Abstract
Hydrodynamics-based delivery has been used as an experimental tool to express transgene in small animals. This in vivo gene transfer method is useful for functional analysis of genetic elements, therapeutic effect of oligonucleotides, and cancer cells to establish the metastatic cancer animal model for experimental research. Recent progress in the development of image-guided procedure for hydrodynamics-based gene delivery in large animals directly supports the clinical applicability of this technique. This review summarizes the current status and recent progress in the development of hydrodynamics-based gene delivery and discusses the future directions for its clinical application.
Keywords: gene therapy, hydrodynamics-based gene delivery, DNA, clinical application
1. Hydrodynamics-Based Gene Delivery in Small Animals
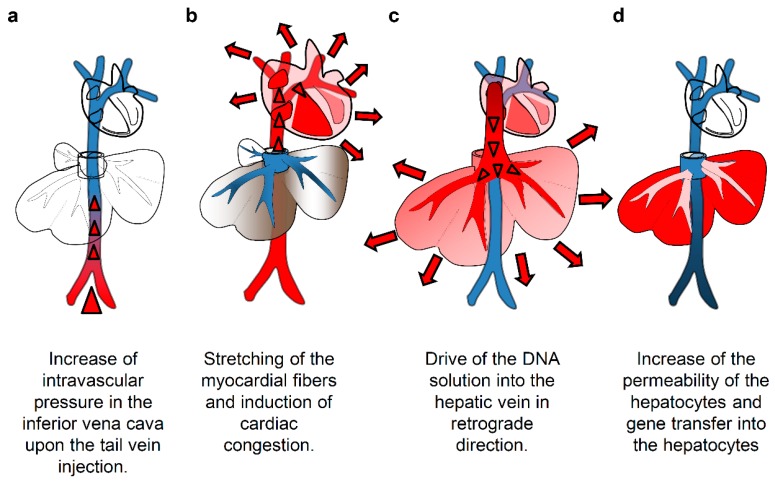
Hydrodynamics-based delivery utilizes hydrodynamic pressure induced by the volume and flow of injection as the driving force to facilitate intracellular gene transfer [1,2]. The original method of hydrodynamic gene delivery (HGD) in small animals involves tail vein injection of plasmid DNA solution in a volume equal to 8%–10% of the body weight (BW) in 5–7 s [1,3]. Among the various organs showing considerably high levels of transgene expression, the liver shows the highest level, and about 40% hepatocytes are transfected by a single injection of less than 50 µg of plasmid DNA [4]. The mechanism of HGD is shown in Figure 1. The injected plasmid solution directly goes to the heart, inducing cardiac congestion and backflow to the inferior vena cava (IVC) and hepatic veins. This solution then reaches the sinusoids; the hydrodynamic pressure expands the liver, enlarges the fenestrae of the endothelium, and forces invagination of the cellular membrane of the hepatocytes to allow the DNA to move into the cytoplasm [5] (Figure 1). In small animals, the opened cellular membrane is smoothly resealed usually within a few minutes, thereby trapping the DNA within the cells. The enlarged liver returns to its original size within 24 h [6]. Hepatic enzymes, including aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase, exhibit a transient increase in the serum after the injection and return to normal levels within a few days [6].
Figure 1.
Mechanism of hydrodynamics-based delivery to the mouse liver. (a) The intravascular pressure in the inferior vena cava increases upon the tail vein injection of the solution; (b) induction of cardiac congestion and accumulation of the solution in the inferior vena cava; (c) flow of the injected solution into the liver in retrograde and pushing blood preexistent toward portal side; (d) pressure-mediated increase of the membrane permeability and transfer of gene into hepatocytes.
Evidence collected thus far shows that HGD has a short-term effect in animals [3]. No long-term effect on animal health has been reported. The significantly higher transgene expression observed in the liver following HGD via the tail vein has been attributed to several features that are unique to the liver, including the presence of fenestrated sinusoids, the absence of a basement membrane, the high capacity of hepatocytes for gene expression, proximity to the IVC, low blood pressure, and the large surface area of hepatocytes facing the lumen. After the procedure, regular blood circulation resumes, the delivered transgene is expressed, and the gene products appear in transfected cells. While the liver shows the highest level of transgene expression in mice, other organs and tissues including kidney, muscles, and even tumors show gene expression, which indicates the applicability of the procedure for various diseases [7,8,9].
