Abstract
Background
Many studies have shown that microRNAs (miRNAs) exhibit altered expression in various cancers and may play an important role as prognostic biomarkers. The present meta-analysis was undertaken to summarize recent studies of the use of microRNA-145 (miR-145) in the assessment of prostate cancer and to analyze the prognostic role of miR-145 for disease-free survival (DFS) outcome.
Methods
The present meta-analysis was performed by searching PubMed with the use of multiple search strategies. Data were extracted from studies examining DFS in patients with prostate cancer who showed lower expression of miR-145. Pooled hazard ratios of miR-145 and 95% confidence intervals were calculated. Four studies with a total of 211 patients were included in this meta-analysis.
Results
For overall DFS, the pooled hazard ratio of lower miR-145 expression in prostate cancer was 0.74 (95% confidence interval: 0.23–2.34, P = 0.001). Thus, lower miR-145 expression was found to significantly predict poorer outcomes.
Conclusions
The present findings suggest that downregulation of the expression of miR-145 might predict poor prognosis in patients with prostate cancer.
Keywords: miR-145, Prognosis, Prostate cancer
Introduction
Prostate cancer (PCa) is the most common malignancy and the third leading cancer-related cause of death among men in the Western world. A projected 233,000 new cases of PCa will be diagnosed and ∼29,480 men will die of the disease in the United States alone in 2014. The mortality of PCa accounts for ∼10% of all cancer deaths.1 Although the 5-year survival rate of PCa is higher in early-stage disease after treatment with surgical resection or androgen deprivation therapy, one-third of treated PCa patients will experience disease recurrence and will progress into castration-resistant PCa, a more aggressive disease, leading to a poor prognosis.2
Although prostate-specific antigen (PSA) levels have been used as a predictor of biochemical progression, clinical progression, and death from PCa, many potentially aggressive PCa tumors may remain undetected in men because of normal PSA values. Accordingly, a need exists to develop biomarkers that can supplement or even replace PSA for the diagnosis and prognostication of PCa.3 Many genes associated with p53 mutations,4–6 PTEN mutations,7–9 PSCA mutations,10 and androgen receptor mutations11 have been investigated as markers of prognosis and disease progression of PCa, but the clinical use of these markers requires further research.
MicroRNAs (miRNAs) are small noncoding RNAs with a length of ∼22 nucleotides that predominantly bind to 3′-untranslated regions of target genes, leading to either translational repression or mRNA degradation. miRNAs play important roles in various biological and metabolic processes, including development, differentiation, signal transduction, cell maintenance, disease, and cancer.12 Recent studies have shown that miR-145 is a tumor-suppressive miRNA that is downregulated in several cancer types, including bladder cancer,13 colon cancer,14–16 breast cancer,17 and ovarian cancer.18 Furthermore, a lower level of miR-145 expression has been found to be predictive of cancer progression. By contrast, the normal tissues in which the cancers originate show good expression of miR-145. Meanwhile, miR-145 was reported to be downregulated in PCa tissues in many studies and to be associated with the prognosis of PCa. However, the results of those studies were inconclusive and might not be powerful enough owing to limited sample sizes. In the present study, we evaluated the relationship between miR-145 expression and disease-free survival (DFS) outcome.
