Abstract
We report the characteristics of relapse, treatment response, and outcomes of 145 elderly patients with multiple myeloma in first relapse after front-line treatment with VMP or VTP. Reappearance of CRAB symptoms (113 patients) and more aggressive forms of disease (32 patients) were the most common patterns of relapse. After second-line therapy, 75 (51.7%) patients achieved at partial response and 16 (11%) complete response (CR). Overall survival was longer among patients receiving VMP as front-line induction (21.4 vs. 14.4 months, P=0.037), in patients achieving CR (28.3 vs. 14.8 months; P=0.04), and in patients without aggressive relapse (28.6 vs. 7.6 months; P=0.0007).
Keywords: Relapsed myeloma, Elderly patients, Patterns of relapse
1. Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy and presents primarily in elderly patients, with a median age at manifestation of approximately 72 years in Europe [1,2]. The number of older patients with this disease is expected to rise over time as a consequence of the increased life expectancy of the normal population. In recent years, the introduction of novel agents such as thalidomide, lenalidomide, and the proteasome inhibitor bortezomib has changed the management of elderly myeloma patients and extended overall survival (OS) times in all age categories supporting the use of modern anti-myeloma therapy independent of age [3,4].
Despite this improvement in OS, MM remains incurable and the majority of patients ultimately relapses and require further therapy. Thus, knowledge of relapse patterns and management of relapsed disease is a critical aspect of MM treatment and an important area of ongoing research [5]. Moreover, the optimal sequence or combination of post relapse therapeutic strategies remains unclear, and information is needed on the efficacy of each treatment, especially in the second-line setting. In this regard, previous reports focused on patients relapsing after conventional chemotherapy or autologous stem cell transplantation [6–8], and such data on elderly patients in the era of novel therapies is limited.
With the aim of understanding whether exposure to novel agents based induction affected the efficacy of subsequent therapy we have conducted a post hoc subgroup analysis of 145 patients with MM in first symptomatic relapse previously included in the GEM2005MAS65 Spanish trial. Front-line therapy in this trial consisted of bortezomib, melphalan, and prednisone (VMP) or bortezomib, thalidomide, and prednisone (VTP).
2. Methods
The Spanish GEM05MAS65 trial lasted from March, 2006 to October, 2008 and included 260 patients from 63 Spanish centers. At study entry, every patient was aged 65 years or older and had newly diagnosed, untreated, symptomatic, measurable MM. These patients had received a homogeneous induction treatment consisting either in bortezomib, melphalan, and prednisone (VMP) or bortezomib, thalidomide, and prednisone (VTP). Design of the study and treatment arms have been extensively described elsewhere [9–11]. Briefly, patients were upfront randomized to receive induction with 6 cycles of VMP or VTP. One hundred and seventy eight patients completed the six induction cycles and were randomly assigned to maintenance therapy with bortezomib plus prednisone (VP, n=87) or bortezomib plus thalidomide (VT, n=91) [9–11].
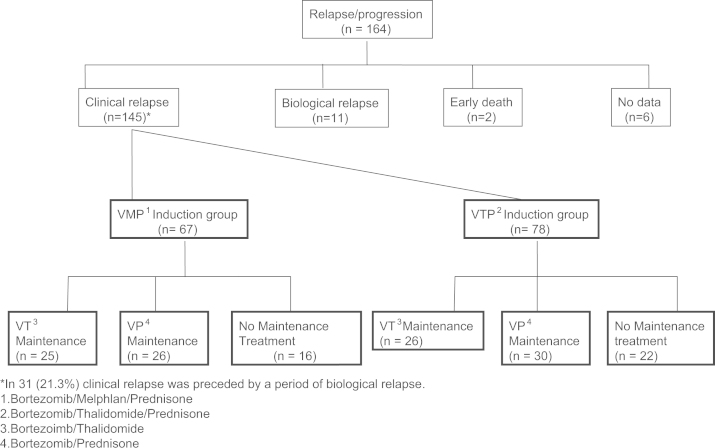
As of December 31st, 2013, 164 patients of the GEM05MAS65 trial had suffered disease relapse or progression. One hundred and forty-five (88%) received second line therapy and form the basis of this study. Nineteen (12%) patients were excluded due to asymptomatic relapse at time of analysis (11 patients), no data at relapse (6 patients) and early death after relapse without receiving second-line therapy (2 patients) (Fig. 1).
