Abstract
Combining resting-state functional magnetic resonance imaging (fMRI) connectivity and behavioral analysis during sedation, we factored out general effects of the anesthetic drug propofol and a specific index of conscious report, participants’ level of responsiveness. The factorial analysis shows that increasing concentration of propofol in blood specifically decreases the connectivity strength of fronto-parietal cortical loops. In contrast, loss of responsiveness is indexed by a functional disconnection between the thalamus and the frontal cortex, balanced by an increase in connectivity strength of the thalamus to the occipital and temporal regions of the cortex.
Highlights
-
•
It is debated if thalamo-cortical or fronto-parietal circuits induce consciousness.
-
•
We measured functional connectivity of awake and propofol-sedated healthy subjects.
-
•
We factored out effects of drug concentration and arousal level of the participants.
-
•
Fronto-parietal loops are related only to drug concentration in blood.
-
•
In contrast, thalamic connectivity indexes individual’s level of arousal.
1. Introduction
Theories of consciousness differ on whether the induction of consciousness is mainly governed by the thalamo-cortical arousal circuits (the thalamic “off-switch”) (Schroter et al., 2012), or instead by the ignition of a connection between fronto-parietal cortices (cortico-cortical reverberation) (Dehaene and Changeux, 2005). A main experimental difficulty in resolving this debate is that the data on which these theories are examined focus either states of consciousness (coma, sleep, anesthesia, drowsiness, vigilance) (Boly et al., 2012; Boveroux et al., 2010; Magnin et al., 2010; Rees et al., 2002; Sergent et al., 2005) or the contents, processes and behaviors which characterize conscious function (i.e. explicit reports, volitional control, flexible and versatile behavior) (Bekinschtein et al., 2009; Dehaene and Changeux, 2011).
Anesthetic drugs provide a unique tool to study the neuroanatomical substrates of consciousness, since they can produce controlled and reversible perturbations of conscious level. However, the mechanisms by which they suppress consciousness are poorly understood (Breshears et al., 2010). Predominant theories of consciousness propose that consciousness is lost when the sedative agent produces functional disconnection between distant cortical regions, causing a loss of integration capacity (Alkire et al., 2008; Barttfeld et al., 2015; Schroter et al., 2012; Schrouff et al., 2011). While connectivity provides one metric of integration, it remains an incomplete measure, and loss of integration is not always reflected by a reduction in overall connectivity. For example, it has been reported that propofol causes a large drop in connectivity (Barttfeld et al., 2015; Gomez et al., 2013) but propofol-induced anesthesia has also been related to the emergence of hypersynchronous cortical states, both in EEG (Supp et al., 2011) and fMRI (Liu et al., 2014; Stamatakis et al., 2010).
While anesthetic drugs produce controlled loss of consciousness, relating drug dose to neural effects and behavioral correlates is not straightforward, since identical doses may result in different plasma (and presumably brain) levels of drug in different subjects, owing to pharmacokinetic variations. While plasma drug levels correlate better with directly observed brain effects (demonstrated, for example, using functional imaging (Barttfeld et al., 2015; Gomez et al., 2013)) not all observed changes in brain function tightly correlate with behavioral measures of consciousness. This is because the functional changes that constitute the neural signature of the drug include some effects that are not critical to the maintenance of consciousness. Here we seek to differentiate, with resting-state functional connectivity, drug effects that directly modulate consciousness from those that do not, by factoring out functional connectivity changes associated with behavioral measures of consciousness and with plasma drug levels.
2. Materials and methods
2.1. Participants
Twenty-four participants (10 males, 19–52 years old, mean = 34.79, standard deviation = 9.16) participated in this study. We obtained local ethics permission from the Cambridgeshire 2 Regional Ethics Committee and written consent from participants. Volunteers were informed of the risks of propofol administration, such as loss of consciousness and respiratory and cardiovascular depression. Subjects were instructed to close their eyes and think about nothing in particular throughout the acquisition of the resting state BOLD data.
2.2. fMRI acquisition and analysis
Resting-state fMRI data were acquired on a Siemens Trio 3 T scanner (WBIC, Cambridge). Each functional BOLD volume consisted of 32 interleaved, descending, oblique axial slices, 3 mm thick with an interslice gap of 0.75 mm and in-plane resolution of 3 mm, field of view = 192 × 192 mm, repetition time = 2 s, time echo = 30 ms, and flip angle 78. We acquired 156 volumes of BOLD data per session, resulting in a session length of 5.2 min. We also acquired T1-weighted structural images at 1 mm isotropic resolution in the sagittal plane, using an MPRAGE sequence with TR = 2250 ms, TI = 900 ms, TE = 2.99 ms and flip angle = 9°, for localization purposes. fMRI images were preprocessed using SPM5 (Wellcome Institute of Imaging Neuroscience, London, UK) implemented in MatLab (Mathworks, Natick, MA). The first six volumes were discarded to allow for MR signal equilibration. Pre-processing involved slice-timing correction and within-subject realignment to account for head motion. Realignment involved a two-step process, initially aligning all images to the first image acquired and then realigning all images to the mean image of each session using linear transformations. Realigned images were spatially normalized to the MNI space defined by a template image available in SPM5. We used 12-parameter linear affine transformations (translation, rotation, zoom, and shear in x, y, and z directions) as well as a linear combination of three dimensional discrete cosine basis functions for finer non-linear adjustments. The transformation parameters obtained from the spatial normalization of the mean to standard space (MNI) were applied to every functional image acquired during the resting state scanning session. Spatially normalized images were smoothed with an isotropic 8-mm3 full-width half-maximum Gaussian kernel. We removed by regression the six movement parameters resulting from rigid body correction for head motion, the global signal of the entire brain and four nuisance variables obtained from time series from the ventricles ([22, −37, 17] and [−22, −37, 17]) and white matter ([31, −12, 30] and [−31, −12, 30]) regions of interest (ROIs), using the REST toolbox for MatLab (http://restfmri.net/forum/REST). The rationale for removing global signal was the need to compare experimental conditions that are quite different both behaviorally and physiologically. Anesthesia causes a diminution of heart beat rhythm, respiration rhythm, blood pressure and many other confound effects that global signal removal helps to minimize (Ciobanu et al., 2012).
