Abstract
Background
Recent conflicts in Afghanistan and Iraq produced a substantial number of critically wounded service-members. We collected biomarker and clinical information from 73 patients who sustained 116 life-threatening combat wounds, and sought to determine if the data could be used to predict the likelihood of wound failure.
Methods
From each patient, we collected clinical information, serum, wound effluent, and tissue prior to and at each surgical débridement. Inflammatory cytokines were quantified in both the serum and effluent, as were gene expression targets. The primary outcome was successful wound healing. Computer intensive methods were used to derive prognostic models that were internally validated using target shuffling and cross-validation methods. A second cohort of eighteen critically injured civilian patients was evaluated to determine if similar inflammatory responses were observed.
Findings
The best-performing models enhanced clinical observation with biomarker data from the serum and wound effluent, an indicator that systemic inflammatory conditions contribute to local wound failure. A Random Forest model containing ten variables demonstrated the highest accuracy (AUC 0.79). Decision Curve Analysis indicated that the use of this model would improve clinical outcomes and reduce unnecessary surgical procedures. Civilian trauma patients demonstrated similar inflammatory responses and an equivalent wound failure rate, indicating that the model may be generalizable to civilian settings.
Interpretation
Using advanced analytics, we successfully codified clinical and biomarker data from combat patients into a potentially generalizable decision support tool. Analysis of inflammatory data from critically ill patients with acute injury may inform decision-making to improve clinical outcomes and reduce healthcare costs.
Funding
United States Department of Defense Health Programs.
Keywords: Combat trauma, Wound healing, Clinical decision support, Decision analysis, Inflammation
Highlights
-
•
We analyzed biomarker and clinical data to predict the likelihood of wound failure.
-
•
We found that systematic inflammatory conditions contribute to local wound failure.
-
•
This response is comparable between combat wounded and civilian patients.
-
•
This response can be measured and translated into clinical decision support tools.
-
•
These predictive models will benefit both military and civilian health systems.
1. Introduction
During the last decade of conflict in Afghanistan and Iraq, our military health system (MHS) treated a large number of critically-wounded servicemen and women (Casualty Report). Many of them sustained systemic polytrauma, mangled extremities and traumatic amputations from blasts. These devastating injuries pushed the physiologic reserves of these generally young, previously healthy patients to the extreme. Thanks to the combined effects of body armor, tourniquets, tactical combat casualty care, aggressive resuscitation techniques, and a robust trauma system, many service members who would have died in previous conflicts survived to reach tertiary care facilities (Elster et al., 2013, Sheridan et al., 2014). Because these survivors posed substantial reconstruction and rehabilitation challenges, we organized a coordinated effort to characterize the physiologic response of military patients to these devastating injuries, and determine if particular biomarkers predict perioperative complications.
Early in the conflicts, we noted that severely-injured patients demonstrate systemic inflammatory dysregulation and relative immunosuppression (Hawksworth et al., 2009). This suggests that the native inflammatory system, geared toward mitigating less severe injuries, is ill-equipped to regulate the massive physiologic insults produced by blast injuries (Hawksworth et al., 2009). Complications such as delayed wound healing and dehiscence (Forsberg et al., 2008), venous thromboembolism (Gillern et al., 2011), and ventilator-associated pneumonia (Landrum and Murray, 2008) occurred more frequently than expected; and unanticipated outcomes such as heterotopic ossification (Potter et al., 2007, Forsberg et al., 2009) and angioinvasive fungal infections (Warkentien et al., 2012) were frequently observed. In fact, even highly experienced military surgeons had difficulty risk-stratifying their patients' wounds (Forsberg et al., 2014) because the conventional manner of visually assessing wounds (Bartlett, 2003, Stromeyer, 1862, Moorhead, 1942a, Selcer, 2008) was inadequate.
The timing of wound closure is important. If a combat wound is closed prematurely, it is more likely to dehisce. When this happens, the injured service member requires additional surgical procedures that can jeopardize life, limb or residual limb length. However, if a wound is closed unnecessarily late, the delay and additional procedures prolong the patient's hospital stay, delay rehabilitation, and increase the risk that the patient will develop a hospital-acquired infection or other complication. In the hopes of optimizing care, we sought to characterize each patient's physiologic response to injury, with the goal of developing a decision support tool to guide the timing of wound closure.
2. Materials and Methods
2.1. Selection of Patients
Study participants were screened and treated at Walter Reed National Military Medical Center (WRNMMC), Bethesda, MD, between January 2007 and January 2012. Each candidate participant had been evacuated from Iraq or Afghanistan after sustaining a combat-related injury to one or more extremities. All had at least one extremity wound > 75 cm2 treated en route with negative pressure wound therapy.
To get a preliminary sense of the validity and generalizability of our findings, we also enrolled a civilian comparison group of patients who were treated in the Marcus Trauma Center of Grady Memorial Hospital, a civilian Level I trauma center in Atlanta, GA. Both groups, military and civilians, were enrolled using a common IRB approved study protocol.
2.2. Human Subject Considerations
Inclusion criteria for this study as at least one extremity wound > 75 cm2 treated with negative pressure wound therapy without immune or connective tissue disorders. Details of the consenting process are outlined below.
2.3. Walter Reed National Military Medical Center (Study Number 352334)
This study was reviewed and approved by the Walter Reed National Military Medical Center Institutional Review Board. We consider combat casualties to be a vulnerable patient population. Because of this, every effort was made to eliminate the appearance of military rank, authority, or the perception of coercion during the enrollment process. Patients were identified by the manifest of incoming combat casualties from overseas. Each prospective study participant received a standardized briefing by one of three research associates specifically trained in the informed consent process. Informed consent was obtained for each study participant. For those who were unconscious, or otherwise unable to communicate, we obtained informed consent from the patient's legally authorized representative in accordance with local and federal regulations. Patients enrolled by this method were re-consented after their cognitive status improved, after first being given the opportunity to withdraw from the study. Each study participant was given the opportunity to withdraw from the study at regular intervals, coinciding with the timing of sample collection.
