Abstract
At the end of mitosis, cells typically complete their division with cytokinesis. In certain tissues however, incomplete cytokinesis can give rise to cells that remain connected by intercellular bridges, thus forming a syncytium. Examples include the germline of many species, from fruitfly to humans, yet the mechanisms regulating syncytial formation and maintenance is unclear, and the biological relevance of syncytial organization remains largely speculative. To better understand these processes, we recently used the germline of Caenorhabditis elegans as a model for syncytium development. Analysis of the germline syncytial architecture throughout development revealed that it arises progressively during larval growth and that it relies on the activity of 2 actomyosin scaffold proteins of the Anillin family. Our work also showed that the gonad can sustain elastic deformation when under mechanical stress and that this property may be conferred by the malleability of syncytial openings. We suggest that elasticity and resistance to mechanical stress constitutes a general property of syncytial tissues.
Keywords: anillin, germline development, cytokinesis, syncytiogenesis
Introduction
Healthy mammalian cells are typically mononucleated and physically separate their duplicated DNA and cytoplasmic content into the 2 daughter cells after mitosis. This step, termed cytokinesis, depends on the synchronized activities of cell cycle regulators, microtubules and the cortical actin cytoskeleton to build and localize an equatorial actomyosin contractile ring, the cytokinetic ring. Ingression of the cytokinetic ring between the 2 segregated sets of chromosomes allows the separation of the daughter cells. In some cases however, cells do not complete cytokinesis and thus form a syncytium, as in germ cells in many species and some physiologically polyploid cells such as mammalian hepatocytes.1,2 Impaired cytokinesis and polyploidy are also often observed in cancer cells where they are thought to favor genetic instability.3-5 One important challenge in cell biology is to understand the physiological mechanisms by which some animal cells undergo controlled incomplete cytokinesis without deleterious consequences such as aneuploidy. Here we discuss our recent findings on syncytial organization using the Caenorhabditis elegans gonad as a model.6 Our work revealed the importance of balancing the activities of 2 proteins of the Anillin-family of actin scaffold proteins to stabilize contractile rings in an open form and suggested a novel interesting role in the resistance of syncytial structures to mechanical stress.
The C. elegans Gonad as a Model for Syncytium Formation and Organization
During development of the C. elegans embryo, the unique germline precursor blastomere, termed P4, divides at approximately the 100 cells stage to give birth to the 2 primordial germ cells (GCs) Z2 and Z3. These cells remain mitotically quiescent until the mid-L1 larval stage, when they start to proliferate. GC mitotic proliferation is sustained throughout larval development until animals complete the last larval stage, when GCs initiate meiosis and gametogenesis. The germline of an adult C. elegans hermaphrodite consists of more than 1000 nuclei organized in 2 gonad arms that form tubular syncytia, in which all GCs surround a central cytoplasm core, termed rachis. Each GC is connected to the rachis through a stable opening, referred to as rachis bridge, that is stabilized by a ring (Fig. 1). As is the case for other syncytia, rachis bridge rings are enriched in the actomyosin regulators that are typically involved in cytokinetic ring formation and ingression such as the non-muscle myosin NMY-2, the 2 Anillin proteins ANI-1 and ANI-2, and the centralspindlin complex components CYK-4 and ZEN-4.6-8 Interestingly, depletion of some of these proteins and other regulators of actomyosin contractility results in severe disorganization of gonad architecture, such as absence of GC partitions and multinucleation.9 This suggests parallels in the organization of stable rachis bridge rings and cytokinetic rings.
Figure 1.