2.3. Behavioral experiment
All participants performed a semantic categorization task inside the MRI scanner. Findings from the functional imaging obtained during the execution of the task are reported elsewhere (Adapa et al., 2014) Here we focus on the resting state data obtained during the same session. Words used in each of the four scanning runs were pseudo-randomly drawn from a set of 280 items (140 living items, e.g. tiger, birch, and 140 non-living items, e.g. table, stone) in subsets of 40 items (20 living, 20 non-living), matched for relevant psycholinguistic variables (word frequency, length, imageability, acoustic amplitude and familiarity). Participants heard spoken words with assignment of items to sedation levels counter-balanced over participants. Subjects were asked to respond using a button box whether presented words referred to living or non-living items. For each participant and session, we measured the percentage of missed responses across all conditions. This measure was used as a regressor for the resting-state fMRI connectivity data.
2.4. Propofol administration
Sedation is a pharmacologically induced, reversible state, characterized by dose-related impairment of cognitive functions. Progressively increasing sedation results eventually in general anesthesia, or “drug-induced loss of consciousness” during which patients are not rousable, even by painful stimulation (Chernik et al., 1990). In our experiment propofol was administered using a computer controlled intravenous infusion (Marsh et al., 1991), aiming to achieve two target plasma drug levels: low sedation and moderate sedation. There were always two trained anesthesiologists present during data acquisition, and observed the volunteer from the MRI control room and on a video link that showed the volunteer in the scanner. Heart rate, electrocardiogram (ECG) and pulse oximetry were continuously monitored using an MR-compatible multiparameter monitor (Precess, In Vivo Corp, Orlando, FL, USA). Non-invasive systemic blood pressure was measured intermittently during the study, but was suspended during scanning. In all volunteers two blood samples (2 × 1 ml) were taken at each sedation level for later measurement of plasma propofol concentrations with high performance liquid chromatography (HPLC). At a propofol plasma target concentration of 0.6 µg/ml (mean [SD] measured plasma concentration 0.38 [0.27]) all volunteers displayed a lethargic response to their name spoken in a normal tone (corresponding with an Observer's Assessment of Alertness/Sedation Scale (OAA/S) score of 4, or a Ramsay score of 2). At a propofol plasma target concentration of 1.2 µg/ml (mean [SD] measured plasma concentration 0.61 [0.32] µg/ml), all volunteers were more deeply sedated and responded only after their name was called loudly (corresponding with an OAA/S score of 3, or a Ramsay score of 3). At the recovery stage, the mean plasma concentration was 0.27 [0.07] µg/ml. The mean propofol level in blood per person at a given sedation level is the mean of two blood samples, collected at the beginning and at the end of each experimental run that included the resting-state scanning and the semantic task experiment.
2.5. fMRI analysis
Analyses were done using MatLab (The MathWorks Inc., Natick, MA). We used a previously defined set of ROIs (Dosenbach et al., 2010) composed of 141 ROIs comprising five functional systems (Fronto-parietal (FP), Cingulo-opercular (OP), Default Mode Network (DMN), Sensorimotor (SM) and Occipital (OC) (Fig. S1)). We built ROIs as 5-mm sphere around each ROI coordinate. For each ROI, a time-series was extracted for each subject and each sedation state, using the Marsbar software package (http://marsbar.sourceforge.net), averaging all gray matter voxels within a sphere (a few white matter voxels were left aside for some ROIs). These regional fMRI time-series were then used to construct a 141-node functional connectivity network for each subject and sedation state (Fig. S2). We used wavelet analysis to construct correlation matrices from the time-series. We applied a maximum overlap discrete wavelet transform (MODWT) to each of the time-series to decompose them into the following three frequency components: scale 1 (0.13 to 0.25 Hz), scale 2 (0.06 to 0.12 Hz), and scale 3 (0.01 to 0.05 Hz) (Barttfeld et al., 2013; Supekar et al., 2008). All subsequent analyses were done based on the scale 3 component, whose frequency lies in the typical range of slow frequency fluctuations of the Default network (0.009 < f < 0.08).