Materials and methods
Search strategy
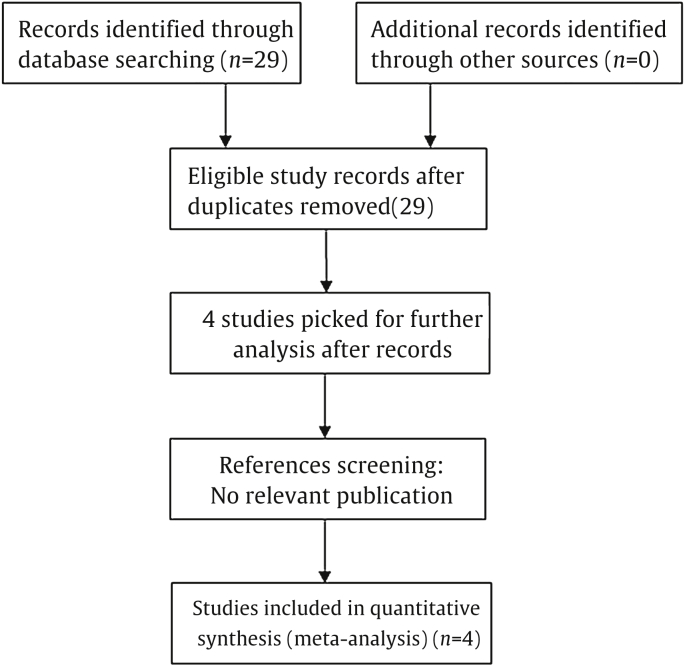
This meta-analysis was carried out in accordance with the guidelines of the Meta-analysis of Observational Studies in Epidemiology Group.19 The PubMed and Medline databases were searched for the last time in May 2014, and no lower date limit was used. Only reviews published in English were evaluated. The following search strategy was used: (“microRNA-145” OR “miR-145” OR “miRNA-145”) AND (“prostate cancer” OR “prostate carcinoma” OR “PCa”) AND (”prognosis” OR “prognosis”). Eligible studies were included in the meta-analysis if they met the following criteria: (1) they had to discuss patients with PCa; (2) they had to measure miR-145 expression in tumor tissue; (3) they had to investigate DFS outcome or the correlation between miR-145 expression and clinical variables; and (4) the method of miR-145 detection had to be the same. Articles were excluded on the basis of any of the following criteria: (1) they were review articles, letters, or laboratory articles; (2) they were not in English; (3) they lacked key information for calculation by use of methods established by Parmar et al,20 Tierney et al21; Williamson et al,22 and (4) they were repeat studies that included the same author and the same samples from the same patients as in a study already included. Two reviewers (X.Z. and J.W.) independently evaluated the titles and abstracts of the identified articles in duplicate. A flow diagram of the study selection process is presented in Fig. 1.
Fig. 1.
Study selection process.
Data extraction
Eligible papers were reviewed independently by two investigators. Data were extracted from each study according to the selection criteria mentioned above. We extracted the primary information, including multivariate analysis, Kaplan–Meier survival analysis, P values, and hazard ratios (HRs), independently. Further data extracted from the studies included the first author's name, year of publication, origin of the study population, size of the study population, tumor node metastasis (TNM) stage, method of detecting miR-145, the PSA level, cutoff value, and duration of follow-up. HR values < 1 were considered indicative of significant associations with poor outcome and HR values > 1 indicative of significant associations with good outcome. Disagreements were resolved by discussion. All data were subject to consensus.
Statistical analysis
Heterogeneity was assessed by using Q statistics (P < 0.10 was considered heterogeneous). Any significant heterogeneity among the studies was resolved by using the random-effects model. Otherwise, the fixed-effects model was used. The I2 statistic, which measures the percentage of the total variation across studies that is due to heterogeneity rather than to chance, was also assessed.23 The effect of miR-145 expression on DFS was estimated by using forest plots. A pooled HR was calculated by using a fixed-effects model or a random-effects model as appropriate. A pooled HR < 1 indicated poor prognosis for the groups with lower miR-145 expression and was considered statistically significant if the 95% CI did not overlap 1. Publication bias was evaluated by using the funnel plot and Begg's test, and P > 0.05 was considered indicative of a lack of publication bias.24 All analyses were performed by using STATA version 12.0 (Stata Corporation, College Station, TX, USA). A P value < 0.05 was considered to be statistically significant.
Results
Study characteristics
Four studies were identified as eligible for full-text review.24–27 These eligible studies were published between 2009 and 2013. One study evaluated patients from the United States,25 one evaluated patients from China,26 one evaluated patients from Korea,27 and one evaluated patients from Greece.28 These studies included a total of 211 patients with a mean number of 52.6 patients per study. These four eligible studies were all retrospective cohort studies. The method of miR-145 detection was all quantitative real-time polymerase chain reaction. miR-145 expression levels were measured in tumor tissue. The mean length of follow-up ranged from 19.4 months to 82 months. Characteristics of the eligible studies are summarized in Table 1.
Table 1.
