Abstract
Pituicytoma is an extremely rare neoplasm derived from pituicytes, which are glial cells in the posterior lobe of the pituitary gland. A malignant pituicytoma was found in the intracranial cavity of a 55-week-old male Sprague-Dawley rat. Macroscopically, the tumor was located on the sphenoid bone and involved the pituitary gland. The tumor was composed of sheets of fusiform cells with spindle- or pleomorphic-shaped nuclei and abundant eosinophilic cytoplasms. The cells were arranged in a whirling or irregular growth pattern. Some tumor cells were bizarre multinucleated giant cells with cytoplasmic eosinophilic hyaline droplets. Many tumor cells were strongly positive for vimentin and glial fibrillary acidic protein, and some cells were positive for ED-1 and S-100. These findings closely resembled those of a giant cell glioblastoma derived from the pituitary gland, suggesting anaplastic pituicytoma. From our review of the literature, we believe this is the first report of a spontaneous malignant pituicytoma in a rodent.
Keywords: glioblastoma, immunohistochemistry, giant cells, pituicytoma, pituitary gland, rats
The incidence of spontaneous intracranial tumors is very low in rats, with the exception of pituitary neoplasms1, 2. Spontaneous tumors of the pituitary gland are one of the most common neoplasms in aging Sprague-Dawley (SD) rats, with most pituitary tumors arising from the pars distalis. The histological and immunohistochemical characteristics of pars distalis tumors have been well described in rats3, and a few reports of tumors arising from the pars intermedia in rodents have been published4. However, there are only a few papers describing the morphology of tumors arising from the posterior lobe, because these tumors are extremely rare5, 6. Intrinsic tumors of the posterior lobe include the pituicytoma, craniopharyngioma and ganglioneuroma6,7,8. In humans, the pituicytoma is a well-differentiated neoplasm classified as a World Health Organization (WHO) grade I tumor9. It is defined as a solid, circumscribed, low-grade spindle cell astrocytic tumor in adults that originates in the posterior pituitary or its stalk10. In rodents, only a few cases of pituicytoma have been reported in older rats6, 11. In the present report, we describe a spontaneously occurring giant cell glioblastoma (malignant astrocytoma, high-grade) derived from the pituitary gland of an older SD rat that was suggestive of malignant pituicytoma. This lesion was investigated by histopathological and immunohistochemical techniques to establish its cellular composition.
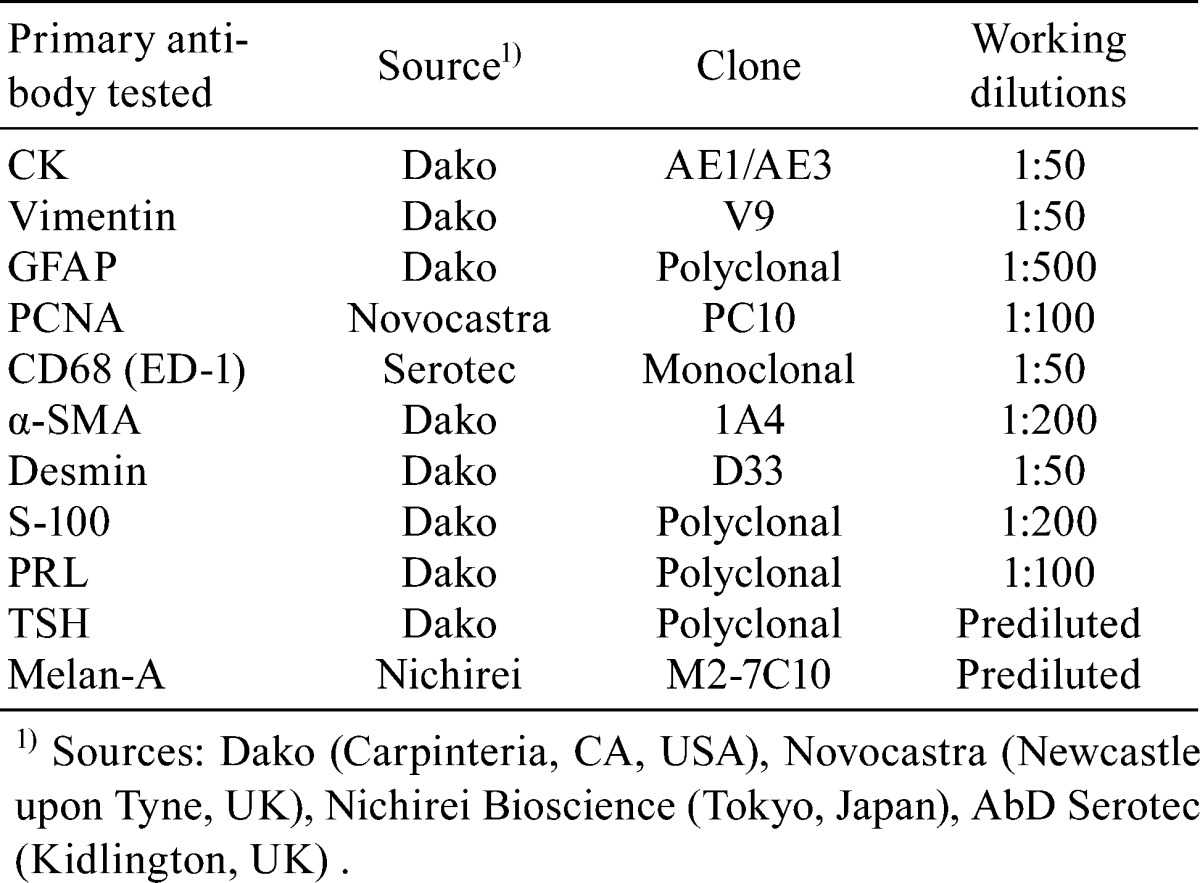