2.4. Grady Memorial Hospital (Study Number 00058229)
This study was reviewed and approved by the Emory University Institutional Review Board and the Grady Hospital Research Oversight Committee. Patients were identified by one of the co-investigators during attendance at general surgery morning reports, attendance of trauma bay resuscitations, review of surgical operative logs and referrals from other admitting surgeons. Other admitting surgeons were contacted to ensure they are in agreement with patient enrollment prior to approach of the patient. Patients were informed that they could withdraw from the study at any time and for any reason without penalty or adjustments in their routine care. All patients with wounds that met inclusion criteria were approached by study personnel and the patient and/or the patient's family were engaged in a detailed discussion about the aims of the study and the potential risks and benefits of study participation. Potential subjects were also informed that wounds seen in the civilian setting would be analyzed and compared to similar findings in military patients with war wounds. For patient's unable to give personal consent for this study, the next-of-kin or legally authorized representative were approached and consent sought after appropriate discussion as described.
Any candidate study participant found to have a confounding co-morbid condition, such as an immune or connective tissue disorder, was excluded. To guide the timing of closure, surgeons at both used the conventional “4C's”: color, consistency, contractility when stimulated, and the capacity to bleed when incised (Bartlett, 2003, Bowyer, 2006).
2.5. Demographics
We collected a comprehensive set of demographic and clinical information, including gender, age, body mass index (BMI), tobacco use, mechanism of injury, Injury Severity Score (ISS), time from injury, units of transfused blood products, and associated neurovascular or traumatic brain injuries. At the time of each surgical débridement, we also recorded the patient's APACHE II score, wound size, and time from injury. Each wound was followed for a minimum of six weeks following surgical closure. Successful closure was defined as greater than 90% split thickness skin graft acceptance, the absence of infection (Sherertz et al., 1992), and the absence of dehiscence following delayed primary closure. Dehiscence was defined as a reopened wound that required additional surgical treatment within 30 days of closure or coverage.
2.6. Sample Collection
Serum and wound effluent samples were collected at the time of each débridement procedure, which occurred approximately every 48–72 h until wound closure or coverage was performed (Hawksworth et al., 2009). Up to three wounds per patient were monitored.
After each débridement, a representative tissue biopsy was taken from the central portion of each wound. For transcript analysis, an aliquot (30–35 mg) was used for RNA extraction, which was subsequently validated using spectroscopic (NanoDrop) and gel-electrophoretic (Agilent Bioanalyzer 2100) techniques prior to being converted to complementary DNA (cDNA).
2.7. Gene Transcript Analysis
Wound biopsies were analyzed for 190 inflammatory and wound healing genes. RNA extracted from each biopsy was required to exhibit an A260/A280 ratio ≥ 1.6, an RNA Integrity Number ≥ 8, and an 18S/16S rRNA peak ratio ≥ 1.7. Qualification of cDNA was achieved using real-time qPCR targeting 18S (required CT in the range 7–10 using a threshold of 0.1). Qualified cDNA samples were loaded to a low-density array (Applied Biosystems) containing target specific primers and probe. A maximum CT of 35 cycles was used and individual gene transcript expressions were normalized to 18S rRNA. Relative quantitation was evaluated using the 2−∆∆Ct method (Livak and Schmittgen, 2001).
At the final débridement, the qPCR data was evaluated by generating an Ingenuity iReport for Isoform-level Human RNA-Sequence Data (IPA®, QIAGEN Redwood City, California, USA). For these analyses, we used a threshold p-value of 0.05 and fold change of 1.5. The endogenous controls were MAPK14, NFKB1, TGFBR1, 18 s, and GAPDH; the 18 s had score of 0.87 and GAPDH 1.158 as described by Vandesompele et al. (Vandesompele et al., 2002).
2.8. Protein Analysis
Serum and wound effluent proteins were quantified using a Luminex 100/200 IS xMAP Bead Array Platform (Millipore Corp, Billerica, MA) as previously described (Hawksworth et al., 2009). Thirty-two cytokines, chemokines and growth factors were quantified using a Human Cytokine 30-plex panel supplemented with a custom Human 2-plex panel (Life Technologies; Cat. No LHC6003 and LCP0002, Grand Island, NY). Data were categorized as related to the initial débridement, the penultimate débridement, and the final débridement.
2.9. Statistics, Data Modeling, and Cost Analysis
Both parametric statistical and machine learning methodologies (Bayesian Belief Networks — BBN; Random Forest Analysis — RF; logistic regression using Least Absolute Shrinkage and Selection Operator — LASSO) were used to analyze the data. The goal was to estimate the likelihood of wound failure, while incorporating target shuffling, cross validation, and decision analysis. Additionally, a cost-savings analysis, for both civilian and military health systems, was also developed.
We performed univariate analysis to identify general relationships between wound outcome and serum, effluent and gene expression targets. Statistical differences between these continuous variables and wound outcome were evaluated using the Mann–Whitney U test and the post hoc Tukey–Kramer assessment. The levels of significance were adjusted using the false discovery rate method.
