis will be critical in selecting appropriate host-directed therapy. Overall, these host-directed compounds provide a novel strategy for antituberculosis therapy.
Drugs that potentiate existing host immune defenses, or evoke new ones, can limit the infectivity and pathogenicity of mycobacteria. Some of these drugs are already FDA-approved and may be readily translated as adjunctive therapies for tuberculosis.
Frustrated by the limitations of traditional antimycobacterial therapies, researchers in the tuberculosis (TB) community have focused on the possibility of modulating the host immune response as adjunctive therapy. Particularly in the case of multidrug-resistant (MDR) or extensively drug-resistant TB (XDR-TB), host-directed therapies provide a largely untapped approach as adjunctive anti-TB therapies, either to directly increase the ability of the host immune system to effectively eliminate mycobacteria or to limit collateral tissue damage associated with infection that can result in morbidity and mortality. New attention has focused on exploring host-directed therapies, and there has been a spike in reviews articulating fundamental principles; each aspect of the host response can conceivably be modulated in a way that maximizes bacterial killing while minimizing inflammatory tissue damage (Hawn et al. 2013; Zumla et al. 2013, 2014; Kaufmann et al. 2014; Wilkinson 2014). These approaches are still somewhat speculative, and there are only a few actively used host-directed drugs in the field. However, there is growing sentiment that alternative approaches are needed and that manipulation of host immunity coupled to a greater understanding of biological mechanisms hold new promise in the anti-TB armamentarium. Many compounds under active investigation have already been approved by the United States Food and Drug Administration (FDA) and thus might lead to accelerated clinical trials and implementation.
ADVANTAGES TO HOST-DIRECTED APPROACHES
Antibiotic resistance is a major public health concern for TB, even for effectively administered therapeutics, and MDR-TB and XDR-TB are serious problems worldwide (Shah et al. 2007; Gandhi et al. 2010; WHO 2010). In part, this is because of long therapies that sometimes are not completed (Castelnuovo 2010). In addition and despite the long generation time of Mycobacterium tuberculosis (Mtb), there is consistent accumulation of alleles that confer resistance to a variety of drugs during the course of a human infection (Sacchettini et al. 2008). Antibiotic-resistant alleles classically involve mutation of a direct antibiotic target and arise relatively frequently, in spite of the long generation time of Mtb, and may also arise from increased expression of membrane transporters (Ioerger et al. 2013). Studies based on whole-genome sequencing in cynomolgus macaques revealed an in vivo mutation rate on the order of 10−10 mutations per bacterial generation (Ford et al. 2011). Notably, mutation rates were comparable in latent disease, and DNA lesions identified suggested that oxidative damage might be a driving force increasing mutational load in vivo. Furthermore, East Asian lineage strains had close to a 10-fold increase in mutation rate relative to standard strains from the Euro-American lineage (Ford et al. 2013). Via whole-genome sequencing of an outbreak strain in humans, the mutation rate in active TB was similar, but latent TB was reduced (Colangeli et al. 2014). In any event, the number of antibiotic-resistant strains is burgeoning and can lead to rapid depletion of options for physicians and health care workers and the development of circulating drug-resistant strains (Gandhi et al. 2006). In theory, host-directed therapies bypass many of the problems associated with pathogen-directed therapies (Schwegmann and Brombacher 2008). They offer to potentiate existing immune defenses against Mtb or evoke new ones. Because host therapies target host proteins, it is much less likely that bacteria will generate a mutation that directly abrogates compound binding.
HOST-DIRECTED THERAPIES IN CLINICAL HUMAN TRIALS OR PRECLINICAL ANIMAL STUDIES
Anti-Inflammatory Therapies
So far, most host-directed therapies are still theoretical. One major exception is the use of broadly acting corticosteroids. TB infections in humans induce classic inflammatory responses (Kaufmann and Dorhoi 2013), and, as in other infectious diseases, it is the balance between immunopathology and insufficient inflammation that may determine disease severity and outcome (Casadevall and Pirofski 2003). Broadly acting glucocorticoids, such as dexamethasone and prednisone, have been used in a number of trials and are the standard-of-care for some severe forms of TB. They represent a relatively accessible and inexpensive approach to limiting inflammation, which can be a prime cause of morbidity and mortality. They have proved particularly effective in cases of TB meningitis (Schoeman et al. 1997; Thwaites et al. 2004; Prasad and Singh 2008) and have been adopted as standard-of-care for TB pericarditis and meningitis (Hakim et al. 2000; Mayosi et al. 2002; Thwaites et al. 2009). A recent meta-analysis of clinical trials using corticosteroids showed a 17% reduction in mortality across 41 clinical trials (Critchley et al. 2013).
The detrimental effects of inflammation in the human host are crystallized in TB-IRIS (immune reconstitution inflammatory syndrome), a paradoxical worsening of TB and inflammatory symptoms with reconstitution of the immune system associated with highly active antiretroviral therapy (HAART) (Dhasmana et al. 2008; Meintjes et al. 2008). A randomized controlled trial for adjunctive prednisone showed amelioration of symptoms for TB-IRIS (Meintjes et al. 2010). More generally, the morbidity associated with the inflammatory symptoms of IRIS reflects a generally detrimental inflammatory state that can be induced by TB infection (Marais et al. 2009). It remains to be investigated, as described below, whether corticosteroids could be generally beneficial in pulmonary TB, or perhaps among a subset of cases with particularly high levels of inflammation.
As host-directed therapies, however, these more general suppressors of inflammation may lead to secondary complications including immunosuppression. Knowing the basic biology of mycobacterial pathogenesis, as heterogeneous as that might be, may guide the search for more effective and specific host-directed therapies.
Modulation of Inflammation by Phosphodiesterase Inhibitors
cAMP plays a key role in both inflammation and tumor necrosis factor (TNF) production, as cAMP produced in host cells can lead to modulation of TNF levels. Therefore, cAMP levels are tightly regulated by host phosphodiesterases (PDEs). However, bacterial adenylyl cyclases can promote cAMP production in host cells (Agarwal et al. 2009). Based on the effectiveness of thalidomide in treating severe leprosy reactions (erythema nodosum leprosum), it was proposed that the modulation of extreme inflammation/TNF levels via thalidomide might be host-beneficial (Corral and Kaplan 1999). Thalidomide has been used in clinical pediatric trials of adjunctive therapy for TB meningitis, and the limitation of TNF production is thought to be key (Schoeman et al. 2000, 2004). However, the extreme teratogenic properties of thalidomide make it particularly dangerous (Kim and Scialli 2011), and other modulators of TNF-mediated pathologies in TB would be preferable.
One promising host-directed approach is the set of PDE inhibitors that appear to act directly on host cAMP levels. The PDE4 inhibitor CC-3052, a thalidomide analog, increased the effectiveness of isoniazid in a mouse model through reduced TNF production (Koo et al. 2011; Subbian et al. 2011a,b). Similarly, in two different mouse models, the FDA-approved PDE3 inhibitor cilostazol, which inhibits a separate class of PDEs reduced time to sterilization during antibiotic short course therapy by 1 mo (from 6 mo) (Maiga et al. 2012). Inhibition of PDE4 in a mouse model by different drugs resulted in worse outcomes in mice, but this may also be consistent with increased growth in the PDE-inhibited environment (the PDE4 inhibitor CC-3052 in rabbits was shown to result in slightly increased burden as monotherapy) and may suggest that adjunctive use could still benefit the host (Maiga et al. 2013). Fundamentally, these therapies aim to restrict immunopathology arising from TNF excess while allowing many of the antimycobacterial properties of host cells to remain intact.
Eicosanoid Modulation
Intriguingly, cAMP levels also impinge directly on production of host eicosanoids, lipid mediators of inflammation that are increasingly recognized as important modulators of the host immune response in mycobacterial infection. Eicosanoids may be central to the control of inflammatory responses during infection with pathogenic mycobacteria. The balance of these pro- and anti-inflammatory lipid mediators may lead to either uncontrolled inflammation or insufficient production of protective inflammatory mediators.