The present case was a 55-week-old male Crl:CD(SD) rat. The rat was one of 50 untreated male rats that were examined for investigation of historical control data. These animals were purchased from Charles River Laboratories Japan, Inc. (Kanagawa, Japan), at 10 weeks of age and were housed in plastic cages (one rat per cage) in a room maintained at 23 ± 3°C and 30 to 70% humidity with 12 h light and dark cycles in a research laboratory at Maruho Co., Ltd. The rats had free access to a CRF-1 pellet diet (Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water. The animal in question was routinely monitored for clinical signs twice a week and was weighed once a week before sacrifice. It was anesthetized with isoflurane (Escain®; Mylan Inc., Tokyo, Japan) and sacrificed by abdominal aortic transection. A complete necropsy was conducted, and over 40 organs, including an intracranial mass detected during the necropsy, were fixed in 10% neutral buffered formalin. The tumor mass along with the sphenoid bone was decalcified in 5% ethylenediaminetetraacetate, embedded in paraffin, sectioned into 3 μm slices and stained with hematoxylin and eosin (H&E). Periodic acid-Schiff (PAS), Masson’s trichrome (MT) and phosphotungstic acid hematoxylin (PTAH) were also applied for characterizing an eosinophilic hyaline droplet observed in the tumor. Sequential sections from the mass were labeled with antibodies for pancytokeratin (CK) as an epithelial cell marker, vimentin as a mesenchymal cell marker, glial fibrillary acidic protein (GFAP) as a glial cell marker, proliferating cell nuclear antigen (PCNA) as a proliferating cell marker, CD68 (ED-1) as a macrophage marker, smooth muscle actin (SMA) and desmin as muscle cell markers, S-100 as a Schwann cell and adipocyte marker, prolactin (PRL) and thyroid stimulating hormone (TSH) as markers of hormone-producing cells in the anterior pituitary lobe and melan-A as a melanocyte marker. All antibodies are listed in Table 1. Immunohistochemical staining was carried out using the labeled streptavidin-biotin method. Sections were deparaffinized, hydrated and blocked for endogenous peroxidases. Heat-induced antigen retrieval was performed for CK, vimentin, PCNA, GFAP and melan-A. Trypsin treatment was performed for ED-1. A streptavidin-biotin staining kit (LSAB; Dako, Carpinteria, CA, USA) was used according to the manufacturer’s instructions to identify the antigen-antibody complexes. The reaction products were visualized with 3-3′-diaminobenzidine tetrahydrochloride. The Ethics Committee for Animal Experiments of Maruho Co., Ltd., approved the experimental protocol and all animal procedures, and all procedures were in accordance with our guidelines for animal experimentation and those of the Japanese Association for Laboratory Animal Science.