Fig. 1.
Flow diagram of relapsed patients.
2.1. Definitions
Response to salvage therapy and clinical relapses were evaluated according to the International Myeloma Working Group (IMWG) criteria, but near complete response (nCR) category, as defined by disappearance of monoclonal protein at routine electrophoresis but positive immunofixation, was added [12]. Biological relapse was defined as progressive, asymptomatic increase in M-component and clinical relapse was defined as evidence of organ dysfunction and reappearance of CRAB features. For the purpose of this article, aggressive relapse was considered when the patient presented extramedullary plasmacytomas, plasma cell leukemia or severe renal failure requiring hemodialysis at time of relapse.
2.2. Statistical analysis
The proportions of patients with a given set of characteristics were compared by the chi-square test or by the Fisher exact test. The chi-square and Fisher exact tests were also used, as appropriate, to compare overall response, complete response (CR), and nCR between both groups. The duration of PFS was calculated from the start of the second line treatment to new disease progression, death from any cause, or reference date (December 31, 2013). Patients who were alive and discontinued the study without evidence of disease progression were censored at the last evaluation for assessment of PFS. OS was calculated as the time from start of the second line treatment until death from any cause, or censored at the last reference date. PFS, and OS were plotted according to the Kaplan–Meier product-limit method with comparisons made by the log-rank test. All patients were followed until death or reference date (December 31, 2013). All statistical analyses were performed with version 3.0.1 of R software (The R Project for Statistical Computing) [13].
3. Results
3.1. Characteristics of patients at relapse
Median age at time of relapse in the overall series was 74.4 years (range, 66–87.5) and 68 (46.9%) patients were ≥75 years. Median time between diagnosis and relapse was 16.4 months (range, 3–67.5). Overall, 67 patients had received front-line therapy with VMP and 78 received induction with VTP. Clinical and laboratory characteristics at time of relapse were well balanced between both groups of patients (Table 1). Twenty-five and 26 patients in VMP received maintenance with VP or VT, respectively. In the VTP induction group maintenance consisted on VP in 26 patients and VT in 30 patients. Finally, 38 patients did not receive any type of maintenance because they progressed before to be randomized to maintenance or due to front-line induction-associated side effects.
Table 1.
Type of front-line induction regimen and characteristics of patients at first relapse.
| Charac teristic | No. (%) | Median (range) | VMP Induction n=67 (%) | VTP Induction n=78 (%) |
|---|---|---|---|---|
| Male | 69 (47.6) | 33 (49.3) | 36 (46.2) | |
| Age (years) | 74.4 (66 –87.5) | 74.5 (66–87.5) | 74.3 (66.3–87.2) | |
| M protein | ||||
| IgG | 80 (57.6) | 38 (60.3) | 42 (55.3) | |
| IgA | 43 (30.9) | 20 (31.7) | 23 (30.3) | |
| Light chain | 16 (11.5) | 5 (7.9) | 11 (14.5) | |
| ISS stage | ||||
| 1 | 35 (29.7) | 15 (28.3) | 20 (30.8) | |
| 2 | 46 (39) | 22 (41.5) | 24 (36.9) | |
| 3 | 37 (31.3) | 16 (30.2) | 21 (32.3) | |
| Creatinine>2 (mg/dl) | 13 (9) | 3 (4.5) | 10 (13) | |
| Hemoglobin (mg/dl) | 11.4 (6.5–15.3) | 11.5 (6.7–14.7) | 11.4 (6.5–15.3) | |
| Calcium>10.5 (mg/dl) | 6 (4.2) | 3 (4.5) | 3 (3.9) | |
| Plasma cell leukemia | 4 (2.8) | 2 (3) | 2 (2.6) | |
| Extramedullary plasmacytoma | 27 (18.6) | 12 (17.9) | 15 (19.2) | |
| Hemodialysis | 1 (0.7) | 0 (0) | 1 (1.3) | |
| Type of relapse | ||||
| Biological | 31 (21) | 16 (24) | 15 (19) | |
| Clinical | 82 (57) | 37 (55) | 45 (58) | |
| Aggressive | 32 (22) | 14 (21) | 18 (23) | |
| Interval diagnosis-relapse (months) | 16.4 (0.03–67.5) | 19.2 (0–67.5) | 15.0 (0–65.2) | |
VMP: Bortezomib, Melphalan, and Prednisone; VTP: Bortezomib, Melphalan, and Thalidomide.