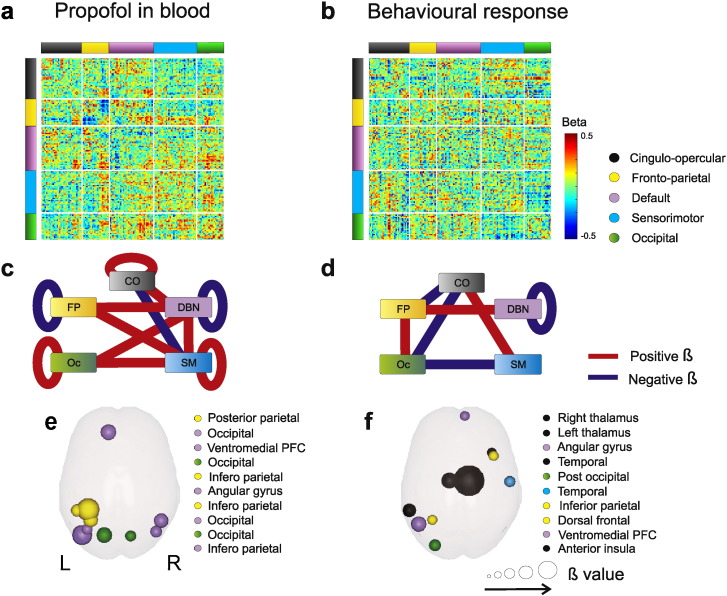
For each sedation state s and participant p we measured a 141 × 141 connectivity matrix Cs,p (Fig. S2). The matrix entry Cs,p(i,j) indicates the temporal correlation of the average fMRI signal from the voxels in each ROI i and j, which henceforth is referred to as functional connectivity. To study functional connectivity changes associated with propofol concentration and responsiveness, we conducted an across-subjects multivariate linear regression, using the least squares method, between each entry ij of the connectivity matrix Cs,p(i,j), drug concentration as well as rate of missed responses as a proxy for the level of consciousness of each participant, and participant's age as a regressor of no interest. Using reaction times as a regressor instead of missed responses produced qualitatively the same results (see Fig. S3). This way we obtained two matrices Br(i,j) (Fig. 1a, b), one per regressor of interest r, in which each entry ij represents the dependence or beta (β) value for the connectivity between ROI(i) and ROI(j), and drug level (Fig. 1a) and responsiveness (Fig. 1b). To search for statistically significant effects of both regressors on functional connectivity, we first calculated the average β coefficient matrix ’r, a 5 × 5 matrix resulting from all possible pairings between resting state networks for each Br matrix. Statistical differences between the entries of r, matrices were assessed through a permutation analysis (Efron and Tibshirani, 1994) to test the hypothesis of a dependence between each regressor's variability and connectivity values. For each ROI pair we shuffled each regressor's values across participants 5000 times, each time calculating the β-values for the shuffled regressor. We set the threshold for significance at a p-value = 0.05, Bonferroni corrected for multiple comparisons (15 unique comparisons between ’r entries × 2 regressors). In Fig. 1c, d we plot as red edges (positive β value) and blue edges (negative β value) between system pairs, all z-scores that survived multiple comparisons correction.
Fig. 1.
a-b) Matrix of β-values showing dependences of functional brain connectivity with propofol level (a) and number of missed responses (b). c-d) Reduced connectivity matrix r for propofol (c) and missed responses (d). Blue connections represent negative Bn,m values, indicating a decrease in connectivity between systems n and m as the regressor increases, while red connections represent significantly positive Bn,m values, indicating a connectivity increase between systems n and m as regressor increases. e-f) Top ten ROIs with the highest average connectivity changes associated with propofol (e) and number of missed responses (f).
To construct Fig. 1e, f, we calculated for each ROI the average change in connectivity over all 4 sedation states associated with each regressor, as
where Br is the matrix of Fig. 1a, b, and N = 141 (i.e. number of ROIs). We averaged the absolute β value of a ROI j to all other ROIs because we were only interested in the amount of change in connectivity produced by propofol and number of misses. We ranked all ROIs in descending values of averaged β value br,j, and plotted into glass brains the top 10 ROIs for each regressor (Fig. 1e, f). Sphere size on the glass brains indicates the corresponding value.
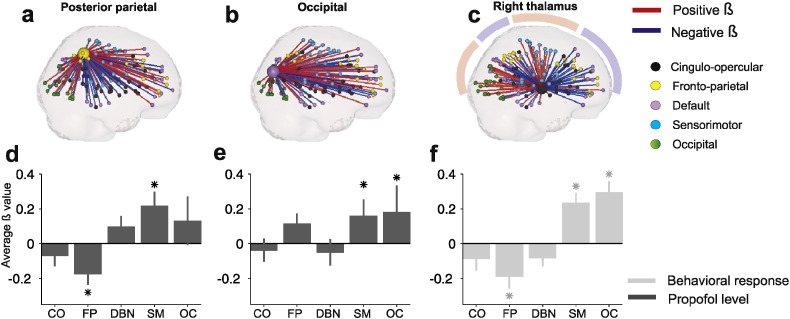
To construct Fig. 2d–f we searched for significant br,j values. Since br,j values approximate a Gaussian distribution, we normalized each br,j value
and obtained the corresponding pr,j to each zr,j, that is, the cumulated probability in the normalized Gaussian distribution at zr,j. We identified those ROIs whose connectivity is selectively affected by one regressor and not the other, that is whose ppropofol is significant and whose pbehavior is not, and vice-versa.
