Abstract
PI3Kδ inhibitors such as idelalisib are providing improved therapeutic options for the treatment of chronic lymphocytic leukaemia (CLL). However under certain conditions, inhibition of a single PI3K isoform can be compensated by the other PI3K isoforms, therefore PI3K inhibitors which target multiple PI3K isoforms may provide greater efficacy. The development of compounds targeting multiple PI3K isoforms (α, β, δ, and γ) in CLL cells, in vitro, resulted in sustained inhibition of BCR signalling but with enhanced cytotoxicity and the potential for improve clinical responses. This review summarises the progress of PI3K inhibitor development and describes the rationale and potential for targeting multiple PI3K isoforms.
Keywords: CLL, PI3K, Idelalisib, Duvelisib
Highlights
-
•
Inhibition of PI3K may still be effective in CLL patients resistant to ibrutinib.
-
•
Functional redundancy between PI3K isoforms may be a mechanism of drug resistance.
-
•
Targeting multiple PI3K isoforms can increase cytotoxicity against CLL cells.
1. Introduction
B cell receptor (BCR) activation and subsequent downstream signalling is pivotal for maintenance and proliferation of chronic lymphocytic leukaemia (CLL) leading to tumour progression [1]. Therefore targeting the BCR and associated pathways is attractive for CLL and other BCR driven B cell malignancies. These BCR signals are mediated via a series of key kinases including SYK, BTK and PI3K, and inhibitors such as entospletinib (SYK inhibitor), ibrutinib (BTK inhibitor) and idelalisib (PI3Kδ inhibitor) are showing clinical efficacy, and are likely to replace standard chemotherapy regimens for the treatment of CLL. So far, with limited follow up, ibrutinib and idelalisib have shown clear efficacy in suppressing tumour progression but have not been curative. A minority of treated patients who go on to develop resistance to ibrutinib have extremely poor outcomes with a median survival of 3.1 months after discontinuation [2]. However once resistance to ibrutinib occurs, PI3K inhibitors may still be therapeutically effective [3]. This review will therefore focus on PI3K inhibitors in CLL.
PI3K, via phosphorylation of the inositol lipid phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), forms the second messenger molecule phosphatidylinositol (3,4,5)-trisphosphate (PI(3,4,5)P3) which recruits and activates pleckstrin homology domain containing proteins, leading to downstream signalling events crucial for proliferation, survival and migration. Class I PI3K enzymes consist of four distinct catalytic isoforms, PI3Kα, PI3Kβ, PI3Kδ and PI3Kγ. The PI3Kδ and PI3Kγ isoforms are expressed predominantly in leucocytes, whereas the PI3Kα and PI3Kβ isoforms are ubiquitously expressed [4].
PI3K becomes activated upon ligation of a number of chemokine and cytokine receptors expressed by CLL cells and following BCR ligation [5,6]. PI3K mediated signalling is known to be constitutively activated in CLL [6] and patients with more progressive disease [IGHV unmutated (U-CLL)] show significantly greater PI3K expression compared to less progressive disease [IGHV mutated (M-CLL)] [7].
2. Pharmacological inhibition of PI3Kδ in CLL
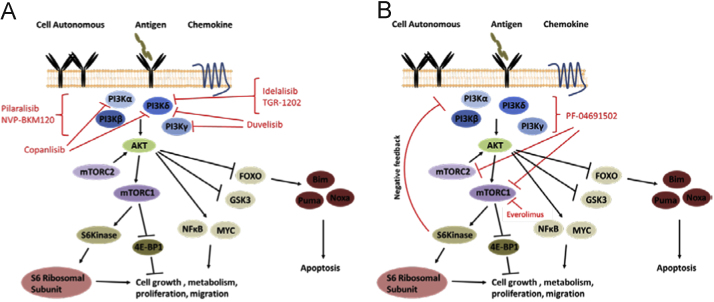
The crucial role of PI3Kδ in normal B cell biology was identified using genetic and pharmacological studies [4] and its haematopoietic restricted expression has made it an attractive target for therapeutic intervention in haematological malignancies (Fig. 1A).
Fig. 1.
