Abstract
Iron-deficient individuals experience a loss of appetite that can be restored with iron supplementation. It has been proposed that iron influences the satiety hormone leptin; however, a direct link between iron and leptin has remained elusive. In this issue of the JCI, Gao and colleagues demonstrate an inverse relationship between adipocyte iron and leptin that is mediated by iron-dependent activation of cAMP-responsive element binding protein (CREB), the transcription factor that represses leptin transcription. Together, the results of this study provide a mechanistic connection between dietary iron and the appetite-regulating hormone leptin.
Looking for the link between iron and appetite
Iron is an essential metal that is required for many metabolic processes; therefore, it is reasonable to hypothesize that iron acquisition and overall nutrition are coordinated. A previous study identified a link between iron deficiency and decreased appetite in primary school-aged Kenyan children and demonstrated that iron supplementation improved both growth and appetite (1); however, the mechanism by which iron mediates this effect is poorly understood. It has been proposed that decreased levels of the hormone leptin, which regulates food intake, might account for increased appetite during treatment for iron deficiency. In a small study of young children, Topaloglu and colleagues found no association between plasma leptin levels and appetite during iron therapy, although this study did confirm a direct correlation between food intake and serum levels of the iron-storing protein ferritin (2). Moreover, leptin levels only correlated with BMI, leading Topaloglu et al. to conclude that the iron supplementation–associated increase in appetite was leptin independent. A later report proposed that alterations in the levels of other hormones might be related to diminished appetite observed in iron-deficient individuals (3).
In this issue, Gao and colleagues extend the search to identify the link between iron levels and appetite (4). While the link between leptin and iron seemed tenuous, Gao et al. reasoned that leptin might indeed be involved in mediating iron-associated effects on appetite. Evaluation of a cohort of patients with serum ferritin levels within the normal range revealed an inverse correlation between serum ferritin, which serves as a surrogate indicator of body iron stores, and serum levels of leptin. Serum iron levels were also inversely related to serum leptin levels in a cohort with type 2 diabetes or metabolic syndrome. However, there was no association between ferritin and BMI. While it is not clear why the results of Topaloglu and colleagues differ from the observations of Gao et al., there are several possibilities. For example, the effect of iron levels on leptin could have been missed in the earlier study because a smaller number of subjects were examined (2, 4). Additionally, these two studies focused on different populations, and iron-deficient infants and children may respond differently than adults.
CREB provides a connection
Gao and colleagues followed up on their observation that ferritin and leptin were associated with studies in murine models and cultured cells. In animals fed a high-iron diet, both leptin mRNA and serum leptin levels were decreased. The high-iron diet resulted in a two-fold increase in hepatic iron levels and more than a three-fold increase in adipocyte iron. Moreover, adipocyte-specific deletion of the iron exporter ferroportin, which resulted in iron loading in adipocytes, reduced serum leptin levels. Conversely, in a murine model of hereditary hemochromatosis, adipocyte iron levels were decreased and leptin levels were increased. The same inverse correlation between adipocyte iron status and leptin mRNA expression was also observed in an adipocyte cell line.
Gao and colleagues determined that increased cellular iron causes an increase in phosphorylation of cAMP-responsive element binding protein (CREB) that is independent of the canonical CREB activators cyclic AMP and calcium. CREB phosphorylation was associated with inhibition of leptin transcription via CREB binding at sites that were detected in the putative leptin promoter (Figure 1). Moreover, expression of a dominant-negative CREB protein in cultured adipocytes rendered the leptin promoter insensitive to iron status.
Figure 1. The transcription factor CREB connects iron status to leptin expression in adipocytes.
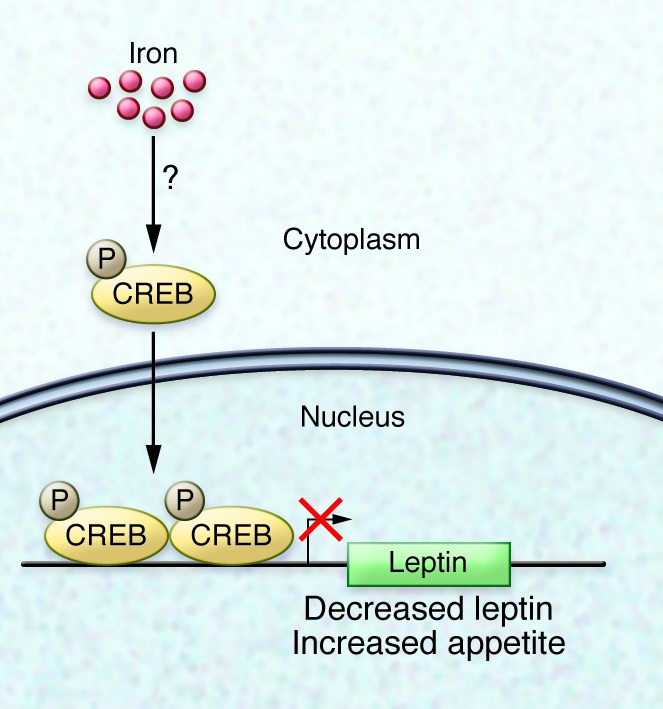
Elevation of intracellular iron in adipocytes results in cAMP- and calcium-independent phosphorylation of CREB. Phosphorylated CREB binds 2 sites within the putative leptin promoter and inhibits transcription. Decreased leptin, in turn, increases appetite.
