Abstract
In 2008, Novartis Animal Health developed a new class of anthelmintics, the amino-acetonitrile derivatives (AAD) of which monepantel is the most prominent compound. Monepantel was designed for the treatment of sheep against the parasitic nematode Haemonchus contortus. Because monepantel acts through a different mechanism, it is effective against nematodes that have acquired resistance to long-standing anthelmintics. In order to benefit from a maximum lifespan and efficacy of this new compound, the mode of action of monepantel needs to be understood. Studies on the model nematode Caenorhabditis elegans led to the identification of at least one target of monepantel: the monovalent cation channel ACR-23. Here we comment on the effects of monepantel on C. elegans and on the development of resistant parasitic nematode strains.
Keywords: acr-23, anthelmintic, C. elegans, monepantel, motility, parasite
Introduction: Monepantel, a Member of a New Class of Anthelmintics
Parasitic nematodes cause substantial loss of productivity in farming animals and pose a serious threat to the health of pets. To cope with worm infections, the chemical industry has developed several classes of anthelmintics that have been used in the field for decades. However, over the years and especially after repeated usage, resistant nematode strains have appeared. With the development of AADs, it is possible to fight against multidrug-resistant strains.1 Older anthelmintics have various modes of action such as the nicotinic agonists (levamisole and pyrantel) which cause spastic paralysis in nematodes; the acetylcholinesterase inhibitors (haloxon); the gamma-amino-butyric acid agonists (Piperazine); glutamate-gated chloride channel stimulators (ivermectin) which lead to paralysis of pharyngeal pumping and the benzimidazoles (abendazole and melbendazole), which bind to β-tubulin consequently inhibiting microtubule formation.2 Ivermectin-, benzimidazole- and levamisole-resistant C. elegans have been shown to be sensitive to AADs strongly suggesting that monepantel functions via a different mode of action and therefore targets genes that have not yet undergone selective pressure.1
The Ion Channel ACR-23 is a Target of Monepantel in C. elegans
Like many parasitic nematodes, wild-type C. elegans is sensitive to monepantel.3 However monepantel has little or no efficacy against certain parasitic nematodes like Taenia ovis, Esophagostomum venulosum, or the free-living nematode Pristionchus pacificus, which lacks the monepantel receptor.4,5
C. elegans is not a parasite, but a nematode that can easily and conveniently be maintained in culture. With its short life cycle and well-established tools for genetic analysis, it is the perfect organism to screen for mutants that are resistant to monepantel. Such a screen led to the identification of the nicotinic acetylcholine receptor superfamily member ACR-23.1 In AAD-resistant mutants, the ACR-23 protein has lost all or part of its activity, indicating that it is a major target of monepantel.1,3 Interestingly, acr-23 cRNAs reconstitute a monepantel-sensitive current in Xenopus oocytes. We found that choline and monepantel act as agonists on ACR-23 and therefore proposed that ACR-23 forms a monepantel-sensitive channel that is permeable to monovalent cations.3 We also suggested that the anthelmintic blocks the channel in its open configuration and found ACR-23 to be mainly expressed in body wall muscle cells. We therefore proposed that monepantel blocks muscle function by continuous depolarization of the membrane of muscle cells.3
More recently, glycine betaine was identified as the natural ligand of ACR-23.6 Moreover, in this study monepantel was shown to boost betaine action, resulting in spastic paralysis. This study, however, proposes that ACR-23 acts in neurons rather than in muscles where ACR-23 is predominantly expressed.3,6 In any case, the nervous system is tightly coupled to the muscular system via the neuromuscular junctions, so they can possibly function in tandem with regards to ACR-23, therefore accounting for the discrepancies observed in the two studies.
Expression of ACR-23 and Mobility Defects of acr-23 Mutants
We found that an acr-23::gfp fusion reporter construct, which could fully rescue acr-23 mutants, was predominantly expressed in the body wall muscle cells and in some cells of the tail, which we did not identify.3 A more recent report showed that a reporter construct driven by the acr-23 promoter was mainly expressed in the mechanosensory neurons PLM, PVM, AVM and ALM and in body wall muscle cells.6 In our transgenic strain, we can observe two cells expressing acr-23 in the tail, but their position and shape neither correspond to the two PLM, nor to cells of the body wall muscles. The absence of expression in the tail mechanosensory neurons with the protein fusion construct indicates that while the acr-23 promoter is functional and able to rescue acr-23 null mutants, the gene product is post-transcriptionally downregulated. As a consequence, it might be present at levels that are not visible in neurons.