3.2. Characteristics of relapse
Reappearance of CRAB symptoms was the most frequently type of relapse and it was observed in 82 (56.6%) patients while 31 (21.4%) patients presented a previous phase of biological relapse before developing MM-related symptoms. Median (range) time between biological and clinical relapse was 5.1 (2–24) months. Thirty-two (22%) patients presented some type of aggressive relapse. No association was found between the pattern of relapse (aggressive vs. non-aggressive) and the type of induction therapy administered at the time of diagnosis.
3.2.1. Treatment for relapse
Median time between disease relapse or progression and initiation of therapy was 17 days in the VMP (range, 0–1755) and 12 days (range, 0–711) in the VTP group of patients (P non significant). Relapse therapy was not predefined in the GEM2005MAS65 protocol. Lenalidomide-based combinations were the most commonly regimens administered (78 patients: 57.2% and 50% in the VMP and VTP arms, respectively). Retreatment with bortezomib-containing regimens were administered as rescue therapy in 25 (17.3%) patients, eleven (16.4%) in the VMP group and 14 (18%) in the VTP groups. Thirty-four (23.5%) patients received conventional chemotherapy-containing regimens (12 (8.2%) in the VMP group and 22 (15.2%) in the VTP group); finally, 8 (5.5%) patients received supportive care as the only therapy for myeloma after relapse (5 in the VMP group and 3 in the VTP group). Table 2 shows the therapy regimens used to treat first relapse.
Table 2.
Type of treatment administered at first relapse.
| Type of therapy | Total (%) | Front-line treatment |
Post induction maintenance |
|||
|---|---|---|---|---|---|---|
| VMP | VTP | VP | VT | None | ||
| n=67 (%) | n=78 (%) | n=51 (%) | n=56 (%) | n=38 (%) | ||
| Bortezomib-based | 25 (17.3) | 11 (16.4) | 14 (18) | 8 (15.7) | 10 (17.9) | 7 (18.4) |
| Lenalidomide-based | 78 (53.8) | 39 (58.2) | 39 (50) | 28 (54.9) | 37 (66.1) | 13 (34.2) |
| Conventional chemotherapy | 34 (23.5) | 12 (17.9) | 22 (28.2) | 12 (23.5) | 7 (12.5) | 15 (39.5) |
| Supportive care | 8 (5.5) | 5 (7.5) | 3 (3.8) | 3 (5.9) | 2 (3.6) | 3 (7.9) |
VMP: Bortezomib, Melphalan, and Prednisone; VTP: Bortezomib, Melphalan, and Thalidomide; VP: Bortezomib and Prednisone; VT: Bortezomib and Thalidomide.
3.3. Response according to induction therapy
One hundred and thirty-one patients who relapsed were evaluable for response to rescue therapy while 14 patients died shortly after relapse and could not be evaluated. According to the front-line regimen administered, no differences in the rate of partial response or better was observed in patients receiving VMP (37 patients, 55.2%) or VTP (38 patients, 48.7%). Investigator-reported best responses to second line therapies are summarized in Table 3.
Table 3.
Best response to rescue therapy.