2. Experimental Applicability of Hydrodynamic Gene Delivery
Because of its simplicity, high efficiency, and reproducibility, hydrodynamics-based delivery has been utilized in scientific research for the delivery of DNA, small interfering RNA (siRNAs), proteins, small compounds, viral vectors, and even cancer cells. Recently, Li et al. reported the utility of the procedure for the development of mouse models of multiple metastatic tumors in the liver, kidney, and lung [10]. Hydrodynamics-based delivery of murine melanoma cells (B16-F1), murine breast cancer cells (4T1), and murine renal cell carcinoma (RENCA) resulted in liver, kidney, and lung metastatic tumors, whereas slow injection of these cells resulted in tumors only in the lung [10]. In HGD, the only requirement for gene delivery is a plasmid containing the expression cassette. Persistent gene expression is dependent on the regulatory element in the plasmid, and modifications in this region, such as a combination of albumin promoter and alpha-fetoprotein enhancer introns, can significantly contribute to sustained gene expression [11]. HGD is also useful for delivering large DNA fragments [12] of therapeutic genes as tested in small animal models for various disease models including hepatitis, hematological disorders, metabolic disease, infectious disease, muscle disease, and malignant diseases [4,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. HGD is also an effective tool in studies of genetic immunization and treatment of viral infection [47,48]. In addition, it has been used in the establishment of small animal disease models for hepatitis, liver fibrosis, liver cancer, sepsis, and lipidemia [49,50,51,52,53,54,55].
Based on the promising results of gene expression studies conducted using small rodents, efforts have been made to apply this method to clinical gene therapy. Establishment of safe and reproducible procedures is an essential step in the project. Key factors that must be addressed include the following: (1) reduction in the injection volume, (2) therapeutic effect, and (3) reproducibility of the procedure that can be performed by any physician. To address these points, we have developed an image-guided, computer-assisted HGD procedure and have shown its safe and effective use for gene delivery in the liver and muscles of pigs and dogs [56,57,58,59].
3. Clinical Applicability of Image-Guided Hydrodynamic Gene Delivery
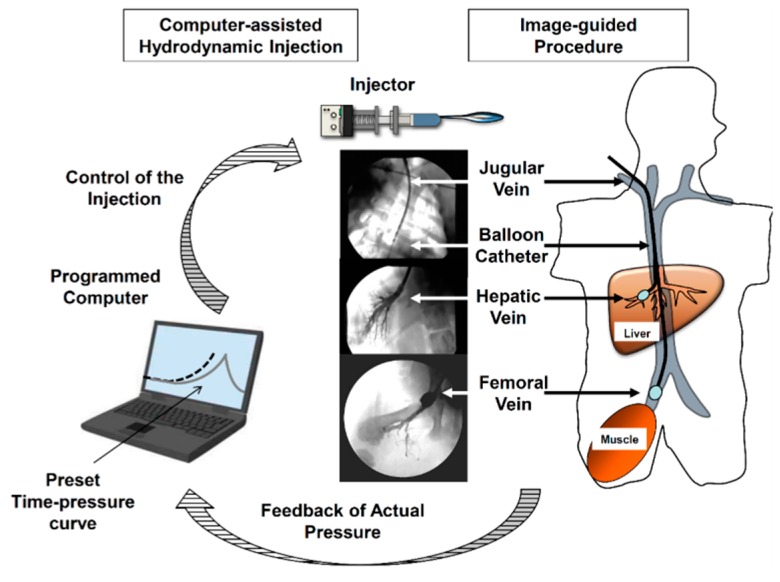
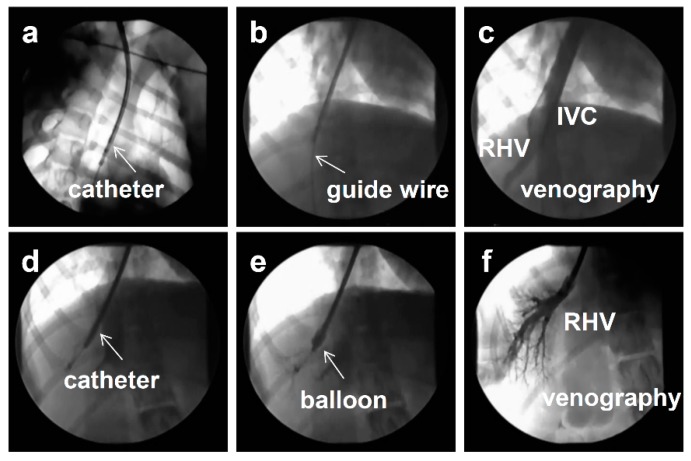
The image-guided procedure was developed to reduce the injection volume (Figure 2), because an injection volume of approximately 10% of the BW (~5 L for a 50-kg man) is considered impractical and unsafe. Some modifications of the original procedure have been reported. For example, Eastman et al. demonstrated that a volume of 15 mL/kg can be safely injected into an isolated liver in rabbits [60]. Similar results were reported by several studies using small pigs as an animal model [61,62,63]. However, due to procedural difficulties, the reproducibility of the procedure and the efficiency of gene delivery remained unclear. To further extend the clinical applicability of the procedure, Kamimura et al. performed site-specific gene delivery to the livers [56] and muscles [57] of 20-kg pigs by combining with a clinically well-established X-ray image-guided method of catheter insertion at the site of target organs [56,57]. The procedure involved insertion of a short sheath into the jugular vein, followed by insertion of a balloon catheter (Figure 3a) into the target lobular hepatic vein or femoral vein using a guide wire (Figure 3b–d). The balloon was then inflated by injecting a small amount of the phase contrast medium (Figure 3e). Thereafter, obstruction of blood flow was verified by injecting a small volume of the phase contrast medium into the vasculature through the catheter (Figure 3f), followed by HGD. The injection was given specifically to a localized area in the liver, enabling a significant reduction in the injection volume from 10% of the BW to 1.25% of the BW in pigs [56,58]. Target-specific gene delivery was confirmed at the area near the injection site. The procedure has also been applied to gene delivery in the