We then performed logistic regression using LASSO, and machine learning using RF and BBN modeling techniques. Two RF models were created; one that evaluated all features, and one that contained only the top 10 features after ranking variable importance using a RF filter. The goal was to develop models designed to estimate the likelihood of healing for an individual wound, based on clinical, systemic and localized wound information contained within each record. With 157 variables and 27 events (failed wounds), we mitigated the risk of overfitting the final model to the training set using 10-fold cross validation, as well as by a variety of methods unique to each modeling process. For the BBN, a proprietary machine-learning algorithm (FasterAnalytics, DecisionQ Inc., Washington DC, USA) uses a scoring formula that balances goodness of fit against robustness using a parsimony metric. The RF models employ the random subspace method (Ho, 1998) and the LASSO method (Tibshirani, 1997) inherently minimizes overfitting through L1 regularization, via penalization of the loss function.
In order to ensure the relationships identified by each modeling technique were not due to chance alone, we used a target shuffling technique (Nisbet et al., 2009). Briefly, we compared each model built with the original training data against a permuted null distribution containing 1000 iterations with the wound outcomes (healed or dehisced) shuffled randomly among each of the records. Iterated cross-validation was used to evaluate the likelihood of sample bias resulting from a single round of k-fold cross-validation. For each model, the mean area under the curve (AUC) of the receiver operator characteristic (ROC) curves for all 1000 iterations was reported.
The methodology used to develop, validate, and update these models conforms to the Transparent Reporting of multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) guidelines.
2.10. Accuracy and Decision Analysis
We first assessed accuracy using 10-fold Cross-validation. The data were randomized into 10 matching train-and-test sets. Each training set comprised 90% of records and model development to include variable and coefficient selection were performed on each unique training set. A corresponding test set comprised the remaining 10% of records. Each matching set was unique to ensure there was no overlapping information between sets. I.e. Each test set contained true unknowns, and none of the information comprising the test set was used to develop the corresponding models.
For most surgical problems, the consequences of undertreatment are generally less desirable than those associated with overtreatment. Decision Curve Analysis (DCA) (Vickers and Elkin, 2006) allows one to evaluate model performance by weighing the relative consequences of a falsely positive or negative result. This technique allows one not only to compare a variety of classifiers directly, but is also critical prior to recommending the model(s) be used clinically. We used FasterAnalytics™ (DecisionQ, Washington, DC, USA) for the BBN modeling, and R© Version 3.0 (R Core Team) for all other data processing, curation and analysis.
2.11. Economic Impact Analysis
We estimated cost savings that might be gained through implementation of this clinical decision support tool across the US healthcare system. In doing so, we conducted an extensive medical and public policy literature review of over 200 peer-reviewed papers. We focused on those papers that provided insight into the care of open wounds associated with trauma and an inpatient hospital stay. The content was drawn from the National Center for Biotechnology Information's PubMed Central® databases and U.S. Library of Congress. In addition, we obtained healthcare outcome data, such as the number of relevant medical cases in North America, Western Europe, and Australia. Moreover, since open wounds can be associated with a very large variety of medical conditions, we focused on those that specifically required some level of critical care. The cost savings associated with Hospital Acquired Infections was also determined as a function of hospital length of stay (HLOS). Extending our analysis to the Department of Defense's Military Health System (MHS), we retroactively applied our national cost-savings figures to patients with extremity injuries sustained during Operation Iraqi Freedom and Operation Enduring Freedom (population data provided by the US Army Institute of Surgical Research — Joint Theater Trauma Registry). Cost-savings associated with a reduction in operating room visits were also factored into our model.
2.12. Role of the Funding Source
Funding was provided by the United States Department of Defense Health Programs. JAF, BKP and EAE are active duty service members and performed this work as part of their official duties. All authors had complete access to all of the data and had responsibility for the decision to submit for publication.
3. Results
3.1. Combat-wounded Patients Require Significant Resources
During the study period, 410 consecutive injured service members were screened for enrollment. Of these, 75 met inclusion criteria and provided informed consent. After enrollment, one participant requested to be withdrawn from the study, and one was lost to follow-up. This left 73 participants with 116 unique wounds who underwent 399 individual débridements. The majority of our subjects were injured by blasts from improvised explosive devices; the vast majority had an Injury Severity Score of 16 (Interquartile Range IQR 4,28), and most required a lengthy hospital stay, with about one in four staying longer than 7 weeks. Twenty-three percent of wounds failed after initial closure. The demographic profile of enrollees is summarized in Table 1.
Table 1.
Patient demographics and wounds reflective of the military patient population.
| Number (%) or median (IQR) | |
|---|---|
| Patients (n = 73) | |
| Age (years) | 22 (18.26) |
| Gender | |
| Male | 73 (100) |
| Injury Severity Score | 16 (4,28) |
| Traumatic brain injury | |
| Yes | 47 (64.4) |
| No | 20 (27.4) |
| Unknown | 6 (8.2) |
| Hospital length of stay (days) | 26 (4,48) |
| ICU length of stay (days), 32 patients | 3 (1,7.5) |
| Number of wounds | |
| 1 | 38 (52.1) |
| 2 | 27 (37) |
| 3 | 8 (11) |
| Wounds (n = 116) | |
| Mechanism of Injury | |
| Blast | 106 (91.4) |
| Crush | 1 (0.9) |
| GSW | 9 (7.8) |
| Wound location | |
| Lower Extremity | 97 (83.6) |
| Upper Extremity | 19 (16.4) |
| Wound surface area (cm2) | 225 (43,407) |
| Wound type | |
| Amputation | 15 (12.9) |
| Lower extremity | 12 (10.3) |
| Upper extremity | 3 (2.6) |
| Fasciotomy | 24 (20.7) |
| Lower extremity | 21 (18.1) |
| Upper extremity | 3 (2.6) |
| Open fracture | 12 (10.3) |
| Lower extremity | 9 (7.8) |
| Upper extremity | 3 (2.6) |
| Traumatic soft tissue injury | 65 (56) |
| Lower extremity | 55 (47.4) |
| Upper extremity | 10 (8.6) |
| Injury to closure (days) | 10 (5.75,14.25) |
| Hospital arrival to closure (days) | 5 (1,9) |
| Wound failure | |
| Dehisced | 27 (23.3) |
| Healed | 89 (76.7) |
3.2. Systemic Responses Appear to be a key Driver of Outcome
An analysis of systemic and local cytokines and chemokines suggest that failed wound closure was associated with substantial systemic inflammation. Univariate comparisons demonstrated that serum IL-7, RANTES, VEGF, IFN-γ, IL-10, EOTAXIN, MCP-1, IL-6, IL-2R, HGF, IL-2, IL-17 and EGF and the Effluent IL-7, IL-6, IFN-γ, IL-2R, IL-4, and IP-10 differed significantly (adjusted p < 0.05) in wounds that ultimately failed, compared to those that healed uneventfully. Thirteen of these 19 inflammatory proteins are serum markers that regulate the systemic inflammatory response to injury.
