Abstract
Reactive oxygen species (ROS) play a central role in estrogen deficiency-induced bone loss. We previously identified and characterized a novel member of the Peroxiredoxin (PRX) like 2 family that we called PAMM: Peroxiredoxin Activated in M-CSF stimulated Monocytes, a redox regulatory protein that modulates osteoclast differentiation in vitro. In this study, we report increased PAMM expression in H2O2-treated cells and in bones from ovariectomized (OVX) mice 4 weeks after surgery, models for oxidative stress in vitro and in vivo, respectively. We also detected increased PAMM abundance and phosphorylated Akt in OVX mice treated with estrogen. In addition, Wortmannin, a specific PI3Kinase inhibitor and Rapamycin, an inhibitor of the PI3Kinase/Akt pathway, blocked Akt phosphorylation and stimulation of PAMM expression by M-CSF. These results indicate that M-CSF-induced PAMM expression is mediated by Akt phosphorylation. Our data also suggest that estrogen-induced PAMM expression is mediated by phosphorylation of Akt. These findings point to PAMM as a potential candidate for Akt-mediated protection against oxidative stress.
Abbreviations: PAMM, Peroxiredoxin Activated in M-CSF stimulated Monocytes; M-CSF, macrophage colony-stimulating factor; RANKL, Receptor activator of nuclear factor kappa-B ligand; TRAP, tartrate-resistant acid phosphatase; Nrf2, nuclear factor-erythroid 2-related factor 2
Keywords: PAMM, Antioxidant, Oxidative stress, Bone loss, Ovariectomy
Graphical abstract
Highlights
-
•
We previously identified, characterized and proved PAMM is a novel antioxidant protein that regulates osteoclast formation and activity via modulation of ROS production in vitro.
-
•
PAMM expression is induced by oxidative stress in vivo and in vitro.
-
•
PAMM expression in osteoclasts is stimulated by estrogen and is mediated by Akt phosphorylation.
-
•
PAMM may be a potential candidate for Akt-mediated protection against oxidative stress and bone loss induced by estrogen deficiency.
1. Introduction
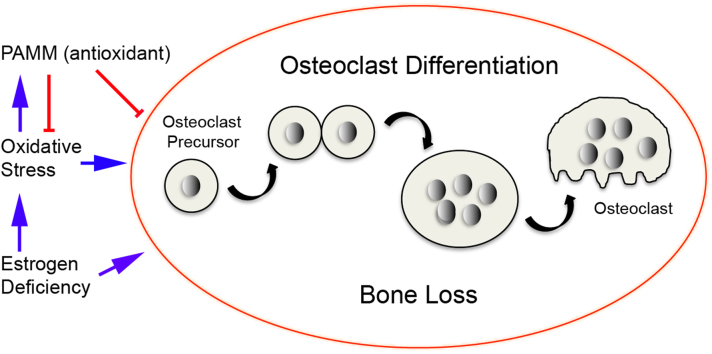
Bone homeostasis is influenced by decreases in defenses against oxidative stress as well as increases in the production of reactive oxygen species (ROS) [1]. At the cellular level, defects in bone remodeling caused by oxidative stress are associated with decreased osteoblast and osteoclast numbers and decreased bone formation rate as well as increased osteoblast and osteocyte apoptosis [2]. Aging and loss of sex steroids also result in increased oxidative stress as reflected by an increase in the levels of reactive oxygen species, and decreased activity of glutathione reductase—an enzyme that catalyzes the reduction of glutathione disulfide (GSSG) to glutathione (GSH) [3–5]. Lean et al. demonstrated that estrogen deficiency contributes to bone loss by lowering thiol antioxidants in osteoclasts [6]. Estrogen, on the other hand, can exert beneficial effects in other tissues by suppressing the generation of ROS [7]. The same changes in oxidative stress can be acutely reproduced by ovariectomy in female rats [8]. For instance, the levels of lipid peroxidation (LPO) and hydrogen peroxide (H2O2) were increased and enzymatic antioxidants including superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione S transferase (GST) were decreased in ovariectomized animals when compared to sham-operated control rats [9]. Ovariectomy not only causes an increase in the levels of ROS, but also a reduction in the levels of the enzymes that regenerate the reduced forms of glutathione and thioredoxin in bone marrow [6]. Moreover, levels of both antioxidants as well as their regenerative enzymes are rapidly normalized by treatment with 17-β estradiol [10]. Finally, treatment with antioxidants can prevent estrogen-deficiency bone loss while drugs that reduce thiol antioxidants cause bone loss [10]. Taken together, these observations show that estrogen deficiency causes bone loss, at least in part, by lowering antioxidant levels in bone. The presence of estrogen, on the other hand, may prevent bone loss by enhancing bone antioxidant defenses. López-Grueso et al. provided evidence that estrogen replacement therapy (ERT) after ovariectomy in animals can prevent oxidative stress and metabolic alterations [11].