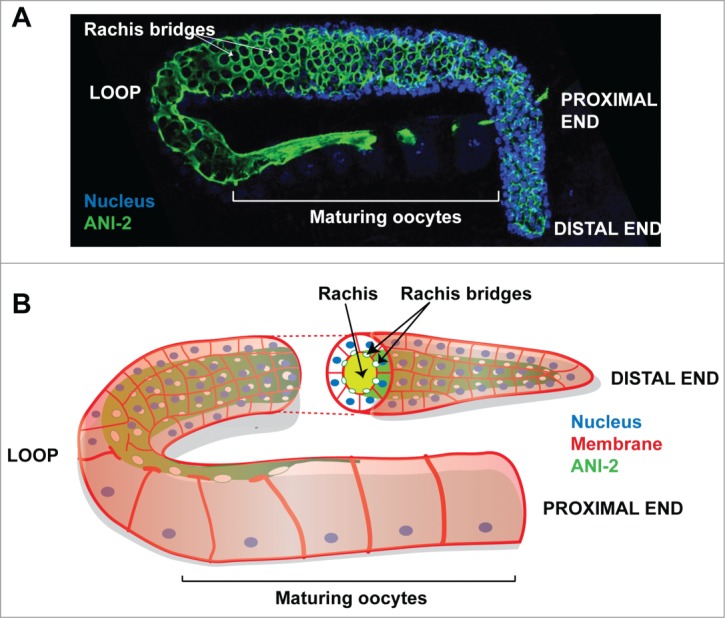
Organization of the C. elegans hermaphrodite gonad (A) Confocal projection of a fixed wild-type hermaphrodite C. elegans gonad arm stained with ANI-2 (highlighting the rachis in green) and DAPI (highlighting DNA in blue). Each rachis bridge is open to the rachis and is stabilized by a ring enriched in ANI-2 and other contractility regulators. (B) Schematic representation of a wild-type hermaphrodite gonad arm. A virtual transverse section of the germline is depicted, showing the rachis and its delineating ANI-2-enriched cortex.
Our recent work in C. elegans revealed that the Anillin proteins ANI-1 and ANI-2 participate in cytokinesis of somatic blastomeres and biogenesis of the germline syncytium.6,10 The nematode protein ANI-1 possesses all the motifs of Drosophila and mammalian Anillin and could, in principle, bind actin, myosin and all the other regulators mentioned above; it is thus considered as the C. elegans canonical Anillin. ANI-2 is a shorter Anillin isoform that lacks the N-terminal domains predicted to bind actin and myosin, but still possesses the C-terminal domains predicted to interact with RhoA, CYK-4, ECT-2, microtubules and septins. While ANI-1 is localized at the cytokinetic ring of all somatic cells and is required for proper asymmetric cytokinetic ring ingression, ANI-2 is mainly enriched at rachis bridges and its levels are minimal in embryonic somatic blastomeres (Fig. 1).6,7 This led us to investigate in more details the role of ANI-2 in the development of the syncytial germline.
Syncytium Biogenesis: From Nucleation to Maturation
During cytokinesis, dividing cells typically form a transient intercellular bridge, known as the midbody, that gets pinched off during cellular abscission. In cells undergoing incomplete cytokinesis however, abscission does not complete and the midbody matures into a stable bridge interconnecting the 2 daughter cells.11 The spatiotemporal analysis of ANI-2 localization during C. elegans embryonic development revealed that it is undetectable in early blastomeres and becomes enriched specifically in the P4 germline blastomere. Interestingly, upon division of P4, ANI-2 accumulates and persists at the midbody, suggesting that its loading and stabilization between the 2 primordial GCs serves as nucleating event in syncytial organization. Despite this early accumulation of ANI-2, we could not detect exchange of a fluorescent marker between Z2 and Z3, suggesting that the 2 cells do not share cytoplasm and, thus, are not syncytial at this stage. This indicates that if rachis bridges are nucleated upon P4 division, they require further maturation to allow cytoplasmic exchange. This is compatible with the observation, made by electron microscopy, that syncytial organization of the germline only becomes apparent at the second larval stage.12
Analysis of ANI-2 localization during larval development revealed that it remains enriched at the apical side of GCs and at rachis bridge rings at all stages, including in adult animals. Interestingly, we found that rachis bridge diameter increases as animals progress through larval stages. These results suggest that rachis bridge maturation occurs progressively during larval development and is independent from rachis bridge nucleation. Such decoupling could be analogous to Drosophila, where ring canals and cytoplasmic bridges connecting GCs increase in diameter as GCs progress through gametogenesis.13 While an increase in cell size could in principle account for the increase in rachis bridge diameter, we found that the size of C. elegans GCs remains largely constant during larval development (unpublished results). It will be interesting to determine the mechanism that allows the increase of rachis bridge diameter, as this may inform on the regulation of their maturation. It has been shown that the tight bundling of actin filaments to one another is key to ring canal expansion and stabilization during Drosophila oogenesis.13 While there are similarities of composition between C. elegans cytokinetic rings and rachis bridge rings, whether a process such as this promotes rachis bridge maturation has yet to be determined.