Schematic representation of the PI3K/mTOR signalling pathway with pharmacological agents in pre-clinical/clinical development for CLL indicated. (A) PI3K activation by receptor ligation induces re-localisation and activation of AKT (amongst other proteins not shown) which in turn initiates downstream signalling events crucial for CLL survival and proliferation. PI3K inhibitors in pre-clinical development, clinical trials or approved for CLL treatment are indicted. mTOR exists in two complexes; mTORC1 which phosphorylates S6 kinase and 4E-BP1 (eukaryotic translation initiation factor 4E-binding proteins) thereby promoting translation and protein synthesis and mTORC2 which phosphorylates and thus enhances the activation of AKT. (B) S6 kinase is activated downstream of PI3K and mTORC1 and promotes ribosomal translational activity. S6 kinase also acts in a negative feedback loop to constrain further PI3K mediated signalling. Selective inhibition of mTORC1 (for example by everolimus as indicated) abrogates S6 kinase mediated negative feedback mechanisms and leads to enhancement of PI3K mediated signalling and AKT activation. This effect is thought to have limited the efficacy of mTOR inhibitors alone in the clinic for various cancers. Use of a dual PI3K/mTOR inhibitor (for example PF-04691502 as indicated) prevents this amplification of PI3K signalling by preventing the phosphorylation of AKT by mTORC2 and by directly inhibiting PI3K.
Idelalisib preferentially inhibits PI3Kδ and has recently gained approval for the treatment of relapsed/refractory CLL. It has been evaluated in a phase I clinical trial in 54 CLL patients with relapsed/refractory disease; nodal shrinkage and overall survival were obtained in 81% and 72% patients respectively [8]. In a phase III clinical trial, idelalisib combined with the anti-CD20 antibody rituximab significantly improved progression free survival (81%) and overall survival (91%) in relapsed CLL patients (n=220) compared to placebo plus rituximab [9]. Commonly observed adverse events in patients taking idelalisib included pneumonia, rash and diarrhoea [8], however idelalisib and rituximab demonstrated an acceptable safety profile with no significant increase overall in adverse events compared to placebo plus rituximab [9].
Idelalisib demonstrates a dual mechanism of action by inhibiting pro-survival signalling pathways [6], and, like other kinase inhibitors, leads to re-localisation of tumour cells by blocking ingress into and promoting egress out of the lymph node into the blood. Release from the protective lymph environment into blood renders CLL cells more susceptible to apoptosis. PI3Kδ is expressed by all leucocytes including T cells, raising the possibility that the therapeutic effect of idelalisib may, at least in part, be due to effects on the surrounding immune cells in addition to direct effects on CLL cells [10]. Intriguingly, IL-4 protects against idelalisib induced apoptosis in vitro [6], indicating that microenvironmental influences may protect CLL cells against PI3K inhibitors and that co-inhibition of the function of surrounding cells may be an important factor in successful treatment.
Ongoing clinical trials with idelalisib are examining the combination with other agents; including rituximab, ofatumumab, obinutuzumab and bendamustine. Furthermore, a recent publication showed that combination of idelalisib with ibrutinib is synergistic, indicating potential benefit from combined or sequential therapy [11]. In addition to idelalisib, development of other PI3Kδ inhibitors for the treatment of lymphoid malignancies is ongoing including TGR-1202, a novel PI3Kδ inhibitor with significant differences in its chemical structure compared to idelalisib and with lower reported incidences of colitis in patients. TGR-1202 is currently in phase I clinical trials, with significant nodal responses observed in 88% of relapsed/refractory CLL patients to date (clinicaltrials.gov, NCT01767766).
Duvelisib (IPI-145) targets both PI3Kδ and PI3Kγ isoforms [12] and induced apoptosis in CLL samples in vitro, abrogated bone marrow stromal cell-mediated survival, inhibited BCR mediated signalling and chemotaxis in response to CXCL12 [13]. Importantly, duvelisib also killed CLL cells that were resistant to ibrutinib [3], this may hold true with other PI3K inhibitors, and could form an important strategy for treating patients refractory to ibrutinib. Duvelisib has completed phase I clinical trials in which 89% of patients showed a reduction (≥50%) of enlarged lymph nodes and 47% patients showed an overall response to the drug [14]. Duvelisib is now in a number of clinical trials for CLL, including in combination with anti-CD20 antibodies and in patients refractory to ibrutinib (clinicaltrials.gov, NCT02292225, NCT01871675).
Although these results are extremely promising, the long term effects of PI3Kδ inhibition in patients are unknown. Will the disruption of regulatory T cell function over a number of years lead to increased risk of developing autoimmune disorders? Furthermore, increased incidences of colitis have been reported in patients treated with idelalisib, and although the exact cause is unknown, increased colitis also occurred in a murine model where PI3Kδ kinase activity was disrupted. Moreover, the PI3Kδ isoform is expressed by epithelial cells and is known to have a crucial role in lumen formation [15]. This challenges the concept of restricted usage of PI3Kδ to haematological cells and therefore raises potential concerns for the effect of PI3Kδ inhibitors on epithelial tissues; however patient responses to these agents in the short term may outweigh any potential long term effects.