In our study, we found that starved acr-23 mutants moved more rapidly and in straighter trajectories than wild-type starved worms.3 Using the same allele, locomotion defects have also been reported in another study in which well-fed acr-23 mutants were less mobile upon starvation than wild type.6 Although both reports clearly state that in the presence of monepantel, acr-23 mutants are more mobile than wild type, this observation is not very clear in the absence of the drug.3 We therefore propose that the opposite mobility phenotypes, which have been observed in the two studies, may be caused by different experimental conditions.7
Dynamics of the Response to Monepantel
Anthelmintics act in different manners on nematodes. They can, for instance, cause muscle relaxation, as does ivermectin, which triggers flaccid paralysis.8 Other anthelmintics cause muscle hyper contraction (spastic paralysis) as observed with levamisole and monepantel.1,2 It is well established that monepantel acts as an anthelmintic. In fact, with C. elegans there is a dose-dependent effect of monepantel on larval development and general mobility.1,3 However, adult animals seem to be more tolerant as reflected by the ability of occasional escapers that are still able to lay eggs and to crawl for days on plates containing 1 or 20 μM monepantel.3 Anthelmintics are often referred to as being lethal to nematodes. However, whether monepantel actually kills C. elegans or parasitic worms remains an open question. This could be addressed by verifying how fast the drug acts, how long its effects remain, and whether nematodes are able to recover from exposure to monepantel once the drug has been cleared away.
C. elegans can live on solid or in liquid medium, but the motion patterns are quite different: the S-shaped movement for crawling becomes a C-shaped movement for swimming.9 The two types of movement therefore require different neuromuscular inputs and might be differently affected by monepantel. Therefore, subtle defects in locomotion, which are not visible on agar plates, could be captured with C. elegans moving in liquid medium. In the absence of monepantel we found only minor differences in that acr-23(cb27) mutants were slightly more active than wild type as reflected by the number of body thrashes per minute (Fru and Puoti, unpublished data). However in the presence of the drug, wild-type C. elegans cultured in liquid medium were very rapidly affected by monepantel, and surprisingly even acr-23 null mutants were paralyzed by the compound (Fru and Puoti, unpublished data). Because ACR-23 is missing in the mutants, the observed sensitivity to monepantel could be caused by another receptor. If so, this receptor could be identified through a mutagenesis screen for the rescue of the swimming defect in an acr-23 null mutant background. Alternatively, the effect of monepantel on swimming could be stronger due to metabolic differences resulting from the two modes of movement.
Interestingly, C. elegans larvae kept on monepantel for one day mostly developed into well-moving fertile adults once shifted to plates without the anthelmintic. However after longer exposure the recovery rate was weaker (Fru and Puoti, unpublished data). Therefore, the larvae even if paralyzed by the compound, can recover after having been exposed to monepantel for a short time. We have not tested recovery with parasitic nematodes, but at least Haemonchus contortus could behave similarly to C. elegans in that monepantel paralyses both species.3,10 We speculate that susceptible parasitic nematodes that are eliminated from the intestine after drenching are able to survive and to lay eggs once on the pasture. In the case of Haemonchus contortus, such eggs can develop into L3 larvae and infect other animals.11
How Does Resistance Appear?
Like resistance to antibiotics, resistance to anthelmintics arises through mutations in the parasite's genome.3,12 In fact, with C. elegans, selection on monepantel allowed the isolation of allele acr-23(cb27) as a spontaneous mutation, without the addition of mutagen.1 Similarly, mutations in the homolog mptl-1 were isolated from AAD-resistant H. contortus species.13 Since monepantel does not kill C. elegans, one can easily imagine that under constant selective pressure, the more tolerant individuals are given the possibility to escape and to reproduce. With parasitic nematodes such as H. contortus, paralysis would result in its elimination through the feces. If similarly to C. elegans, larvae of parasitic nematodes were able to recover after removal of monepantel, they would survive once eliminated in the dung and exposed to rain. The recovery of these individuals therefore represents an additional source of infection. Fortunately, such escapers only represent a minute proportion of the population of free-living parasites. In fact, a large part of a population of parasitic nematodes does not reside in the host, but on pasture.14 This population, which is in principle not subjected to the anthelmintic, provides a gene pool of susceptible worms to dilute resistant genes.15 As a consequence, susceptible worms that are able to recover will contribute to increase of individuals in a refugium.