muscles of pigs. Gene delivery parameters, including injection site, flow rate, and volume, have been optimized and the clinical applicability of the procedure in terms of safety and efficiency has been shown in large pigs [58] and dogs [59]. Using optimized conditions, human alpha-1 antitrypsin gene expression was achieved in pig liver for more than two months after gene delivery [58]. Physiological parameters, including heart rate, blood pressure, oxygen saturation, and body temperature, remained normal during and after hydrodynamic injection in these animal studies [59]. In addition, no changes in BW were observed in dogs during the experimental period, which included three hydrodynamic injections over 6 weeks at 2-week time intervals. Following liver-targeted HGD, PCR was used to assess systemic distribution of the plasmid DNA; results showed no gene transfer to cells beyond the target organ [58,59]. In addition, no allergic symptom or change in the animal condition was observed following repeated HGD using the same genes [58,59]. These results support the clinical applicability of the image-guided HGD procedure in terms of its safety and efficiency for gene delivery.
Figure 2.
Schematic presentation of computer-assisted and image-guided hydrodynamics-based delivery. Balloon catheter is located at the proper section of hepatic vein (for gene transfer to the liver) or femoral vein (for gene transfer to skeletal muscle). Pressure upon the hydrodynamics-based delivery is transduced from the sensor placed inside of the target vasculature to the computer. Then the computer starts the injection and control the injection speed according to the real-time pressure. The photo images represent the visualization of vascular structure at the insertion site using phase contrast medium (Modified from [64] with permission. Copyright Future Medicine Ltd. 2015).
Figure 3.
Image-guided procedure for hydrodynamics-based delivery. (a) A short sheath is inserted into the jugular vein, followed by an insertion of a balloon catheter. (b) Site-specific insertion of guide-wire into the target lobular hepatic vein. (c,d) Confirmation of target hepatic vein using a small amount of phase contrast. RHV, right hepatic vein, IVC, inferior vena cava. (e) Inflation of the balloon on the tip of the catheter to block the backflow. (f) Verification of the obstruction of blood flow by injecting a small volume of the phase contrast medium into the vasculature through the catheter.
4. Clinically Applicable Reproducible Procedure
To achieve clinical reproducibility in terms of the therapeutic effect, development of an automatic computer-controlled injection device is essential. Previous studies have identified the injection time–intravascular pressure curve as the key parameter for HGD [6]. Suda et al. developed a first-generation computer-controlled injection device using CO2 gas as a physical force to control the time–pressure curve of HGD [65]. The intravascular pressure in the animal body upon injection is transmitted to the computer through the pressure detector placed at the tip of the injection catheter. The computer turns the sustained injection CO2 pressure on or off according to the detected intravascular pressure to achieve the desired level of intravascular pressure or to stop the injection once the level is achieved [65]. The injector has shown reproducible gene delivery to muscle and liver in pigs [56,57]. Kamimura et al. have recently developed a new electric motor-driven injector [59,66]. The injector self-adjusts the injection power depending on the real-time feedback of the actual pressure in the body and reproduces the preloaded time–pressure curves installed in the computer prior to the injection. The major advantage of using this injector is that it eliminates the risk of CO2 embolism or unexpected high intravascular pressure that can occur due to the sustained injection CO2 pressure and damage the injection-targeted organs [59,66]. Recently, it has been tested in large animals and shown to be safe, reproducible, and efficient for gene delivery in dogs [59]. Combination of an image-guided procedure and a computer-controlled injection device will allow physicians to apply this procedure in the clinic (Figure 2).