We cloned and characterized a novel antioxidant protein: Peroxiredoxin Activated in M-CSF stimulated Monocytes (PAMM) [12]. Expression of PAMM is increased in CD14+ monocytes stimulated with M-CSF (Macrophage colony-stimulating factor) and decreases as M-CSF – stimulated cells undergo RANKL (Receptor activator of nuclear factor kappa-B- ligand) – dependent osteoclast differentiation. PAMM has a CXXC motif, which is essential for its redox functions. It is expressed in bone, brain, liver, and kidney. Our previous studies showed that expression of wild-type PAMM in cells resulted in an increased GSH/GSSG ratio and also protected cells from hydrogen peroxide (H2O2)-induced oxidative stress, indicating that PAMM has antioxidant activity. In addition, PAMM overexpression inhibited RANKL-induced osteoclast formation in vitro [12]. ROS stimulate osteoclast differentiation and function whereas free radical scavengers and antioxidants have the opposite effect. PAMM regulates osteoclast formation and activity via modulation of ROS production. These findings provided evidence that PAMM is a redox regulatory protein that modulates osteoclast differentiation in vitro, and also support a novel paradigm in which estrogen deficiency results in modified expression of PAMM in osteoclast precursors, allowing an increase in ROS, which ultimately leads to increased osteoclast formation and bone resorption.
In this study we examined the role of PAMM in ovariectomy-induced bone loss. We sought to determine if oxidative stress induces the redox-regulating activity of PAMM following ovariectomy and in an established in vitro model of oxidative stress. We also assessed the impact of estrogen treatment on PAMM expression following ovariectomy.
2. Materials and methods
2.1. Reagents
Antibodies against β-actin (no. 4967), Phospho-Akt (Ser473) (no.9271) were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Anti-C10orf58 (PAMM) antibody (HPA009025), 17β-estradiol (E2) (E8515), corn oil (C8267), H2O2 (H1009). Wortmannin (W1628) and Rapamycin (R8781) were purchased from Sigma-Aldrich Inc. (St. Louis, MO). The high affinity estrogen receptor antagonist ICI 182,780 was purchased from TOCRIS Bioscience (Minneapolis, MN-Cat no. 1047).
2.2. Mice and ovariectomy
All procedures were approved by the Forsyth Institute IACUC. Twelve-weeks-old female Swiss Webster mice were purchased from Charles River Laboratories (Wilmington, MA). Bilateral ovariectomy (OVX, n=12) was performed under general anesthesia (150 mg/kg Ketamine combined with 10 mg/kg Xylazine). Sham ovariectomy (Sham, n=12) was similarly performed but with externalization and replacement of the ovaries. Beginning one day after surgery OVX animals were injected subcutaneously with vehicle (corn oil, C8267, Sigma-Aldrich, n=6) or with replacement doses of E2 (E8515, Sigma-Aldrich, 30 ng/g/day, n=6) once daily for four weeks. Sham-operated animals received daily subcutaneous injections of vehicle for four weeks. Body weight was recorded weekly for all groups. For each animal the right femur was fixed in 4% paraformaldehyde and prepared for histological analysis and the left distal femur was used to obtain protein lysates for GSH/GSSG ratio measurement and western blot analysis as described below.
2.3. In vivo assessment of bone mineral density (BMD)
Dual Energy X-ray Absorptiometry (DXA) scanning was performed using a Lunar PIXImus Scanner (Lunar PIXImus2, software version 1.4X) immediately prior to surgery (12 weeks of age) and 4 weeks after OVX/Sham. Total BMD (excluding the head) and distal femur BMD were measured. For this, mice were positioned on their abdomens with the hindlimb externally rotated. The hip and knee were flexed to 110° with the ankle in neutral position. A region of interest (16×17 pixels) at the distal femoral metaphysis was chosen for analysis.
2.4. Cell culture
Human peripheral blood mononuclear cells (PBMCs) were isolated from PBS-diluted blood (1/1, vol/vol) by centrifugation on a Histopaque-1077 (Sigma-Aldrich) density gradient. CD14+ selection was performed by using the Human CD14 Selection Cocktail (StemCell Technologies, catalog no. 18058). CD14+ PBMCs were cultured for 4 days in α-MEM/10% FBS supplemented with 150 ng/ml human M-CSF (PeproTech Inc.) with or without 100 nM Wortmannin [13] or Rapamycin [14]. For certain experiments cells were incubated for 20 minutes with hydrogen peroxide (H2O2) at the indicated concentrations. In the estrogen replacement experiments, estrogen was stripped from the FBS by incubation twice in 0.25% dextran-coated charcoal (Sigma-Aldrich, St. Louis, MO) for 30 min at 45 °C. E2 (1 nM) and/or ICI 182,780 (100 nM) [6] were added for estrogen replacement or estrogen inhibition, respectively.