3.3. The Local Wound Environment Contributes to Wound Failure
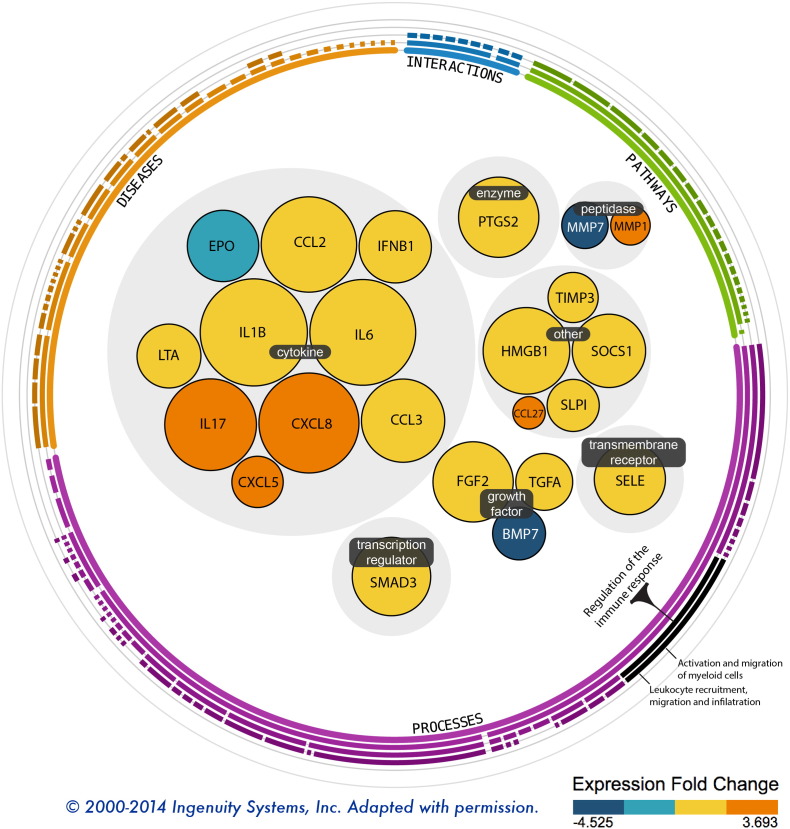
Univariate analysis of the wound gene expression data demonstrated twenty-three transcripts that were differentially expressed in the wounds that failed, compared to those that healed uneventfully. These transcripts are predominately involved in cell migration, leukocyte recruitment, and regulation of the immune response. Fig. 1 depicts these genes, grouped by molecular function.
Fig. 1.
This Ingenuity functional map depicts 23 genes differentially expressed in the 19 wounds that failed, compared to those that went on to heal uneventfully.
3.4. Mathematical Modeling Produced Clinically Promising Decision Support Tools
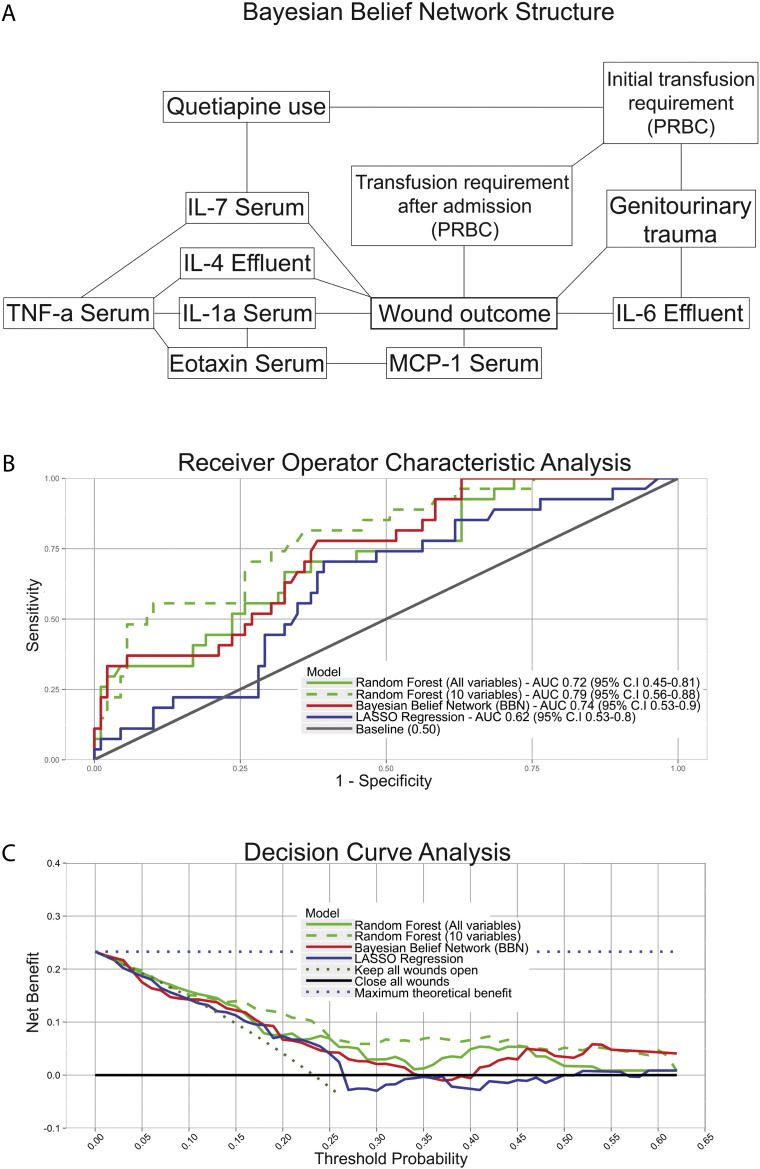
Each modeling method yielded prognostic information. The BBN, represented graphically (Fig. 2a), revealed seven features that are most closely related to the wound outcome, Serum IL7, Effluent IL4, Serum IL1a, Serum MCP-1, Effluent IL-6, Genitourinary trauma, and the transfusion requirement after admission. After measuring accuracy (Fig. 2b), the RF model designed to select the 10 important features was most accurate (AUC 0.79; 95% C.I. 0.57–0.91) followed by the BBN (12 features) (AUC 0.74; 95% C.I. 0.53–0.90) and the RF model designed to consider all 157 features contained within each record (AUC 0.72; 95% C.I. 0.47–0.83). The LASSO model (AUC 0.62; 95% C.I. 0.53–0.8) performed worst of all models and required 8 variables. Throughout the modeling process, we sought to mitigate the risk of overfitting whenever possible. Our efforts to do so are described in the Statistics, Data Modeling, and Cost Analysis section, above.
Fig. 2.
The Bayesian Belief Network can be represented graphically, as demonstrated in Panel A. Receiver Operator Characteristic Analysis and Decision Curve Analysis are depicted in Panels B and C, respectively.
All models demonstrated positive net benefit, indicating they are preferable to assuming that all patients or no patients undergo wound closure (Fig. 2c) at the penultimate débridement. The BBN and RF models were associated with the highest net benefit at some point along the curve, indicating that either could be used clinically, depending