Abstract
Background
To evaluate tumor-associated macrophages (TAMs) infiltration and microvessel density as possible prognostic factors related to prostate cancer (PCa) progression.
Methods
Immunostaining of TAMs in prostate biopsy specimens was performed using a monoclonal antibody CD68 and microvessel density (MVD) using von Willebrand factor (vWF) from 25 specimens with high-grade prostatic intraepithelial neoplasia (HGPIN) and 25 specimens with PCa after transurethral resection of the prostate (TURP). Six microscopic (×200) fields were selected for TAM counting and six microscopic (×100) fields were selected for MVD counting around the cancer foci. Association between age, preoperative prostate-specific antigen (PSA), pathologic Gleason sum (GS), TAM, MVD, extracapsular extension, and metastasis were assessed using Pearson/Spearman, Student t test/Mann-Whitney U test and one-way analysis of variance/Kruskal-Wallis test.
Results
The mean of age, PSA, TAMs, and MVD were 69.1 ± 9.9, 67.1 ± 92.4, 26.2 ± 11.9, and 31.4 ± 14.0, respectively, from 50 specimens with PCa and HGPIN. Increasing TAMs number was not correlated with increasing MVD number and there was no significant mean difference statistically (P > 0.05) in TAMs and MVD although the mean of TAMs number was higher in PCa versus HGPIN but significant in PSA level (P < 0.001). In PCa specimens, age, PSA, TAMs, and MVD number were higher in patients with metastatic and extracapsular extension, but not significant statistically (P > 0.005). There was no correlation between TAMs and MVD (P > 0.001).
Conclusions
TAMs and MVD had increased PCa but did not provide independent prognostic value. Increasing numbers of TAMs was not always followed by an increase in MVD. HGPIN is the most likely precursor for PCa.
Keywords: High-grade prostatic intraepithelial neoplasia, Microvessel density, Prostate cancer, Tumor-associated macrophage
Introduction
Prostate cancer (PCa) is the second most common disease and the sixth leading cause of death in males, around 14% (903,500) of the total new cancer cases of the disease and 6% (258,400) of the total deaths caused by cancer in men at the end of 2008.1 The cause of initiation and progression of PCa is not yet recognized; some studies suggest that genetic factors, race, diets, and environment factors play an important role in the development of the disease.2,3
Tumor-associated macrophages (TAM) played a significant biological role in initiation and progressivity of tumor. However, the clinical significance of TAM in various cancers is not yet determined. This research was designed to determine whether infiltration of TAM is a predictor of a disadvantageously pathological parameter and a poor prognosis in men undergoing radical prostatectomy (RP) for PCa. This research was consistent with previous research, which reported a higher level of TAM in malignant PCa compared to the benign tissue and higher Gleason score. The level of TAM was higher than in prostatic intraepithelial neoplasia (PIN) compared with benign tissue. The higher Gleason score containing a higher number of TAM was also compared with the lower Gleason score. Although the mechanisms of TAM promoting the development and the progression of prostate cancer is not known, animal studies in vivo properties showed that mobilization and infiltration of TAM played a key role in the development and the progression of PCa, and a histopathological study showed that the level of TAM positively correlated with the microvessel density (MVD).4
With PCa, in addition to tumor grading, vascular invasion is also evaluated, because the presence of malignant cells in the vessels increases the risk of pelvic metastasis. The theory of tumor dependence growth in angiogenesis is directed by scientists to focus on angiogenesis inhibition as a method to control the growth of neoplastic cell. The number of angiogenesis tumors can be measured quantitatively with MVD techniques. In this technique, endothelial cells have an immune reaction by immunohistochemical means, and then are counted with an optical microscope. Some studies have indicated the correlation between MVD and risk of tumor invasion on prostate and breast cancer. Additionally, correlation between MVD, vascular invasion, nuclear pleomorphism, and proliferation has been observed. Although association between MVD and the level of survival sometimes seems to be controversial, many scientists have suggested MVD as a prognostic and a predictive factor.5
MVD, which increased in PCa tissues caused by proliferation of neovessels and the increase of MVD was associated with the prognosis and development of PCa, and metastasis, the degree of the disease, and the survival rate. Thus, visualization of MVD can improve detection and cancer grading. The Gleason score is based on the microscopic characterization of PCa. This is the most commonly used system for PCa grading and is an important factor in the formulation therapy schedule and the prognosis of PCa. Evaluation of a tumor is significant in regarding detection and characterization. The study of MVD in PCa showed the relationship between the increase MVD with the carcinoma and with the higher tumor grade.6
The risk of finding PCa in a subsequent biopsy with HGPIN is 15 times greater than in biopsies without PIN. HGPIN shares many biochemical and genetic changes with cancer.7 Our objective was to evaluate TAMs infiltration and microvessel density in HGPIN and PCa.