2.5. Real-time PCR analysis
Total RNA was extracted from cells and tissues using TRIAZOL (Invitrogen). For RT-PCR, 2 μg of RNA was reverse transcribed into cDNA with SuperScript II (Invitrogen, no. 18064-022). Real-time reverse transcription polymerase chain reaction was conducted with iQ™ SYBR® Green supermix (Bio Rad, Hercules, CA, USA) in a 20 μl reaction volume containing 1 μl of a 1 μl of first-strand reaction product and 1 μl of a 500 nM gene-specific upstream and downstream primer mix. Amplification and analysis of cDNA fragments was performed using a CFX Connect™ Real-Time PCR Detection System (Bio Rad, Hercules, CA, USA). Cycling conditions were as follows: initial denaturation at 95 °C for 10 min, followed by 40 cycles consisting of a 15 s denaturation interval at 95 °C, and a 1 min annealing and extension interval at 60 °C. Amplification of human GAPDH mRNA, the housekeeping gene, was used as an endogenous control to normalize expression results. Levels of mRNA expression were measured as threshold levels (Ct) and normalized with the individual GAPDH control Ct values. PAMM and Nrf2 mRNA abundance were presented as relative quantification (RQ). The primer sequences were as follows: human pamm (C10orf58) sense, 5’-ATA GAC CTG AAA ACA CTG GA-3’, and antisense, 5’-GCA GCT TCC TCT CGA CAG AG-3’; human Nrf2 sense, 5′-GAG AGC CCA GTC TTC ATT GC-3′, and antisense, 5′-TGC TCA ATG TCC TGT TGC AT-3′; human GAPDH, sense, 5’-AAT CCC ATC ACC ATC TTC CA-3’, and antisense, 5’-TGG ACT CCA CGA CGT ACT CA-3’.
2.6. Western blot analysis
The left distal femur of OVX and SHAM mice was resected, flash frozen in liquid nitrogen, and homogenized by hand in Novex® Tris–Glycine SDS Sample Buffer (Invitrogen). Cell protein lysates were generated by resuspending cells in the sample buffer. Equivalent amounts of protein were loaded onto Novex Tris–Glycine SDS gels (Invitrogen), electrophoresed, and transferred to nylon membranes. After blocking, membranes were incubated with the indicated primary antibodies overnight at 4 °C, washed in TBST buffer to remove unbound antibody, and incubated with secondary antibody for 1 h at room temperature. Chemiluminescent signal was detected with LUMIGLO reagents A and B (Cell Signaling Technology; Danvers, MA; no. 7003) and blots were then exposed to X-ray film.
2.7. Histology and TRAP staining
Femurs were fixed in cold 4% paraformaldehyde, decalcified in cold 14% EDTA, embedded in paraffin, sectioned at 5 μm and placed on charged slides (Manco Inc., Avon, OH). Osteoclast activity was detected by tartrate-resistant acid phosphatase (TRAP) staining. Slides were deparaffinized and rehydrated through graded ethanol to distilled water. TRAP solution (0.1 M acetic acid buffer solution; naphthol AS-MX phosphate; fast red Violet LB salt; N,N-dimethylformamide; Sigma-Aldrich Inc.) was applied and the sections were incubated at 37 °C for 30 min or until the control was developed. The slides were then rinsed in distilled water, counterstained with 0.02% Fast Green for 30 s, rinsed with water, dehydrated through graded alcohols, and mounted. Images were acquired with a LEICA DMLS optical microscope equipped with a LEICA DC 100 digital imaging system.
2.8. Immunohistochemistry
Immunohistochemistry was performed on deparaffinized bone sections according to standard protocols. Briefly, sections were incubated with primary antibodies at the indicated dilutions in a humidified chamber overnight at 48 °C. The following day, the slides were washed 3×10 min in 1×PBS+0.1% (vol/vol) Tween-20. Sections were then incubated for 1 h at room temperature with the biotinylated secondary antibody (100 μ/section, diluted 1:250 to 1:750 in blocking buffer) in a humidified chamber. Finally, slides were washed 3×10 min in 1×PBS and incubated with one drop of ABC (Ready-to-use Vectastain ABC Kit, Vector Laboratories, Inc., Burlingame, CA), incubated for 30 min at room temperature, washed 3×5 min in 1×PBS, and incubated for 2–10 min with DAB solution until signal developed. Images were acquired with a LEICA DMLS optical microscope equipped with a LEICA DC 100 digital imaging system.