Characteristics of four studies included in the present meta-analysis.a)
| Author | Origin of population | Age (yr) | N | Stage | PSA (ng/mL) | Cut off | Sampling | Method | Survival analysis | Hazard ratios | Follow-up (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qing et al26 2010 | China | 71.6 | 106 | T1-T4 | – | ΔCt method | tumor | qRT-PCR | DFS | Reported | 82 |
| Kang et al27 2012 | Korea | 64.7 | 73 | T2a-T3b | 7.5 | ΔCt method | tumor | qRT-PCR | DFS | Reported | 19.4 |
| Avgeris et al28 2013 | Greece | 65 | 62 | T2a-T3b | – | 45th percentile | tumor | qRT-PCR | DFS | Reported | 75 |
| Schaefer et al25 2009 | USA | 63 | 76 | T2a-T3b | 6.7 | Median | tumor | qRT-PCR | DFS | Reported | 50 |
DFS, disease-free survival; –, not mentioned; PSA, prostate specific antigen; qRT-PCR, quantitative real-time PCR.
The studies included here are all retrospective cohort studies with different groups of patients.
Meta-analysis results
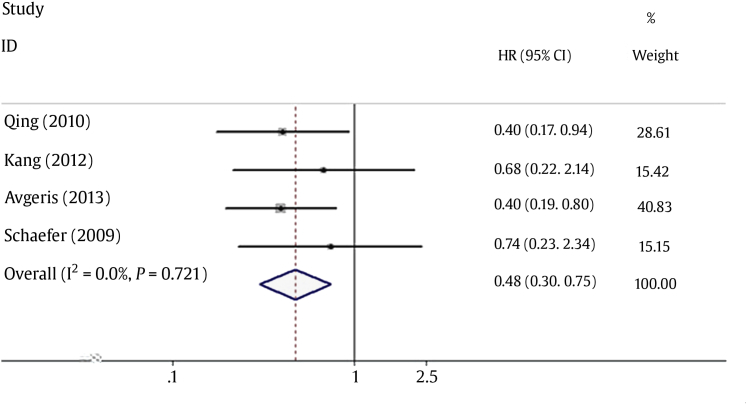
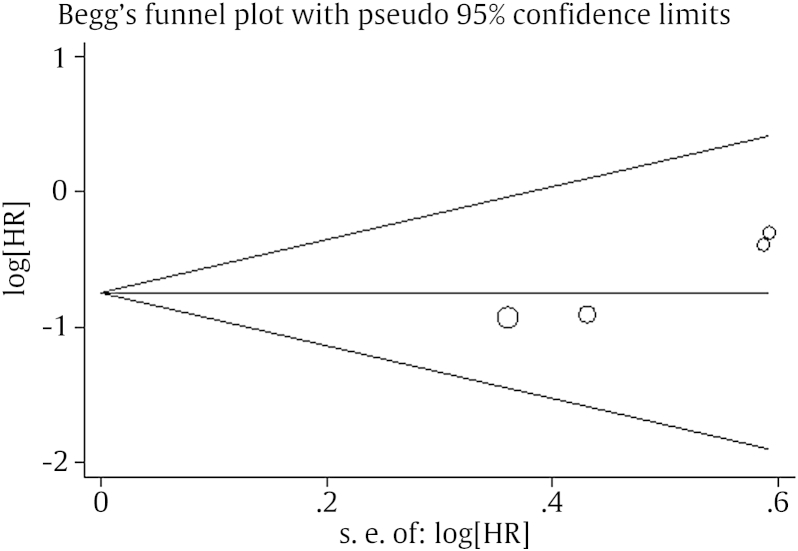
As shown in Table 1, all four studies reported the HR and 95% CI directly. No heterogeneity was detected between the studies, as indicated by evaluation of the relationship between the reduced miR-145 level and DFS (I2 = 0.0%, P = 0.721). Therefore, a pooled HR and its 95% CI were calculated by use of a fixed-effects model. Forest plots of the individual HR estimates and the results of the meta-analysis are presented in Fig. 2. According to these results, lower levels of miR-145 expression were significantly predictive of poor DFS. The pooled HR was 0.74 (95% CI: 0.23–2.34, P = 0.001). Publication bias was evaluated by using funnel plots and Begg's tests. No significant publication biases were observed in this meta-analysis (P = 0.089; Fig. 3).