Materials and methods
This research was a prospective analytic study with the cross-sectional design to examine the correlation between TAMs and MVD as well as the PCa progression. The patients in whom PCa was diagnosed at Sardjito Hospital from 2009 to 2011 were selected. Patients with prostate preparation after a well-processed treatment (on the evaluation of Hematoxylin and eosin stain (HE)), were examined for prostate specific antigen (PSA), and inspection bone scan/scintigraphy/bone survey was performed about 2 weeks after the treatment of transurethral resection of the prostate (TURP) and the size of prostate was measured through transabdominal/transrectal ultrasonography (TAUS/TRUS) incorporated in the research. In our hospital, RP was rarely done during this period (2 patients). Therefore, The RP specimen was excluded.
The extracapsular extension, seminal vesicle invasion, and lymph node metastasis were assessed by magnetic resonance imaging (MRI) and/or multi-slice computer tomography (MSCT). The prostate preparation was taken from the result of core biopsy, prostate preparation with the diagnosis besides PIN high-grade and prostate adenocarcinoma, prostate adenocarcinoma preparation consisted of prostate small tumor focus (<5 spacious point), damaged paraffin block lost or impossible to cut off with microtome, preparation that did not meet the requirements for the immunohistochemical examination and the patient who had no any completely medical record data were excluded.
TAMs comprise the macrophages in stroma tissues (peritumoral) assessed through immunohistochemical examination using antibodies of anti-CD68 through screening all tumor areas and determined six hot spot areas (area with the most populous of positive CD68) with a weak enlargement (×50), chosen under a microscope at ×200 magnification, and made the average value of the 6 areas around the cancer foci. MVD was an indicator of angiogenesis assessed using average microvessel count (AMC) methods with considering the expression of vWF at endhotelial cell with immunohistochemical examination using antibody of vWF. This method was performed by determining six areas straddling the border between the tumor and normal tissues with strong enlargement (×100), and then the mean of positive expression of vWF positive was made. Association between age, preoperative PSA, pathologic Gleason score (GS), TAM, MVD, extracapsular extension, and metastasis were assessed using Pearson/Spearman, Student t/Mann-Whitney U tests, and the one-way analysis of variance/Kruskal-Wallis test.