2.9. Measurement of GSH/GSSG ratio
The GSH/GSSG ratio was measured with the glutathione reductase/5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) assay kit (BIOXYTECHR GSH/GSSG-412TM, Catalog number 21040, OXIS International, Beverly Hills, CA) from bone protein lysates according to the manufacturer's instructions. Briefly, total glutathione (GSHt) and GSSG concentrations were derived from GSH and GSSG standard curves and converted to nmol/mg of protein. Reduced GSH concentrations were found by subtracting GSSG from total glutathione. Finally, the GSH/GSSG ratio was calculated by dividing the difference between total glutathione and GSSG concentrations by the GSSG concentration (ratio=GSHt–2 (GSSG) / GSSG) [15].
2.10. Statistical analysis
Data are presented as means plus or minus SDs from at least three independent experiments. Statistical significance was determined by using one-way analysis of variance (ANOVA) followed by the Student t test (*p<0.05; **p<0.01).
3. Results
3.1. Ovariectomy increases PAMM abundance and Akt phosphorylation in Bone
Sham animals were administered vehicle. Body weight was recorded weekly for all groups. Body composition and bone mineral density (BMD) were monitored to confirm effective ovariectomy (OVX) and E2 replacement therapy. Uterine weight was recorded at the time of sacrifice for all animals. All animals gained weight similarly during the 4-week treatment period (Table 1) and there were no significant differences in weight gain based on experimental group. The mean uterine weight in the OVX/Vehicle group was significantly lower (p<0.01) than the other groups 4 weeks after surgery (Table 1).
Table 1.
Body weight and uterine weight (Grams).
|
Body weight |
Uterine weight (4 weeks after OVX) | ||
|---|---|---|---|
| Week 0 | Week 4 | ||
| OVX/Vehicle | 23.67±1.92 | 29.62±1.84 | 0.03±0.006** |
| OVX/E2 | 24.67±2.89 | 31.13±1.73 | 0.15±0.02 |
| Sham/Vehicle | 23.47±2.21 | 28.62±1.27 | 0.14±0.03 |
Values are mean ± SD. *, indicates the significant differences at the level of p<0.05.
Indicates the significant differences at the level of p<0.01.
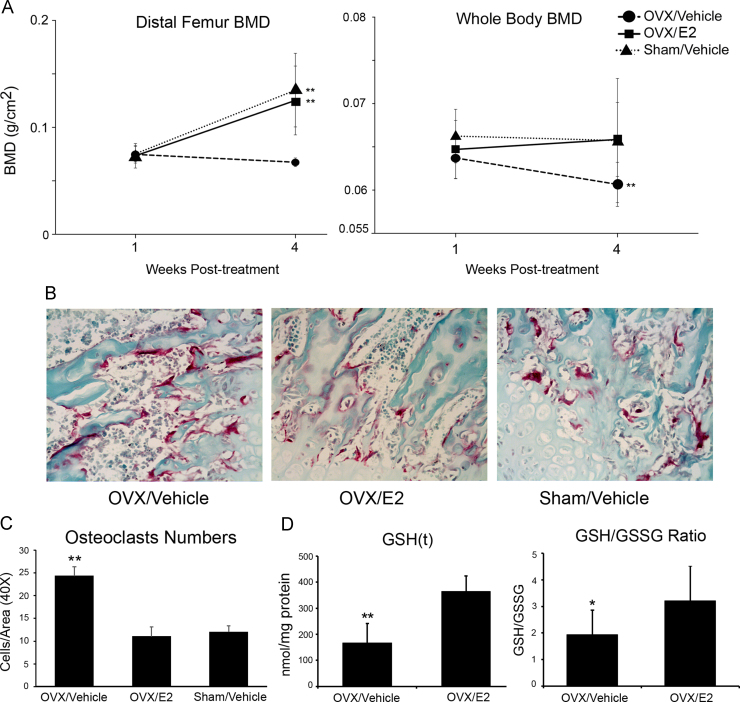
Distal femur bone mineral density (BMD) and whole body BMD (excluding the head) were assessed in all groups immediately prior to OVX/Sham and 4 weeks after OVX/Sham. There were no significant differences in baseline distal femur or total body BMD between the groups (p>0.05). Over the 4-week period, distal femur BMD increased significantly in the OVX/E2 and the Sham/Vehicle groups (70.5% and 79%, respectively) while distal femur BMD decreased significantly in the OVX/Vehicle group (9.7%). Similarly, there was no detectable difference in whole body BMD in the OVX/E2 and the Sham/Vehicle groups while there was a significant decline in the OVX/Vehicle group. As a result, both distal femur and total body BMD were significantly lower in the OVX/Vehicle group (0.068 g/cm2 and 0.060 g/cm2, respectively) compared to the OVX/E2 (0.125 g/cm2 and 0.065 g/cm2) and Sham/Vehicle group (0.134 g/cm2 and 0.065 g/cm2) after 4 weeks of treatment (**p<0.01) (Fig. 1A). TRAP staining was performed of sections through the distal femur and osteoclasts were quantified as the number of TRAP positive cells per high-powered field (Fig. 1B). There were significantly fewer osteoclasts in the OVX/E2 and SHAM/Vehicle groups compared to the OVX/Vehicle group (**p<0.01, Fig. 1C) confirming that estrogen replacement inhibited ovariectomy-induced osteoclast differentiation. Moreover, decreased GSH(t) and GSH/GSSG ratio were detected in the OVX/Vehicle group (p<0.05, Fig. 1D), confirming oxidative stress in ovariectomized mice.