Virulent mycobacteria induce production of anti-inflammatory lipoxins, including Lipoxin A4 (LXA4) during high-dose mouse infections and in cell culture macrophage infections (Bafica et al. 2005; Chen et al. 2008; Divangahi et al. 2009). LXA4 favors necrotic cell death in cell culture models in part by limiting production of PGE2, which is required for plasma membrane repair and membrane integrity (Chen et al. 2008; Divangahi et al. 2009). By promoting necrotic cell death, LXA4 and the limitation of PGE2 production drive bacterial expansion to the detriment of the host.
In a mouse high-dose infection model, protection was noted for Mtb-infected mice deficient in Alox5, the gene encoding the enzyme responsible for both production of the LTA4 intermediate upstream of the proinflammatory eicosanoid LTB4 and important in the production of the anti-inflammatory lipoxin LXA4 (Bafica et al. 2005). The ability of zileuton, a clinically used asthma drug, to block lipoxin production was suggested initially as a host-directed therapy that might improve TB outcomes (Bafica et al. 2005).
In the zebrafish-Mycobacterium marinum model, a forward genetic screen for susceptibility loci identified the enzyme Lta4h as critical in controlling mycobacterial disease. Namely, when the lta4h gene was mutated, the LTA4 intermediate was shunted to anti-inflammatory lipoxins, and this lipoxin excess was found to modulate TNF levels, thereby limiting the ability of the host to restrict mycobacterial infection (Tobin et al. 2010, 2012).
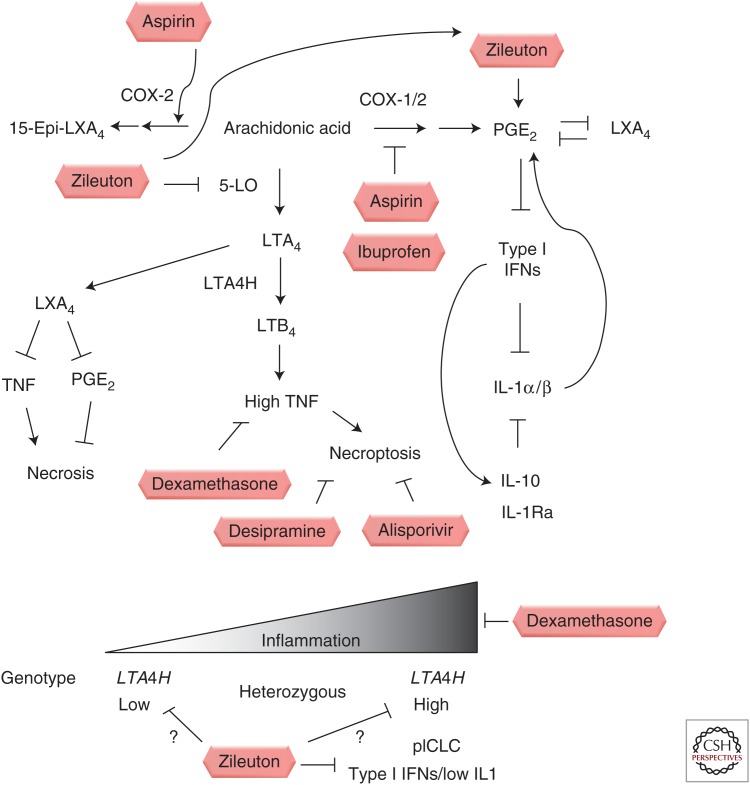
How relevant are these findings in mammalian and zebrafish models to human disease? In two human cohorts, one in Vietnam and one in Nepal, there was an association of heterozygosity at the LTA4H locus with disease severity, in both TB meningitis and leprosy (Tobin et al. 2010). Two copies of a functional, high-activity promoter variant led to excess inflammation, presumably caused by excess production of proinflammatory, TNF-promoting eicosanoids, such as LTB4. In contrast, two copies of the low-activity promoter variant altered the balance into what is thought to be an excessively anti-inflammatory state (Tobin et al. 2012). Remarkably, in a retrospective analysis of a randomly controlled trial for adjunctive dexamethasone therapy in TB meningitis, the entirety of the beneficial effect was associated with the group of high-activity homozygotes, whereas the low-activity homozygotes derived no benefit from adjunctive therapy (Thwaites et al. 2004; Tobin et al. 2012). These findings led to a model in which balanced inflammation was optimal, and which predicted that different genotypes drove more severe disease for fundamentally different reasons (Fig. 1).
Figure 1.
Schematic of opportunities for host-directed therapies modulating eicosanoid balance. Clinically approved drugs that modulate eicosanoid production and that have been tested in animal models of mycobacterial infection are shown in boxes. Some drugs, such as aspirin, may act at multiple points in the eicosanoid pathway, whereas others, such as zileuton, may result in shunting effects. Bottom panel depicts human genotypes associated in a Vietnamese cohort with increased or decreased production of LTA4H and differential responsiveness to dexamethasone adjunctive therapy as well as a mouse model of disease in IL-1 pathway-deficient mice or animals with elevated type I interferons.
Eicosanoids, with their well-known roles in inflammation and their emerging roles in TB pathogenesis, thus provide an important host-specific target. The findings at the level of innate immunity extend also to downstream effects on the adaptive immune response, with impaired antigen cross-presentation, suggesting that the eicosanoids control mycobacterial infection at a number of levels (Divangahi et al. 2010).
In the high inflammatory state, LTA4H excess leads to increased TNF levels and engages a pathway of programmed necrosis in zebrafish macrophages (Tobin et al. 2012). Although higher TNF levels lead to early improved control of infection in individual macrophages, RIP1/RIP3-mediated necroptosis ultimately leads to necroptotic cell death that liberates the infecting mycobacteria to grow extracellularly, relatively unrestrained (Roca and Ramakrishnan 2013). In a thorough genetic analysis, the generation of mitochondrial reactive oxygen species was shown to lead initially to increased intracellular killing of mycobacteria, but then to macrophage necroptotic cell death via two parallel pathways—ceramide production mediated through acid sphingomyelinase and regulation of mitochondrial cyclophilin D (Roca and Ramakrishnan 2013). By understanding these processes in molecular detail, two host-directed, clinically approved drugs targeting each of these pathways were shown to rescue the hypersusceptibility induced by high TNF levels. Desipramine targets acid sphingomyelinase (Elojeimy et al. 2006). Alisporivir, currently in phase III clinical trials, targets cyclophilin D interactions with the mitochondrial permeability transition pore complex, a crucial step in mitochondrial destruction (Vandenabeele et al. 2010; Quarato et al. 2012). Because TNF initially promotes increased intracellular control, combination of higher TNF levels with inhibition of programmed necrosis resulted in a transformation of a more severe disease phenotype to greater resistance (Roca and Ramakrishnan 2013).
Finally, modulation of PGE2 itself in the context of interleukin-1 (IL-1)-deficient mice and type I interferon (IFN) induction provides a promising host-directed therapy. In a mouse infection model using mice deficient in IL-1 signaling or with excess type I IFN production, conditions that may be particularly relevant to patients with more severe disease (Mayer-Barber et al. 2011), zileuton and PGE2 administration had host-beneficial effects (Mayer-Barber et al. 2014). Inhibition of the enzyme 5-lipooxygenase (5-LO) via the asthma drug zileuton, is proposed to shunt eicosanoid production toward increased PGE2 production, which in the context of high-inflammatory states driven by type I IFNs, can suppress detrimental type I IFN production, partially rescues mortality, and reduces bacterial burden (Mayer-Barber et al. 2014). Thus, multiple clinically approved drugs that act on eicosanoid balance and displayed in Figure 1 appear to have potential as host-directed therapies. Moreover, many of the host-directed therapies described above have genotype-specific effects that depend on eicosanoid balance in zebrafish, mice, and humans.