Fig. 2.
a–c) ROIs whose connectivity significantly changed with propofol (a, b) and number of missed responses (c). Each ROI is linked to all other ROIs, a blue line represents negative β-values, red lines represent positive β-values. d–f) Average β between ROIs posterior parietal (d), occipital (e) and Right thalamus (f) with all other functional systems, for the significant regressor. S.E.M. represents standard error of the mean.
To quantify those changes in connectivity between these ROIs and functional systems, we collapsed all Br (i,rois) across all ROI values corresponding to the same functional system (Fig. 2d–f). For each system n and each significant ROI from Fig. 1d–f we obtained the mean β value for connections linking system n and the ROI (B(:,j)), for the regressor significantly affecting that ROI. For example, the mean β value of connections linking the thalamic ROI with SM system is the average of 33 β values, since SM is composed by 33 ROIs. This average β value reflects the average dependence of connections between the systems and the ROIs. The standard error of the mean (S.E.M.) was obtained through a jackknife procedure (Miller, 1974): we obtained the S.E.M. by repeating the regression N − 1 times (being N the number of subjects), each time excluding a different subject from the analysis. S.E.M. is then calculated as . Statistical analysis was performed following a permutation test as previously described. Significant threshold was set at p = 0.001, Bonferroni corrected for multiple comparisons (5 functional systems × 3 times, one for each significant ROI, Fig. 2d–f).
To further determine the differential effect of drug concentration and capacity to respond on functional connectivity (since the measures are non-independent), we tested for differences in overlapping correlations (Meng et al., 1992). Overlapping correlation allows the quantification of possible relationships between correlation of Y and X1 and correlation of Y and χ2, taking into account the fact that X1 and χ2 are correlated. To calculate the overlapping correlation for each significant ROI (Right Thalamus and Posterior Parietal), we first measured for each sedation state s the average connectivity matrix s, a 5 × 5 matrix resulting from all possible pairings between systems. We computed the correlation coefficient between the two regressors of interest (drug concentration and missed responses) across all subjects and sessions. We tested whether the correlation between each ROI and a functional system is affected by both or only one regressor.
2.6. Assessment of correlation between regressors
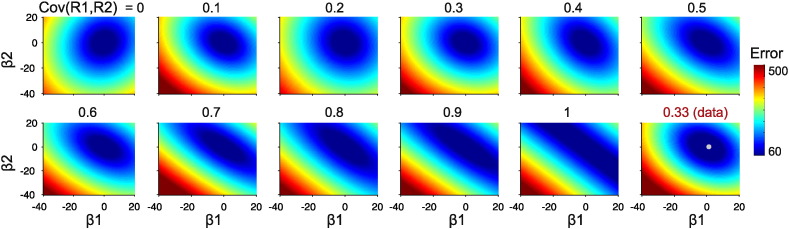
To ensure that correlation between our regressors of interest (propofol level and responsiveness) does not impede us from disentangling their actual contribution to functional connectivity we performed a theoretical analysis, running a simulation to explore the landscape of possible β values, in order to quantify how good and reliable our current estimation of their values is. We constructed artificial regressors (drugsim and misssim) controlling the amount of covariance between them, from 0 to 1. We used these regressors to calculate a simulated connectivity, as Ysim = α + β1 × drugsim + β2 × misssim + noise, with β1 and β2 being the actual β values calculated for that ROI pair.
We calculated the MSE comparing Ysim to the actual data (Y), and repeated this procedure for a broad range of β values (minimizing α value independently for each calculation) in order to construct a landscape of β values.
3. Results
As participants entered a state of drowsiness, response times to simple binary decisions became extremely long (although this remained highly variable between subjects) and eventually a subset of participants stopped responding to some or all trials (proportion of missed responses no sedation, mean [SD] = 0.06 [0.14]; missed responses sedation level 1 = 0.02 [0.03]; missed responses sedation level 2 = 0.19 [0.28]; missed responses recovery = 0.03 [0.05]). Propofol concentration in blood and percentage of missed responses varied widely between participants. As expected, these two measures correlated significantly (correlation coefficient = 0.33, p < 0.01), but with sufficient dispersion to assure reliable regressions to both factors simultaneously. The low correlation value testifies the coarseness of drug level as an index of conscious function and justifies the acquisition of behavioral responses to factor out the effect of drug concentration per se and the behavioral markers of consciousness.
Next we sought to analyze how propofol level and responsiveness impact functional connectivity between ROIs. Both regressors revealed simultaneous increases and decreases in connectivity between different systems (as opposed to a global decrease in connectivity) (Fig. 1c, d). A set of connections showed the same dependency with pharmacological and behavioral markers of drowsiness. For example DMN within-connectivity (considering the connectivity of all the ROIs of the DMN system among themselves) decreased both with increasing drug concentration and number of misses. In contrast, other connections (for example within FP connectivity) showed a decrease in connectivity with higher drug levels, displaying no covariance with behavioral markers of awareness. FP−OC connectivity showed an increase only associated with behavior.