Table 1. Antibodies Used in This Study.
The animal grew normally until 51 weeks of age, at which point its body weight was 651 g; it then wasted to 553 g at 55 weeks of age. The animal displayed clinical abnormalities such as lacrimation, bradypnea and decreased spontaneous motility. On gross examination, a gray-white mass 10×10×15 mm in size was observed on the sphenoid bone that involved the pituitary gland and trigeminal nerve (Fig. 1a). The mass was easily separated from the brain but was not removed from the sphenoid bone, and the base of the brain had a smooth surface. The majority of the other organs and tissues were macroscopically normal, though the intracranial mass did cause compression and deformation of the bottom of the cerebrum (Fig. 1b).
Fig. 1.
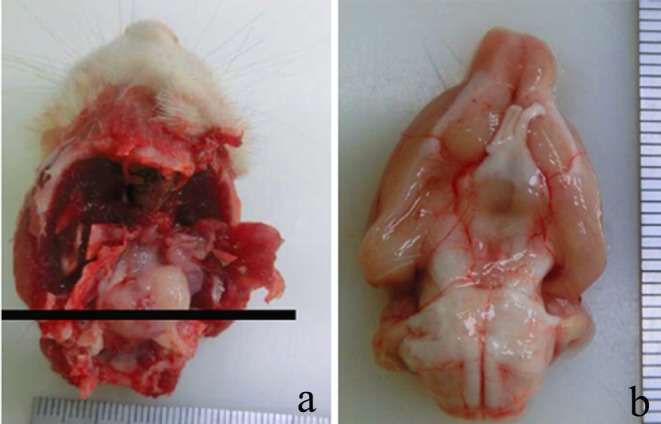
(a) A gray-white mass was located on the sphenoid bone and involved the pituitary gland and trigeminal nerve. The solid line indicates the plane of section. (b) The bottom of the cerebrum was compressed and deformed by the intracranial mass. Scale: mm.
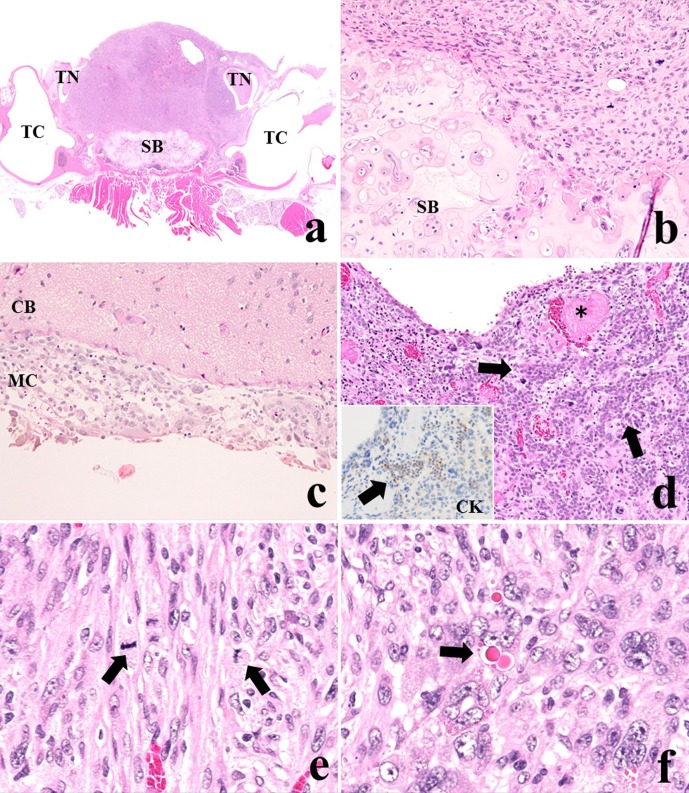
Careful histopathological observation revealed that the tumor cells had partially invaded the sphenoid bone and the meninx of the cerebrum but did not invade the trigeminal nerves (Fig. 2a–c) or metastasize to any other organs. Small nests of native anterior cells were sporadically seen in the tumor with congestion and thrombosis (Fig. 2d) and were positive for