| VMP Inductionn=67 (%) |
VTP Inductionn=78 (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Bz-based no. (%) | Len-based no. (%) | CC no. (%) | SC no. (%) | Bz-based no. (%) | Len-based no. (%) | CC no. (%) | SC no. (%) | |
| CR | 0(0) | 7(18.4) | 0 | 0 | 2 (14.3) | 6(15.4) | 1 (4.8) | 0 |
| nCR | 3(27.3) | 11(28.9) | 0 | 0 | 2 (14.3) | 9 (23.1) | 4 (19) | 0 |
| PR | 3(27.3) | 9(23.7) | 4 (40) | 0 | 1 (7.1) | 10 (25.6) | 3(14.3) | 0 |
| SD | 3 (27.3) | 6 (15.8) | 1 (10) | 0 | 4 (28.6) | 4 (10.3) | 5 (23.8) | 0 |
| NE | 0 (0) | 1 (2.6) | 2 (16.7) | 2 (40) | 0 (0) | 0 (0) | 1 (4.5) | 1 (33.3) |
| PD | 2 (18.2) | 3 (7.9) | 4 (40) | 3 (60) | 4 (28.6) | 6 (15.4) | 6 (28.6) | 2 (100) |
| ORR | 6 (54.5) | 27 (69.2) | 4 (40) | 0 | 5 (35.7) | 25 (64.1) | 8 (38.1) | 0 |
VMP: Bortezomib, Melphalan, and Prednisone; VTP: Bortezomib, Melphalan, and Thalidomide; CC: Conventional chemotherapy; SC: Supportive care; CR: Complete response; nCR: Near complete response, PR: Partial response; SD: Stable disease; NE: Not evaluable; PD: Progressive disease; ORR: Overall response rate (comprises partial response or better).
3.4. Response according to maintenance therapy
When considering the responses observed according to the type of maintenance therapy administered, partial response or better was observed in 28 (56%) patients receiving VP and in 32 (58%) patients receiving VT.
3.5. Response according to rescue therapy
Partial response or better was achieved in 52 (67%) patients after lenalidomide-based combinations and in 11 (44%) patients receiving bortezomib-based therapy (P=0.04). Twelve (35%) out of the 34 patients receiving conventional chemotherapy achieved at least partial response. Complete responses were observed in 13 (25%) and in 2 (18%) patients receiving lenalidomide-based and bortezomib-based therapy, respectively.
3.6. Progression-free survival
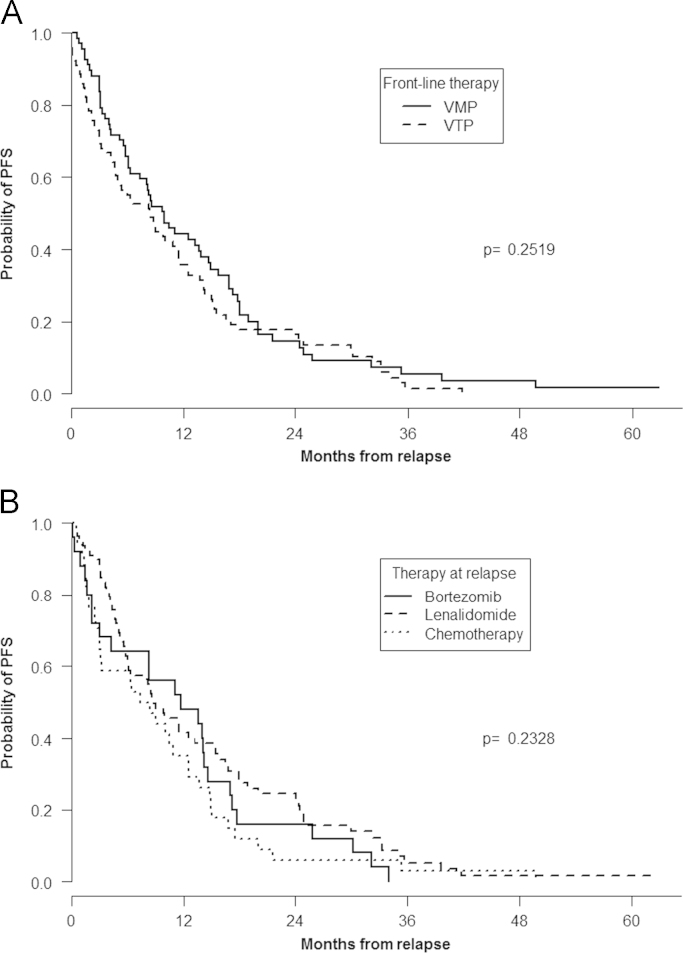
The median follow-up from the time of disease relapse or progression was 14.3 months and 136 patients (94%) have progressed or died after relapse. The median PFS after relapse in the overall series was 8.84 (95% confidence interval [CI]: 6.37–11.53) months and it was 9.92 (95% CI: 7.36–14.7) and 8.49 (95% CI: 4.96–11.5) months among patients receiving induction with VMP and VTP, respectively (Fig. 2A). According to maintenance therapy, PFS was 8.5 and 8.9 months among patients receiving VP or VT and 8.7 months in those patients who did not receive maintenance (P non significant). Median PFS was, 11.73 (95% CI: 4.24–17), 8.84 (95% CI: 6.18–15.3), and 7.90 (95% CI: 3.15–12.5) for patients receiving bortezomib-, lenalidomide-, and chemotherapy-based therapies at time of relapse, respectively (Fig. 2B). No significant differences were observed in PFS among patients in the VMP and VTP groups according to the type of therapy administered at relapse (data not shown).