How do Nematodes Develop Resistance to Monepantel?
A genetic screen for resistance to monepantel led to the identification of 44 alleles in C. elegans, of which 27 correspond to mutations in the coding sequence of acr-23.1,3 The remaining 17 mutants have not been investigated so far, but could represent new targets that lead to resistance to monepantel. We complemented acr-23 mutant alleles by expressing wild-type acr-23.3 Remarkably, we also found that alleles, which do not correspond to mutations in the acr-23 open reading frame were rescued by wild-type copies of acr-23.3 Consequently, at least some of the 17 additional alleles might affect genes that either control ACR-23 expression or activity. In Fig. 1, we highlight several possibilities on how C. elegans could develop resistance to monepantel. Firstly, expression of acr-23 could be impaired by mutations in the acr-23 promoter or in other regulators that control the transcription of this gene. Secondly, mutations in the acr-23 open reading frame abolish or decrease ACR-23 function. Such mutations have been characterized to date.1,3,6 Thirdly, nematodes can also acquire resistance to monepantel through mutations in accessory proteins that assemble ACR-23 subunits. RIC-3 represents an example of such a chaperone.3,16 It is also possible that ACR-23 receptors need another protein to be fully functional. This factor is indicated by a question mark. However, to account for the results obtained through the electrophysiological analyses, such a factor would need to be functionally conserved in Xenopus oocytes.3,6 Of course, other possibilities exist. Betaine is a natural product, which is used as an anthelmintic.17 It shares a common receptor (ACR-23) with monepantel and has SNF-3 as its transporter. Consequently, snf-3 loss-of-function mutants have increased extracellular accumulation of betaine and are hypersensitive to betaine, a condition that could be attenuated by mutations in acr-23.6 It is worth mentioning that the study of Peden et al. showed that monepantel and betaine had a synergistic effect on the paralysis of C. elegans. Therefore, rare gain-of-function alleles of snf-3 could increase the clearance of betaine from the extracellular space, thus leading to increased resistance to monepantel.6 The protein denoted “Rm” represents a putative receptor to monepantel, which acts on swimming motion rather than on crawling. Nevertheless, if mutated, protein Rm does not necessarily confer resistance to monepantel, as long as the ACR-23 protein is functional.
Figure 1.
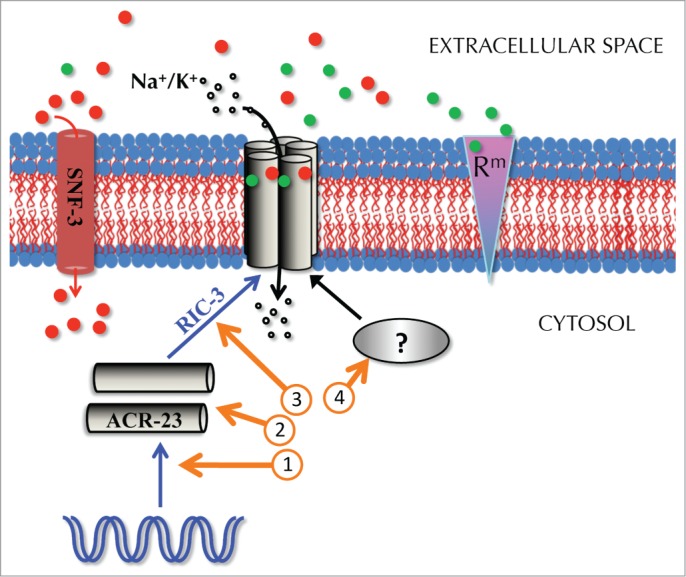
How C. elegans could develop resistance to monepantel. The ACR-23 receptor is represented by a homopentamer that transports monovalent cations (open circles) into the cytosol. ACR-23 is activated by betaine (filled red circles) and monepantel (filled green circles). Betaine is also transported across the membrane by SNF-3. The synthesis of a functional ACR-23 receptor involves several steps, which if inactivated, could result in partial or full resistance to monepantel. Such steps involve gene expression (circled “1”) and assembly of the receptor (“3”), for example by the chaperone RIC-3. In fact, ric-3 mutants are moderately resistant to monepantel.2 Mutations in the ACR-23 protein result in resistance to monepantel (“2”). Additional factors could be required for a fully functional ACR-23 receptor (“4”). Blue arrows indicate the synthesis pathway of the ACR-23 receptor. “Rm” represents a putative additional receptor to monepantel. The representation of the proteins in one single membrane tract should not imply that they are expressed in the same cell.