Fig. 2.
Forest plot of the relationship between downregulation of miRNA-145 level and disease-free survival in patients with prostate cancer. CI, confidence interval; HR, hazard ratio; miRNA, microRNA.
Fig. 3.
Funnel plot of lower miRNA-145 expression and disease-free survival among patients with prostate cancer. S. E. HR, hazard ratio; miRNA, microRNA.
Discussion
PCa is a slow-growing malignant tumor of the male reproductive system. In most cases, PCa is infiltrative and tumor cells are scattered within the normal prostate stroma, making the tumor content in each sample quite different. Therefore, an urgent need exists to identify novel miRNAs as tools or markers for prediction of aggressive PCa.
miR-145 was previously found to be downregulated in many solid tumors compared with adjacent nontumor tissue, including in PCa.29 Although miR-145 levels in PCa tissues are generally low compared with those in nontumor tissues, the variations in miR-145 expression are large, with a 10- to 30-fold range in tumor tissues.29,30 This phenomenon suggests that expression of miR-145 within prostate tumor tissues may display a heterogeneous pattern that can be used as a biomarker to differentiate PCa patients on the basis of tumor aggressiveness. However, the results of the few previous studies that have explored the role of miR-145 deregulation in the prediction of PCa prognosis were inconsistent.
The study by Avgeris et al28 clearly supported the tumor-suppressor role of miR-145 and highlighted its clinical utility in PCa. In that study, the downregulation of miR-145 expression in PCa was further correlated with a higher Gleason score, late-stage and larger diameter tumors, and higher pretreatment and follow-up serum PSA levels. These findings illustrate the central role of miR-145 loss in disease progression and support the potential use of miR-145 for improvement of prognostication. Chen et al26 analyzed PCa and benign prostate tissue samples and showed significantly decreased miR145 expression in PCa samples (P < 0.001), particularly in those with tumor progression, and suggested that miR-145 may play a major role in PCa prognosis. By contrast, Kang et al27 did not observe an association between miR-145 and clinicopathologic parameters of PCa (HR: 0.679, 95% CI: 0.215–2.143), indicating that these types of miRNA do not have prognostic value for PCa patients. These results are paradoxically consistent with results from previous studies. Schaefer et al25 performed miRNA profiling in 79 PCa tissues using quantitative real-time polymerase chain reaction to evaluate the potential of using miRNA as a prognostic marker. They reported that expression of miR-96 was associated with Gleason score and biochemical tumor recurrence after radical prostatectomy but that decreased miR-145 expression was not significantly associated with prognosis of PCa (HR: 0.74, 95% CI: 0.23–2.34). Overall, the prognostic role of miR-145 in PCa remains a puzzle.
The present meta-analysis is the first systematic evaluation of the relationship between the miR-145 expression level and the prognosis of PCa. Downregulation of miR-145 expression was found to be predictive of poor DFS among PCa patients in this meta-analysis. The pooled HR of DFS was 0.48 (95% CI: 0.30–0.75). The differences were found to be statistically significant and no significant heterogeneity was observed during the meta-analysis (I2 = 0%).
The biological function of miR-145 may affect the relationship between miR-145 expression and cancer outcome. The downregulation of miR-145 in different types of tumors suggests its role in controlling cell proliferation, migration, and invasion.31 It has been reported that the lower expression of miR-145 is due to the methylation of the promoter region or p53 mutation of miR-145 in PCa cell lines.32 Target gene searches have revealed that the pro-apoptotic gene, TNFSF10, is significantly upregulated by overexpression of miR-145.30 Fuse et al33 identified that miR-145 can act as a tumor suppressor through the direct control of FSCN1 expression in PCa cell lines. In addition, Hart et al34 showed that the proto-oncogene ERG is a target of miR-145. Both downregulation of the tumor suppressor miR-145 and upregulation of the ERG oncoprotein appear to be relevant for the development of PCa.34 Here we show that miR-145, an miRNA whose main role was shown to be vascular smooth muscle cell maintenance35,36 and human embryonic stem cell differentiation,37 could be useful in the prognostication of PCa as single biomarker or in combination.