5. Conclusions and Future Perspectives
Significant progress has been made in a variety of gene delivery systems to date. While viral vectors are highly effective and have been used in approximately 67% of clinical trials, the possibility of carcinogenesis and development of an immune response remain the largest hurdles to clinical application [64,67,68,69]. For nonviral vector-based gene delivery used in 24% of clinical trials, a variety of compounds have been used; however, their effectiveness in gene delivery remains significantly lower than that of viral vectors. Gene delivery methods using a physical approach, including needle injection, ballistic DNA injection, electroporation, sonoporation, photoporation, magnetofection, and HGD, are a part of the nonviral vector-based gene delivery approach; these are relatively new and have demonstrated their potential to transfer DNAs directly into the cells in the target organs.
Among these methods, HGD has been extensively studied in small animals. The simplicity and effectiveness of the approach has encouraged scientists and physicians to further develop hydrodynamics-based gene therapy. The recent progress in image-guided catheterization-based procedures has enabled physicians to perform HGD to specific target organs. This site-specific gene delivery with reduced injection volume and the development of computer-controlled injection devices enables safe, reproducible, and efficient gene delivery. Recently, a study using liver-targeted computer-controlled HGD showed no change in systemic inflammatory cytokines after injection, whereas an increase in myokines was observed due to the stretching of the vascular structure upon injection [59]. These results support the clinical applicability of HGD, and recently the more clinically applicable computer-controlled injector has been developed. The pre-clinical trial in the large animals using the new injector is ongoing (unpublished data). Further efforts must be made to develop the procedure in non-human primates as a human model and to establish plasmids that are clinically suitable for long-term gene expression.
Acknowledgements
This work was supported in part by Grant-in-Aid for Scientific Research from Japanese Society for the Promotion of Sciences 22890064, 23790595, 26860354, and Takara Bio Research Grant from JSGT to Kenya Kamimura and 26293175 to Shuji Terai.
Author Contributions
Kenya Kamimura, Takeshi Yokoo, Hiroyuki Abe, Yuji Kobayashi, Yoko Shinagawa, Kohei Ogawa, and Ryosuke Inoue performed the experiments. Shuji Terai analyzed the data; Kenya Kamimura prepared manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Liu F., Song Y., Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 2.Zhang G., Budker V., Wolff J.A. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum. Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 3.Zhang G., Gao X., Song Y.K., Vollmer R., Stolz D.B., Gasiorowski J.Z., Dean D.A., Liu D. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004;11:675–682. doi: 10.1038/sj.gt.3302210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang G., Song Y.K., Liu D. Long-term expression of human alpha1-antitrypsin gene in mouse liver achieved by intravenous administration of plasmid DNA using a hydrodynamics-based procedure. Gene Ther. 2000;7:1344–1349. doi: 10.1038/sj.gt.3301229. [DOI] [PubMed] [Google Scholar]
- 5.Suda T., Liu D. Hydrodynamic gene delivery: Its principles and applications. Mol. Ther. 2007;15:2063–2069. doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- 6.Suda T., Gao X., Stolz D.B., Liu D. Structural impact of hydrodynamic injection on mouse liver. Gene Ther. 2007;14:129–137. doi: 10.1038/sj.gt.3302865. [DOI] [PubMed] [Google Scholar]