Fig. 2.
Post relapse progression-free survival according to front-line treatment (A) and (B) progression-free survival depending on the type of therapy administered at relapse.
3.7. Overall survival
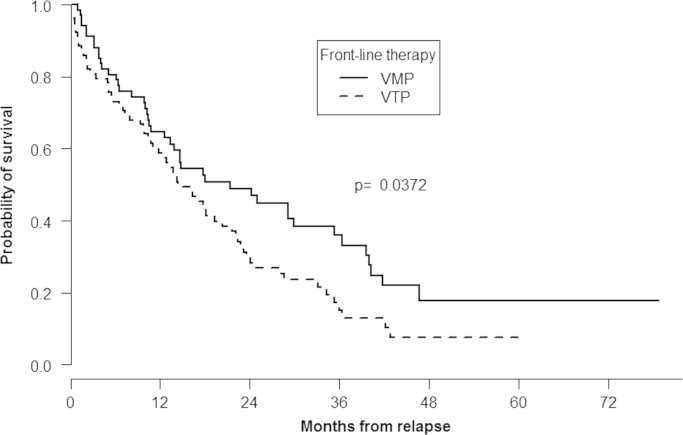
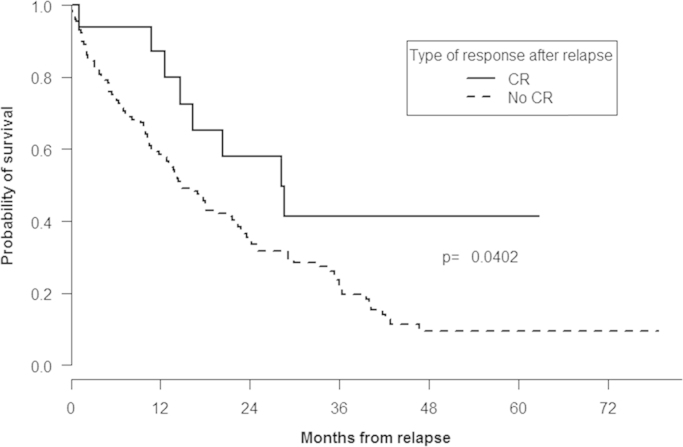
At the time of analysis, 108 (74.4%) patients have died, including 44 (65.6%) and 64 (82%) in the VMP and VTP arms, respectively. Median OS from start of subsequent therapy in the overall series of patients was 17 months (95% IC: 13.8–22.4). OS from time of relapse was significantly longer among patients receiving front-line therapy with VMP, 21.4 (95% IC: 13.8–36.4) months when compared with those in the VTP group, 14.4 (95% IC: 11.9–21.7) months (P= 0.037; Fig. 3). Likewise, OS was also significantly longer among patients achieving CR after relapse when compared with patients who did not achieve CR (28.3 vs. 14.8 months; P=0.04) (Fig. 4).
Fig. 3.
Overall survival according to front-line treatment.
Fig. 4.
Overall survival according to the type of response achieved after relapse.