CK (inserted illustration). The tumor was composed of sheets of fusiform cells with spindle- or pleomorphic-shaped nuclei and abundant eosinophilic cytoplasms (Fig. 2e). Many mitotic figures were visible in the tumor cells. The tumor cells were arranged in whirling and storiform patterns or in an irregular growth pattern. None of the tumor cells were arranged in parallel arrays with nuclear palisades, formed pseudo-glandular structures or were pseudopalisading around a necrotic area. Bizarre multinucleated giant cells were frequently observed within the tumor; some of these cells included eosinophilic hyaline droplets (EHDs) in their cytoplasms (Fig. 2f). EHDs were brightly eosinophilic, variably sized round inclusions. These cells were diffusely distributed in the tumor tissue. EHDs were positive for PAS, stained deep purple with PTAH and brightly red after MT staining (Fig. 3), similarly to EHDs reported in human glioblastoma12.
Fig. 2.
(a) The mass almost replaced the pituitary gland with invasion to the sphenoid bone (SB) but did not infiltrate the trigeminal nerves (TN) and tympanic cavities (TC) (×12.5). (b) Tumor cells invaded the sphenoid bone. (c) Tumor cells invaded the meninx of the cerebrum (MC) but not the parenchyma of the cerebrum (CB). (d) Small nests of native anterior cells were observed in the tumor (arrows) with congestion and thrombosis (*) (×100). Native anterior tissues were positive for CK (×200, inserted illustration). (e) The