- 7.Kamimura K., Liu D. Physical approaches for nucleic acid delivery to liver. AAPS J. 2008;10:589–595. doi: 10.1208/s12248-008-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamimura K., Suda T., Zhang G., Liu D. Advances in gene delivery systems. Pharm. Med. 2011;25:293–306. doi: 10.1007/BF03256872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonamassa B., Hai L., Liu D. Hydrodynamic gene delivery and its applications in pharmaceutical research. Pharm. Res. 2011;28:694–701. doi: 10.1007/s11095-010-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J., Yao Q., Liu D. Hydrodynamic cell delivery for simultaneous establishment of tumor growth in mouse lung, liver and kidney. Cancer Biol. Ther. 2011;12:737–741. doi: 10.4161/cbt.12.8.16442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wooddell C.I., Reppen T., Wolff J.A., Herweijer H. Sustained liver-specific transgene expression from the albumin promoter in mice following hydrodynamic plasmid DNA delivery. J. Gene Med. 2008;10:551–563. doi: 10.1002/jgm.1179. [DOI] [PubMed] [Google Scholar]
- 12.Hibbitt O.C., Harbottle R.P., Waddington S.N., Bursill C.A., Coutelle C., Channon K.M., Wade-Martins R. Delivery and long-term expression of a 135 kb LDLR genomic DNA locus in vivo by hydrodynamic tail vein injection. J. Gene Med. 2007;9:488–497. doi: 10.1002/jgm.1041. [DOI] [PubMed] [Google Scholar]
- 13.Anavi S., Hahn-Obercyger M., Margalit R., Madar Z., Tirosh O. A novel antihypoglycemic role of inducible nitric oxide synthase in liver inflammatory response induced by dietary cholesterol and endotoxemia. Antioxid. Redox Signal. 2013;19:1889–1901. doi: 10.1089/ars.2012.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rydz N., Swystun L.L., Notley C., Paterson A.D., Riches J.J., Sponagle K., Boonyawat B., Montgomery R.R., James P.D., Lillicrap D. The C-type lectin receptor CLEC4M binds, internalizes, and clears von Willebrand factor and contributes to the variation in plasma von Willebrand factor levels. Blood. 2013;121:5228–5237. doi: 10.1182/blood-2012-10-457507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H.C., Zhao H., Chen J., Wu W.L., Wang H.L., Jiao G.J., Chen Y.Z. Role of recombinant plasmid pEGFP-N1-IGF-1 transfection in alleviating osteoporosis in ovariectomized rats. J. Mol. Histol. 2013;44:535–544. doi: 10.1007/s10735-013-9498-3. [DOI] [PubMed] [Google Scholar]
- 16.Gao M., Zhang C., Ma Y., Bu L., Yan L., Liu D. Hydrodynamic delivery of mIL10 gene protects mice from high-fat diet-induced obesity and glucose intolerance. Mol. Ther. 2013;10:1852–1861. doi: 10.1038/mt.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Y., Liu D. Hydrodynamic delivery of adiponectin and adiponectin receptor 2 gene blocks high-fat diet-induced obesity and insulin resistance. Gene Ther. 2013;20:846–852. doi: 10.1038/gt.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang H., Wang Y., Li G., Zhang L., Zhang G.W., Liao Y.C., Hanawa H., Zou J. Effect of hydrodynamics-based delivery of IL-18BP fusion gene on rat experimental autoimmune myocarditis. Clin. Exp. Med. 2014;14:397–408. doi: 10.1007/s10238-013-0260-7. [DOI] [PubMed] [Google Scholar]
- 19.Fukushima M., Hattori Y., Tsukada H., Koga K., Kajiwara E., Kawano K., Kobayashi T., Kamata K., Maitani Y. Adiponectin gene therapy of streptozotocin-induced diabetic mice using hydrodynamic injection. J. Gene Med. 2007;11:976–985. doi: 10.1002/jgm.1104. [DOI] [PubMed] [Google Scholar]
- 20.Shigekawa M., Hikita H., Kodama T., Shimizu S., Li W., Uemura A., Miyagi T., Hosui A., Kanto T., Hiramatsu N., et al. Pancreatic STAT3 protects mice against caerulein-induced pancreatitis via PAP1 induction. Am. J. Pathol. 2012;181:2105–2113. doi: 10.1016/j.ajpath.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 21.Lu S.L., Tsai C.Y., Luo Y.H., Kuo C.F., Lin W.C., Chang Y.T., Wu J.J., Chuang W.J., Liu C.C., Chao L., et al. Kallistatin modulates immune cells and confers anti-inflammatory response to protect mice from group a streptococcal infection. Antimicrob. Agents Chemother. 2013;57:5366–5372. doi: 10.1128/AAC.00322-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen I.Y., Paulmurugan R., Nielsen C.H., Wang D.S., Chow V., Robbins R.C., Gambhir S.S. A titratable two-step transcriptional amplification strategy for targeted gene therapy based on ligand-induced intramolecular folding of a mutant human estrogen receptor. Mol. Imaging Biol. 2014;16:224–234. doi: 10.1007/s11307-013-0673-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G., Wooddell C.I., Hegge J.O., Griffin J.B., Huss T., Braun S., Wolff J.A. Functional efficacy of dystrophin expression from plasmids delivered to mdx mice by hydrodynamic limb vein injection. Hum. Gene Ther. 2010;21:221–237. doi: 10.1089/hum.2009.133. [DOI] [PubMed] [Google Scholar]