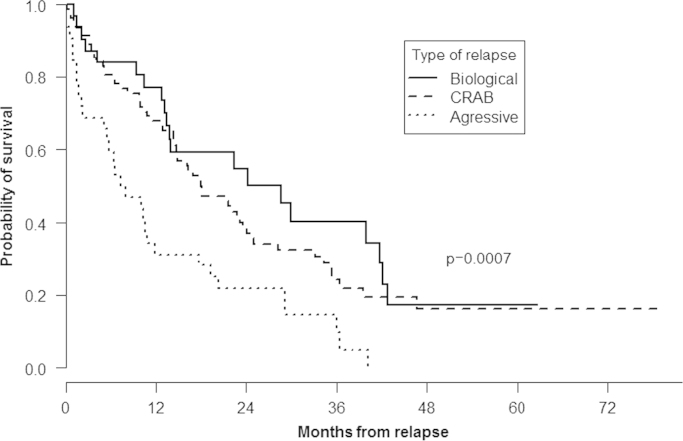
When the type of relapse was considered, median OS was, respectively 28.6 (95% CI: 14–43), 18.1 (95% CI: 15–25), and 7.6 (95% CI: 5–18) months after asymptomatic, clinical, and aggressive relapse (P=0.0007) (Fig. 5). Finally, no differences in OS were observed according to the type of treatment administered at relapse or depending on the type of maintenance therapy administered (data not shown).
Fig. 5.
Overall survival according to the type of relapse.
4. Discussion
In our study, including 145 elderly patients with MM in first relapse after front-line treatment including new drugs, both the type of relapse and the achievement of CR post relapse were the main factors affecting outcome, while the type of regimen administered to treat relapse had a minor impact on post relapse evolvement.
In this study, every patient received front-line therapy with bortezomib as part of the GEM2005MAS65 trial and 50% of them also received thalidomide during the induction regimen. It has been hypothesized that by the exposure to novel agents in relapsed or refractory multiple MM, more resistant subclones may survive under the selective stress of the agents, explaining the more aggressive forms of relapse [14]. Our findings, however, do not support this hypothesis. In fact, an asymptomatic increase in the monoclonal component along with the reappearance of the classical CRAB symptoms were the most commonly forms of relapse (113 patients, 78%) and only 32 (22%) patients developed aggressive relapse. This pattern of relapse is similar to that observed in younger patients who received induction with conventional chemotherapy followed by autologous transplantation [6–8]. Thus, the type of relapse might be more related with disease biology such as the intraclonal heterogeneity recently reported in MM [15,16]. However, additional studies are needed to try to clarify whether exposure to regimens including one or more novel drugs during induction will increase the risk of more resistant disease at time of relapse.
The overall and CR rate after relapse were 51.7% and 11%, respectively and not influenced by the type of regimen administered in the front-line therapy (VMP or VTP). However, achievement of CR after relapse was associated to a significantly longer OS when compared with those patients who obtained less than CR (28.3 vs. 14.8 months; P=0.04). In newly diagnosed MM patients an association between achieving deep levels of remission and long-term survival has been proved confirming this parameter as a valid surrogate marker of the treatment efficacy [18] but the value of the depth of response in the relapse setting has been less investigated [19]. Our results, however strongly suggest that CR should also be a valid objective of any therapeutic strategy even in patients in relapse.
Median PFS after first relapse in the overall series was 8.84 months with no differences between VMP and VTP and shorter than the corresponding figure observed with the use of lenalidomide and dexamethasone in first relapse by other groups [17]. However, it must be noted that these results were achieved at a time when novel agents were rarely used upfront, and results might be different in patients who received novel agents as first-line treatment like those included in our study. Although direct comparisons are not possible, the PFS observed in our study was similar to that recently reported with bortezomib and dexamethasone [20] but shorter than that observed with different three-drug regimens such as bortezomib, dexamethasone, and panobinostat [20] or lenalidomide, dexamethasone, and carfilzomib [21] suggesting that a triple combination may be more effective for this subgroup of patients. However, it should also be highlighted that median age at time of relapse was much higher in our series than in other studies dealing with MM patients in relapse a fact that also may explain the shorter duration of PFS observed in our study [14,17,20,21].