on the surgeon's threshold probability—the point at which he or she would become indecisive about whether to close a particular wound. However, the RF model with 10 features provided the highest net benefit over the broadest range of threshold probabilities, indicating that it may be best for clinical use. These results suggest if the only wounds closed were those with a less than a 25% risk of failure, out of every 100 patients, 88 wounds would be closed sooner, 12 wounds would undergo additional débridement(s) prior to closure, and there would be 12 fewer wound failures than if surgeons used standard practice.
3.5. Lessons Learned from Military Trauma Apply to Civilians
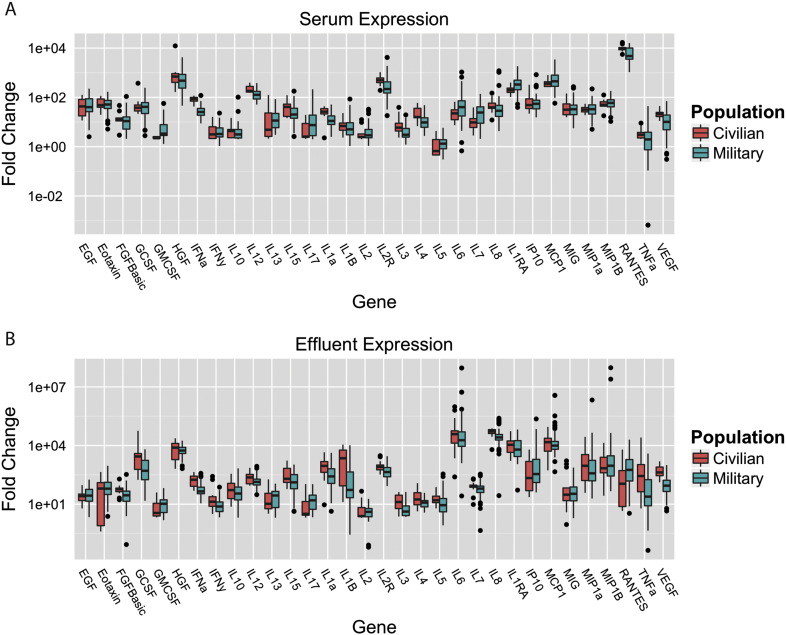
To determine if the biologic responses seen in our military cohort apply to civilian trauma patients, we analyzed a second set of 18 subjects with 27 significant extremity wounds who underwent 49 surgical débridements. Most of these injuries were due to blunt trauma; none were related to blast. Four (15%) of the closed wounds failed. Of note, civilian patients with wound failure exhibit similar distributions in HLOS as those derived from our military sample. Fig. 3 depicts the similarities between the concentrations of the majority of serum and effluent biomarkers in military and civilian patients (Fig. 3). Due to differences in sample analysis platform, direct external model validation was not possible; however, these preliminary findings suggest that future validation of our existing model using civilian patients will be feasible and such tools will be relevant for both populations.
Fig. 3.
The comparison of inflammatory mediators in the serum and effluent of military and civilian patients demonstrates similar distributions. We observed more variability, however, in the concentrations of these proteins in the military patients.
4. Discussion
Blast injuries and other combat-associated wounds present unique challenges to healthcare providers and institutions. We found that comprehensive biological assessment, coupled with advanced mathematical techniques, can be used to generate predictive models that may help surgeons minimize wound-related complications, decrease hospital lengths of stay and lower costs.