Fig. 1.
Bone loss and oxidative stress are induced by ovariectomy. (A) Bone density (distal femur and whole body) was determined by DXA immediately prior to surgery and 4 weeks after surgery. Decreased BMD was determined in the OVX/Vehicle group compared to the OVX/E2 and Sham/Vehicle groups (**p<0.01). (B) TRAP staining was performed on sections through the distal femur from OVX and sham-operated animals with/without estrogen replacement to quantify osteoclasts (TRAP positive cells). (C) Osteoclasts were counted per area (40X) in all groups. Results are expressed as mean +/− SD. We detected fewer osteoclasts in the OVX/E2 and SHAM groups (**p<0.01) compared to the OVX/Vehicle group. (D) Decreased total GSH (**p<0.01) and GSH/GSSG (*p<0.05) ratio from bone protein lysates is detected in the OVX/Vehicle group showing that oxidative stress in OVX mice.
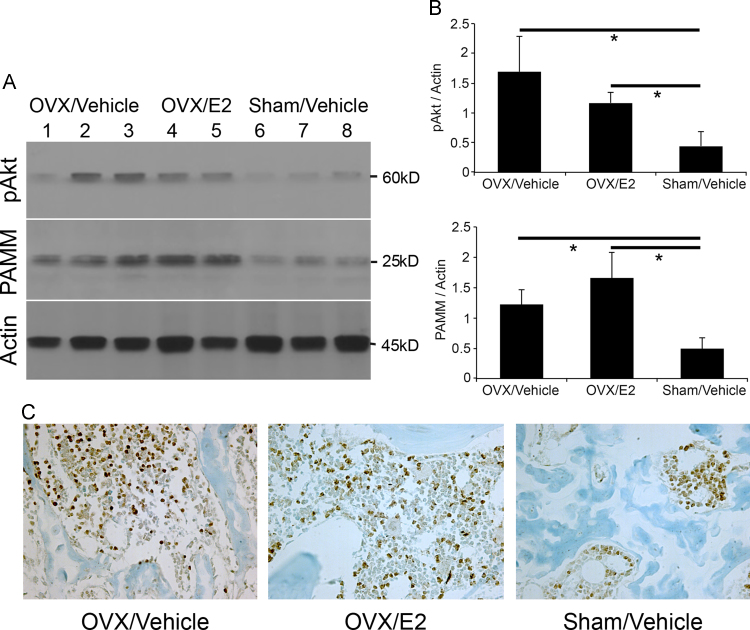
Activation of the PI3K/Akt/NF-κB signaling pathway is essential for osteoclast differentiation from monocytic precursors, osteoclast proliferation, osteoclast survival, and osteoclastic bone resorbing activity [12,13,16]. Akt also provides a survival signal that protects cells from apoptosis induced by growth factor withdrawal and oxidative stress [14]. We hypothesized that ovariectomy would result in both Akt activation and increased PAMM expression in bone. We therefore determined Akt activation in bone by assessing Ser 473 phosphorylation status by western blot analysis. We detected increased Akt phosphorylation in protein lysates from the distal femur in the OVX/Vehicle and OVX/E2 groups compared to the Sham/Vehicle group (p<0.05). Similarly, we found significantly increased PAMM protein expression in the OVX/Vehicle and OVX/E2 groups compared to the Sham/Vehicle group (p<0.05) (Fig. 2A and B). Expression of PAMM at the distal femur was also detected by immunohistochemistry 4 weeks after OVX or Sham-operated mice (Fig. 2C).
Fig. 2.
Ovariectomy increases PAMM abundance and Akt phosphorylation in bone. (A) Western blot analysis of protein extracts from distal femur shows increased PAMM expression and Akt phosphorylation in OVX/Vehicle group (n=3, lanes 1, 2, and 3) and OVX/E2 group (n=2, lanes 4 and 5) compared to Sham/Vehicle mice (n=3, lanes 6, 7, and 8). (B) PAMM and pAkt are increased in OVX group compare with Sham group (*p<0.05). There is a trend toward increased PAMM expression with E2 treatment in OVX mice. (C) Expression of PAMM at the distal femur was also detected by immunohistochemistry 4 weeks after OVX or Sham-operated mice.