In MM, the optimal sequence or combination of the different therapeutic regimens available remains unclear and information is needed on the efficacy of each treatment after various prior therapies. Although a substantial number of different regimens and schedules were used for the treatment of first relapse, lenalidomide-based were the most commonly used combinations for treatment at relapse (53.8% of patients) and 17.3% were retreated with bortezomib at time of relapse. The overall response rate was higher among patients receiving rescue therapy with lenalidomide when compared with bortezomib combinations (67% and 44%, respectively; P=0.04) but similar CR rate in both groups of patients. Interestingly, no differences in PFS were observed among both groups of patients despite the fact that every patient had been previously exposed to bortezomib. These findings suggest that, retreatment with the same class of drugs on which patients have previously responded may be effective and represents a feasible and effective treatment option for patients with relapsed MM who previously responded to these drugs and a potential alternative to initiating subsequent line therapy with a different class of agents.
In our study, OS after relapse was also significantly prolonged in those patients receiving front-line therapy with VMP, confirming the superiority of this regimen when compared with VTP, a finding previously reported for the whole series of patients [11].
As expected, our results showed a significantly shorter OS among patients with more aggressive forms of relapse (7.6 months) when compared with those patients presenting clinical (18.1 months) or biological (28.6 months) relapse suggesting that alternative, more efficacious rescue therapies are clearly needed in this subgroup of patients.
It is well-recognized that relapse can take different forms, and several groups have described various relapse patterns. However, the majority of these reports focused on patients relapsing after conventional chemotherapy or autologous stem cell transplantation and data about relapse patterns in elderly patients receiving front-line treatment with new drugs are limited [6,7]. Our results, however, showed a significantly shorter OS among patients with more aggressive forms of relapse (7.6 months) when compared with those patients presenting clinical (18.1 months) or biological (28.6 months) relapse suggesting the utility of these classifications and that alternative, more efficacious rescue therapies are clearly needed for these subgroups of patients.
This study has some limitations. We investigated only the type of regimen administered at relapse and no data about doses or schedule of the drugs used were available. In this regard, heterogeneity of the ageing process is characterized by marked variability in the rate of functional deterioration, both between and within individuals. Therefore, individualized management, tailored to differences in functional capacity, life expectancy, and social and economic support, is needed to better define and optimal treatment strategy for these patients [22,23].
In conclusion, our study shows that patterns of relapse in elderly patients receiving front line therapy including novel agents are similar to that observed after conventional chemotherapy with patients with more aggressive forms of relapse doing poorly. Our results also show that PFS after relapse is similar regardless the induction regimen (VMP or VTP) administered at diagnosis and the type of treatments administered at relapse. Finally, a significantly longer post relapse OS was observed among patients receiving VMP at induction and in the subgroup of patients achieving CR after relapse.
Authors contributions
Aurelio Lopez, Javier de la Rubia wrote the paper; Maria Victoria Mateos was the principal investigator and co-ordinated the research; Marta Valero participated in the data collection; Jose Ignacio Lorenzo performed the statistical analysis; Albert Oriol, Joaquin Martinez, Montserrat Perez, Rafael Martinez, Raquel de Paz, Miguel Granell, Felipe De Arriba, Maria Jesus Blanchard, Francisco Javier Peñalver, Jose Luis Bello, Maria Luisa Martin,and Joan Bargay, recruited the patients; Joan Blade, Juan Jose Lahuerta, Jesus Francisco San Miguel co-ordinated the research. The authors report no potential conflicts of interest.
Footnotes
Trial registration: www.clinicaltrials.gov as #NCT00443235.