tumor was composed of sheets of fusiform cells with spindle- or pleomorphic-shaped nuclei and abundant eosinophilic cytoplasms. Many mitotic figures were seen in the tumor cells (arrows) (×400). (f) Bizarre multinucleated giant cells with eosinophilic hyaline droplets were observed (arrow) (×400). H&E staining.
Fig. 3.
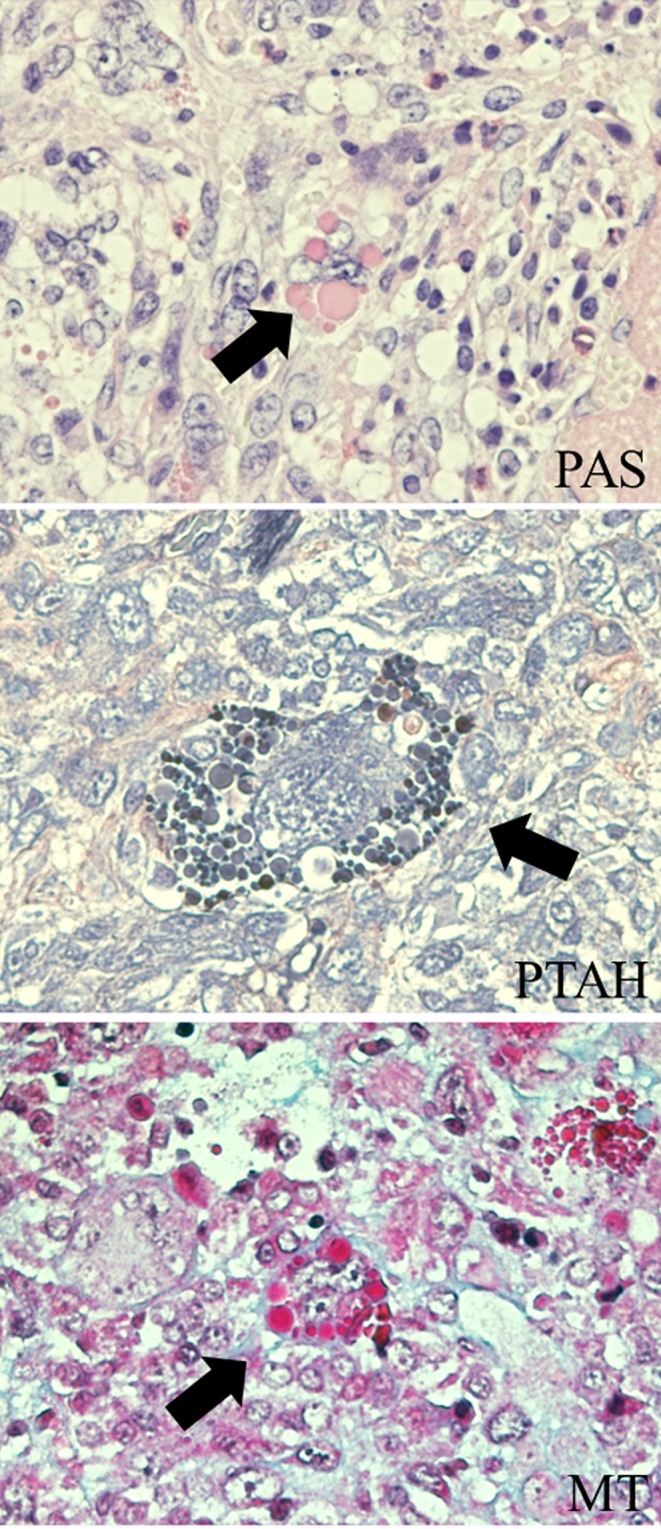
Special staining for multinucleated giant cells with EHDs. EHDs were positive for PAS, deep purple after PTAH and brightly red after MT staining (arrows).
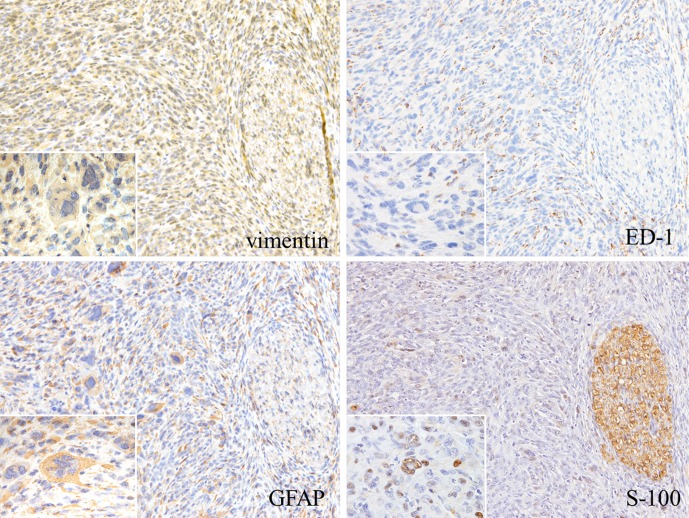
The results of the immunohistochemistry staining are summarized in Table 2. The tumor cell cytoplasms were strongly positive for vimentin and GFAP. Some tumor cell nuclei were weakly positive for S-100 (Fig. 4). These results indicated that the tumor cells were astrocytic in origin. Weak positivity for ED-1 was observed in the cytoplasms of some tumor cells, most likely indicating phagocytic activity in the tumor cells. The multinucleated giant cells showed reactivity similar to the mononuclear tumor cells, except for their reaction to ED-1 (Fig. 4). All types of tumor cells, including the giant cells, were strongly positive for PCNA, indicating high proliferative activity. No immunoreactivity was observed for α-SMA, desmin, PRL, TSH or melan-A in any of the tumor cell types. The native anterior cells were positive for CK and PRL (data not shown).
Table 2. Results of Immunohistochemical Staining.