3.2. M-CSF stimulation of PAMM expression requires Akt phosphorylation
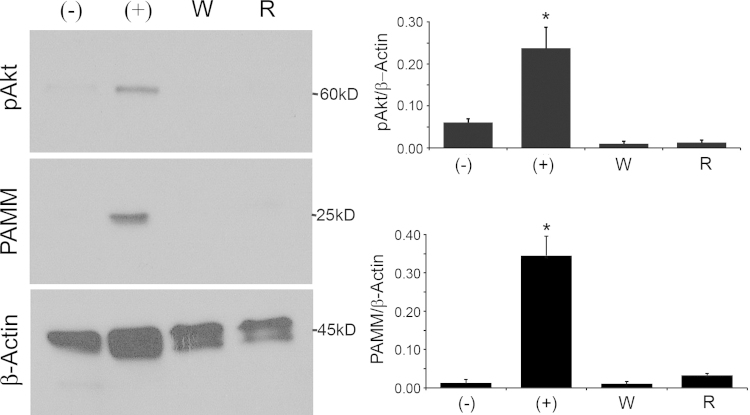
We have previously shown that M-CSF induces PAMM expression in cultured CD14+ peripheral blood mononuclear cells (PBMCs) [12]. We next determined if M-CSF stimulation of PAMM expression required Akt phosphorylation, which requires PI3Kinase activity. We stimulated human CD14+ PBMCs with M-CSF and determined PAMM abundance and phosphorylated Akt in the presence of Wortmannin or Rapamycin. Wortmannin is a specific PI3Kinase inhibitor and Rapamycin is an inhibitor of the Akt/mTOR pathway, that acts downstream of Akt. We found that both Wortmannin and Rapamycin blocked M-CSF induced PAMM expression via inhibited phosphorylation of Akt (Fig. 3).
Fig. 3.
M-CSF stimulation of PAMM expression requires Akt phosphorylation. Human CD14+ PBMCs were stimulated with M-CSF (+) and Wortmannin (W) or Rapamycin (R) for 4 days. M-CSF induced PAMM expression and phosphorylation of Akt (Ser 473). Wortmannin and Rapamycin, on the other hand, blocked M-CSF-induced Akt phosphorylation and PAMM expression. (−): Untreated control cells. (+): Cells stimulated with 150 ng/mL M-CSF. W: Cells stimulated with M-CSF and 100 nM Wortmannin. R: Cells stimulated with M-CSF and 100 nM Rapamycin.
3.3. Estrogen stimulates M-CSF induced PAMM Expression in preosteoclasts
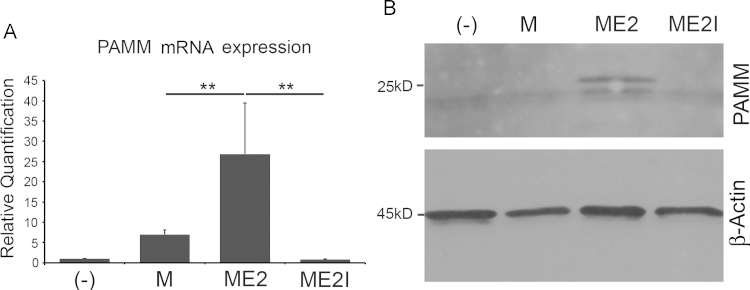
We then determined if estrogen was required for M-CSF induced PAMM expression. We cultured human CD14+ PBMCs for 4 days in α-MEM/10% charcoal stripped FBS, 150 ng/mL human M-CSF (PeproTech Inc.) and E2 with/without ICI 182,780, an estrogen antagonist. RT-PCR analysis of RNA isolated from the cells demonstrated that estrogen increased pamm gene abundance while ICI 182,780 decreased it (Fig. 4A). Western blot analysis of protein extracts confirmed these results (Fig. 4B).
Fig. 4.
Estrogen deficiency blocks M-CSF-induced PAMM expression in preosteoclasts. Human CD14+ PBMCs were cultured for 4 days in α-MEM/10% charcoal stripped FBS supplemented with 150 ng/mL human M-CSF (PeproTech Inc.) (M) and 1 nM E2 (ME2, M-CSF+E2) or with 100 nM ICI 182,780 (ME2I, M-CSF+E2+ICI). (A) Total RNA was extracted from the cultured cells and analyzed by qPCR. Real time PCR analysis demonstrates that, compared with the cells treated with M-CSF alone (M), PAMM abundance is increased in cells treated with M-CSF and Estradiol (ME2), and significantly decreased in the presence of ICI 182,780 (ME2I). (B) Western blot analysis from total cell protein lysates shows that PAMM expression is detected in estrogen-treated cells (ME2). No PAMM band can be observed in ICI 182,782-treated cells (ME2I).