For this study, we used demographic, injury, clinical, proteomic, transcriptomic information and machine learning techniques to estimate the likelihood of wound failure in 73 severely injured combat casualties. We did this by using a set of individually low sensitivity/low specificity biomarkers coupled with clinical data to create high sensitivity/high specificity estimations that can inform surgeons when to close wounds to maximize the likelihood of healing and minimize the risk of dehiscence. Improving medical decision-making and critical care in this manner offers several potential benefits (Chen et al., 2013), including minimizing complications, improving outcomes, accelerating recovery and reducing costs. Moreover, our findings suggest that our observations may be generalized to civilian injuries (Biddinger et al., 2013).
Although our findings are promising, this study has limitations. Blast wounds produce highly diverse injury patterns, and our military patient population is comprised of young, previously healthy individuals. It is possible that models generated using data from these patients may not apply to older individuals or patients who have sustained other types of trauma. Our finding that a relatively small sample of civilian trauma patients mounts similar physiologic responses to injury suggests our approach may be applicable outside military populations, but this must be verified in larger samples of patients. Even more important, our study is limited by its relatively small sample size, which can be troublesome considering the large number of variables analyzed in each record. In such situations, it can be difficult to detect meaningful patterns in the data, which also increases the risk of overfitting the models and producing overly optimistic measures of accuracy. By reducing the features in the model using machine learning to identify relevant features, and randomizing the training data using target shuffling (Nisbet et al., 2009), we decreased the risk of overfitting and helped ensure the patterns detected by each modeling approach were unlikely to be due to chance. An additional concern is that wound failure was unequally distributed throughout the dataset. Given the relatively small number of failed wounds, it is statistically easier to identify patterns associated with normal wound healing than less common outcomes such as wound failure. Despite these concerns, we achieved our goal of developing models to estimate the likelihood of wound failure.
Past contributions of military medicine to civilian health care are numerous and substantive. They include improvements in ground and aeromedical medical evacuation (Larrey, 1814, Connor, 2010), regulation (Anon., 1937), open treatment of severe wounds (Moorhead, 1942a, Moorhead, 1942b, Gould, 1917), the combination of surgical débridement with local and systemic use of antibiotics to reduce surgical site infection and sepsis (Moorhead, 1942a, Moorhead, 1942b, Meleney, 1948, Neushul, 1993), the application of vascular surgical techniques (Rich and Hughes, 1969), and, more recently, renewed use of tourniquets and other modalities to achieve field hemostasis (Lakstein et al., 2003). Each of these advances was catalyzed by one or more armed conflicts that stimulated quantum improvements in the treatment of severely injured patients. Most of these medical advances are now ubiquitous in modern civilian health care or, in the case of tourniquets, rapidly becoming so (Biddinger et al., 2013).
Efforts to apply advanced analytics to support clinical decision making have the potential to transform healthcare, as dramatically as advanced analytics have transformed the financial, manufacturing, logistics and telecommunications industries (Brown et al., 2011). In addition to reducing lengths of stay and therefore inpatient costs, precision medicine can improve patient outcomes and save lives. Increasing the efficiency may be particularly important in ICUs, which represent only 10% of inpatient beds, but 20–35% of hospital operating costs (Krell, 2010).
We are not the first group to attempt to apply knowledge gained in armed conflict to benefit future trauma victims. For example, the practice of quantitative bacterial cultures gained popularity after animal studies demonstrated the deleterious effects of high bacterial counts on split thickness skin graft healing (Liedberg et al., 1955). The technique was further developed for use in blast and burn wounds during the Vietnam era to estimate the likelihood of wound healing (Robson and Heggers, 1969, Krizek and Robson, 1975). Though the technique was successfully translated to the civilian setting in the treatment of burns, the use of quantitative culturing to predict the clinical course of blast wounds was lost. By reaffirming this observation that clinical decisions can be guided by biologic data in trauma patients, and demonstrating its utility in a preliminary sample of civilian trauma patients, we seek to reintroduce civilian practitioners to the value of this approach using modern assays and analytical capabilities.
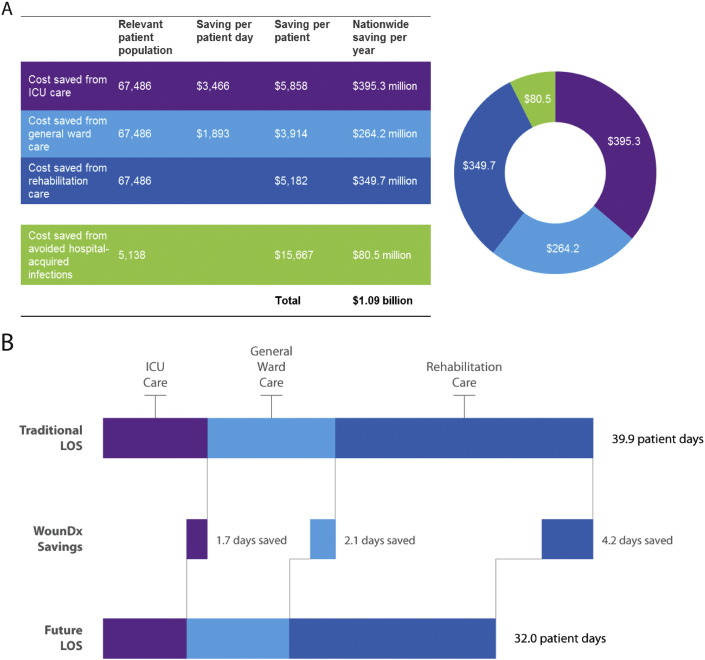
If the clinical decision support models we describe perform equally well in larger samples of patients, they may reduce the rate of open wound dehiscence to 5%. It this were accomplished, it would represent a 68% improvement over current practice in the US (Hostetler et al., 2006). In order to gauge the economic impact of improved decision making in critically injured patients, we performed an analysis on cost savings from reduced need for inpatient ICU care, general ward care, outpatient rehabilitation, and reduced exposure to hospital-acquired infections. Considering the 67,486 patients with traumatic extremity wounds, our approach could save the US healthcare system approximately $1 billion (£670 million) per year, primarily by reducing ICU, general ward, and rehabilitation stays by 1.7, 2.1, and 4.2 days respectively (Fig. 4). Additionally, if the data we report had been available during the past decade of conflict, this approach we describe could decrease treatment costs in the military health system by more than $470 million (£314 million).