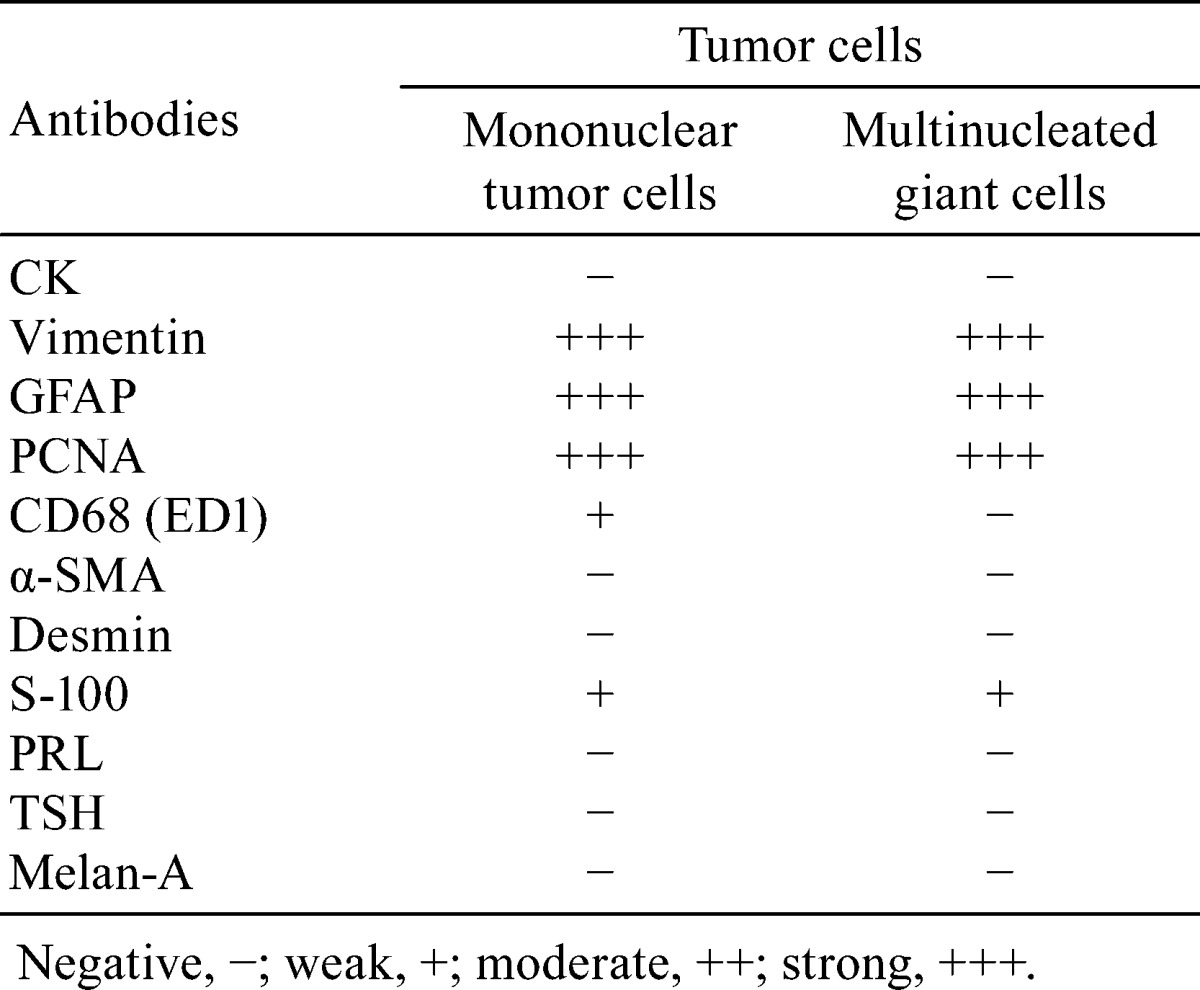
Fig. 4.
Immunohistochemical staining of tumor cells. The cytoplasms of the tumor cells were strongly immunoreactive for vimentin and GFAP. Some tumor cell nuclei were positive for S-100, and the existing nerve tissue was strongly positive for S-100. The cytoplasm of some tumor cells was immunoreactive for ED-1(×100). Many multinucleated giant cells (inserts) were also positive for vimentin and GFAP, and some of them had nuclei that were weakly positive for S-100. However, no cells were positive for ED-1 (arrows) (×400, enlarged illustration).