Nonsteroidal Anti-Inflammatory Drugs
Aspirin is a well-tested and effective administered drug with low risk in adults. In addition to its effect as a COX-1/COX-2 inhibitor that limits production of prostaglandins, it has been shown to modify COX-2 to produce the aspirin-triggered lipoxins (Claria and Serhan 1995), potent proresolving lipids that have in vivo effects on neutrophil recruitment and inflammatory cytokines (Morris et al. 2009). In addition to modulating inflammation through classical COX-dependent inhibition of prostaglandins, it may actively promote the proresolving signals mediated by lipoxins, and it has been used as a therapy in the zebrafish model of hyperinflammatory states during mycobacterial infection (Tobin et al. 2012). In the case of TB driven by hyperinflammatory genotypes or status, the production of these lipoxins may exert proresolving effects and limit disease pathology. However, in the case of insufficient inflammation, the effects may be different in skewing the balance of PGE2 and LXA4. In a mouse model with a predominant type I INF response, for example, PGE2 amplification is thought to promote host survival in the context of a type I INF predominant innate immune response, and so aspirin might have unwanted effects under conditions that recapitulate this model (Mayer-Barber et al. 2014). Two clinical trials assessing the effect of aspirin on TB meningitis outcomes were inconclusive, with one trial suggesting a significant benefit (p = 0.03) of adjunctive therapy on survival. However, it would be interesting to examine the effects of adjunctive aspirin administration on different genotypes and inflammatory states, given what literature on eicosanoid balance has suggested (Misra et al. 2010; Schoeman et al. 2011).
Additionally, using the C3HeB/FeJ mouse model of TB (Pan et al. 2005), in which caseation necrosis occurs, ibuprofen treatment given as a monotherapy starting 4 wk postinfection reduced burden and increased survival (Vilaplana et al. 2013). The precise basis of these effects, other than a hypothesized suppression of detrimental inflammation, remains to be seen. Future studies could address if reducing hyperinflammatory damage is sufficient or if research should focus on specific pathways engaged, including the eicosanoids and aspirin-triggered lipoxins.
Imatinib
As noted with therapies to modulate eicosanoids, the straightest line to host-directed therapy may be to identify drugs that are already FDA-approved. One promising example from this work comes from use of the ABL tyrosine kinase inhibitor imatinib mesylate, known commercially as Gleevec. Previously, it had been shown that imatinib was able to inhibit poxvirus pathogenesis via limitation of actin motility of the cell-associated enveloped virions (Reeves et al. 2005). In mycobacterial infections with both Mtb and the closely related M. marinum, there appear to be both cell autonomous and cell nonautonomous beneficial effects of inhibition, with inhibition of ABL resulting in increased killing and promoting acidification of the mycobacterial phagosome (Napier et al. 2011; Bruns et al. 2012). Mice treated with imatinib had fewer granuloma lesions and less bacterial load in infected organs, for both Mtb and M. marinum infections (Napier et al. 2011). When coadministered with first-line drugs, imatinib had a synergistic therapeutic effect (Napier et al. 2011). In addition, monocytes from imatinib-treated patients showed more acidic vacuoles by LysoSensor staining (Bruns et al. 2012). As Gleevec is soon to be off patent, this may be an effective and viable therapy for cases of both drug-resistant and drug-susceptible Mtb.
Alteration of Lipid Metabolism
Statins provide an interesting additional avenue for a host-directed therapy, as widely used drugs that have proven safe and effective (Ray et al. 2010). Parihar et al. (2014) have shown increased promotion of phagosome maturation and autophagy via reduction of cholesterol levels, both processes that are thought to be host-beneficial in the context of mycobacterial infection. Because mycobacteria hijack host cholesterol as a carbon source on IFN-γ activation (Pandey and Sassetti 2008; Griffin et al. 2012), limitation of cholesterol levels may also influence bacterial persistence, although it is unclear to what extent altering these levels would have a meaningful effect on disease progression in humans. Epidemiological studies of TB susceptibility or progression among individuals taking statins have not yet been achieved, in part owing to largely nonoverlapping populations.
Pathogenic mycobacteria are thus able to reprogram lipid balance within host cells for their own benefit. Notably, in addition to animal models, this has been observed in human lung tissue in profiling of laser-capture microdissections of human caseating granulomas from resected lung (Kim et al. 2010) and in decreased phagosomal lipolysis (Podinovskaia et al. 2013). Virulent but not avirulent Mtb (as well as Bacillus Calmette–Guérin [BCG]) reprograms lipid balance within infected macrophages in cell culture, leading to a foamy cell phenotype with extensive lipid droplets that is thought to benefit the infecting bacterium (D’Avila et al. 2006; Singh et al. 2012; Podinovskaia et al. 2013). During infection, glycolysis is shifted toward ketone body synthesis, and the product d-3-hydroxybutyrate (DHB) then activates the G-protein-coupled receptor GPR109A; this then leads to a feedback mechanism that promotes cAMP signaling and limits lipolysis (Singh et al. 2012). By targeting this pathway with mepenzolate bromide, a GPR109A inhibitor, bacterial burden was reduced in cell culture and in mouse in vivo models (Singh et al. 2012).
Other pathways controlling lipid composition are also targets for intervention. PPAR-γ is a canonical ligand-activated nuclear receptor that modulates fatty acid metabolism and storage as well as playing previously underappreciated roles in macrophage immune response (Kiss et al. 2013). Notably, in the generation of foamy macrophages, which have high levels of PPAR-γ expression, Mtb and manLAM activate PPAR-γ through a mannose receptor-dependent pathway, and knockdown in human macrophages or inhibition leads to decreased mycobacterial growth (Almeida et al. 2009; Rajaram et al. 2010). Knockdown of either PPAR-γ or testicular receptor 4 (TR4) resulted in reduced bacterial burden in macrophages, with modulation of CD36, IL-10 production, and phagolysosome fusion as possible mechanisms (Mahajan et al. 2012). There are at least three FDA-approved compounds pioglitazone, rosiglitazone, and treprostinil with activity directed at PPAR-γ (Kiss et al. 2013; Rask-Andersen et al. 2014). Finally, other nuclear receptors such as TR4, which may be activated by keto-mycolic acid, may also play important roles in mediating foamy macrophage formation (Mahajan et al. 2012; Dkhar et al. 2014). Thus, these candidate compounds and others modulating these pathways also provide prospective adjunctive therapies.
Vitamin D
Vitamin D has long been proposed to have important host-beneficial effects in TB, including immunomodulatory effects. In human macrophages, TLR2-mediated or INF-γ-mediated activation of cyp27b1 leads to conversion of 25-hydroxyvitamin D3 (25(OH)D3) to bioactive 1,25-dihydroxyvitamin D3 (Liu et al. 2006; Krutzik et al. 2008; Fabri et al. 2011). In contrast to rodent models, the primary antimycobacterial effector function on TLR stimulation of this conversion event lies in the generation of antimicrobial peptides (Liu et al. 2006). IFN-γ antimicrobial activity can be mediated through activation of CYP27B1. More recently, 1,25-dihydroxyvitamin D (1,25D) was shown to enhance IL-1β transcription in macrophages and induce antimicrobial peptide production in cocultured lung epithelial cells (Verway et al. 2013). Finally, vitamin D has been shown to induce autophagy in cell culture via production of LL-37 (Yuk et al. 2009) (see autophagy section below), thus linking to an additional host-protective process that might also contribute to bacterial control in vivo. In addition to its antimicrobial effects on the host macrophage, subgroup analysis of a clinical trial showed that vitamin D as an adjunctive therapy may reduce hyperinflammatory responses and thus may contribute in multiple ways to improved outcomes (Coussens et al. 2012).