How to Optimize the Efficiency of Monepantel?
A recent report describes the outbreak of resistant nematode species in goat after extensive usage of monepantel.18 In this particular case, most individuals were treated simultaneously for 17 consecutive times with monepantel. The authors of the report conclude that such intense drenching leaves a very small population of susceptible worms able to reproduce and dilute the pool of resistant mutants. In addition, the metabolism in goat is more robust than in sheep and might have contributed to shorten the life span of the active compound.18
As with other anthelmintics, a maximum efficiency time for monepantel can only be obtained through alternated treatments with compounds that act on various targets. Although a large part of a population of parasitic nematodes lives in the pasture, only the individuals that are taken up by the host are able to reproduce. Therefore, in addition to alternating anthelmintics, a refugium of susceptible parasitic nematodes should be maintained to compete with the gene pool of resistant mutants.15 On the side of the pharmaceutical industry, resistance to monepantel could be countered by the development of new anthelmintics that are more stable in the host, or that target additional genes, which are implicated in the activity of ACR-23. The identification and characterization of an additional receptor to monepantel could yield new insights for the design of anthelmintics for the years to come.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Lucien Rufener, Jacques Bouvier, and Ronald Kaminsky from the Novartis Centre de Recherche Santé Animale in St. Aubin, for the generous gift of monepantel.
References
- 1. Kaminsky R, Ducray P, Jung M, Clover R, Rufener L, Bouvier J, Weber SS, Wenger A, Wieland-Berghausen S, Goebel T, et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature 2008; 452(7184):176-80; PMID:18337814; http://dx. doi: 10.1038/nature06722 [DOI] [PubMed] [Google Scholar]
- 2. Martin RJ. Modes of action of anthelmintic drugs. Vet J 1997; 154:11-34; PMID:9265850; http://dx.doi.org/ 10.1016/S1090-0233(05)80005-X [DOI] [PubMed] [Google Scholar]
- 3. Rufener L, Bedoni N, Baur R, Rey S, Glauser DA, Bouvier J, Beech R, Sigel E, Puoti A. acr-23 Encodes a monepantel-sensitive channel in Caenorhabditis elegans. PLoS Pathog 2013; 9:e1003524; PMID:23950710; http://dx.doi.org/ 10.1371/journal.ppat.1003524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bustamante M, Steffan PE, Bonino Morlán J, Echevarria F, Fiel CA, Cardozo H, Castells D, Hosking BC. The efficacy of monepantel, an amino-acetonitrile derivative, against gastrointestinal nematodes of sheep in three countries of southern Latin America. Parasitol Res 2009; 106(1):139-44; PMID:19789895; http://dx. doi: 10.1007/s00436-009-1638-z [DOI] [PubMed] [Google Scholar]
- 5. Rufener L, Keiser J, Kaminsky R, Mäser P, Nilsson D. Phylogenomics of ligand-gated ion channels predicts monepantel effect. PLoS Pathog 2010; 6:e1001091; PMID:20838602; http://dx.doi.org/ 10.1371/journal.ppat.1001091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peden AS, Mac P, Fei YJ, Castro C, Jiang G, Murfitt KJ, Miska EA, Griffin JL, Ganapathy V, Jorgensen EM. Betaine acts on a ligand-gated ion channel in the nervous system of the nematode C. elegans. Nat Neurosci 2013; 16:1794-801; PMID:24212673; http://dx.doi.org/ 