Our case was characterized by sheets of fusiform cells with spindle- or pleomorphic-shaped nuclei and abundant eosinophilic cytoplasms, bizarre multinucleated giant cells with EHDs, invasion into neighboring tissues, high proliferative activity and strong positivity for vimentin and GFAP. The tumor was composed of GFAP-positive cells, suggesting an astrocytic origin. Astrocytic tumors in rodents are classified as low-grade (well-differentiated), medium-grade (anaplastic) and high-grade (glioblastoma or glioblastoma multiforme) tumors according to the International Harmonization of Nomenclature and Diagnostic Criteria (INHAND) and the WHO International Histological Classification of Tumors of the Nervous System of Domestic Animals13. Our findings suggest that this tumor was a high-grade astrocytoma (glioblastoma multiforme). Giant cell glioblastoma, a variant of glioblastoma multiforme, is a rare neoplasm characterized by predominance of bizarre multinucleated giant cells with abundant eosinophilic cytoplasms and GFAP positivity in humans9, 14, 15. The EHDs reported in human glioblastoma are positive for PAS, PTAH, and MT staining12, similar to our results. The EHDs in glioblastoma are defined as lysosomal structures by ultrastructural studies12. Additionally, astrocytic tumors are positive for S-100 and ED-116, similar to the results of our case.
The pituitary gland, especially the posterior lobe, is composed of numerous capillaries that are supported by modified glial cells known as pituicytes17. Pituicytes are elongated uni- or bipolar cells that display prolongation of the cytoplasm into one or more processes; these cells are also strongly positive for GFAP18. The tumor in the current study was located on the sphenoid bone and involved the pituitary gland. The tumor cells invaded the sphenoid bone and the meninx of the cerebrum without cellular transition to the brain parenchyma or trigeminal nerve. These findings suggest that the present case was a malignant astrocytic tumor that originated from the pituitary gland. Based on our findings, this case may have originated from pituicytes in the posterior lobe of the pituitary gland, although the primary development of this case was unclear. Craniopharyngiomas are also intrinsic tumors of the posterior lobe; they originate from remnants of Rathke’s pouch and consist of tubular or glandular structures8. However, our case had no tubular or glandular structures and no positivity for CK.
In general, a pituicytoma is defined as a low-grade spindle cell astrocytic tumor in adults that originates in the posterior pituitary or its stalk10. In previous cases in rats6, cystic or pseudoglandular structures and pseudopalisading of tumor cells around a vein (perivascular arrangement) were observed, and mitotic figures were sporadically detected (Table 3). In contrast, our case did not show a specific morphological pattern like that above but instead had many mitotic figures and bizarre multinucleated giant cells.
Table 3. Comparison between Previous Cases and Our Case.
In conclusion, morphological and immunohistochemical findings for our case closely resembled those of a giant cell glioblastoma, suggesting anaplastic pituicytoma. From our review of the literature, we believe this to be the first report of a spontaneous malignant pituicytoma in a rodent.
Acknowledgments
The authors would like to thank Drs. Susan Elmore of the Cellular and Molecular Pathology Branch, National Institutes of Environmental Health Sciences, National Toxicology Program, USA, and Dr. James P. Morrison of Charles River Laboratories, Pathology Associates, USA, for their review of this case and the members of the Kansai Conference of Toxicologic Pathology, Japan, for critical discussion.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors have no conflicts of interest in connection with this paper.
References
- 1.Yoshizawa K, Oishi Y, Makino N, Suzuki J, Matsumoto M, Yamauchi K, Fujihira S, and Fujii T. Malignant schwannoma of the intracranial trigeminal nerve in a 19-week-old female Sprague-Dawley rat. J Toxicol Pathol. 9: 107–112. 1996. [Google Scholar]
- 2.Nakazawa M, Tawaratani T, Uchimoto H, Kawaminami A, Ueda M, Ueda A, Shinoda Y, Iwakura K, Kura K, and Sumi N. Spontaneous neoplastic lesions in aged Sprague-Dawley rats. Exp Anim. 50: 99–103. 2001. [DOI] [PubMed] [Google Scholar]
- 3.Sandusky GE, Van Pelt CS, Todd GC, and Wightman K. An immunocytochemical study of pituitary adenomas and focal hyperplasia in old Sprague-Dawley and Fischer 344 rats. Toxicol Pathol. 16: 376–380. 1988. [DOI] [PubMed] [Google Scholar]
- 4.Oishi Y, Matsumoto M, Yoshizawa K, Fujihira S, Tsubura A, and Morii S. Spontaneous pituitary adenoma of the pars intermedia in mice and rats: histopathological and immunocytochemical studies. J Toxicol Pathol. 5: 223–231. 1992. [Google Scholar]
- 5.Pituicytoma (B) Pituitary gland. From the goRENI website: http://www.goreni.org.