The presence of ANI-2 at the onset of germline formation and its persistence at rachis bridges throughout larval development suggested that it plays a role in rachis bridge nucleation and/or maturation. Analysis of rachis bridge organization in ani-2 mutant animals revealed that the majority of GCs had no detectable rachis bridge. A minority of GCs (30%) had well defined rachis bridges but, as opposed to controls, their diameter did not increase during larval development. This suggests that ANI-2 is not essential for rachis bridge nucleation but that its presence is crucial during rachis bridge maturation to promote their opening.
Syncytial Maintenance Depends on a Balance Between Anillin Activities
Phenotypic analysis of early embryonic development previously suggested that ANI-2, lacking the predicted N-terminal actin- and myosin-binding domains found in ANI-1, may function as negative regulator of contractility.10 ANI-1, like ANI-2, is enriched at GC rachis bridges throughout C. elegans development suggesting that the 2 Anillin proteins function in germline organization. As reported in our recent article,6 we observed that depletion of ANI-1 by RNAi did not cause severe defects in gonad organization but resulted in an increase in the diameter of rachis bridges, an increase in rachis width and a delay in oocyte cellularization (Fig. 2). Interestingly, these defects are opposite to those observed in ani-2 mutants and many of the phenotypes of ani-2 mutants were partly suppressed by RNAi depletion of ANI-1. This suggests that the defects in ani-2 mutant animals result from an increase in ANI-1 activity and that the 2 Anillin proteins counteract each other to regulate the rachis bridge stability and germline syncytial organization. What regulates the balance of activity between the 2 Anillins is currently unclear. The two proteins do not control each other's accumulation at rachis bridges, and thus this balance may rely on the potential capacity of ANI-2 to “titrate” one or many actomyosin regulators required for ANI-1 function in organizing or stabilizing contractile networks. Interestingly, phenotypic profiling screens in the C. elegans gonad have shown that depletion of several proteins, including known regulators of actomyosin contractility, results in defects reminiscent of those observed in ani-2 mutants.9 Whether these proteins work with ANI-2 to regulate syncytial organization is not known, but future analyses of the molecular composition of rachis bridge rings and of the mutual dependence of these proteins for their proper localization will enhance our understanding of rachis bridge nucleation/maturation and of syncytial organization.
Figure 2.
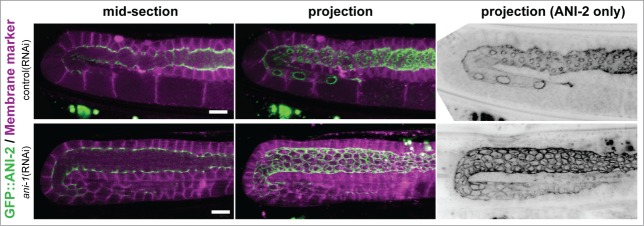
ANI-2 localization in control and ANI-1-depleted animals. Confocal mid-sections and projections of live wild-type adult hermaphrodite gonads treated with control(RNAi) or ani-1(RNAi) and expressing GFP::ANI-2 (green) and a membrane marker (magenta). Rachis diameter is significantly increased and GC rings are significantly larger in ani-1(RNAi) animals compared to control. Scale bars, 10 μm.