Results
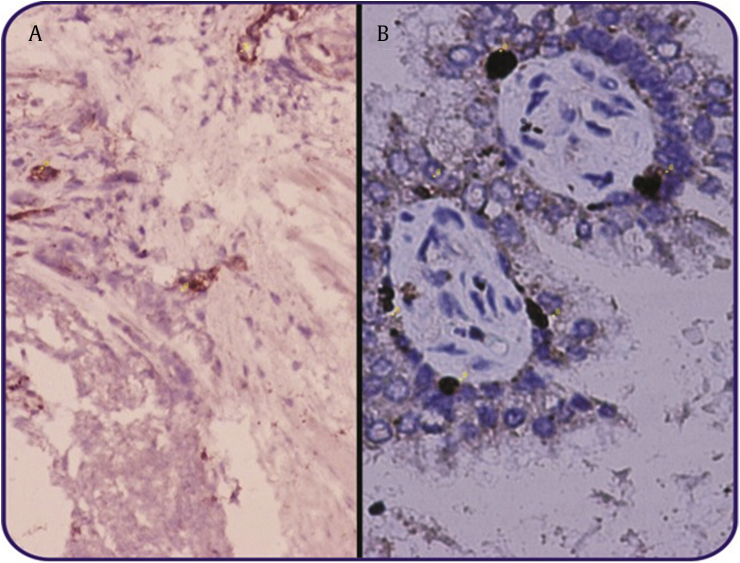
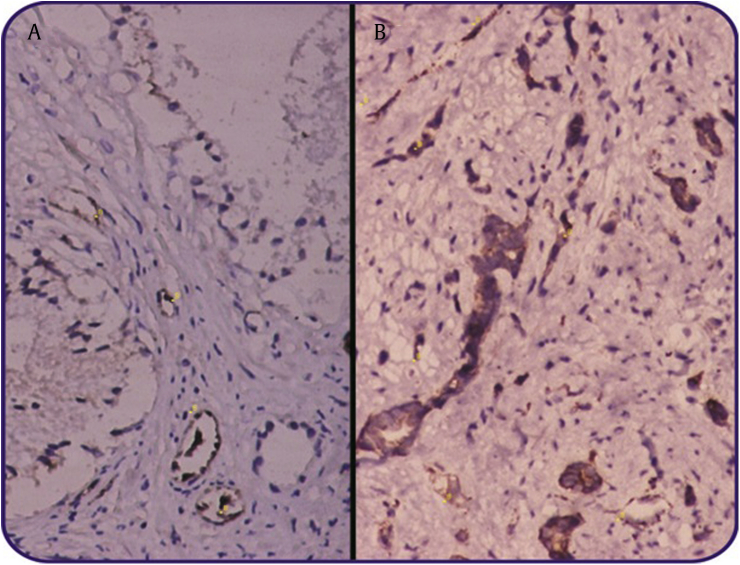
This study had been carried out using 50 specimens consisting of 25 specimens of prostate cancer and 25 specimens of HGPIN. TAMs assessment was performed with immunohistochemical staining of prostate specimens using monoclonal antibody of CD68 and the evaluation of MVD was through immunohistochemical staining of prostate specimens using the monoclonal antibody of vWF (Fig. 1 and 2). From this research, obtained average age, PSA, TAMs, and MVD in all samples were 69.1 ± 9.9, 67.1 ± 92.4, 2 ± 11.9, and 31.4 ± 14.0 respectively. In prostate cancer, there were 11 patients (44%) experiencing metastasis, 7 patients (28%) with extracapsular extension, and 14 patients (56%) with Gleason score ≥8 (Table 1).
Fig. 1.
Coloration of immunohistochemical TAMs to specimens using monoclonal antibody of CD68 ×200. (A) High-grade PIN, (B) Prostate Cancer. TAM, tumor-associated macrophages; PIN, prostatic intraepithelial neoplasia.
Fig. 2.
Coloration of immunohistochemical MVD to specimens using monoclonal antibody von Willebrand factor (vWF) ×100. (A) High-grade PIN, (B) Prostate Cancer. MVD, microvessel density; PIN, prostatic intraepithelial neoplasia.
Table 1.
Characteristics of research variable.
| Variables | |
|---|---|
| Age (mean ± SD, median) | 69.1 ± 8.4 (70.5) |
| PSA (mean ± SD, median) | 67.1 ± 92,4 (34.4) |
| TAMs (mean ± SD, median) | 26,2 ± 11.9 (24) |
| MVD (mean ± SD, median) | 31.4 ± 14.0 (27) |
| Metastasis (N, %) | |
| Yes | 11 (44) |
| No | 14 (56) |
| Extracapsular extension (N, %) | |
| Yes | 7 (28) |
| No | 18 (72) |
| Gleason score (N, %) | |
| ≤6 | 4 (16) |
| 7 | 7 (28) |
| ≥ 8 | 14 (56) |
MVD, microvessel density; PSA, prostate-specific antigen; SD, standard deviation; TAMs, tumor-associated macrophages.