Because this is an inexpensive intervention, it is a particularly appealing host-directed therapy, and a number of clinical studies, including randomized controlled trials, have been performed. Genetic associations and serum concentration measurements have suggested associations of vitamin D production with susceptibility to TB (Wilkinson et al. 2000; Ustianowski et al. 2005; Wejse et al. 2007). Initially, clinical trials of vitamin D supplementation gave mixed results (Morcos et al. 1998; Nursyam et al. 2006; Wejse et al. 2009). However, in the most extensive clinical trial performed to date, there was no overall statistically significant effect on time to sputum clearance (Martineau et al. 2011). On stratification of the patient population by vitamin D receptor genotype, it was suggested that one class of homozygotes derived benefit from the therapy (Martineau et al. 2011). The functional consequence of this human variant is not known, but it would be interesting to follow up in further and perhaps larger trials, including the use of vitamin D supplementation for investigation of drug-resistant strains of Mtb.
IDENTIFICATION OF NEW HOST-DIRECTED THERAPIES
Cell-Culture-Based Screens
Cell-culture-based screens showed in principle that limitation of host gene products can dramatically restrict mycobacterial growth, particularly in cell-autonomous assays. Genome-wide screens, first in Drosophila S2 cells using Mycobacterium fortuitum with a Drosophila RNA interference (RNAi) library (Philips et al. 2005), revealed a number of gene products promoting phagocytosis and restricting bacterial growth. Using Mtb infection and a small interfering RNA (siRNA) screen in murine macrophages, first for kinases and phosphatases and then in a genome-wide screen in human macrophages, an important host-based set of genes with resistance phenotypes on inhibition identified wide targets, including autophagic networks (Jayaswal et al. 2010; Kumar et al. 2010). Thus, in principle, host genes that allow mycobacteria to replicate intracellularly could be targeted, although few chemical inhibitors of these hits were well known.
Originally identified in an RNAi cell-culture-based screen for Salmonella typhimurium growth restriction, AKT1 inhibition and a host-directed chemical inhibitor of AKT1 were sufficient to limit Mtb intracellular growth (Kuijl et al. 2007). Both AKT1 and AKT2 also appeared in one of the host siRNA screens, confirming a role for AKT1 as host-permissive within macrophages and suggesting that its inhibition might be effective as a host-directed therapy (Kumar et al. 2010).
More recently, these screens have evolved to relatively high-throughput chemical screens, often coupled to cellular phenotypes. Using a library of FDA-approved small molecules, combined with an siRNA screen and high-throughput imaging of BCG infection, two compounds were identified that reduced burden and modulated autophagy. Nortriptyline and prochlorperazine edisylate thus represent host-directed drugs that are already approved (Sundaramurthy et al. 2013).
Similarly, Stanley et al. (2014) identified host-targeting small molecules that had no effect on in vitro–grown Mtb but restricted bacterial intracellular growth. Known drugs identified included fluoxetine, which induced autophagy in cell culture, and gefitinib, an inhibitor of epidermal growth factor receptor (EGFR), which was also shown to reduce bacterial replication in an in vivo mouse model of acute infection (Stanley et al. 2014).
Here, one of the translational questions is the degree to which cell-culture assays serve as a proxy for the complexity of the human immune response. However, chemical screens in cell culture with FDA-approved compounds may prove to be a powerful shortcut to de novo drug development.
Autophagy
One striking result from many of the cell-culture screens is a significant number of hits involving autophagic pathways (Kumar et al. 2010; Sundaramurthy et al. 2013). Autophagy is thought to play an important role in restriction of a number of intracellular pathogens and is the subject of active research that seeks to target this process for host-directed therapies (Rubinsztein et al. 2012; Shoji-Kawata et al. 2013). In Mtb infections, autophagy restricts mycobacterial replication and may be triggered by a number of cues, including IFN-γ and extracellular DNA sensing via STING (stimulator of interferon genes) (Gutierrez et al. 2004; Watson et al. 2012). Induction of IRGM, which is required for control in a mouse model, may drive autophagy in some cases (MacMicking et al. 2003; Singh et al. 2006). Mice deficient in central autophagy pathway components such as ATG5 have both higher bacterial burden and increased inflammation, which appears in a cell-autonomous manner (i.e., is not merely an effect of higher burden) (Castillo et al. 2012). This suggests that autophagic processes are induced and can limit mycobacterial infections but that they are normally inhibited at least partially by pathogenic mycobacteria and that full induction of autophagy can have dramatic host-beneficial effects.
It remains elusive how these cell-autonomous processes will translate into animal models, when multiple cell types are exposed to a drug. Generally, animals defective in autophagic processes have defects in mycobacterial control, but few whole-animal rescue experiments have been performed.
Protein Kinase as a Potential Host Target
Double-stranded RNA (dsRNA)-dependent protein kinase (PKR) was shown to be an enzyme in which its absence in mice results in improved control of bacterial burden and thus meets a clear notion of a host-directed therapy, and chemical inhibitors are being developed (Bryk et al. 2011; Wu et al. 2012). Again, by targeting these host proteins, overall burden is reduced. New compounds have not yet been examined for off-target effects in whole animals, although proof of principle using a mouse knockout in live animals is a promising beginning.
MODELS OF IMMUNOPATHOLOGY
Cell-culture models, although amenable to higher-throughput experimentation, can be limited to assessing largely cell-autonomous effects on burden. The reality of TB as an in vivo disease implies that models of host are similarly important. However, even well-established versatile models like the mouse can differ in their outcomes depending on strain differences. This has led to the adoption of less genetically and immunologically tractable models that may recapitulate better the full spectrum of physiology and pathology, including primates, guinea pigs, mini-pigs, and rabbits (Flynn 2006). The combination of host-directed therapies with these models will give insights into what may ultimately prove most effective in humans and allows the observation of different cell types in a whole organism.
Neutrophils
Although the tuberculous granuloma is largely defined by the presence of epithelioid macrophages, neutrophils are clearly present at both early and later stages of infection where they play multiple roles (Canetti 1955; Eum et al. 2010). Transcriptional profiling of whole blood revealed a neutrophil-associated signature in active cases (Martineau et al. 2007; Berry et al. 2010). These roles include both host-protective functions—in the zebrafish mycobacterial infection model, they are important in mycobacterial killing within the granuloma through NADPH-oxidase-dependent mechanisms, notably after the initial formation of the granuloma (Yang et al. 2012). However, neutrophil-mediated inflammation appears to play an important host-detrimental role in later infection (Desvignes and Ernst 2009; Nandi and Behar 2011). Epithelial-produced CXCL5 mediates recruitment of CXCR2-positive neutrophils. Knockout mice of either the ligand or receptor are relatively protected in a high-dose mouse infection model (Nouailles et al. 2014). Thus, the balance of sufficient neutrophil function and limitation of immunopathology may be critical. Similarly, in a mouse infection model, deletion of miR-223 leads to dysregulated inflammation and recruitment of neutrophils (Dorhoi et al. 2013). These data suggest that host modulation of microRNAs (miRNAs) might form another basis for host-directed therapies. Mtb lipomannan has been shown in macrophages to regulate the balance of proinflammatory and anti-inflammatory miRNAs (mir-155 and mir-125b), respectively, to modulate TNF production (Rajaram et al. 2011). Because of considerable clinical interest in modulation of miRNAs, this might form a future approach, but delivery technologies in humans or in the context of TB require further investigation (Iannaccone et al. 2014).
Matrix Metalloproteases
Matrix metalloproteases, key remodeling enzymes in the human lung, may also play a role in immunopathology of TB. Induced in human TB and in whole-animal models, MMP-1 is a canonical matrix metalloprotease that is found in the human TB granuloma (Elkington et al. 2011b). Although not present in mice, human MMP-1 expressed transgenically in the mouse drives alveolar destruction in lung granulomas and greater collagen breakdown (Elkington et al. 2011a). Potential routes for modulating the collagenase MMP-1 include a specific collagenase inhibitor, Ro32-3555, currently in trials for rheumatoid arthritis (Hemmings et al. 2001; Elkington et al. 2011b).