10.1038/nn.3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glauser DA. How and why Caenorhabditis elegans uses distinct escape and avoidance regimes to minimize exposure to noxious heat. Worm 2013; 2:e27285; PMID:24744986; http://dx.doi.org/ 10.4161/worm.27285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ardelli BF, Stitt LE, Tompkins JB, Prichard RK. A. comparison of the effects of ivermectin and moxidectin on the nematode Caenorhabditis elegans. Vet Parasitol 2009; 165:96-108; PMID:19631471; http://dx.doi.org/ 10.1016/j.vetpar.2009.06.043 [DOI] [PubMed] [Google Scholar]
- 9. Pierce-Shimomura JT, Chen BL, Mun JJ, Ho R, Sarkis R, McIntire SL. Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc Natl Acad Sci U S A 2008; 105(52):20982-7; PMID:19074276; http://dx. doi: 10.1073/pnas.0810359105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rufener L, Baur R, Kaminsky R, Mäser P, Sigel E. Monepantel allosterically activates DEG-3/DES-2 channels of the gastrointestinal nematode Haemonchus contortus. Mol Pharmacol 2010; 78:895-902; PMID:20679419; http://dx.doi.org/ 10.1124/mol.110.066498 [DOI] [PubMed] [Google Scholar]
- 11. Wanyangu SW, Karimi S, Mugambi JM, Bain RK. Availability of Haemonchus contortus L3 larvae on pasture at Kiboko: a semi-arid warm agro-climatic zone in Kenya. Acta Trop 1997; 68:183-9; PMID:9386793; http://dx.doi.org/ 10.1016/S0001-706X(97)00090-9 [DOI] [PubMed] [Google Scholar]
- 12. Sandgren A, Strong M, Muthukrishnan P, Weiner BK, Church GM, Murray MB. Tuberculosis drug resistance mutation database. PLoS Med 2009; 6:e2; PMID:19209951; http://dx.doi.org/ 10.1371/journal.pmed.1000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rufener L, Mäser P, Roditi I, Kaminsky R. Haemonchus contortus acetylcholine receptors of the DEG-3 subfamily and their role in sensitivity to monepantel. PLoS Pathog 2009;5(4):e1000380; PMID:19360096; http://dx. doi: 10.1371/journal.ppat.1000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grosz DD, Eljaki AA, Holler LD, Petersen DJ, Holler SW, Hildreth MB. Overwintering strategies of a population of anthelmintic-resistant Haemonchus contortus within a sheep flock from the United States Northern Great Plains. Vet Parasitol 2013; 196(1-2):143-52; PMID: 23433645; http://dx. doi: 10.1016/j.vetpar.2013.01.026 [DOI] [PubMed] [Google Scholar]
- 15. Besier RB. Refugia-based strategies for sustainable worm control: factors affecting the acceptability to sheep and goat owners. Vet Parasitol 2012; 186:2-9; PMID:22197747; http://dx.doi.org/ 10.1016/j.vetpar.2011.11.057 [DOI] [PubMed] [Google Scholar]
- 16. Halevi S, McKay J, Palfreyman M, Yassin L, Eshel M, Jorgensen E, Treinin M., The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J 2002; 21:1012-20; PMID:11867529; http://dx.doi.org/ 10.1093/emboj/21.5.1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jenkins T, Blunden G, Wu Y, Hankins SD, Gabrielsen BO. Are the reductions in nematode attack on plants treated with seaweed extracts the result of stimulation of the formaldehyde cycle? Acta Biol Hung 1998; 49:421-7; PMID:10526988 [PubMed] [Google Scholar]
- 18. Scott I, Pomroy WE, Kenyon PR, Smith G, Adlington B, Moss A. Lack of efficacy of monepantel against Teladorsagia circumcincta and Trichostrongylus colubriformis. Vet Parasitol 2013; 198:166-71; PMID:23953148; http://dx.doi.org/ 10.1016/j.vetpar.2013.07.037 [DOI] [PubMed] [Google Scholar]