Univariate analysis
The increase of TAMs was not related to the increase of MVD with Spearman test (P = 0.103) and there was no mean difference between in the number of TAMs and MVD, between PCa and HGPIN, though TAMs mean was higher on PCa than HGPIN (27.6 ± 14.2 vs. 24.7 ± 9.3). Nevertheless, there were differences in mean of PSA between PCa and HGPIN with P < 0.001 (117.5 + 106.8 versus 27.1 –16.6 ±) (Table 2).
Table 2.
Univariate analysis between prostate cancer versus high-grade prostatic intraepithelial neoplasia.
| Variable | Prostate cancer | High-grade PIN | P |
|---|---|---|---|
| Age (mean ± SD, median) | 69.4 ± 9.9 (71) | 68.8 ± 6.8 (68) | 0.817a) |
| PSA (mean ± SD, median) | 117.5 ± 106.8 (87.6) | 16.6 ± 27.1 (9.3) | <0.001b) |
| TAMs (mean ± SD, median) | 27.6 ± 14.2 (26) | 24.7 ± 9.3 (24) | 0.393a) |
| MVD (mean ± SD, median) | 31.1 ± 16.4 (26) | 31.7 ± 11.6 (28) | 0.392b) |
MVD, microvessel density; PIN, prostatic intraepithelial neoplasia; PSA, prostate-specific antigen; SD, standard deviation; TAMs, tumor-associated macrophages.
Independent t test.
Mann-Whitney U test.
In the cancer prostate group, based on the incident of metastasis (yes vs. no), age (70.9 ± 11.8 vs 68.1 ± 8.4), PSA (143.5 ± 135.9 vs. 97.0 ± 76.1), TAMs (32.2 ± 16.3 vs. 24.1 ± 11.7) and MVD (38.3 ± 20.5 vs. 25.4 ± 9.7), overall data had mean score higher on metastasic patient, but statistically not significant (P > 0.05) (Table 3).
Table 3.
Univariate analysis on prostate cancer based on the incident of metastasis.
| Variable | Yes | No | P |
|---|---|---|---|
| Age (mean ± SD, median) | 70.9 ± 11.8 (75) | 68.1 ± 8.4 (70.5) | 0.501a) |
| PSA (mean ± SD, median) | 143.5 ± 135.9 (100) | 97.0 ± 76.1 (68.8) | 0.379b) |
| TAMs (mean ± SD, median) | 32.2 ± 16.3 (29) | 24.1 ± 11.7 (24.5) | 0.160a) |
| MVD (mean ± SD, median) | 38.3 ± 20.5 (33) | 25.4 ± 9.7 (22.5) | 0.112a) |
MVD, microvessel density; PSA, prostate-specific antigen; SD, standard deviation; TAMs, tumor-associated macrophages.
Independent t-test.
Mann-Whitney U test.
In the PCa group, based on the extracapsular extension (yes vs. no), age (72.3 ± 10.3 vs. 68.2 ± 9.8), PSA (148.2 + 131.9 vs. 105.5 ± 96.9) and TAMs (32.3 ± 22.2 vs. 25.8 ± 9.9), all data had mean score higher on patients with extracapsular extension but statistically not significant (P > 0.05). Mean of MVD (27.7 ± 15.2 vs. 32.4 ± 17) was higher in patients who did not experience the extracapsular extension, but statistically not significant (P > 0.05). (Table 4).
Table 4.