Remaining questions
The work by Gao et al. raises several interesting questions. First, while mice on a high-iron diet were shown to have lower leptin levels and eat more chow than animals fed standard chow, the body weights of iron-fed mice were not increased. Additionally, measurements of fat and lean body mass in high-iron diet–fed mice were indistinguishable from animals on normal chow. Similarly, mice with increased adipocyte iron due to adipocyte-specific inactivation of ferroportin showed a leptin-associated change only in eating behavior, but not in other metrics of body size and composition. Together, these observations fit with the lack of association between ferritin and BMI described in human subjects. However, it is not clear why increased food consumption does not translate into measurable differences in body parameters.
Second, the link between iron and CREB phosphorylation is still unknown. Others have reported that decreased intracellular iron is associated with phosphorylation or activation of CREB in a variety of cell types, possibly related to decreased reactive oxygen species (5–7), but the precise molecular details remain obscure. There is abundant evidence that cellular iron status not only influences gene expression posttranscriptionally through interactions with iron regulatory elements in noncoding regions of a limited set of mRNAs, but iron deficiency also causes increased transcription of some genes that are important in iron homeostasis (8). The mechanism for transcriptional induction in response to iron deficiency has been elusive, and CREB phosphorylation might be part of the answer. More work will need to be done to fully sort this relationship out.
Third, CREB has many targets, and it is likely that there are other downstream effects of iron-related changes in CREB activity. Iron-dependent regulation of CREB function has potential to be particularly important in cells that experience a wide physiological range in iron-storage capacity, such as intestinal epithelial cells, tissue macrophages, and hepatocytes. The broader implications of such an effect remain to be elucidated.
While there is substantial literature associating iron overload with diabetes and insulin resistance, the molecular mechanisms are incompletely understood. Clearly, the interaction between iron homeostasis and systemic energy metabolism is complex, and the report by Gao et al. contributes one additional piece to the puzzle.
Acknowledgments
N.C. Andrews is supported by NIH grant R01 DK089705.
Footnotes
Conflict of interest: The author is a member of the Board of Directors of Novartis AG.
Reference information:J Clin Invest. 2015;125(9):3422–3423. doi:10.1172/JCI83193.
See the related article beginning on page 3681.
References
- 1.Lawless JW, Latham MC, Stephenson LS, Kinoti SN, Pertet AM. Iron supplementation improves appetite and growth in anemic Kenyan primary school children. J Nutr. 1994;124(5):645–654. doi: 10.1093/jn/124.5.645. [DOI] [PubMed] [Google Scholar]
- 2.Topaloglu AK, Hallioglu O, Canim A, Duzovali O, Yilgor E. Lack of association between plasma leptin levels and appetite in children with iron deficiency. Nutrition. 2001;17(7–8):657–659. doi: 10.1016/s0899-9007(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 3.Isguven P, Arslanoglu I, Erol M, Yildiz M, Adal E, Erguven M. Serum levels of ghrelin, leptin, IGF-I, IGFBP-3, insulin, thyroid hormones and cortisol in prepubertal children with iron deficiency. Endocr J. 2007;54(6):985–990. doi: 10.1507/endocrj.K07-031. [DOI] [PubMed] [Google Scholar]
- 4.Gao Y, et al. Adipocyte iron regulates leptin and food intake. J Clin Invest. 2015;125(9):3681–3691. doi: 10.1172/JCI81860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Ye S, Fujiwara T, Manolagas SC, Zhao H. Steap4 plays a critical role in osteoclastogenesis in vitro by regulating cellular iron/reactive oxygen species (ROS) levels and cAMP response element-binding protein (CREB) activation. J Biol Chem. 2013;288(42):30064–30074. doi: 10.1074/jbc.M113.478750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaman K, et al. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J Neurosci. 1999;19(22):9821–9830. doi: 10.1523/JNEUROSCI.19-22-09821.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi EY, et al. Transcriptional regulation of IL-8 by iron chelator in human epithelial cells is independent from NF-κB but involves ERK1/2- and p38 kinase-dependent activation of AP-1. J Cell Biochem. 2007;102(6):1442–1457. doi: 10.1002/jcb.21367. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, et al. Systemic regulation of Hephaestin and Ireg1 revealed in studies of genetic and nutritional iron deficiency. Blood. 2003;102(5):1893–1899. doi: 10.1182/blood-2003-02-0347. [DOI] [PubMed] [Google Scholar]