The Syncytial Architecture of the Gonad Favors Resistance to Mechanical Stress
One of the striking phenotypes associated with the loss of ani-2 is a germ cell multinucleation defect that initiates upon entry into adulthood and progresses in severity from then on. This is a striking phenotype as the germline of ani-2 mutants is largely morphologically normal until then, except for a decrease in rachis bridge diameter and in rachis width. Our analysis revealed that the multinucleation defect results from a collapse of GC partitions and is not a consequence of severe endoreplication or cytokinetic failure, suggesting that it is of non-cell autonomous origin.
One of the processes that begins when hermaphrodites enter adulthood is oogenesis, an event during which oocyte growth is sustained by cytoplasmic streaming in the rachis.14 We found that multinucleation is dependent on oogenesis, as multinucleated compartments were reduced in conditions where oogenesis was absent, such as in male gonads (which form only sperm) and in gonads depleted of GLD-1/2 (which contain only mitotic GCs). We postulated that the cytoplasmic streaming that occurs during oogenesis could generate mechanical stress at rachis bridges, and that in absence of ANI-2 this stress could be sufficient to provoke the collapse of partitions between GCs. To test this hypothesis, we imaged adult hermaphrodite gonads during ovulation, when entry of the oocyte into the spermatheca is accompanied by an important deformation and stretching of the rachis. Interestingly, we found that this stretching is transient and is accompanied by an equally transient increase in the diameter of the most proximal rachis bridge. This indicates that the gonad has elastic properties and further suggests that rachis bridges account for its elastic deformation. Importantly, this elastic response upon ovulation was lost in animals partially depleted of ANI-2, suggesting that ANI-2 is important to confer elastic properties to the gonad. Our results suggest a model in which the presence of ANI-2 at rachis bridge rings allows their deformation, which in turns, confers elastic properties to the whole tissue and allows it to sustain deformation and thus compensate for mechanical stress. Tissue elasticity and resistance to mechanical stress could be a conserved feature of syncytia. For instance, recent work on murine syncytiotrophoblasts grown in culture revealed that their cortex is more elastic than that of the mononucleated trophoblasts from which they are derived.15 While much remains to be done to fully understand the precise mechanism by which syncytia are formed and maintained, our work suggests that the differential expression and/or regulation of contractility regulators such as ANI-1 and ANI-2 may provide the necessary tools to begin addressing this important question.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Haglund K, Nezis IP, Stenmark H. Structure and functions of stable intercellular bridges formed by incomplete cytokinesis during development. Commun Integr Biol [Internet] 2011. [cited 2013 Mar 26]; 4:1-9. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3073259&tool=pmcentrez&rendertype=abstract; PMID:21509167; http://dx.doi.org/ 10.4161/cib.13550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Margall-Ducos G, Celton-Morizur S, Couton D, Bregerie O, Desdouets C. Liver tetraploidization is controlled by a new process of incomplete cytokinesis. J Cell Sci [Internet] 2007. [cited 2013 Jun 11]; 120:3633-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17895361; http://dx.doi.org/ 10.1242/jcs.016907 [DOI] [PubMed] [Google Scholar]