The present meta-analysis had some limitations. Firstly, only a few studies have specifically regarded miR-145 and the prognosis of PCa. Secondly, the cutoff values differed in the different studies. A consistent cutoff value needs to be determined in future studies. Thirdly, in this meta-analysis, downregulation of miR-145 expression was found to have a prognostic role in PCa, but it was not possible to confirm miR-145 as an independent predictive factor. Recently, researchers have suggested that a set of miRNAs might have a stronger predictive effect than a single miRNA.38 Fourthly, the expression of miR-145 was detected in tumor tissue samples but not in serum or plasma. However, circulating prognostic markers were found to be more valuable than tissue markers in cancer patients.
In summary, the present meta-analysis showed the downregulation miR-145 expression levels to be closely associated with poor prognosis in PCa patients. More multi-center clinical investigations with larger sample sizes should be conducted to confirm these findings.
Conflicts of interest
The authors have nothing to disclose.
References
- 1.Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics. 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Prostate Cancer Trialists' Collaborative Group Maximum androgen blockade in advanced prostate cancer: an overview of the randomized trials. Lancet. 2000;355:1491–1498. [PubMed] [Google Scholar]
- 3.Bradford T.J., Tomlins S.A., Wang X., Chinnaiyan A.M. Molecular markers of prostate cancer. Urol Oncol. 2006;24:538–551. doi: 10.1016/j.urolonc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Bookstein R., MacGrogan D., Hilsenbeck S.G., Sharkey F., Allred D.C. p53 is mutated in a subset of advanced-stage prostate cancers. Cancer Res. 1993;53:3369–3373. [PubMed] [Google Scholar]
- 5.Navone N.M., Labate M.E., Troncoso P., Pisters L.L., Conti C.J., von Eschenbach A.C. p53 mutations in prostate cancer bone metastases suggest that selected p53 mutants in the primary site define foci with metastatic potential. J Urol. 1999;161:304–308. [PubMed] [Google Scholar]
- 6.Voeller H.J., Sugars L.Y., Pretlow T., Gelmann E.P. p53 oncogene mutations in human prostate cancer specimens. J Urol. 1994;151:492–495. doi: 10.1016/s0022-5347(17)35000-0. [DOI] [PubMed] [Google Scholar]
- 7.Feilotter H.E., Nagai M.A., Boag A.H., Eng C., Mulligan L.M. Analysis of PTEN and the 10q23 region in primary prostate carcinomas. Oncogene. 1998;16:1743–1748. doi: 10.1038/sj.onc.1200205. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H., Freije D., Nusskern D.R., Okami K., Cairns P., Sidransky D. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998;58:204–209. [PubMed] [Google Scholar]
- 9.Wang S.I., Parsons R., Ittmann M. Homozygous deletion of the PTEN tumor suppressor gene in a subset of prostate adenocarcinomas. Clin Cancer Res. 1998;4:811–815. [PubMed] [Google Scholar]
- 10.Reiter R.E., Gu Z., Watabe T., Thomas G., Szigeti K., Davis E. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci U S A. 1998;95:1735–1740. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taplin M.E., Bubley G.J., Shuster T.D., Frantz M.E., Spooner A.E., Ogata G.K. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. New Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 12.Meister G. miRNAs get an early start on translational silencing. Cell. 2007;131:25–28. doi: 10.1016/j.cell.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Ichimi T., Enokida H., Okuno Y., Kunimoto R., Chiyomaru T., Kawamoto K. Identification of novel microRNA targets based on microRNA signatures in bladder cancer. Int J Cancer. 2009;125:345–352. doi: 10.1002/ijc.24390. [DOI] [PubMed] [Google Scholar]
- 14.Akao Y., Nakagawa Y., Naoe T. MicroRNA-143 and-145 in colon cancer. DNA Cell Biol. 2007;26:311–320. doi: 10.1089/dna.2006.0550. [DOI] [PubMed] [Google Scholar]