Fig. 4.
Cost analysis over the US healthcare system suggests that the use of this clinical decision support tool may afford yearly savings of $1.09B (£730 million) across the US Healthcare system, by reducing ICU, general ward, and rehabilitation stays by 1.7, 2.1, and 4.2 days respectively.
In summary, this study demonstrates that it is not merely the physical destructive nature related to the mechanism of injury in wounds, but the resulting inflammatory response that dictates wound outcome. In addition, by combining biomarker data with clinical observations, we were able to generate highly predictive algorithms to help surgeons identify when to close or otherwise cover wounds in high risk military and civilian populations. Doing so may minimize the risk of wound failure and maximize the likelihood of uneventful healing. If further validated, consistently applying this approach would improve surgical outcomes, allow trauma patients to spend less time in intensive care, and reduce healthcare costs.
Acknowledgments
We thank Stacia Moreno, Fred Gage and Felipe Lisboa for coordinating study enrollment and sample processing. We also thank Richard Barth, BS and Trevor Brown, PhD for helpful data assembly, Ms. Elizabeth Silvius for data analysis, as well as Felix Chang, MBA and Arnaud Belard, MBA for performing the civilian and military business case analyses.
Footnotes
US Government disclaimer:
U.S. Government Disclosure: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of the Army, the Department of Defense, nor the U.S. Government. JAF, BKP and EAE are military service members and this work was prepared as part of their official duties. Title 17 U.S.C §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties. Research activities leading to the development of this manuscript were funded by the Department of Defense – Defense Health Program – Joint Program Committee 6 (USUHS HT9404-13-1-0032 and USUHS HU0001-15-2-0001).
Author contributions:
Conception and design of the study: JAF, EAE and ADK.
Acquisition of data: EAE, JAF, BKP and CJD.
Analysis and interpretation of data: EAE, JAF, BKP, MBW, AV and ADK.
Drafting the article: EAE, JAF and MBW.
Critical revision for important intellectual content: BKP, MBW, AV, CJD, ADK and EAE.
Final approval of the article: JAF, BKP, MBW, AV, CJD, ADK and EAE.
References
- Anon When to lock the stable. N. Engl. J. Med. 1937;217:960. [Google Scholar]
- Bartlett C.S. Clinical update: gunshot wound ballistics. Clin. Orthop. Relat. Res. 2003:28–57. doi: 10.1097/00003086-200303000-00005. [DOI] [PubMed] [Google Scholar]
- Biddinger P.D., Baggish A., Harrington L. Be prepared—the Boston Marathon and mass-casualty events. N. Engl. J. Med. 2013;368:1958–1960. doi: 10.1056/NEJMp1305480. [DOI] [PubMed] [Google Scholar]
- Bowyer G. Débridement of extremity war wounds. J. Am. Acad. Orthop. Surg. 2006;14:S52–S56. doi: 10.5435/00124635-200600001-00012. [DOI] [PubMed] [Google Scholar]
- Brown B., Chui M., Manyika J. Are you ready for the era of ‘big data’. McKinsey Q. 2011 http://www.mckinsey.com/insights/strategy/are_you_ready_for_the_era_of_big_data (October: 1–12) [Google Scholar]
- Casualty Report Casualty Report. http://www.defense.gov/news/casualty.pdf Casualty Report. (accessed Nov 3, 2014)
- Chen L.M., Kennedy E.H., Sales A., Hofer T.P. Use of health IT for higher-value critical care. N. Engl. J. Med. 2013;368:594–597. doi: 10.1056/NEJMp1213273. [DOI] [PubMed] [Google Scholar]
- Connor R. Air & Space Magazine Smithsonian. 2010. Medevac from Luzon. (published online July 1) [Google Scholar]
- Elster E.A., Butler F.K., Rasmussen T.E. Implications of combat casualty care for mass casualty events. JAMA. 2013;310:475–476. doi: 10.1001/jama.2013.167481. [DOI] [PubMed] [Google Scholar]
- Forsberg J., Elster E., Andersen R. Correlation of procalcitonin and cytokine expression with dehiscence of wartime extremity wounds. 2008;90:580–588. doi: 10.2106/JBJS.G.00265. [DOI] [PubMed] [Google Scholar]
- Forsberg J.A., Pepek J.M., Wagner S. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J. Bone Joint Surg. Am. 2009;91:1084–1091. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- Forsberg J.A., Potter B.K., Polfer E.M., Safford S.D., Elster E.A. Do inflammatory markers portend heterotopic ossification and wound failure in combat wounds? Clin. Orthop. Relat. Res. 2014;472:2845–2854. doi: 10.1007/s11999-014-3694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillern S.M., Sheppard F.R., Evans K.N. Incidence of pulmonary embolus in combat casualties with extremity amputations and fractures. J. Trauma. 2011;71:607–613. doi: 10.1097/TA.0b013e3182282574. [DOI] [PubMed] [Google Scholar]