- 3. Davoli T, de Lange T. Telomere-driven tetraploidization occurs in human cells undergoing crisis and promotes transformation of mouse cells. Cancer Cell [Internet] 2012. [cited 2014 Aug 26]; 21:765-76. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3376354&tool=pmcentrez&rendertype=abstract; PMID:22698402; http://dx.doi.org/ 10.1016/j.ccr.2012.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ganem NJ, Cornils H, Chiu S-Y, O’Rourke KP, Arnaud J, Yimlamai D, Théry M, Camargo FD, Pellman D. Cytokinesis failure triggers hippo tumor suppressor pathway activation. Cell [Internet] 2014. [cited 2014 Aug 14]; 158:833-48. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0092867414008204; PMID:25126788; http://dx.doi.org/ 10.1016/j.cell.2014.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fujiwara T, Bandi M, Nitta M, Ivanova E V, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature [Internet] 2005. [cited 2014 Sep 8]; 437:1043-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16222300; PMID:16222300; http://dx.doi.org/ 10.1038/nature04217 [DOI] [PubMed] [Google Scholar]
- 6. Amini R, Goupil E, Labella S, Zetka M, Maddox AS, Labbé J-C, Chartier NT. C. elegans anillin proteins regulate intercellular bridge stability and germline syncytial organization. J Cell Biol [Internet] 2014. [cited 2014 Sep 8]; 206:129-43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24982432; PMID:24982432; http://dx.doi.org/ 10.1083/jcb.201310117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maddox AS, Habermann B, Desai A, Oegema K. Distinct roles for two C. elegans anillins in the gonad and early embryo. Development [Internet] 2005; 132:2837-48. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&=15930113; PMID:15930113; http://dx.doi.org/ 10.1242/dev.01828 [DOI] [PubMed] [Google Scholar]
- 8. Zhou K, Rolls MM, Hanna-Rose W. A postmitotic function and distinct localization mechanism for centralspindlin at a stable intercellular bridge. Dev Biol [Internet] 2013. [cited 2013 Jun 16]; 376:13-22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23370148; PMID:23370148 [DOI] [PubMed] [Google Scholar]
- 9. Green RA, Kao H-L, Audhya A, Arur S, Mayers JR, Fridolfsson HN, Schulman M, Schloissnig S, Niessen S, Laband K, et al. A high-resolution C. elegans essential gene network based on phenotypic profiling of a complex tissue. Cell [Internet] 2011. [cited 2013 Aug 13]; 145:470-82. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3086541&tool=pmcentrez&rendertype=abstract; PMID:21529718; http://dx.doi.org/ 10.1016/j.cell.2011.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chartier NT, Salazar Ospina DP, Benkemoun L, Mayer M, Grill SW, Maddox AS, Labbé J-C. PAR-4/LKB1 mobilizes nonmuscle myosin through anillin to regulate C. elegans embryonic polarization and cytokinesis. Curr Biol [Internet] 2011. [cited 2013 Mar 26]; 21:259-69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21276723; PMID:21276723 [DOI] [PubMed] [Google Scholar]
- 11. Greenbaum MP, Ma L, Matzuk MM. Conversion of midbodies into germ cell intercellular bridges. Dev Biol [Internet] 2007. [cited 2013 Mar 4]; 305:389-96. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2717030&tool=pmcentrez&rendertype=abstract; PMID:17383626; http://dx.doi.org/ 10.1016/j.ydbio.2007.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev Biol [Internet] 1976; 49:200-19. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20026024; PMID:943344; http://dx.doi.org/ 10.1016/0012-1606(76)90267-0 [DOI] [PubMed] [Google Scholar]
- 13. Tilney LG, Tilney MS, Guild GM. Formation of actin filament bundles in the ring canals of developing Drosophila follicles. J Cell Biol [Internet] 1996. [cited 2014 Oct 29]; 133:61-74. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2120773&tool=pmcentrez&rendertype=abstract; PMID:8601-614; http://dx.doi.org/ 10.1083/jcb.133.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolke U, Jezuit EA, Priess JR. Actin-dependent cytoplasmic streaming in C. elegans oogenesis. Development [Internet] 2007; 134:2227-36. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17507392; PMID:17507392; http://dx.doi.org/ 10.1242/dev.004952 [DOI] [PubMed] [Google Scholar]
- 15. Zeldovich VB, Clausen CH, Bradford E, Fletcher D. a, Maltepe E, Robbins JR, Bakardjiev AI. Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog [Internet] 2013. [cited 2014 Sep 8]; 9:e1003821. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3861541&tool=pmcentrez&rendertype=abstract; PMID:24348256; http://dx.doi.org/ 10.1371/journal.ppat.1003821 [DOI] [PMC free article] [PubMed] [Google Scholar]