3.4. Oxidative stress induces PAMM and Nrf2 mRNA expression in vitro
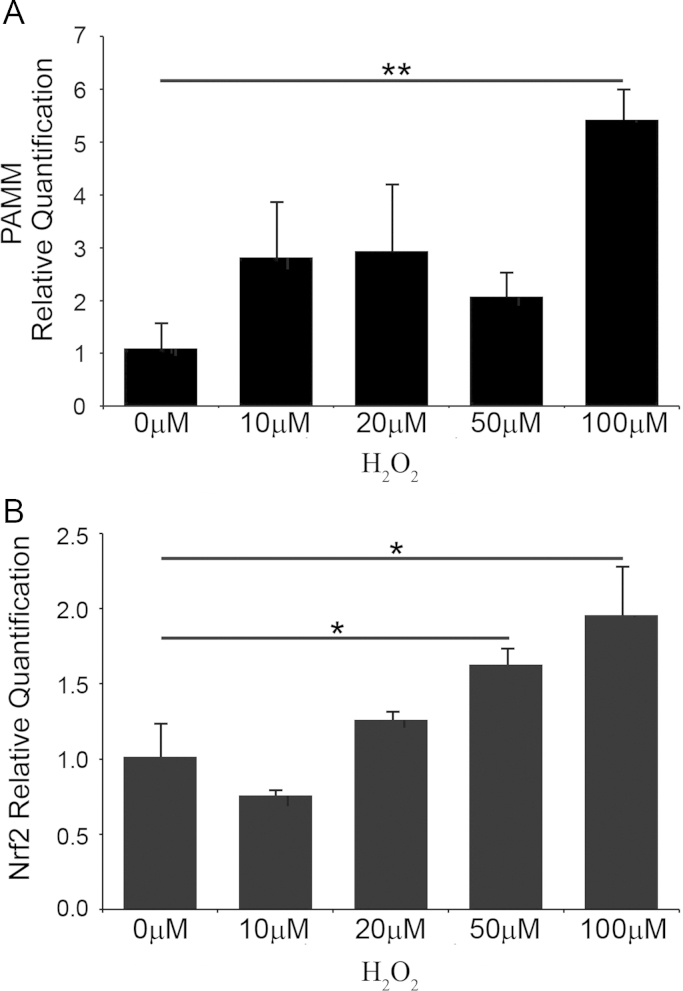
Ovariectomy causes both estrogen-deficiency and oxidative stress [5]. We observed increased PAMM expression in long bones of mice from the OVX/Vehicle group suggesting that upregulation of PAMM was induced by oxidative stress in vivo. To confirm this observation at the cellular level, we treated human PBMCs with H2O2 for 20 min, after which we replaced the H2O2-containing medium with the fresh medium without H2O2 [17,18]. Total RNA was extracted at 24 h after treatment and analyzed for PAMM mRNA expression by RT PCR. We found significantly increased PAMM mRNA abundance in human PBMCs treated with 100 µM H2O2, (Fig. 5A). Nuclear factor-erythroid 2-related factor 2 (Nrf2) is a transcription factor that regulates the cellular response to oxidative stress by stimulating expression of several antioxidant proteins [19]. We hypothesized that H2O2-induced PAMM expression was associated with H2O2-induced Nrf2 mRNA expression. Indeed, we found a significant increase in Nrf2 mRNA in cells treated with 50 µM H2O2 (Fig. 5B), confirming our hypothesis.
Fig. 5.
Antioxidant stress induces PAMM mRNA expression in human PBMCs. Human peripheral blood mononuclear cells (PBMCs) were treated with increasing concentrations of H2O2 (0, 10, 20, 50 and 100 µM) for 20 min. Total RNA was extracted 24 h after treatment, reverse transcribed into cDNA and analyzed by real-time PCR. (A) PAMM mRNA abundance is significantly increased by treatment with 100 µM H2O2 in human PBMC. (B) Nrf2 mRNA abundance is significantly increased by treatment with 50 and 100 µM H2O2 in human PBMC compared with untreated cells.
4. Discussion
In this study, we used ovariectomy in mice, a well-established model system of bone loss induced by estrogen deficiency and a model system of oxidative stress in vivo, to examine the role of PAMM during pathological bone loss. We also stimulated human PBMC with hydrogen peroxide (H2O2) to study oxidative stress and PAMM expression in vitro. We detected increased PAMM abundance in bones from ovariectomized mice 4 weeks after surgery. We also found increased abundance of PAMM and Nrf2 mRNA in H2O2-treated cells compared to untreated control cells. We demonstrated that blocking estrogen receptor inhibited M-CSF-induced PAMM expression in preosteoclasts. These results suggest that PAMM may be part of the cellular antioxidant response designed to maintain redox homeostasis.