Univariate analysis on prostate cancer based on the extracapsular extension.
| Variable | Yes | No | P |
|---|---|---|---|
| Age (mean ± SD, median) | 72.3 ± 10.3 (77) | 68.2 ± 9.8 (71) | 0.369a) |
| PSA (mean ± SD, median) | 148.2 ± 131.9 (120) | 105.5 ± 96.9 (64.5) | 0.412b) |
| TAMs (mean ± SD, median) | 32.3 ± 22.2 (34) | 25.8 ± 9.9 (25) | 0.482a) |
| MVD (mean ± SD, median) | 27.7 ± 15.2 (22) | 32.4 ± 17 (27.5) | 0.544a) |
MVD, microvessel density; PSA, prostate-specific antigen; SD, standard deviation; TAMs, tumor-associated macrophages.
Independent t test.
Mann-Whitney U test.
For mean of age, TAMs and MVD was not higher than in the Gleason score, except for PSA which had higher mean for patients with higher Gleason score (Table 5). On Spearman correlation testing, no correlation was found between TAMs and MVD on prostate cancer with metastasis (P = 0.157) and the extracapsular extension (P = 1.492).
Table 5.
Univariate analysis on prostate cancer based on the gleason score.
| Variable | ≤6 | 7 | ≥8 | P |
|---|---|---|---|---|
| Age (mean ± SD, median) | 72.3 ± 3.2 (71) | 68.3 ± 12.4 (66) | 69.1 ± 10.3 (74) | 0.818a) |
| PSA (mean ± SD, median) | 52.9 ± 67.9 (28.4) | 78.1 ± 52.9 (50) | 155.6 ± 122.5 (129.9) | 0.092b) |
| TAMs (mean ± SD, median) | 23.8 ± 5.7 (25) | 33.4 ± 18.9 (38) | 25.9 ± 13.1 (23.5) | 0.448a) |
| MVD (mean ± SD, median) | 33.3 ± 5.4 (34) | 32.0 ± 13.5 (29) | 30.0 ± 19.9 (21.5) | 0.441a) |
MVD, microvessel density; PSA, prostate-specific antigen; SD, standard deviation; TAMs, tumor-associated macrophages.
One-way analysis of variance.
Kruskal-Wallis test.
Discussion
The increase of TAM infiltration had been associated with the pathological characteristics and poor prognosis in various cancers, including breast, colorectal, and bladder cancer. However, in other research, an infiltration of TAM was associated with prognosis or did not have the prognostic value in colorectal cancer and breast cancer. Similarly, the clinical significance of TAM in the development of prostate cancer and survival has not been clear. Two previous studies indicated that the increase of TAM infiltration was associated with survival-specific cancer that is associated with a worse survival, whereas the other study found that the increase of TAM infiltration in prostate tumor was a predictor of patient survival rates. These studies had been restricted by the small sample size and the lack of treatment modality uniformity, so that it was difficult to draw a conclusion about the significance of TAM in prostate cancer; histopathological study demonstrated that the level of TAM was positively correlated with MVD.4
The clinical significance of TAMs and MVD in prostate cancer is not yet determined and is still controversial. This research was designed to determine whether infiltration of TAMs and the increase of MVD were predictors of disadvantageous pathological parameters and poor prognosis in men who are undergoing TURP for PCa. Studies have been performed using 50 specimens, consisting of 25 specimens of PCa and 25 specimens of HGPIN. From this research, mean of age, PSA, TAMs, and MVD in all samples was 69.1 ± 9.9, 67.1 ± 92.4, 26.2 ± 11.9, and 31.4 ± 14.0, respectively. In PCa, 11 patients (44%) experienced metastasis, seven patients (28%) with extrascapsular extension, and 14 patients (56%) with Gleason score ≥ 8. Nonomura et al8 examined TAMs infiltration in 71 patients with prostate cancer and obtained the median of age 74, TAM 22, and PSA 50.1 ng/mL with 21 patients (29.6%) with ≥8 Gleason score and 11 patients (15.5%) with extracapsular extension.