- 24.Watcharanurak K., Nishikawa M., Takahashi Y., Kabashima K., Takahashi R., Takakura Y. Regulation of immunological balance by sustained interferon-gamma gene transfer for acute phase of atopic dermatitis in mice. Gene Ther. 2013;20:538–544. doi: 10.1038/gt.2012.69. [DOI] [PubMed] [Google Scholar]
- 25.Cim A., Sawyer G.J., Zhang X., Su H., Collins L., Jones P., Antoniou M., Reynes J.-P., Lipps H.-J., Fabre J.W. In vivo studies on non-viral transdifferentiation of liver cells towards pancreatic beta cells. J Endocrinol. 2012;214:277–288. doi: 10.1530/JOE-12-0033. [DOI] [PubMed] [Google Scholar]
- 26.Tsai S.M., Wang W.P. Expression and function of fibroblast growth factor (FGF) 7 during liver regeneration. Cell. Physiol. Biochem. 2011;27:641–652. doi: 10.1159/000330073. [DOI] [PubMed] [Google Scholar]
- 27.Zhu C., Li Y., Li W., Wu Q., Gao R. Gene transfer of c-met confers protection against D-galactosamine/lipopolysaccharide-induced acute liver failure. Dig. Dis Sci. 2012;57:925–934. doi: 10.1007/s10620-012-2052-4. [DOI] [PubMed] [Google Scholar]
- 28.Shashidharamurthy R., Machiah D., Bozeman E.N., Srivatsan S., Patel J., Cho A., Jacob J., Selvaraj P. Hydrodynamic delivery of plasmid DNA encoding human FcgammaR-Ig dimers blocks immune-complex mediated inflammation in mice. Gene Ther. 2012;19:877–885. doi: 10.1038/gt.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bu X., Zhou Y., Zhang H., Qiu W., Chen L., Cao H., Fang L., Wen P., Tan R., Yang J. Systemic administration of naked plasmid encoding HGF attenuates puromycin aminonucleoside-induced damage of murine glomerular podocytes. Am. J. Physiol. Renal Physiol. 2011;301:F784–F792. doi: 10.1152/ajprenal.00210.2011. [DOI] [PubMed] [Google Scholar]
- 30.Thomson R., Molina-Portela P., Mott H., Carrington M., Raper J. Hydrodynamic gene delivery of baboon trypanosome lytic factor eliminates both animal and human-infective African trypanosomes. Proc. Natl Acad. Sci. USA. 2009;106:19509–19514. doi: 10.1073/pnas.0905669106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richard M., Arfi A., Seguin J., Gandolphe C., Scherman D. Widespread biochemical correction of murine mucopolysaccharidosis type VII pathology by liver hydrodynamic plasmid delivery. Gene Ther. 2009;16:746–756. doi: 10.1038/gt.2009.36. [DOI] [PubMed] [Google Scholar]
- 32.Lee S., Hong S.W., Choi H.S., Lee L.Y., Nam C., Rhee Y., Chung U., Lim S. Experimental parathyroid hormone gene therapy using OC31 integrase. Endocr. J. 2008;55:1033–1041. doi: 10.1507/endocrj.K08E-040. [DOI] [PubMed] [Google Scholar]
- 33.Okumura A., Saito T., Otani I., Kojima K., Yamada Y., Ishida-Okawara A., Nakazato K., Asano M., Kanayama K., Iwakura Y., et al. Suppressive role of leukocyte cell-derived chemotaxin 2 in mouse anti-type II collagen antibody-induced arthritis. Arthritis Rheum. 2008;58:413–421. doi: 10.1002/art.23215. [DOI] [PubMed] [Google Scholar]
- 34.Miao C.H., Ye X., Thompson A.R. High-level factor VIII gene expression in vivo achieved by nonviral liver-specific gene therapy vectors. Hum. Gene Ther. 2003;14:1297–1305. doi: 10.1089/104303403322319381. [DOI] [PubMed] [Google Scholar]
- 35.Wang C., Delogu S., Ho C., Lee S.A., Gui B., Jiang L., Ladu S., Cigliano A., Dombrowski F., Evert M., et al. Inactivation of Spry2 accelerates AKT-driven hepatocarcinogenesis via activation of MAPK and PKM2 pathways. J. Hepatol. 2012;57:577–583. doi: 10.1016/j.jhep.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochoa M.C., Fioravanti J., Duitman E.H., Medina-Echeverz J., Palazon A., Arina A., Dubrot J., Alfaro C., Morales-Kastresana A., Murillo O., et al. Liver gene transfer of interkeukin-15 constructs that become part of circulating high density lipoproteins for immunotherapy. PLoS ONE. 2012;12:e52370. doi: 10.1371/journal.pone.0052370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukumoto H., Takahashi Y., Ando M., Nishikawa M., Takakura Y. Expression profile-dependent improvement of insulin sensitivity by gene delivery of interleukin-6 in a mouse model of type II diabetes. Mol. Pharm. 2013;10:3812–3821. doi: 10.1021/mp400288e. [DOI] [PubMed] [Google Scholar]