- 15.Schepeler T., Reinert J.T., Ostenfeld M.S., Christensen L.L., Silahtaroglu A.N., Dyrskjøt L. Diagnostic and prognostic microRNAs in stage II colon cancer. Cancer Res. 2008;68:6416–6424. doi: 10.1158/0008-5472.CAN-07-6110. [DOI] [PubMed] [Google Scholar]
- 16.Slaby O., Svoboda M., Fabian P., Smerdova T., Knoflickova D., Bednarikova M. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2008;72:397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- 17.Spizzo R., Nicoloso M.S., Lupini L., Lu Y., Fogarty J., Rossi S. miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-α in human breast cancer cells. Cell Death Diff. 2010;17:246–254. doi: 10.1038/cdd.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nam E.J., Yoon H., Kim S.W., Kim H., Kim Y.T., Kim J.H. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14:2690–2695. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- 19.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.Parmar M.K.B., Torri V., Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson P.R., Smith C.T., Hutton J.L., Marson A.G. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. 2002;21:3337–3351. doi: 10.1002/sim.1303. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 25.Schaefer A., Jung M., Mollenkopf H.J., Wagner I., Stephan C., Jentzmik F. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer. 2010;126:1166–1176. doi: 10.1002/ijc.24827. [DOI] [PubMed] [Google Scholar]
- 26.Chen X., Gong J., Zeng H., Chen N., Huang R., Huang Y. MicroRNA145 targets BNIP3 and suppresses prostate cancer progression. Cancer Res. 2010;70:2728–2738. doi: 10.1158/0008-5472.CAN-09-3718. [DOI] [PubMed] [Google Scholar]
- 27.Kang S.G., Ha Y.R., Kim S.J., Kang S.H., Park H.S., Lee J.G. Do microRNA 96, 145 and 221 expressions really aid in the prognosis of prostate carcinoma? Asian J Androl. 2012;14:752–757. doi: 10.1038/aja.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avgeris M., Stravodimos K., Fragoulis E.G., Scorilas A. The loss of the tumour-suppressor miR-145 results in the shorter disease-free survival of prostate cancer patients. Br J Cancer. 2013;108:2573–2581. doi: 10.1038/bjc.2013.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozen M., Creighton C.J., Ozdemir M., Ittmann M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene. 2008;27:1788–1793. doi: 10.1038/sj.onc.1210809. [DOI] [PubMed] [Google Scholar]
- 30.Zaman M.S., Chen Y., Deng G., Shahryari V., Suh S.O., Saini S. The functional significance of microRNA-145 in prostate cancer. Br J Cancer. 2010;103:256–264. doi: 10.1038/sj.bjc.6605742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sachdeva M., Mo Y.Y. miR-145-mediated suppression of cell growth, invasion and metastasis. Am J Transl Res. 2010;2:170–180. [PMC free article] [PubMed] [Google Scholar]
- 32.Suh S.O., Chen Y., Zaman M.S., Hirata H., Yamamura S., Shahryari V. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis. 2011;32:772–778. doi: 10.1093/carcin/bgr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuse M., Nohata N., Kojima S., Sakamoto S., Chiyomaru T., Kawakami K. Restoration of miR-145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int J Oncol. 2011;38:1093. doi: 10.3892/ijo.2011.919. [DOI] [PubMed] [Google Scholar]
- 34.Hart M., Wach S., Nolte E., Szczyrba J., Menon R., Taubert H. The proto-oncogene ERG is a target of microRNA miR-145 in prostate cancer. FEBS J. 2013;280:2105–2116. doi: 10.1111/febs.12236. [DOI] [PubMed] [Google Scholar]
- 35.Xin M., Small E.M., Sutherland L.B., Qi X., McAnally J., Plato C.F. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elia L., Quintavalle M., Zhang J., Contu R., Cossu L., Latronico M.V. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu N., Papagiannakopoulos T., Pan G., Thomson J.A., Kosik K.S. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 38.Larne O., Martens-Uzunova E., Hagman Z., Edsjö A., Lippolis G., den Berg M.S. miQ—A novel microRNA based diagnostic and prognostic tool for prostate cancer. Int J Cancer. 2013;132:2867–2875. doi: 10.1002/ijc.27973. [DOI] [PubMed] [Google Scholar]