After identifying brain systems whose connectivity distinctively indexes the effect of drug levels and the number of misses in the semantic categorization task, we zoomed in to identify individual ROIs whose connectivity shows greater variance (increases or decreases) with drug and misses. The ROIs showing the highest variation of connectivity with drug belong to the DMN and FP systems, mostly their posterior components (Fig. 1e). In contrast, the ROIs showing the highest variation in connectivity with number of misses were mainly in the CO system (Fig. 1f), with two symmetrical thalamic ROIs (MNI coordinates, [11, −12, 6] and [−12, −12, 6]) ranking in first and second places. This unbiased rank analysis indicates that behavioral markers of awareness are associated with thalamic connectivity, in agreement with the thalamo-cortical arousal circuits (the thalamic “off-switch”) (Alkire et al., 2008) hypothesis.
The main aim of our work was to identify which patterns of connectivity are affected specifically by propofol concentration and which by behavioral markers of conscious function. To this aim, we identified those ROIs whose br values were significantly different from zero for one regressor and not the other. Only the connectivity of two cortical areas (ROIs) varied significantly with propofol level and not with number of misses: Posterior Parietal − [−35, −46, 48] p < 0.05, Bonferroni corrected (141 ROIs producing 141 comparisons) – and Occipital ([−42, −76, 26], p < 0.003). Conversely, only the connectivity of the Right Thalamus — [11, −12, 6], p < 0.05, Bonferroni corrected (141 comparisons) varied significantly with the number of misses, but not with propofol concentration. This analysis shows that these three regions are the only ones (of the 141 candidate ROIs) whose pattern of connectivity to the rest of the brain is affected either by the drug concentration, or by behavioral markers, but not by both. Next, we measured, for these three regions (i = 1, 2, 3), the pattern of connectivity changes to all other ROIs, Br(i,rois) with rois = 1:N (Fig. 2a–c). To quantify the pattern of change of the three identified ROIs we collapsed, all Br(i,rois) across all ROI values corresponding to the same functional system (Fig. 2d–f). The only significant effects observed with increasing propofol concentration in this analysis were reduction in posterior parietal connectivity with the FP system and posterior parietal increased connectivity with the SM system (Fig. 2a, d). On the other hand, complete loss of responsiveness or extremely long RTs were associated with a clear topographical switching thalamic connectivity (Fig. 2c, f), consisting of a disconnection of the right thalamus from the entire frontal (both DMN and FP) and parietal (FP) lobes, and instead increasing its connectivity to the SM and OC systems (shadows in Fig. 2c mark spatial domains of positive and negative β-values). The same pattern was displayed by the mirror thalamic ROI, Left Thalamus, that however did not survive multiple comparisons (Fig. S4).
Our analyses implicitly rely on the assumption that we can differentiate the impact produced by each regressor on connectivity. We recognized that our two regressors of interest (plasma propofol level and behavioral state) were not entirely independent. Consequently, to further determine the differential effect of drug concentration and capacity to respond on functional connectivity, we tested for differences in overlapping correlations (Meng et al., 1992). Right thalamic connectivity was differentially affected by one of the two regressors for connections to the CO system (N = 72; z = 1.87; p < 0.05), FP system (N = 72; z = 1.68; p < 0.05), SM system (N = 72; z = −1.97; p < 0.05), and CO system (N = 72; z = −2.07; p < 0.05). This suggests again that functional connectivity from and to the right thalamus is primarily influenced by responsiveness. In contrast the connectivity of Posterior Parietal ROI showed a differential effect in overlapping correlations of the variables on connections to FP (N = 72; z = 2.60; p < 0.01) and SM (N = 72; z = 2.07; p < 0.05), suggesting that drug concentration is the main variable affecting parietal connectivity.
We also explored the robustness of our results taking into account random perturbations, through the analysis of the landscape of the mean squared error (MSE) of the regression for a broad range of β values. We found that when covariance between regressors is close to zero, a clear and single minimum exists in the β space (Fig. 3). As the covariance between regressors becomes larger, the valley surrounding this minimum becomes less steep, shifting towards a range of values along the identity line at very high covariance, a case in which many combinations of (interchangeable) β values yield an equally low MSE value (Fig. 3). Fig. 3, lower row and last column shows the β value landscape obtained for the real data whose β values were used to construct the artificial data (the white dot marks the actual β value we empirically obtained, that is, the β value obtained for the non-simulated data). The MSE landscape is quite similar to those with an intermediate r value, in agreement with the calculated value of correlation of 0.33 between our experimental regressors. In this situation a multivariate regression can discriminate between alternative assignments of β values in the presence of a single and very clear minimum (Tabachnick and Fidell, 2013).
Fig. 3.
MSE maps as a function of β values from x–x, for increasing covariation between regressors. Last panel shows MSE map for the actual data (white dot marks the β value pair calculated for the actual data).