- Gould A.P. An Address ON MODERN ANTISEPTICS: Delivered at a Meeting of the Hunterian Society on November 7th, 1917. Br. Med. J. 1917;2:677–679. doi: 10.1136/bmj.2.2969.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth J.S., Stojadinovic A., Gage F.A. Inflammatory biomarkers in combat wound healing. Ann. Surg. 2009;250:1002–1007. doi: 10.1097/sla.0b013e3181b248d9. [DOI] [PubMed] [Google Scholar]
- Ho T.K. The random subspace method for constructing decision forests. IEEE Trans. Pattern Anal. Mach. Intell. 1998;20:832–844. [Google Scholar]
- Hostetler S.G., Xiang H., Gupta S. Discharge patterns of injury-related hospitalizations with an acute wound in the United States. Wounds. 2006;18:340–351. [Google Scholar]
- Krell K.E. Critical care medicine growth requires dealing with our ‘perfect storm’ of manpower shortage. Crit. Care Med. 2010;38:1613–1614. doi: 10.1097/CCM.0b013e3181da4edb. [DOI] [PubMed] [Google Scholar]
- Krizek T.J., Robson M.C. Evolution of quantitative bacteriology in wound management. Am. J. Surg. 1975;130:579–584. doi: 10.1016/0002-9610(75)90516-4. [DOI] [PubMed] [Google Scholar]
- Lakstein D., Blumenfeld A., Sokolov T. Tourniquets for hemorrhage control on the battlefield: a 4-year accumulated experience. J. Trauma. 2003;54:S221–S225. doi: 10.1097/01.TA.0000047227.33395.49. [DOI] [PubMed] [Google Scholar]
- Landrum M.L., Murray C.K. Ventilator associated pneumonia in a military deployed setting: the impact of an aggressive infection control program. J. Trauma. 2008;64:S123–S128. doi: 10.1097/TA.0b013e31816086dc. [DOI] [PubMed] [Google Scholar]
- Larrey B.D.J. 1814. Memoirs of Military Surgery, and Campaigns of the French Armies, on the Rhine, in Corsica, Catalonia, Egypt, and Syria; at Boulogne, Ulm, and Austerlitz; in Saxony, Prussia, Poland, Spain, and Austria. [Google Scholar]
- Liedberg N.C.F., Reiss E., Artz C.P. The effects of bacteria on the take of split-thickness skin grafts in rabbits. Plast. Reconstr. Surg. 1955;16:412. doi: 10.1097/00000658-195507000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Meleney F.L. The prevention of infection in accidental wounds. In: Andrus E.C., Bronk D.W., Carden G.A., editors. Advances in Military Medicine. Little, Brown & Co; Boston, MA: 1948. pp. 95–110. [Google Scholar]
- Moorhead J.J. Surgical experience at Pearl Harbor. JAMA. 1942;118:712–714. [Google Scholar]
- Moorhead J.J. War wounds. In: Pugh W.S., Podolsky E., Runes D.D., editors. War Surgery. Philosophical Library; New York, NY: 1942. pp. 24–26. [Google Scholar]
- Neushul P. Science, government and the mass production of penicillin. J. Hist. Med. Allied Sci. 1993;48:371–395. doi: 10.1093/jhmas/48.4.371. [DOI] [PubMed] [Google Scholar]
- Nisbet R., Elder J., IV, Miner G. Handbook of Statistical Analysis Data Mining Applications. Academic Press; 2009. Model evaluation and enhancement; pp. 285–311. [Google Scholar]
- Potter B.K., Burns T.C., Lacap A.P., Granville R.R., Gajewski D.A. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J. Bone Joint Surg. Am. 2007;89:476–486. doi: 10.2106/JBJS.F.00412. [DOI] [PubMed] [Google Scholar]
- R Core Team R: A language and environment for statistical computing. http://www.R-project.org/
- Rich N.M., Hughes C.W. Vietnam vascular registry: a preliminary report. Surgery. 1969;65:218–226. [PubMed] [Google Scholar]
- Robson M.C., Heggers J.P. Bacterial quantification of open wounds. Mil. Med. 1969;134:19–24. [PubMed] [Google Scholar]
- Selcer P. Standardizing wounds: Alexis Carrel and the scientific management of life in the First World War. Br. J. Hist. Sci. 2008;41:73–107. [Google Scholar]
- Sherertz R.J., Garibaldi R.A., Marosok R.D. Consensus paper on the surveillance of surgical wound infections. Am. J. Infect. Control. 1992:263–270. [Google Scholar]
- Sheridan R.L., Shumaker P.R., King D.R., Wright C.D., Itani K.M.F., Cancio L.C. Case records of the Massachusetts General Hospital. Case 15-2014. A man in the military who was injured by an improvised explosive device in Afghanistan. N. Engl. J. Med. 2014;370:1931–1940. doi: 10.1056/NEJMcpc1310008. [DOI] [PubMed] [Google Scholar]
- Stromeyer L. Resection in Gunshot Injuries. J.B. Lippincott & Co; Philadelphia: 1862. Gunshot fractures; pp. 20–32. [Google Scholar]
- Tibshirani R. The lasso method for variable selection in the Cox model. Stat. Med. 1997;16:385–395. doi: 10.1002/(sici)1097-0258(19970228)16:4<385::aid-sim380>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. (RESEARCH0034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers A.J., Elkin E.B. Decision curve analysis: a novel method for evaluating prediction models. Med. Decis. Mak. 2006;26:565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warkentien T., Rodriguez C., Lloyd B. Invasive mold infections following combat-related injuries. Clin. Infect. Dis. 2012;55:1441–1449. doi: 10.1093/cid/cis749. [DOI] [PMC free article] [PubMed] [Google Scholar]