3. Potential therapeutic use of PI3Kα and/or pan-PI3K inhibitors in CLL
PI3Kδ has been the overwhelming target of choice for PI3K inhibition in CLL; however there is mounting evidence for important roles of other PI3K isoforms in CLL and of functional redundancy between PI3K isoforms in leucocytes in general [4]. Amplification of the PIK3CA locus which encodes for the PI3Kα catalytic subunit has been identified in a proportion of CLL patients [16] and may contribute to constitutive PI3K activation in a subset of CLL patients. In normal B cells either PI3Kα or PI3Kδ can mediate tonic BCR signalling and low level AKT phosphorylation, with PI3Kα able to compensate for the absence of PI3Kδ and maintain normal B cell development in the bone marrow [4]. In contrast, agonist-induced AKT phosphorylation is solely PI3Kδ mediated [4]. Given that BCR derived signalling is crucial for CLL pathogenesis, the inhibition of both PI3Kα and PI3Kδ may be superior to inhibition of either isoform alone and may overcome any functional redundancy between isoforms. Interestingly, constitutive PI3Kα activity limited the efficacy of idelalisib in mantle cell lymphoma and the combined inhibition of both PI3Kα and PI3Kδ was required to abrogate constitutive AKT phosphorylation [17].
Pharmacological inhibition of PI3Kα induces apoptosis of CLL cells in vitro [5,18], and inhibits CLL migration towards CXCL12 [5]. The PI3Kα/δ inhibitor copanlisib induces significantly greater apoptosis of CLL cells compared to idelalisib (IC50 of 450 nM and >10 μM, respectively) in vitro and is currently being assessed in phase II clinical trials for a number of haematological malignancies including CLL [19]. In addition, the pan-PI3K inhibitor NVP-BKM120 showed significantly greater cytotoxicity towards CLL cells than idelalisib (IC50 10 μM vs. 40 μM, respectively) [20], indicating that other PI3K isoforms may, at least in part, compensate for PI3Kδ inhibition in terms of CLL survival. Results from a phase I clinical trial using the pan-PI3K inhibitor pilaralisib with 10 CLL patients showed an acceptable safety profile with lymph node shrinkage (≥50%) in 60% patients and progression free survival (≥6 months) in 70% of patients [21]. However, it remains to be seen whether pan-PI3K inhibition improves clinical response over PI3Kδ inhibition alone in a larger patient cohort.
4. Pan-PI3K/mTOR inhibition as a potential therapeutic option in CLL
mTOR is crucial for cellular proliferation and is an attractive therapeutic target; however the use of selective mTOR inhibitors is hampered by disruption of negative feedback mechanisms via S6 kinase and mTORC2 and subsequent over-activation of PI3K and AKT, respectively (Fig. 1B). Dual PI3K/mTOR inhibitors may overcome this effect by directly inhibiting mTOR and preventing over-activation of AKT via simultaneous inhibition of PI3K. mTOR can also be activated by MAPK mediated signalling, therefore the inhibition of PI3K in combination with inhibition of mTOR may lead to greater suppression of this pro-survival pathway than PI3K inhibition alone. This is demonstrated by a disconnect between PI3K/AKT activity and phosphorylation of the mTOR target 4E-BP1 in CLL [22], which indicates that inhibition of PI3K alone would not achieve maximal inhibition of mTOR mediated signalling.
Indeed, CLL cells treated in vitro with the dual PI3K/mTOR inhibitor PF-04691502 or idelalisib plus mTOR inhibitor everolimus, underwent significantly greater apoptosis compared to PI3Kδ or mTOR inhibition alone [23]. PF-04691502 also inhibited chemotaxis, reduced BCR stimulated signalling/survival and significantly prolonged survival in a murine model of CLL [23]. Various PI3K/mTOR inhibitors are progressing through clinical trials for a number of different cancers and may therefore provide a promising novel treatment strategy for CLL and other B cell malignancies.
5. Conclusion
The critical importance of PI3K mediated signalling in CLL pathogenesis is demonstrated by the clinical efficacy and recent approval of the PI3Kδ inhibitor idelalisib. Increased knowledge of the role other PI3K isoforms have in CLL biology is aiding the development of PI3K inhibitors with increased cytotoxicity against CLL cells. A plethora of novel compounds targeting PI3Kδ and/or multiple PI3K isoforms are now in pre-clinical or clinical studies and may provide increased therapeutic options for the treatment of CLL.
Contributions
M.D.B and A.J.S wrote and edited the manuscript.