4. Discussion
Here we show two distinct changes in patterns of functional connectivity during propofol administration in healthy volunteers, both of which stand out above decreases in global connectivity. The first of these appears to reflect a more generalized synaptic depressant effect that scales with propofol concentrations in plasma, but does not accurately reflect the behavioral changes seen in subjects. However, a second set of changes scale specifically with behavioral changes in responsiveness, and arguably reflect drug effects on critical brain connectivity associated with the maintenance of consciousness. Our results support the hypothesis that consciousness is directly governed by thalamic connectivity, rather than by fronto-parietal loops (cortico-cortical reverberation). In the open and highly debated issue of how anesthetics induce loss of response (LOR) and LOC (Alkire et al., 2008; Magnin et al., 2010; Mhuircheartaigh et al., 2010), our results assign – at least for propofol induced sedation – a key role to the thalamus, which, during reduced responsiveness, appears to disconnect from the frontoparietal network (FP) while increasing its functional connectivity to primary sensory areas (OC and SM systems) (Vanlersberghe and Camu, 2008; Ying et al., 2006).
Thalamic connectivity to the cortex is organized based on functional networks (Dosenbach et al., 2010). However, these individual networks do not work in isolation, but instead exchange information and depend on one another to function properly. While our analyses do not imply causality, our results point to the thalamus as a key hub coordinating these modules into a “network of networks”. It has been recently been shown that brain networks in the awake brain are connected through a topology that maximizes stability (Reis et al., 2014): if interconnections are provided by network hubs, and the connections between networks are moderately convergent, the system of networks is stable and robust to failure, a necessary condition for the maintenance of consciousness (Gallos et al., 2012; Gallos et al., 2012; Reis et al., 2014). We hypothesize that sedation disrupts this stable organization, as the thalamus disengages from its role as a fronto-parietal hub, and connects to sensory cortices, giving rise to a complete altered topology.
Propofol prolongs inhibitory postsynaptic currents mediated by GABA-A receptors leading to enhanced inhibitory synaptic transmission, and also influences presynaptic mechanisms of GABAergic transmission (Franks, 2008). Due to the wide distribution of GABA-A receptors in the brain (Pirker et al., 2000), propofol exerts a general hypnotic effect at the local and global levels in the cortex and subcortical structures that could account for the loss of cortico-cortical (fronto-parietal) connectivity revealed in our findings. However, perturbation in the conscious level, as measured by the capacity to respond, might be better related to preserved integration in specific neural networks such as those implicated in sleep and wakefulness. During the transition to sleep the thalamocortical functional connectivity seems to decrease (Spoormaker et al., 2010) leading to a disruption in information transfer and subsequent loss of consciousness. Propofol sedation creates the same scenario (Stamatakis et al., 2010), possibly through specific inhibition of thalamocortical neurons – perhaps via cyclic-nucleotide-gated (HCN) channels – that have shown to be much more sensitive to propofol than the hippocampus or medullar neurons (Ying et al., 2006). Here we show that reduced responsiveness – arguably associated with loss of integration of information − is closely related to changes in thalamocortical, but not cortico-cortical (fronto-parietal) connectivity.
One caveat is worth underlining for this study. Loss of responsiveness is not the same as loss of consciousness, even though sometimes they are used equivalently in the anesthesia literature. For example, certain anesthetics may produce behavioral unresponsiveness but not complete unconsciousness (Alkire et al., 2008). We have used responsiveness as a better proxy for consciousness than simply drug level – as typically done in the literature – but responsiveness is still an indirect (and incomplete) measure of consciousness.
The following are the supplementary data related to this article.
All one hundred one regions of interest (ROIs) used in the analysis.
a) Correlation matrices for all sedation levels. b) Distribution of correlation values per sedation level. c) Right thalamic ROI mean connectivity to the 5 functional systems, for all sedation levels.
a-b) β matrix showing dependences of functional brain connectivity with propofol level (a) and reaction time (b) instead of percentage of missed responses. c-d) Average β-values associated with Posterior Parietal and Right Thalamus, showing that RT indexes connectivity in the same way than missed responses.
All β-values for the behavioral regressor associated with Right and Left Thalamus ROIs. Red lines indicate positive β-values; blue lines indicate negative β-values. A very clear spatial map is observed, with all frontal FP and DMN connected to thalamus through negative β-values, while SM and OC ROIs are connected to thalamus through positive β-values.
Disclosure and conflict of interest
The authors declare no competing financial interests.
Acknowledgments
This work was partially supported by the Human Frontier Science Program and a Wellcome Trust Biomedical research fellowship WT093811MA. PB had a fellowship of the National Research Council of Argentina (CONICET) and was supported by a Human Frontier Science Program research grant. RMA was supported by the Wellcome Trust (grant number 083660/Z/07/Z), the Raymond and Beverly Sackler Studentship, and the Cambridge Commonwealth Trust. EAS was supported by the Stephen Erskine Fellowship, Queens' College Cambridge. DKM was supported by the British Oxygen Professorship of the Royal College of Anaesthetists. The authors would like to thank Jacobo Sitt for the useful suggestions.