Previous research reported a higher level of TAM in malignant PCa compared with benign tissues and the higher Gleason score. The lever of TAM is higher in PIN than in benign tissues. The higher Gleason score containing a higher number of TAM was also compared with the lower Gleason score.4 Nonomura et al8 reported that the median of PSA is 50.1 ng/mL and median of TAM is 22. Survival, based on recurrence rate, was better on patients with < 22 total TAMs than with ≥ 22 total TAMs, and TAMs infiltration had significant relationships with PSA levels, and the Gleason score and stage T in prostate cancer.8 Lissbrant et al9 found that the increase of TAMs could be a nasty predictive factor in prostate cancer patients after undergoing TURP.9 Gannon et al10 investigated some immune cells in the prostate after RP with or without neoadjuvant-reducing androgen therapy, and found that the increase of TAMs was a predictor of biochemical recurrence (PSA) on univariate test but not a predictor on multivariate analysis.10
In this research, the increase in the total of TAMs was not related to the increase of MVD (P = 0.103), no mean difference was found between the total of TAMs and MVD between PCa and HGPIN though mean of TAMs in PCa was higher than in HGPIN, and in the metastasis and in extracapsular extension PCa group. This research was consistent with the study of Gollapudi et al,4 which reported that the mean of TAMs in PCa is higher than in PIN and benign prostate enlargement. But, TAMs infiltration was not predictive of biochemical recurrence (PSA level) after RP.4 The variations noted in TAM levels in these studies compared to our own can partly be explained by different quantification methods and amount of tissue used to determine TAM levels. There is presently no standardized method for quantification of TAM levels, thus making it difficult to compare studies.
In PCa, in addition to the tumor grading, the vascular invasion is evaluated, because the existence of malignant cells in the vessels increases the risk of pelvic bone metastasis. The theory of dependence tumor growth in angiogenesis is directed by scientists to focus on angiogenesis inhabitation as a method to control neoplastic cell growth. The amount of tumor angiogenesis can be measured quantitatively and MVD techniques.5 In PCa, the invasion of vascular toward the development of cancer depends on the extension extracapsular, the inclusion vesika vesicle, tumor size, tumor metastasis to the lymph node, positive surgery limits, and the grade of pathological examination.2,6 The increase of MVD in PCa is similar as TAMs which is debatable.5,6 Previous investigations showed a weak correlation between MVD, the pathological parameters, and PCa exodus.11 Muhammadnejad et al reported that there was a relationship between MVD and vascular invasion, and provide the predictive value in prostate cancer.5 Haese et al12 reported that MVD with immunohistochemical CD31 showed a significant relationship between pathological stage and the higher Gleason score. Wiedner et al13 also reported the relationship between MVD and invasive PCa and metastasis incident with factor VII-related immunohistochemical antigens (F8-RA).
In this research, there was no MVD difference between prostate cancer with HGPIN and in the PCa group with metastasis or extracapsular extension. This was not in accordance with the theory and research. However, this result was same as several studies reporting that MVD is not associated with some parameters of PCa (tumor grade, the vascular and gland lymph invasion, capsular penetration, PSA recurrence and survival).14 Reuben et al15 and Luczynska et al16 reported that the increase of MVD was not related to the tumor stage, the Gleason score, tumor size, and invasion of the tumor to seminal vesicles.