Acknowledgements
We thank Leukaemia and Lymphoma Research (12044 & 12021) for supporting this work, the patients for supplying tissue and the infrastructure support from a CR-UK centre grant (C34999/A18087) and ECMC grant (C24563/A15581).
References
- 1.Stevenson F.K. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011;118(16):4313–4320. doi: 10.1182/blood-2011-06-338855. [DOI] [PubMed] [Google Scholar]
- 2.Jain P. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062–2067. doi: 10.1182/blood-2014-09-603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong S. IPI-145 antagonizes intrinsic and extrinsic survival signals in chronic lymphocytic leukemia cells. Blood. 2014;124(24):3583–3586. doi: 10.1182/blood-2014-07-587279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu. Rev. Immunol. 2013;31:675–704. doi: 10.1146/annurev-immunol-032712-095946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niedermeier M. Isoform-selective phosphoinositide 3’-kinase inhibitors inhibit CXCR4 signaling and overcome stromal cell-mediated drug resistance in chronic lymphocytic leukemia: a novel therapeutic approach. Blood. 2009;113(22):5549–5557. doi: 10.1182/blood-2008-06-165068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman S.E. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kienle D. Distinct gene expression patterns in chronic lymphocytic leukemia defined by usage of specific VH genes. Blood. 2006;107(5):2090–2093. doi: 10.1182/blood-2005-04-1483. [DOI] [PubMed] [Google Scholar]
- 8.Brown J.R. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furman R.R. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2014;370(11):997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali K. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510(7505):407–411. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Rooij M.F. Ibrutinib and idelalisib synergistically target BCR-controlled adhesion in MCL and CLL: a rationale for combination therapy. Blood. 2015;125(14):2306–2309. doi: 10.1182/blood-2014-12-619163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winkler D.G. PI3K-delta and PI3K-gamma inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem. Biol. 2013;20(11):1364–1374. doi: 10.1016/j.chembiol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Balakrishnan K. The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145, overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL. Leukemia. 2015;29(9):1811–1822. doi: 10.1038/leu.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IPI-145 shows promise in CLL patients. Cancer Discov., 4(2), 2014, 136 [DOI] [PubMed]
- 15.Peng J. Phosphoinositide 3-kinase p110delta promotes lumen formation through the enhancement of apico-basal polarity and basal membrane organization. Nat. Commun. 2015;6:5937. doi: 10.1038/ncomms6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown J.R. Integrative genomic analysis implicates gain of PIK3CA at 3q26 and MYC at 8q24 in chronic lymphocytic leukemia. Clin. Cancer Res. 2012;18(14):3791–3802. doi: 10.1158/1078-0432.CCR-11-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyengar S. P110alpha-mediated constitutive PI3K signaling limits the efficacy of p110delta-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood. 2013;121(12):2274–2284. doi: 10.1182/blood-2012-10-460832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Frias M. Isoform-selective phosphoinositide 3-kinase inhibitors induce apoptosis in chronic lymphocytic leukaemia cells. Br. J. Haematol. 2010;150(1):108–111. doi: 10.1111/j.1365-2141.2010.08151.x. [DOI] [PubMed] [Google Scholar]
- 19.Gockeritz E. Efficacy of phosphatidylinositol-3 kinase inhibitors with diverse isoform selectivity profiles for inhibiting the survival of chronic lymphocytic leukemia cells. Int. J. Cancer. 2015 doi: 10.1002/ijc.29579. [DOI] [PubMed] [Google Scholar]
- 20.Amrein L. The phosphatidylinositol-3 kinase I inhibitor BKM120 induces cell death in B-chronic lymphocytic leukemia cells in vitro. Int. J. Cancer. 2013;133(1):247–252. doi: 10.1002/ijc.27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown J.R. Phase I trial of the pan-PI3K inhibitor pilaralisib (SAR245408/XL147) in patients with chronic lymphocytic leukemia (CLL) or relapsed/refractory lymphoma. Clin. Cancer Res. 2015;21(14):3160–3169. doi: 10.1158/1078-0432.CCR-14-3262. [DOI] [PubMed] [Google Scholar]
- 22.Shull A.Y. RPPA-based protein profiling reveals eIF4G overexpression and 4E-BP1 serine 65 phosphorylation as molecular events that correspond with a pro-survival phenotype in chronic lymphocytic leukemia. Oncotarget. 2015;6(16):14632–14645. doi: 10.18632/oncotarget.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blunt M.D. The PI3K/mTOR inhibitor PF-04691502 induces apoptosis and inhibits microenvironmental signaling in CLL and the Emu-TCL1 mouse model. Blood. 2015;125(26):4032–4041. doi: 10.1182/blood-2014-11-610329. [DOI] [PubMed] [Google Scholar]