Other MMPs may play important roles in tissue-specific choreographed responses in which tissue remodeling occurs and new structures are formed. The mycobacterial granuloma itself is thought to be exploited by infecting bacteria to promote growth initially (Ramakrishnan 2012). Deletion of the matrix metalloprotease MMP-9 has been shown in mouse models to reduce burden and granuloma formation and in zebrafish to be induced in an ESX-1/RD-1-dependent manner to drive granuloma formation via interaction with host epithelial cells (Taylor et al. 2006; Volkman et al. 2010). Inhibition of the MMPs has given equivocal results in cancer trials, suggesting that there may be multiple roles at the level of the whole organism (Overall and Lopez-Otin 2002; Parks et al. 2004), but the specific effects of single MMP knockouts or modulation suggest that these could be important targets for host-directed therapy.
OTHER APPROACHES
Immunotherapy
More expensive, host-directed therapies delivering specific cytokines have been an option and some are undergoing clinical trials. Because the IL-12/IFN-γ axis is so critical in Mendelian susceptibilities to mycobacterial disease as well as promoting restriction of mycobacterial growth in animal and cell culture models, the thought emerged that supplementation of IFN-γ held promise as a host immunotherapy (Abel et al. 2014). Indeed, clinical trials with IFN-γ, particularly for MDR-TB, have >15 yr of experience (Condos et al. 1997; Koh et al. 2004; Dawson et al. 2009).
IL-2 immunotherapy has also been proposed but has met with mixed results (Johnson et al. 1997, 2003).
More recently, there has been interest in potential roles for γδ T cells in enhancing immunity. In a nonhuman primate model, researchers have focused on the γδ immune lineage only present in primates; stimulation with IL-2 and a closely related phosphoantigen to that of Mtb (the phosphoantigen Picostim carries a one-carbon difference from mycobacterial (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate [HMBPP]) results in expansion of protective Vγ2 Vδ2 T cells (Chen et al. 2013). Notably, these effects are seen in animals with preexisting disease. The immunotherapy studies in humans and other primates have all been relatively limited with small, but generally measurable and beneficial effects. Immunotherapy may thus represent a potential treatment for particularly hard-to-treat and severe forms of TB.
Dual Host-Targeting and Antimicrobial Functions
Finally, existing antibiotics may also have additional host-directed effects. Clofazimine has in vitro antimycobacterial effects and has been used in combination therapy for Mycobacterium leprae treatment (Arbiser and Moschella 1995). It is in clinical trials for MDR-TB cases, but it may also act through a dual role in modulating the host immune response (Grosset et al. 2013), as it has been suggested to function in part by inducing macrophage apoptosis in the host (Fukutomi et al. 2011). Similarly, existing antimycobacterials such as isoniazid and pyrazinamide may act in part through the induction of host autophagy (Kim et al. 2012). Thus, the strict delineation between host-directed and bacterially directed therapies may not be absolute. More generally, combination effects of drugs on multiple pathways may be important for the full effect of a given drug, particularly when the initial effect is determined empirically and identified in phenotypic screens. Although target identification is critical to understanding the mechanism and to developing new effective compounds, it may be a combination of targets that mediates a full phenotypic effect.
CONCLUDING REMARKS
Only recently embraced by the TB community and funding agencies, the development of host-directed therapies is a promising new and relatively unexplored avenue for attacking a frustratingly intractable disease. However, it is important to note some concerns. In contrast to targeting bacteria directly, whole-animal, wholesale inhibition of host proteins may lead to other consequences. Host proteins may also be used in other contexts, including other organs and other cell types not directly related to mycobacterial infection. Thus, host targeting may have an increased risk for side effects on patients. However, the magic bullet model for TB therapeutics—a drug that targets the bacteria in all of its metabolic states and physiological compartments and is effective in the context of existing public health infrastructure—has clearly not yet been attained. Host-directed therapies open a new trove of possibilities. Repurposing of FDA-approved drugs, some of which show substantial promise in cell-culture models and in in vivo models, may quickly open orthogonal approaches. Choosing among these therapeutic possibilities and fine-tuning them based on a knowledge of mechanism and even host genotypes may result in important new adjunctive therapies that are precisely targeted and highly effective.
ACKNOWLEDGMENTS
The author is grateful to Kim Dohlich for critical reading of this chapter and Ana María Xet-Mull for assistance with figures. The Tobin laboratory is funded through a Mallinckrodt Scholar Award, a Searle Scholar Award, a National Institutes of Health Director’s New Innovator Award (1DP2OD008614-01), a Vallee Foundation Young Investigator Award, a Whitehead Scholar Award, and the Duke University Center for AIDS Research (5P30 AI064518).
Footnotes
Editors: Stefan H.E. Kaufmann, Eric J. Rubin, and Alimuddin Zumla
Additional Perspectives on Tuberculosis available at www.perspectivesinmedicine.org
REFERENCES
- Abel L, El-Baghdadi J, Bousfiha AA, Casanova JL, Schurr E. 2014. Human genetics of tuberculosis: A long and winding road. Philos Trans R Soc Lond B Biol Sci 369: 20130428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal N, Lamichhane G, Gupta R, Nolan S, Bishai WR. 2009. Cyclic AMP intoxication of macrophages by a Mycobacterium tuberculosis adenylate cyclase. Nature 460: 98–102. [DOI] [PubMed] [Google Scholar]
- Almeida PE, Silva AR, Maya-Monteiro CM, Torocsik D, D’Avila H, Dezso B, Magalhaes KG, Castro-Faria-Neto HC, Nagy L, Bozza PT. 2009. Mycobacterium bovis bacillus Calmette-Guerin infection induces TLR2-dependent peroxisome proliferator-activated receptor γ expression and activation: Functions in inflammation, lipid metabolism, and pathogenesis. J Immunol 183: 1337–1345. [DOI] [PubMed] [Google Scholar]
- Arbiser JL, Moschella SL. 1995. Clofazimine: A review of its medical uses and mechanisms of action. J Am Acad Dermat 32: 241–247. [DOI] [PubMed] [Google Scholar]
- Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, Aliberti J. 2005. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest 115: 1601–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, et al. 2010. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466: 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns H, Stegelmann F, Fabri M, Dohner K, van Zandbergen G, Wagner M, Skinner M, Modlin RL, Stenger S. 2012. Abelson tyrosine kinase controls phagosomal acidification required for killing of Mycobacterium tuberculosis in human macrophages. J Immunol 189: 4069–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryk R, Wu K, Raimundo BC, Boardman PE, Chao P, Conn GL, Anderson E, Cole JL, Duffy NP, Nathan C, et al. 2011. Identification of new inhibitors of protein kinase R guided by statistical modeling. Bioorg Med Chem Lett 21: 4108–4114. [DOI] [PubMed] [Google Scholar]
- Canetti G. 1955. The tubercle bacillus in the pulmonary lesion of man: Histobacteriology and its bearing on the therapy of pulmonary tuberculosis, Springer, New York. [Google Scholar]
- Casadevall A, Pirofski LA. 2003. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol 1: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelnuovo B. 2010. A review of compliance to anti tuberculosis treatment and risk factors for defaulting treatment in Sub Saharan Africa. Afr Health Sci 10: 320–324. [PMC free article] [PubMed] [Google Scholar]