- 38.Bulau A.M., Fink M., Maucksch C., Kappler R., Mayr D., Wagner K., Bufler P. In vivo expression of interleukin-37 reduces local and systemic inflammation in concanavalin A-induced hepatitis. Sci. World J. 2011;11:2480–2490. doi: 10.1100/2011/968479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zender L., Hutker S., Liedtke C., Tillmann H.L., Zender S., Mundt B., Waltemathe M., Gosling T., Flemming P., Malek N.P. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc. Natl Acad. Sci. USA. 2003;100:7797–7802. doi: 10.1073/pnas.1330920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang M., Sun R., Wei H., Tian Z. Simultaneous knockdown of multiple ligands of innate receptor NKG2D prevents natural killer cell-mediated fulminant hepatitis in mice. Hepatology. 2013;57:277–288. doi: 10.1002/hep.25959. [DOI] [PubMed] [Google Scholar]
- 41.Zhang G., Marshall A.L., Thomas A.L., Kernan K.A., Su Y., LeBoeuf R.C., Dong X.R., Tchao B.N. In vivo knockdown of nicotinic acetylcholine receptor alpha1 diminishes aortic atherosclerosis. Atherosclerosis. 2011;215:34–42. doi: 10.1016/j.atherosclerosis.2010.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L., Shen H., Li A., Zhang Z., Wang B., Wang J., Zheng X., Wu J., Yang D., Lu M., et al. Inhibition of hepatitis B virus (HBV) gene expression and replication by HBx gene silencing in a hydrodynamic injection mouse model with a new clone of HBV genotype B. Virol. J. 2014;10:214. doi: 10.1186/1743-422X-10-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen S.-W., Zhang X.-R., Wang C.-Z., Chen W.-Z., Xie W.-F., Chen Y.-X. RNA interference targeting the platelet-derived growth factor receptor beta subunit ameliorates experimental hepatic fibrosis in rats. Liver Int. 2008;28:1446–1457. doi: 10.1111/j.1478-3231.2008.01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCaffrey A.P., Meuse L., Pham T.T.-T., Conklin D.S., Hannon G.J., Kay M.A. Gene expression: RNA interference in adult mice. Nature. 2002;418:38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- 45.Tompkins S.M., Lo C.Y., Tumpey T.M., Epstein S.L. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc. Natl Acad. Sci. USA. 2004;101:8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokoi H., Mukoyama M., Nagae T., Mori K., Suganami T., Sawai K., Yoshioka T., Koshikawa M., Nishida T., Takigawa M., et al. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2004;15:1430–1440. doi: 10.1097/01.ASN.0000130565.69170.85. [DOI] [PubMed] [Google Scholar]
- 47.Cui F.D., Asada H., Kishida T., Itokawa Y., Nakaya T., Ueda Y., Yamagishi H., Goji S., Kita M., Imanishi J., et al. Intravascular naked DNA vaccine encoding glycoprotein B induces protective humoral and cellular immunity against herpes simplex virus type 1 infection in mice. Gene Ther. 2003;10:2059–2066. doi: 10.1038/sj.gt.3302114. [DOI] [PubMed] [Google Scholar]
- 48.Lu H.L., Liao F. Melanoma differentiation-associated gene 5 senses hepatitis B virus and activates innate immune signaling to suppress virus replication. J. Immunol. 2013;191:3264–3276. doi: 10.4049/jimmunol.1300512. [DOI] [PubMed] [Google Scholar]
- 49.Sun K., Yang W., Huang Y., Wang Y., Xiang L., Qi J. Leu452His Mutation in Lipoprotein Lipase Gene Transfer Associated with Hypertriglyceridemia in Mice in vivo. PLoS ONE. 2013;8:e75462. doi: 10.1371/journal.pone.0075462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bloom K., Ely A., Mussolino C., Cathomen T., Arbuthnot P. Inactivation of hepatitis B virus replication in cultured cells and in vivo with engineered transcription activator-like effector nucleases. Mol. Ther. 2013;21:1889–1897. doi: 10.1038/mt.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S.I., Shin D., Lee H., Ahn B.-Y., Yoon Y., Kim M. Targeted delivery of siRNA against hepatitis C virus by apolipoprotein A-I-bound cationic liposomes. J. Hepatol. 2009;50:479–488. doi: 10.1016/j.jhep.