References
- Adapa R.M., Davis M.H., Stamatakis E.A., Absalom A.R., Menon D.K. Neural correlates of successful semantic processing during propofol sedation. Hum. Brain Mapp. 2014;35(7):2935–2949. doi: 10.1002/hbm.22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire M.T., Hudetz A.G., Tononi G. Consciousness and anesthesia. Science. 2008;322(5903):876–880. doi: 10.1126/science.1149213. 18988836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barttfeld P., Uhrig L., Sitt J.D., Sigman M., Jarraya B., Dehaene S. Signature of consciousness in the dynamics of resting-state brain activity. Proc. Natl. Acad. Sci. U. S. A. 2015;112(3):887–892. doi: 10.1073/pnas.1418031112. 25561541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barttfeld P., Wicker B., McAleer P., Belin P., Cojan Y., Graziano M., Leiguarda R., Sigman M. Distinct patterns of functional brain connectivity correlate with objective performance and subjective beliefs. Proc. Natl. Acad. Sci. U. S. A. 2013;110(28):11577–11582. doi: 10.1073/pnas.1301353110. 23801762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein T.A., Dehaene S., Rohaut B., Tadel F., Cohen L., Naccache L. Neural signature of the conscious processing of auditory regularities. Proc. Natl. Acad. Sci. U. S. A. 2009;106(5):1672–1677. doi: 10.1073/pnas.0809667106. 19164526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M., Moran R., Murphy M., Boveroux P., Bruno M.A., Noirhomme Q., Ledoux D., Bonhomme V., Brichant J.F., Tononi G., Laureys S., Friston K. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J. Neurosci. 2012;32(20):7082–7090. doi: 10.1523/JNEUROSCI.3769-11.2012. 22593076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveroux P., Vanhaudenhuyse A., Bruno M.A., Noirhomme Q., Lauwick S., Luxen A., Degueldre C., Plenevaux A., Schnakers C., Phillips C., Brichant J.F., Bonhomme V., Maquet P., Greicius M.D., Laureys S., Boly M. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113(5):1038–1053. doi: 10.1097/ALN.0b013e3181f697f5. 20885292 [DOI] [PubMed] [Google Scholar]
- Breshears J.D., Roland J.L., Sharma M., Gaona C.M., Freudenburg Z.V., Tempelhoff R., Avidan M.S., Leuthardt E.C. Stable and dynamic cortical electrophysiology of induction and emergence with propofol anesthesia. Proc. Natl. Acad. Sci. U. S. A. 2010;107(49):21170–21175. doi: 10.1073/pnas.1011949107. 21078987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernik D.A., Gillings D., Laine H., Hendler J., Silver J.M., Davidson A.B., Schwam E.M., Siegel J.L. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J. Clin. Psychopharmacol. 1990;10(4):244–251. 2286697 [PubMed] [Google Scholar]
- Ciobanu L., Reynaud O., Uhrig L., Jarraya B., Le Bihan D. Effects of anesthetic agents on brain blood oxygenation level revealed with ultra-high field MRI. PLOS One. 2012;7(3):e32645. doi: 10.1371/journal.pone.0032645. 22427858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S., Changeux J.P. Ongoing spontaneous activity controls access to consciousness: a neuronal model for inattentional blindness. PLOS Biol. 2005;3(5):e141. doi: 10.1371/journal.pbio.0030141. 15819609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S., Changeux J.P. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70(2):200–227. doi: 10.1016/j.neuron.2011.03.018. 21521609 [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U., Nardos B., Cohen A.L., Fair D.A., Power J.D., Church J.A., Nelson S.M., Wig G.S., Vogel A.C., Lessov-Schlaggar C.N., Barnes K.A., Dubis J.W., Feczko E., Coalson R.S., Pruett J.R., Jr., Barch D.M., Petersen S.E., Schlaggar B.L. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. 20829489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B., Tibshirani R.J. Chapman & Hall; New York: 1994. An Introduction to the Bootstrap. [Google Scholar]
- Franks N.P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 2008;9(5):370–386. doi: 10.1038/nrn2372. 18425091 [DOI] [PubMed] [Google Scholar]
- Gallos L.K., Makse H.A., Sigman M. A small world of weak ties provides optimal global integration of self-similar modules in functional brain networks. Proc. Natl. Acad. Sci. U. S. A. 2012;109(8):2825–2830. doi: 10.1073/pnas.1106612109. 22308319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallos L.K., Sigman M., Makse H.A. The conundrum of functional brain networks: small-world efficiency or fractal modularity. Front. Physiol. 2012;3:123. doi: 10.3389/fphys.2012.00123. 22586406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez F., Phillips C., Soddu A., Boly M., Boveroux P., Vanhaudenhuyse A., Bruno M.A., Gosseries O., Bonhomme V., Laureys S., Noirhomme Q. Changes in effective connectivity by propofol sedation. PLOS One. 2013;8(8):e71370. doi: 10.1371/journal.pone.0071370. 23977030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Li S.J., Hudetz A.G. Increased precuneus connectivity during propofol sedation. Neurosci. Lett. 2014;561:18–23. doi: 10.1016/j.neulet.2013.12.047. 24373986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnin M., Rey M., Bastuji H., Guillemant P., Mauguière F., Garcia-Larrea L. Thalamic deactivation at sleep onset precedes that of the cerebral cortex in humans. Proc. Natl. Acad. Sci. U. S. A. 2010;107(8):3829–3833. doi: 10.1073/pnas.0909710107. 20142493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh B., White M., Morton N., Kenny G.N. Pharmacokinetic model driven infusion of propofol in children. Br. J. Anaesth. 1991;67(1):41–48. doi: 10.1093/bja/67.1.41. 1859758 [DOI] [PubMed] [Google Scholar]