- Castillo EF, Dekonenko A, Arko-Mensah J, Mandell MA, Dupont N, Jiang S, Delgado-Vargas M, Timmins GS, Bhattacharya D, Yang H, et al. 2012. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc Natl Acad Sci 109: E3168–E3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, Serhan CN, Behar SM, Remold HG. 2008. Lipid mediators in innate immunity against tuberculosis: Opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med 205: 2791–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Yao S, Huang D, Wei H, Sicard H, Zeng G, Jomaa H, Larsen MH, Jacobs WR Jr, Wang R, et al. 2013. Phosphoantigen/IL2 expansion and differentiation of Vγ2Vδ2 T cells increase resistance to tuberculosis in nonhuman primates. PLoS Pathog 9: e1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claria J, Serhan CN. 1995. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci 92: 9475–9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangeli R, Arcus VL, Cursons RT, Ruthe A, Karalus N, Coley K, Manning SD, Kim S, Marchiano E, Alland D. 2014. Whole genome sequencing of Mycobacterium tuberculosis reveals slow growth and low mutation rates during latent infections in humans. PLoS ONE 9: e91024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condos R, Rom WN, Schluger NW. 1997. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-γ via aerosol. Lancet 349: 1513–1515. [DOI] [PubMed] [Google Scholar]
- Corral LG, Kaplan G. 1999. Immunomodulation by thalidomide and thalidomide analogues. Ann Rheum Dis 58: I107–I113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens AK, Wilkinson RJ, Hanifa Y, Nikolayevskyy V, Elkington PT, Islam K, Timms PM, Venton TR, Bothamley GH, Packe GE, et al. 2012. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci 109: 15449–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley JA, Young F, Orton L, Garner P. 2013. Corticosteroids for prevention of mortality in people with tuberculosis: A systematic review and meta-analysis. Lancet Infect Dis 13: 223–237. [DOI] [PubMed] [Google Scholar]
- D’Avila H, Melo RC, Parreira GG, Werneck-Barroso E, Castro-Faria-Neto HC, Bozza PT. 2006. Mycobacterium bovis bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: Intracellular domains for eicosanoid synthesis in vivo. J Immunol 176: 3087–3097. [DOI] [PubMed] [Google Scholar]
- Dawson R, Condos R, Tse D, Huie ML, Ress S, Tseng CH, Brauns C, Weiden M, Hoshino Y, Bateman E, et al. 2009. Immunomodulation with recombinant interferon-γ1b in pulmonary tuberculosis. PLoS ONE 4: e6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvignes L, Ernst JD. 2009. Interferon-γ-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity 31: 974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhasmana DJ, Dheda K, Ravn P, Wilkinson RJ, Meintjes G. 2008. Immune reconstitution inflammatory syndrome in HIV-infected patients receiving antiretroviral therapy: Pathogenesis, clinical manifestations and management. Drugs 68: 191–208. [DOI] [PubMed] [Google Scholar]
- Divangahi M, Chen M, Gan H, Desjardins D, Hickman TT, Lee DM, Fortune S, Behar SM, Remold HG. 2009. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol 10: 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM. 2010. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat Immunol 11: 751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhar HK, Nanduri R, Mahajan S, Dave S, Saini A, Somavarapu AK, Arora A, Parkesh R, Thakur KG, Mayilraj S, et al. 2014. Mycobacterium tuberculosis keto-mycolic acid and macrophage nuclear receptor TR4 modulate foamy biogenesis in granulomas: A case of a heterologous and noncanonical ligand-receptor pair. J Immunol 193: 295–305. [DOI] [PubMed] [Google Scholar]
- Dorhoi A, Iannaccone M, Farinacci M, Fae KC, Schreiber J, Moura-Alves P, Nouailles G, Mollenkopf HJ, Oberbeck-Muller D, Jorg S, et al. 2013. MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J Clin Invest 123: 4836–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF, Saraiva L, Pedersen B, Mauri F, Lipman M, et al. 2011a. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest 121: 1827–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkington PT, D’Armiento JM, Friedland JS. 2011b. Tuberculosis immunopathology: The neglected role of extracellular matrix destruction. Sci Transl Med 3: 71ps76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elojeimy S, Holman DH, Liu X, El-Zawahry A, Villani M, Cheng JC, Mahdy A, Zeidan Y, Bielwaska A, Hannun YA, et al. 2006. New insights on the use of desipramine as an inhibitor for acid ceramidase. FEBS Lett 580: 4751–4756. [DOI] [PubMed] [Google Scholar]
- Eum SY, Kong JH, Hong MS, Lee YJ, Kim JH, Hwang SH, Cho SN, Via LE, Barry CE., 3rd. 2010. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 137: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, Lee HM, Krutzik SR, Schenk M, Sieling PA, et al. 2011. Vitamin D is required for IFN-γ-mediated antimicrobial activity of human macrophages. Sci Transl Med 3: 104ra102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JL. 2006. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect 8: 1179–1188. [DOI] [PubMed] [Google Scholar]
- Ford CB, Lin PL, Chase MR, Shah RR, Iartchouk O, Galagan J, Mohaideen N, Ioerger TR, Sacchettini JC, Lipsitch M, et al. 2011. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nat Genet 43: 482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CB, Shah RR, Maeda MK, Gagneux S, Murray MB, Cohen T, Johnston JC, Gardy J, Lipsitch M, Fortune SM. 2013. Mycobacterium tuberculosis mutation rate estimates from different lineages predict substantial differences in the emergence of drug-resistant tuberculosis. Nat Genet 45: 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutomi Y, Maeda Y, Makino M. 2011. Apoptosis-inducing activity of clofazimine in macrophages. Antimicrob Agents Chemother 55: 4000–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. 2006. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368: 1575–1580. [DOI] [PubMed] [Google Scholar]
- Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, van Soolingen D, Jensen P, Bayona J. 2010. Multidrug-resistant and extensively drug-resistant tuberculosis: A threat to global control of tuberculosis. Lancet 375: 1830–1843. [DOI] [PubMed] [Google Scholar]
- Griffin JE, Pandey AK, Gilmore SA, Mizrahi V, McKinney JD, Bertozzi CR, Sassetti CM. 2012. Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem Biol 19: 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosset JH, Tyagi S, Almeida DV, Converse PJ, Li SY, Ammerman NC, Bishai WR, Enarson D, Trebucq A. 2013. Assessment of clofazimine activity in a second-line regimen for tuberculosis in mice. Am J Respir Crit Care Med 188: 608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. 2004. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119: 753–766. [DOI] [PubMed] [Google Scholar]
- Hakim JG, Ternouth I, Mushangi E, Siziya S, Robertson V, Malin A. 2000. Double blind randomised placebo controlled trial of adjunctive prednisolone in the treatment of effusive tuberculous pericarditis in HIV seropositive patients. Heart 84: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawn TR, Matheson AI, Maley SN, Vandal O. 2013. Host-directed therapeutics for tuberculosis: Can we harness the host? Microbiol Mol Biol Rev 77: 608–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings FJ, Farhan M, Rowland J, Banken L, Jain R. 2001. Tolerability and pharmacokinetics of the collagenase-selective inhibitor Trocade in patients with rheumatoid arthritis. Rheumatology 40: 537–543. [DOI] [PubMed] [Google Scholar]
- Iannaccone M, Dorhoi A, Kaufmann SH. 2014. Host-directed therapy of tuberculosis: What is in it for microRNA? Expert Opin Ther Targets 18: 491–494. [DOI] [PubMed] [Google Scholar]
- Ioerger TR, O’Malley T, Liao R, Guinn KM, Hickey MJ, Mohaideen N, Murphy KC, Boshoff HI, Mizrahi V, Rubin EJ, et al. 2013. Identification of new drug targets and resistance mechanisms in Mycobacterium tuberculosis. PLoS ONE 8: e75245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaswal S, Kamal MA, Dua R, Gupta S, Majumdar T, Das G, Kumar D, Rao KV. 2010. Identification of host-dependent survival factors for intracellular Mycobacterium tuberculosis through an siRNA screen. PLoS Pathog 6: e1000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BJ, Bekker LG, Rickman R, Brown S, Lesser M, Ress S, Willcox P, Steyn L, Kaplan G. 1997. rhuIL-2 adjunctive therapy in multidrug resistant tuberculosis: A comparison of two treatment regimens and placebo. Tuber Lung Dis 78: 195–203. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Ssekasanvu E, Okwera A, Mayanja H, Hirsch CS, Nakibali JG, Jankus DD, Eisenach KD, Boom WH, Ellner JJ, et al. 2003. Randomized trial of adjunctive interleukin-2 in adults with pulmonary tuberculosis. Am J Respir Crit Care Med 168: 185–191. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Dorhoi A. 2013. Inflammation in tuberculosis: Interactions, imbalances and interventions. Curr Opin Immunol 25: 441–449. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Lange C, Rao M, Balaji KN, Lotze M, Schito M, Zumla AI, Maeurer M. 2014. Progress in tuberculosis vaccine development and host-directed therapies—A state of the art review. Lancet Respir Med 2: 301–320. [DOI] [PubMed] [Google Scholar]