The absence of MVD correlation on PCa parameters in this research could be caused by several factors involving the small size of sample, the different method of MVD calculation, and the kind of immunohistochemical staining used. Some theories described that MVD was not an indicator to assess an angiogenic process in tumor and is a reflection of the metabolic process of a cancer. This description was also in contrast with a previous theory, which stated that MVD did not represent angiogenesis activities, but only described the distance among tumor capillaries. Oxygen and other nutrition were a limit or how far the distance of vascularization in a tumor was.5,14
Currently, no research is associated with the increase of TAMs and MVD. Nevertheless, in this research, findings suggest that there are associations between the increase of TAMs, MVD in PCa, and HGPIN PCa as well as in metastasis and extracapsular extension of PCa. These findings were not in accordance with the theory described by several studies in the literature about the importance of TAM in PCa and histopathological studies show that the level of TAM positively correlated with MVD. TAM was obtained and kept in the neoplastic tissue by various chemokines and cytokines such as CCL2 and macrophage colony-stimulating factor-CSF. Initially TAM was suspected to have tumoricidal activities, the recent evidence showed that TAM might be involved in the development of cancer because they released cytokine, growth factor, and extracellular matrix protein (e.g., interleukin-6, vascular endothelial growth factor, matrix metalloproteinases) that promote proliferation, angiogenesis, and metastasis tumor.4
HGPIN is the most likely precursor for prostate cancer (CaP). The incidence of HGPIN averages about 9% (range 4–16%) in prostate biopsies, representing 115,000 new cases of HGPIN each year in the United States. It is found predominantly (about 85%) in the peripheral zone of the prostate, much as is CaP The presence of PIN predates CaP by 10 years or more, with low-grade PIN first appearing in men in their 30s. HGPIN is characterized by increased crowding of prostatic cells that have enlarged nuclei and nucleoli and intact to discontinuous basal cell layer.7
In addition, HGPIN shares many biochemical and genetic changes with cancer, including chromosomal instability or abnormality, macrophages, androgen receptor expression, cell proliferation, PSA expression, high molecular weight cytokeratin expression, neovascularization and MVD, progressive loss of differentiation, and markers to defined membrane proteins (such as heparan sulfate proteoglycan: HSPG, type IV collagen). These indicate that basement HGPIN is a heterogeneous entity in the prostate gland and its cancer.7
After HGPIN has been detected in a prostatic biopsy, the risk of finding CaP in a subsequent biopsy is 15 times greater than in biopsies without PIN. The presence of HGPIN in the initial biopsy often results in repeat biopsies. For example, a study found that about one third of men with HGPIN on repeat biopsy have prostate cancer, but others have found variable results. PINs associated with increased MVD may have the potential of progressing to CaP.7 Therefore, we compare the differences of increasing TAMs and MVD in both HGPIN as precancer lession and CaP not included BPH.
This study had several limitations. First, the results may have been influenced by the heterogeneity of patients, immunohistochemical staining techniques, and prostate specimen (only PCa and HGPIN specimen). Second, in our center RP procedure was rare; therefore we could not include the RP specimen. Third, this study had fewer samples compared with the others. Owing to small numbers of patients in our data sets, we were unable to assess for other clinically relevant endpoints. Future studies evaluating subsets of TAMs and MVD with different biological functions may further elucidate the potential role of TAMs and MVD in PCa development and progression.
Conclusion
TAMs and MVD were increased in PCa but could not be used as an independent predictor. The increase of TAMs infiltration was not always followed by the increase of micro-vascularization and in the development of PCa. HGPIN is characterized by increased crowding of prostatic cells that have enlarged nuclei and nucleoli, and intact to discontinuous basal cell layer and shares many biochemical and genetic changes with cancer. We emphasize that our conclusion is tentative and ought to be confirmed in a study of a larger sample size before making a clinical decision. This is the first report to show that a group of molecular markers, when used in concert, can distinguish PINs that are precursors to CaP from those that are not.
Conflicts of interest
None declared.
Acknowledgments
Our thanks go to Dr. Sri Hidayah Nurlela Syafiie, Dr. Iriani Widodo, SpPA (K) and Dr. Sagiri Mangunsudirdjo, SpPA (K) from Department of Anatomical Pathology, Medical Faculty, Gajah Mada University, Yogyakarta who drew the illustrations and gave some advise for this study.