- Meng X.L., Rosenthal R., Rubin D.B. Comparing correlated correlation coefficients. Psychol. Bull. 1992;111(1):172–175. [Google Scholar]
- Mhuircheartaigh R.N., Rosenorn-Lanng D., Wise R., Jbabdi S., Rogers R., Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J. Neurosci. 2010;30(27):9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. 20610743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.G. The JackKnife — a review. Biometrika. 1974;61(1):1–15. [Google Scholar]
- Pirker S., Schwarzer C., Wieselthaler A., Sieghart W., Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101(4):815–850. doi: 10.1016/s0306-4522(00)00442-5. 11113332 [DOI] [PubMed] [Google Scholar]
- Rees G., Kreiman G., Koch C. Neural correlates of consciousness in humans. Nat. Rev. Neurosci. 2002;3(4):261–270. doi: 10.1038/nrn783. 11967556 [DOI] [PubMed] [Google Scholar]
- Reis S.D.S., Hu Y., Babino A., Andrade J.S., Jr., Canals S., Sigman M., Makse H.A. Avoiding catastrophic failure in correlated networks of networks. Nat. Phys. 2014;10(10):762–767. [Google Scholar]
- Schroter M.S., Spoormaker V.I., Schorer A., Wohlschlager A., Czisch M., Kochs E.F., Zimmer C., Hemmer B., Schneider G., Jordan D., Ilg R. Spatiotemporal reconfiguration of large-scale brain functional networks during propofol-induced loss of consciousness. J. Neurosci. 2012;32(37):12832–12840. doi: 10.1523/JNEUROSCI.6046-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrouff J., Perlbarg V., Boly M., Marrelec G., Boveroux P., Vanhaudenhuyse A., Bruno M.A., Laureys S., Phillips C., Pélégrini-Issac M., Maquet P., Benali H. Brain functional integration decreases during propofol-induced loss of consciousness. Neuroimage. 2011;57(1):198–205. doi: 10.1016/j.neuroimage.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Sergent C., Baillet S., Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat. Neurosci. 2005;8(10):1391–1400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- Spoormaker V.I., Schroter M.S., Gleiser P.M., Andrade K.C., Dresler M., Wehrle R., Samann P.G., Czisch M. Development of a large-scale functional brain network during human non-rapid eye movement sleep. J. Neurosci. 2010;30(34):11379–11387. doi: 10.1523/JNEUROSCI.2015-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis E.A., Adapa R.M., Absalom A.R., Menon D.K. Changes in resting neural connectivity during propofol sedation. PLOS One. 2010;5(12):e14224. doi: 10.1371/journal.pone.0014224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Menon V., Rubin D., Musen M., Greicius M.D. Network analysis of intrinsic functional brain connectivity in Alzheimer's disease. PLOS Comput. Biol. 2008;4(6):e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supp G.G., Siegel M., Hipp J.F., Engel A.K. Cortical hypersynchrony predicts breakdown of sensory processing during loss of consciousness. Curr. Biol. 2011;21(23):1988–1993. doi: 10.1016/j.cub.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Tabachnick B.G., Fidell L.S. Pearson; Boston: 2013. Using Multivariate Statistics. [Google Scholar]
- Vanlersberghe C., Camu F. Propofol. Handb. Exp. Pharmacol. 2008;182:227–252. doi: 10.1007/978-3-540-74806-9_11. [DOI] [PubMed] [Google Scholar]
- Ying S.W., Abbas S.Y., Harrison N.L., Goldstein P.A. Propofol block of I(h) contributes to the suppression of neuronal excitability and rhythmic burst firing in thalamocortical neurons. Eur. J. Neurosci. 2006;23(2):465–480. doi: 10.1111/j.1460-9568.2005.04587.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All one hundred one regions of interest (ROIs) used in the analysis.
a) Correlation matrices for all sedation levels. b) Distribution of correlation values per sedation level. c) Right thalamic ROI mean connectivity to the 5 functional systems, for all sedation levels.
a-b) β matrix showing dependences of functional brain connectivity with propofol level (a) and reaction time (b) instead of percentage of missed responses. c-d) Average β-values associated with Posterior Parietal and Right Thalamus, showing that RT indexes connectivity in the same way than missed responses.
All β-values for the behavioral regressor associated with Right and Left Thalamus ROIs. Red lines indicate positive β-values; blue lines indicate negative β-values. A very clear spatial map is observed, with all frontal FP and DMN connected to thalamus through negative β-values, while SM and OC ROIs are connected to thalamus through positive β-values.