References
- 1.Ludwig H., Fritz E., Friedl H.P. Epidemiologic and age dependent data on multiple myeloma in Austria. J. Natl. Cancer Inst. 1982;68:729–733. [PubMed] [Google Scholar]
- 2.Kristinsson S.Y., Landgren O., Dickman P.W. Patterns of survival in multiple myeloma: a population-based study of patients diagnosed in Sweden from 1973 to 2003. J. Clin. Oncol. 2007;25:1993–1999. doi: 10.1200/JCO.2006.09.0100. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S.K., Rajkumar S.V., Dispenzieri A. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kristinsson S.Y., Anderson W.F., Landgren O. Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia. 2014;28:1346–1348. doi: 10.1038/leu.2014.23. [DOI] [PubMed] [Google Scholar]
- 5.Kumar S.K., Therneau T.M., Gertz M.A. Clinical course of patients with relapsed multiple myeloma. Mayo Clin. Proc. 2004;79:867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 6.Alegre A., Granda A., Martinez-Chamorro C. Different patterns of relapse after autologous peripheral blood stem cell transplantation in multiple myeloma: clinical results of 280 cases from the Spanish Registry. Haematologica. 2002;87:609–614. [PubMed] [Google Scholar]
- 7.Lenhoff S., Hjorth M., Turesson I. Intensive therapy for multiple myeloma in patients younger than 60 years. Long-term results focusing on the effect of the degree of response on survival and relapse pattern after transplantation. Haematologica. 2006;91:1228–1233. [PubMed] [Google Scholar]
- 8.Zamarin D., Giralt S., Landau H. Patterns of relapse and progression in multiple myeloma patients after auto-SCT: implications for patients’ monitoring after transplantation. Bone Marrow Transpl. 2013;48:419–424. doi: 10.1038/bmt.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mateos M.V., Oriol A., Martínez-López J. Bortezomib, melphalan, and prednisona versus bortezomib, thalidomide and prednsione as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11:934–941. doi: 10.1016/S1470-2045(10)70187-X. [DOI] [PubMed] [Google Scholar]
- 10.Mateos M.V., Oriol A., Martínez-López J. Maintenance therapy with bortezomib plus thalidomide or bortezomib plus prednisone in elderly multiple myeloma patients included in the GEM2005MAS65 trial. Blood. 2012;120:2581–2588. doi: 10.1182/blood-2012-05-427815. [DOI] [PubMed] [Google Scholar]
- 11.Mateos M.V., Oriol A., Martínez-López J. GEM2005 trial update comparing VMP/VTP as induction in elderlymultiple myeloma patients: do we still need alkylators? Blood. 2014;124:1887–1893. doi: 10.1182/blood-2014-05-573733. [DOI] [PubMed] [Google Scholar]
- 12.Durie B.G.M., Harousseau J.L., San Miguel J. International uniform response criteria for multiplemyeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 13.Team RDC . R Foundation for Statistical Computing; Vienna, Austria: 2011. A Language and Environment for Statistical Computing. [Google Scholar]
- 14.Ahn J.S., Jung S.H., Yang D.H. Patterns of relapse or progression after bortezomib-based salvage therapy in patients with relapsed/refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2014;14:389–394. doi: 10.1016/j.clml.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Morgan G.J., Walker B.A., Davies F.E. The genetic architecture of multiple myeloma. Nat. Rev. Cancer. 2012;12:335–348. doi: 10.1038/nrc3257. [DOI] [PubMed] [Google Scholar]
- 16.Keats J.J., Chesi M., Egan J.B. Clonal competition with alternating dominance in multiple myeloma. Blood. 2012;120:1067–1076. doi: 10.1182/blood-2012-01-405985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stadtmauer E.A., Weber D.M., Niesvizky R. Lenalidomide in combination with dexamethasone at first relapse in comparison with its use as later salvage therapy in relapsed or refractory multiple myeloma. Eur. J. Haematol. 2009;82:426–432. doi: 10.1111/j.1600-0609.2009.01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harousseau J.L., Attal M., Avet-Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114:3139–3146. doi: 10.1182/blood-2009-03-201053. [DOI] [PubMed] [Google Scholar]
- 19.Harousseau J.L., Dimopoulos M.A., Wang M. Better quality of response to lenalidomide plus dexamethasone is associated with improved clinical outcomes in patients with relapsed or refractory multiple myeloma. Haematologica. 2010;95:1738–1744. doi: 10.3324/haematol.2009.015917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.San Miguel J.F., Hungria V.T., Yoon S.S. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed orr elapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15:1195–1206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 21.Stewart A.K., Rajkumar S.V., Dimopoulos M.A. Calfirzomib, lenalidomide and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2015;372:142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 22.Wildes T.M., Rosko A., Tuchman S.A. Multiple myeloma in the older adult: better prospects, more challenges. J. Clin. Oncol. 2014;32:2531–2540. doi: 10.1200/JCO.2014.55.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palumbo A., Bringhen S., Mateos M.V. Geriatric assessment predicts survival and toxicities in elderly myeloma: an International Myeloma Working Group report. Blood. 2015;13:2068–2074. doi: 10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]