References
- 1.Zhu X., Albertsten P.C., Andriole G.L., Roobol M.J., Schroder F.H., Vickers A.J. Risk-based prostate cancer screening. Eur Urol. 2012;61:652–661. doi: 10.1016/j.eururo.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abouassaly R., Thompson I.M., Platz E.A., Klein E.A. Campbell-Walsh urology. 10th ed. Saunders; Philadelphia: 2012. Epidemiology, etiology, and prevention of prostate cancer. [Google Scholar]
- 3.Lee D.H., Jung H.B., Park J.W., Kim K.H., Kim J., Lee S.H. Can't western-based online prostate cancer risk calculators be used to predict prostate cancer after prostate biopsy for the Korean population? Yonsei Med J. 2013;54:665–671. doi: 10.3349/ymj.2013.54.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gollapudi K., Galet C., Grogan T., Zhang H., Zaid J.W., Huang J. Association between the tumor-associated macrophage infiltration, high grade prostate cancer, and biochemical recurrence after radical prostatectomy. Am J Cancer. 2013;3:523–529. [PMC free article] [PubMed] [Google Scholar]
- 5.Muhammadnejad S., Muhammadnejad A., Haddadi M., Oghabian M., Mohagheghi M., Tirgari F. Correlation of microvessel density with nuclear pleomorphism, mitotic count and vascular invasion in breast and prostate cancers at preclinical and clinical levels. Asian Pacific J Cancer Prev. 2013;14:63–68. doi: 10.7314/apjcp.2013.14.1.63. [DOI] [PubMed] [Google Scholar]
- 6.Jiang J., Chen Y., Zhu Y., Yao X., Qi J. Contrast-enhanced ultrasonography for the detection and characterization of prostate cancer: correlation with microvessel density and Gleason score. Clin Radiol. 2011;66:732–737. doi: 10.1016/j.crad.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Sinha A.A., Quast B.J., Reddy P.K., Lall V., Wilson M.J., Qian J. Microvessel density as a molecular marker for identifying high-grade prostatic intraepithelial neoplasia precursors to prostate cancer. Exp Mol Pathol. 2004;77:153–159. doi: 10.1016/j.yexmp.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Nonomura N., Takayama H., Nakayama M., Nakai Y., Kawashima A., Mukai M. Infiltration of tumour-associated macrophagesin prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int. 2010;107:1918–1922. doi: 10.1111/j.1464-410X.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 9.Lissbrant I.F., Stattin P., Wikstrom P., Damber J., Egevad L., Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol. 2000;17:445–451. doi: 10.3892/ijo.17.3.445. [DOI] [PubMed] [Google Scholar]
- 10.Gannon P.O., Poisson A.O., Delvoye N., Lapointe R., Mes-Masson A.M., Saad F. Characterization of the intra-prostatic immune cell infiltration in androgens-deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Tretiakova M., Antic T., Binders D., Kocherginsky M., Liao C., Taxy J. Microvessel density is not increased in prostate cancer: digital imaging of routine based dose sections and tissue microarrays. Hum Pathol. 2013;44:495–502. doi: 10.1016/j.humpath.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Haese A., Dix K., Erbersdobler A., Schlomm T., Walz J., Chun F. Microvessel density is significantly associated with pathologic stage, Gleason score and biochemical recurrence in patients with clinically localized prostate cancer undergoing radical retropubic prostatectomy. Eur Urol Supp. 2008;7:304. [Google Scholar]
- 13.Wiedner N., Carroll P.R., Flax J., Blumenfeld W., Folkman J. Tumor Angiogenesis Correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]
- 14.Payne H., Cornford P. Prostate-specific antigen: an evolving role in diagnosis, monitoring, and treatment evaluation in prostate cancer. Urol Oncol. 2011;29:593–601. doi: 10.1016/j.urolonc.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Rubin M.A., Buyyounouski M., Bagiella E., Sharir S., Neugut A., Benson M. Microvessel density in prostate cancer lack of correlation with the tumor grade, pathologic stage, and clinical outcome. Urology. 1999;53:542–547. doi: 10.1016/s0090-4295(98)00561-5. [DOI] [PubMed] [Google Scholar]
- 16.Luczynska E., Gasinska A., Wilk W. Microvessel density and expression of vascular toward endothelial growth factor in clinically localized prostate cancer. Police J Pathol. 2013;1:33–38. doi: 10.5114/pjp.2013.34601. [DOI] [PubMed] [Google Scholar]