- Kim JH, Scialli AR. 2011. Thalidomide: The tragedy of birth defects and the effective treatment of disease. Toxicol Sci 122: 1–6. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Wainwright HC, Locketz M, Bekker LG, Walther GB, Dittrich C, Visser A, Wang W, Hsu FF, Wiehart U, et al. 2010. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med 2: 258–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Lee HM, Shin DM, Kim W, Yuk JM, Jin HS, Lee SH, Cha GH, Kim JM, Lee ZW, et al. 2012. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe 11: 457–468. [DOI] [PubMed] [Google Scholar]
- Kiss M, Czimmerer Z, Nagy L. 2013. The role of lipid-activated nuclear receptors in shaping macrophage and dendritic cell function: From physiology to pathology. J Allergy Clin Immunol 132: 264–286. [DOI] [PubMed] [Google Scholar]
- Koh WJ, Kwon OJ, Suh GY, Chung MP, Kim H, Lee NY, Kim TS, Lee KS. 2004. Six-month therapy with aerosolized interferon-γ for refractory multidrug-resistant pulmonary tuberculosis. J Korean Med Sci 19: 167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo MS, Manca C, Yang G, O’Brien P, Sung N, Tsenova L, Subbian S, Fallows D, Muller G, Ehrt S, et al. 2011. Phosphodiesterase 4 inhibition reduces innate immunity and improves isoniazid clearance of Mycobacterium tuberculosis in the lungs of infected mice. PLoS ONE 6: e17091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. 2008. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol 181: 7115–7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijl C, Savage ND, Marsman M, Tuin AW, Janssen L, Egan DA, Ketema M, van den Nieuwendijk R, van den Eeden SJ, Geluk A, et al. 2007. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature 450: 725–730. [DOI] [PubMed] [Google Scholar]
- Kumar D, Nath L, Kamal MA, Varshney A, Jain A, Singh S, Rao KV. 2010. Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. Cell 140: 731–743. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, Taylor GA, McKinney JD. 2003. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science 302: 654–659. [DOI] [PubMed] [Google Scholar]
- Mahajan S, Dkhar HK, Chandra V, Dave S, Nanduri R, Janmeja AK, Agrewala JN, Gupta P. 2012. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARγ and TR4 for survival. J Immunol 188: 5593–5603. [DOI] [PubMed] [Google Scholar]
- Maiga M, Agarwal N, Ammerman NC, Gupta R, Guo H, Maiga MC, Lun S, Bishai WR. 2012. Successful shortening of tuberculosis treatment using adjuvant host-directed therapy with FDA-approved phosphodiesterase inhibitors in the mouse model. PLoS ONE 7: e30749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiga M, Ammerman NC, Maiga MC, Tounkara A, Siddiqui S, Polis M, Murphy R, Bishai WR. 2013. Adjuvant host-directed therapy with types 3 and 5 but not type 4 phosphodiesterase inhibitors shortens the duration of tuberculosis treatment. J Infect Dis 208: 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais S, Wilkinson RJ, Pepper DJ, Meintjes G. 2009. Management of patients with the immune reconstitution inflammatory syndrome. Curr HIV/AIDS Rep 6: 162–171. [DOI] [PubMed] [Google Scholar]
- Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. 2007. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest 117: 1988–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, Packe GE, Moore-Gillon JC, Darmalingam M, Davidson RN, et al. 2011. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: A double-blind randomised controlled trial. Lancet 377: 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, Sher A. 2011. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity 35: 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzalez J, Derrick SC, Shi R, Kumar NP, Wei W, et al. 2014. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 511: 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayosi BM, Ntsekhe M, Volmink JA, Commerford PJ. 2002. Interventions for treating tuberculous pericarditis. Cochrane Database Syst Rev 2002: CD000526. [DOI] [PubMed] [Google Scholar]
- Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, Elliott JH, Murdoch D, Wilkinson RJ, Seyler C, et al. 2008. Tuberculosis-associated immune reconstitution inflammatory syndrome: Case definitions for use in resource-limited settings. Lancet Infect Dis 8: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes G, Wilkinson RJ, Morroni C, Pepper DJ, Rebe K, Rangaka MX, Oni T, Maartens G. 2010. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 24: 2381–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra UK, Kalita J, Nair PP. 2010. Role of aspirin in tuberculous meningitis: A randomized open label placebo controlled trial. J Neurol Sci 293: 12–17. [DOI] [PubMed] [Google Scholar]
- Morcos MM, Gabr AA, Samuel S, Kamel M, el Baz M, el Beshry M, Michail RR. 1998. Vitamin D administration to tuberculous children and its value. Boll Chim Farm 137: 157–164. [PubMed] [Google Scholar]
- Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, Newson J, Bellingan G, Gilroy DW. 2009. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol 183: 2089–2096. [DOI] [PubMed] [Google Scholar]
- Nandi B, Behar SM. 2011. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J Exp Med 208: 2251–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier RJ, Rafi W, Cheruvu M, Powell KR, Zaunbrecher MA, Bornmann W, Salgame P, Shinnick TM, Kalman D. 2011. Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe 10: 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouailles G, Dorhoi A, Koch M, Zerrahn J, Weiner J, 3rd, Fae KC, Arrey F, Kuhlmann S, Bandermann S, Loewe D, et al. 2014. CXCL5-secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis. J Clin Invest 124: 1268–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nursyam EW, Amin Z, Rumende CM. 2006. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones 38: 3–5. [PubMed] [Google Scholar]
- Overall CM, Lopez-Otin C. 2002. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat Rev Cancer 2: 657–672. [DOI] [PubMed] [Google Scholar]
- Pan H, Yan BS, Rojas M, Shebzukhov YV, Zhou H, Kobzik L, Higgins DE, Daly MJ, Bloom BR, Kramnik I. 2005. Ipr1 gene mediates innate immunity to tuberculosis. Nature 434: 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Sassetti CM. 2008. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci 105: 4376–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar SP, Guler R, Khutlang R, Lang DM, Hurdayal R, Mhlanga MM, Suzuki H, Marais AD, Brombacher F. 2014. Statin therapy reduces the Mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J Infect Dis 209: 754–763. [DOI] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. 2004. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 4: 617–629. [DOI] [PubMed] [Google Scholar]
- Philips JA, Rubin EJ, Perrimon N. 2005. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science 309: 1251–1253. [DOI] [PubMed] [Google Scholar]
- Podinovskaia M, Lee W, Caldwell S, Russell DG. 2013. Infection of macrophages with Mycobacterium tuberculosis induces global modifications to phagosomal function. Cell Microbiol 15: 843–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K, Singh MB. 2008. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev 2008: CD002244. [DOI] [PubMed] [Google Scholar]
- Quarato G, D’Aprile A, Gavillet B, Vuagniaux G, Moradpour D, Capitanio N, Piccoli C. 2012. The cyclophilin inhibitor alisporivir prevents hepatitis C virus-mediated mitochondrial dysfunction. Hepatology 55: 1333–1343. [DOI] [PubMed] [Google Scholar]
- Rajaram MV, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. 2010. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor γ linking mannose receptor recognition to regulation of immune responses. J Immunol 185: 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram MV, Ni B, Morris JD, Brooks MN, Carlson TK, Bakthavachalu B, Schoenberg DR, Torrelles JB, Schlesinger LS. 2011. Mycobacterium tuberculosis lipomannan blocks TNF biosynthesis by regulating macrophage MAPK-activated protein kinase 2 (MK2) and microRNA miR-125b. Proc Natl Acad Sci 108: 17408–17413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan L. 2012. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol 12: 352–366. [DOI] [PubMed] [Google Scholar]