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 52.Chang J., Sigal L.J., Lerro A., Taylor J. Replication of the human hepatitis delta virus genome is initiated in mouse hepatocytes following intravenous injection of naked DNA or RNA sequences. J. Virol. 2001;75:3469–3473. doi: 10.1128/JVI.75.7.3469-3473.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang K.L., Hung K.C., Chang W.T., Li E.I.C. Establishment of an early liver fibrosis model by the hydrodynamics-based transfer of TGF-beta1 gene. Comp. Hepatol. 2007;6:9. doi: 10.1186/1476-5926-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tward A.D., Jones K.D., Yant S., Cheung S.T., Fan S.T., Chen X., Kay M.A., Wang R., Bishop J.M. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc. Natl Acad. Sci. USA. 2007;104:14771–14776. doi: 10.1073/pnas.0706578104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wesche-Soldato D.E., Lomas-Neira J., Perl M., Chung C.-S., Ayala A. Hydrodynamic delivery of siRNA in a mouse model of sepsis. Methods Mol. Biol. 2008;442:67–73. doi: 10.1007/978-1-59745-191-8_5. [DOI] [PubMed] [Google Scholar]
- 56.Kamimura K., Suda T., Xu W., Zhang G., Liu D. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol. Ther. 2009;17:491–499. doi: 10.1038/mt.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamimura K., Guisheng Z., Liu D. Image-guided, intravascular hydrodynamic gene delivery to skeletal muscle in pigs. Mol. Ther. 2010;18:93–100. doi: 10.1038/mt.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamimura K., Suda T., Zhang G., Aoyagi Y., Liu D. Parameters Affecting Image-guided, Hydrodynamic Gene Delivery to Swine Liver. Mol. Ther. Nucleic Acids. 2013;2:e128. doi: 10.1038/mtna.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamimura K., Kanefuji T., Yokoo T., Abe H., Suda T., Kobayashi Y., Zhang G., Aoyagi Y., Liu D. Safety assessment of liver-targeted hydrodynamic gene delivery in dogs. PLoS ONE. 2014;24:e107203. doi: 10.1371/journal.pone.0107203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eastman S.J., Baskin K.M., Hodges B.L., Chu Q., Gates A., Dreusicke R., Anderson S., Scheule R.K. Development of catheter-based procedures for transducing the isolated rabbit liver with plasmid DNA. Hum. Gene Ther. 2002;13:2065–2077. doi: 10.1089/10430340260395910. [DOI] [PubMed] [Google Scholar]
- 61.Yoshino H., Hashizume K., Kobayashi E. Naked plasmid DNA transfer to the porcine liver using rapid injection with large volume. Gene Ther. 2006;13:1696–1702. doi: 10.1038/sj.gt.3302833. [DOI] [PubMed] [Google Scholar]
- 62.Alino S.F., Herrero M.J., Noguera I., Dasi F., Sanchez M. Pig liver gene therapy by noninvasive interventionist catheterism. Gene Ther. 2007;14:334–343. doi: 10.1038/sj.gt.3302873. [DOI] [PubMed] [Google Scholar]
- 63.Fabre J.W., Grehan A., Whitehorne M., Sawyer G.J., Dong X., Salehi S., Eckley L., Zhang X., Seddon M., Shah A.M. Hydrodynamic gene delivery to the pig liver via an isolated segment of the inferior vena cava. Gene Ther. 2008;15:452–462. doi: 10.1038/sj.gt.3303079. [DOI] [PubMed] [Google Scholar]
- 64.Kamimura K., Suda T., Liu D. Advances and Challenges in the Delivery of Oligonucleotide-Based Therapies. Future Science; London, UK: 2015. Hydrodynamic gene delivery. In Press. [Google Scholar]
- 65.Suda T., Suda K., Liu D. Computer-assisted hydrodynamic gene delivery. Mol. Ther. 2008;16:1098–1104. doi: 10.1038/mt.2008.66. [DOI] [PubMed] [Google Scholar]
- 66.Yokoo T., Kamimura K., Suda T., Kanefuji T., Oda M., Zhang G., Liu D., Aoyagi Y. Novel electric power-driven hydrodynamic injection system for gene delivery: safety and efficacy of human factor IX delivery in rats. Gene Ther. 2013;20:816–823. doi: 10.1038/gt.2013.2. [DOI] [PubMed] [Google Scholar]
- 67.Ginn S.L., Alexander I.E., Edelstein M.L., Abedi M.R., Wixon J. Gene therapy clinical trials worldwide to 2012 - an update. J. Gene Med. 2013;15:65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 68.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 69.Check E. Second cancer case halts gene-therapy trials. Nature. 2003;421:305. doi: 10.1038/421305a. [DOI] [PubMed] [Google Scholar]