- 6.Satoh H, Iwata H, Furuhama K, and Enomoto M. Pituicytoma: primary astrocytic tumor of the pars nervosa in aging Fischer 344 rats. Toxicol Pathol. 28: 836–838. 2000. [DOI] [PubMed] [Google Scholar]
- 7.Sundeep C, Mark JH, and Richard P. Endocrine glands. In: Toxicologic Pathology, Nonclinical Safety Assessment. SS Pritam, AP James, FH Jerry, and G Chirukandath (eds). CRC Press, Florida. 655–716. 2013. [Google Scholar]
- 8.MacKenzie WF, and Boorman GA. Pituitary gland. In: Pathology of the Fischer Rat: Reference and Atlas. GA Boorman, SL Eustis, MR Elwell, CA Montgomery Jr., and WF MacKenzie (eds). Academic Press, San Diego. 485–500. 1990. [Google Scholar]
- 9.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, and Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 114: 97–109. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brat DJ, Scheithauer BW, Fuller GN, and Tihan T. Newly codified glial neoplasms of the 2007 WHO Classification of Tumours of the Central Nervous System: angiocentric glioma, pilomyxoid astrocytoma and pituicytoma. Brain Pathol. 17: 319–324. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlton WW, and Gries CL. Pituicytoma, neurohypophysis, rat. In: Monographs on Pathology of Laboratory Animal, Endocrine System, 2nd ed. TC Jones, CC Capen, U Mohe (eds). Springer-Varlag, Berlin Heidelberg. 94–97. 1996. [Google Scholar]
- 12.Sasaki A, Yoshida T, Kurihara H, Sasaki T, and Nakazato Y. Glioblastoma with large numbers of eosinophilic hyaline droplets in neoplastic astrocytes. Clin Neuropathol. 20: 156–162. 2001. [PubMed] [Google Scholar]
- 13.Kaufmann W, Bolon B, Bradley A, Butt M, Czasch S, Garman RH, George C, Gröters S, Krinke G, Little P, McKay J, Narama I, Rao D, Shibutani M, and Sills R. Proliferative and nonproliferative lesions of the rat and mouse central and peripheral nervous systems. Toxicol Pathol. 40(Suppl): 87S–157S. 2012. [DOI] [PubMed] [Google Scholar]
- 14.Kozak KR, and Moody JS. Giant cell glioblastoma: a glioblastoma subtype with distinct epidemiology and superior prognosis. Neuro-oncol. 11: 833–841. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleihues P, Burger PC, Aldape KD, Brat DJ, Biernat W, and Bigner DD. Glioblastoma. In: WHO Classification of Tumours of the Central Nervous System, 4th ed. DN Louis, H Ohgaki, OD Wiestler, WK Cavenee (eds). IARC Press, Lyon. 33–49. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber K, Garman RH, Germann PG, Hardisty JF, Krinke G, Millar P, and Pardo ID. Classification of neural tumors in laboratory rodents, emphasizing the rat. Toxicol Pathol. 39: 129–151. 2011. [DOI] [PubMed] [Google Scholar]
- 17.Charles CC. The pituitary. In: Endocrine System. TC Jones, U Mohr, and RD Hunt (eds). Spronger-Verlag, New York. 106–107. 1983. [Google Scholar]
- 18.Kwon MJ, and Suh YL. Pituicytoma with unusual histological features. Pathol Int. 61: 598–602. 2011. [DOI] [PubMed] [Google Scholar]