- Rask-Andersen M, Masuram S, Schioth HB. 2014. The druggable genome: Evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication. Annu Rev Pharmacol Toxicol 54: 9–26. [DOI] [PubMed] [Google Scholar]
- Ray KK, Seshasai SR, Erqou S, Sever P, Jukema JW, Ford I, Sattar N. 2010. Statins and all-cause mortality in high-risk primary prevention: A meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch Intern Med 170: 1024–1031. [DOI] [PubMed] [Google Scholar]
- Reeves PM, Bommarius B, Lebeis S, McNulty S, Christensen J, Swimm A, Chahroudi A, Chavan R, Feinberg MB, Veach D, et al. 2005. Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases. Nat Med 11: 731–739. [DOI] [PubMed] [Google Scholar]
- Roca FJ, Ramakrishnan L. 2013. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 153: 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Codogno P, Levine B. 2012. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 11: 709–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchettini JC, Rubin EJ, Freundlich JS. 2008. Drugs versus bugs: In pursuit of the persistent predator Mycobacterium tuberculosis. Nat Rev Microbiol 6: 41–52. [DOI] [PubMed] [Google Scholar]
- Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR. 1997. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics 99: 226–231. [DOI] [PubMed] [Google Scholar]
- Schoeman JF, Springer P, Ravenscroft A, Donald PR, Bekker LG, van Rensburg AJ, Hanekom WA, Haslett PA, Kaplan G. 2000. Adjunctive thalidomide therapy of childhood tuberculous meningitis: Possible anti-inflammatory role. J Child Neurol 15: 497–503. [DOI] [PubMed] [Google Scholar]
- Schoeman JF, Springer P, van Rensburg AJ, Swanevelder S, Hanekom WA, Haslett PA, Kaplan G. 2004. Adjunctive thalidomide therapy for childhood tuberculous meningitis: Results of a randomized study. J Child Neurol 19: 250–257. [DOI] [PubMed] [Google Scholar]
- Schoeman JF, Janse van Rensburg A, Laubscher JA, Springer P. 2011. The role of aspirin in childhood tuberculous meningitis. J Child Neurol 26: 956–962. [DOI] [PubMed] [Google Scholar]
- Schwegmann A, Brombacher F. 2008. Host-directed drug targeting of factors hijacked by pathogens. Sci Signal 1: re8. [DOI] [PubMed] [Google Scholar]
- Shah NS, Wright A, Bai GH, Barrera L, Boulahbal F, Martin-Casabona N, Drobniewski F, Gilpin C, Havelkova M, Lepe R, et al. 2007. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis 13: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji-Kawata S, Sumpter R, Leveno M, Campbell GR, Zou Z, Kinch L, Wilkins AD, Sun Q, Pallauf K, MacDuff D, et al. 2013. Identification of a candidate therapeutic autophagy-inducing peptide. Nature 494: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SB, Davis AS, Taylor GA, Deretic V. 2006. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science 313: 1438–1441. [DOI] [PubMed] [Google Scholar]
- Singh V, Jamwal S, Jain R, Verma P, Gokhale R, Rao KV. 2012. Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host Microbe 12: 669–681. [DOI] [PubMed] [Google Scholar]
- Stanley SA, Barczak AK, Silvis MR, Luo SS, Sogi K, Vokes M, Bray MA, Carpenter AE, Moore CB, Siddiqi N, et al. 2014. Identification of host-targeted small molecules that restrict intracellular Mycobacterium tuberculosis growth. PLoS Pathog 10: e1003946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbian S, Tsenova L, O’Brien P, Yang G, Koo MS, Peixoto B, Fallows D, Dartois V, Muller G, Kaplan G. 2011a. Phosphodiesterase-4 inhibition alters gene expression and improves isoniazid-mediated clearance of Mycobacterium tuberculosis in rabbit lungs. PLoS Pathog 7: e1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbian S, Tsenova L, O’Brien P, Yang G, Koo MS, Peixoto B, Fallows D, Zeldis JB, Muller G, Kaplan G. 2011b. Phosphodiesterase-4 inhibition combined with isoniazid treatment of rabbits with pulmonary tuberculosis reduces macrophage activation and lung pathology. Am J Pathol 179: 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaramurthy V, Barsacchi R, Samusik N, Marsico G, Gilleron J, Kalaidzidis I, Meyenhofer F, Bickle M, Kalaidzidis Y, Zerial M. 2013. Integration of chemical and RNAi multiparametric profiles identifies triggers of intracellular mycobacterial killing. Cell Host Microbe 13: 129–142. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Hattle JM, Dreitz SA, Troudt JM, Izzo LS, Basaraba RJ, Orme IM, Matrisian LM, Izzo AA. 2006. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect Immun 74: 6135–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, Nguyen QH, Nguyen TT, Nguyen NH, Nguyen TN, et al. 2004. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 351: 1741–1751. [DOI] [PubMed] [Google Scholar]
- Thwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J, British Infection S. 2009. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect 59: 167–187. [DOI] [PubMed] [Google Scholar]
- Tobin DM, Vary JC Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, Hagge DA, Khadge S, King MC, Hawn TR, et al. 2010. The lta4 h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 140: 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, Ko DC, Zou Y, Bang ND, Chau TT, et al. 2012. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 148: 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustianowski A, Shaffer R, Collin S, Wilkinson RJ, Davidson RN. 2005. Prevalence and associations of vitamin D deficiency in foreign-born persons with tuberculosis in London. J Infect 50: 432–437. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. 2010. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat Rev Mol Cell Biol 11: 700–714. [DOI] [PubMed] [Google Scholar]
- Verway M, Bouttier M, Wang TT, Carrier M, Calderon M, An BS, Devemy E, McIntosh F, Divangahi M, Behr MA, et al. 2013. Vitamin D induces interleukin-1β expression: Paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog 9: e1003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaplana C, Marzo E, Tapia G, Diaz J, Garcia V, Cardona PJ. 2013. Ibuprofen therapy resulted in significantly decreased tissue bacillary loads and increased survival in a new murine experimental model of active tuberculosis. J Infect Dis 208: 199–202. [DOI] [PubMed] [Google Scholar]
- Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. 2010. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science 327: 466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RO, Manzanillo PS, Cox JS. 2012. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 150: 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wejse C, Olesen R, Rabna P, Kaestel P, Gustafson P, Aaby P, Andersen PL, Glerup H, Sodemann M. 2007. Serum 25-hydroxyvitamin D in a West African population of tuberculosis patients and unmatched healthy controls. Am J Clin Nutr 86: 1376–1383. [DOI] [PubMed] [Google Scholar]
- Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, Andersen PL, Glerup H, Sodemann M. 2009. Vitamin D as supplementary treatment for tuberculosis: A double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 179: 843–850. [DOI] [PubMed] [Google Scholar]
- WHO. 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. World Health Organization, Geneva. [Google Scholar]
- Wilkinson RJ. 2014. Host-directed therapies against tuberculosis. Lancet Respir Med 2: 85–87. [DOI] [PubMed] [Google Scholar]
- Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. 2000. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: A case-control study. Lancet 355: 618–621. [DOI] [PubMed] [Google Scholar]
- Wu K, Koo J, Jiang X, Chen R, Cohen SN, Nathan C. 2012. Improved control of tuberculosis and activation of macrophages in mice lacking protein kinase R. PLoS ONE 7: e30512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L. 2012. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe 12: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. 2009. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 6: 231–243. [DOI] [PubMed] [Google Scholar]
- Zumla A, Nahid P, Cole ST. 2013. Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov 12: 388–404. [DOI] [PubMed] [Google Scholar]
- Zumla AI, Gillespie SH, Hoelscher M, Philips PP, Cole ST, Abubakar I, McHugh TD, Schito M, Maeurer M, Nunn AJ. 2014. New antituberculosis drugs, regimens, and adjunct therapies: Needs, advances, and future prospects. Lancet Infect Dis 14: 327–340. [DOI] [PubMed] [Google Scholar]