In previous studies, we showed that PAMM over-expression prevented the reduction of GSH/GSSG induced by RANKL and abolished RANKL-induced osteoclast differentiation in RAW 264.7 cells. In human PBMCs, M-CSF induced PAMM expression, while addition of RANKL resulted in decreased PAMM expression [12]. These findings indicated that RANKL-induced osteoclast formation requires down-regulation of PAMM. Hyeon et al. [19] obtained similar results while studying the role of Nrf2 during osteoclast differentiation. In fact, RANKL-induced osteoclast differentiation and bone resorption activity was increased in Nrf2-deficient osteoclast-precursors cells compared to wild-type cells. Moreover, Nrf2 loss resulted in reduced production of several antioxidant enzymes and glutathione. Importantly, Nrf2 loss (just like PAMM loss) led to an increase in intracellular ROS level and the oxidized-to-reduced glutathione ratio. The authors finally conclude that Nrf2 (alike PAMM) inhibits RANKL-induced osteoclast differentiation by regulating the cellular redox status. This raises the intriguing idea that PAMM is a novel target of Nrf2, induced by oxidative stress. Studies aimed at clarifying these connections are currently under way.
We report in this study that M-CSF stimulation of PAMM expression requires Akt phosphorylation, via PI3Kinase activity. The PI3K/Akt signaling pathway is essential for RANKL-RANK mediated osteoclastic differentiation [20]. This pathway is also known to be involved in osteoclastogenesis induced by oxidative stress [21]. Akt is a downstream effector of phosphatidylinositol 3 kinase (PI3K) and is a critical mediator of cell proliferation and survival in a variety of cell types [20]. PI3Kinase activity is required for Akt activation [22,23]. Akt provides a survival signal that protects cells from apoptosis induced by growth factor withdrawal and oxidative stress [24]. This pathway involves the activity of PI3P and is, therefore, wortmannin-sensitive. Akt is activated by phospholipid at Thr 308 by PDK1 [25] and by phosphorylation within the carboxy terminus at Ser473. Activated Akt can, in turn, directly phosphorylate mTOR in a rapamycin-sensitive way [26,27]. Glantschnig et al. found that M-CSF induced activation of Akt by phosphorylating residues Thr308 and Ser473 [16].
Our data show that ovarietcomy increases PAMM expression and suggests a novel mechanistic link between ROS and enhanced bone resorption. While estrogen replacement blocked both osteoclast differentiation and bone loss, it did not block PAMM expression in bone. This may be indicative of increased numbers of PAMM-expressing pre-osteoclasts following OVX that do not terminally differentiate into bone-resorbing osteoclasts due to estrogen treatment. PAMM was originally identified as a gene up-regulated in osteoclasts by both M-CSF and RANKL. We found that M-CSF alone was able to induce PAMM expression in monocytes [12]. Indeed, addition of RANKL resulted in slightly decreased PAMM expression in cell culture. Estrogen inhibits osteoclast differentiation whereas M-CSF/RANKL induces it. However, both stimuli enhanced PAMM expression in osteoclast precursors. This observation suggests that PAMM expression might play a dual role in osteoclastogenesis: inhibiting osteoclast differentiation while stimulating proliferation of osteoclast precursors by regulating the levels of intracellular ROS. Indeed, estrogens have been shown to negatively regulate the generation of osteoclast precursors [28–30]. Taken together, our in vivo and in vitro findings suggest that estrogen may exert its bone-sparing effect by supporting expression of PAMM, which prevents osteoclast precursors from differentiating into resorbing osteoclasts.
PAMM is a redox regulatory protein that modulates osteoclast differentiation in vitro. It can be induced by oxidative stress in vitro and in vivo. PAMM expression in osteoclasts precursors is stimulated by estrogen. Moreover, estrogen stimulation of PAMM expression is mediated by phosphorylation of Akt. Our results also suggest that PAMM is a potential candidate for Akt-mediated protection against oxidative stress. Therefore, our findings provide evidence of a novel mechanism linking cellular redox status and bone homeotasis and may suggest new approaches to the prevention and/or treatment of human bone diseases by targeting molecules, like PAMM, that modulate cellular redox status in bone cells.
Acknowledgments
We would like to give our thanks to Ms. Justine Dobeck for technical assistance and Subbiah Yoganathan for expertize with animal experimentation. This study partially received support from National Natural Science Foundation of China (81060074).
Contributor Information
Yan Xu, Email: yxu@forsyth.org.
Leslie R. Morse, Email: leslie.morse@mgh.harvard.edu.
Raquel Assed Bezerra da Silva, Email: raquel@forp.usp.br.
Dianhua Wang, Email: wangdianhuakm@126.com.
Ricardo A. Battaglino, Email: rbattaglino@forsyth.or.
References
- 1.Wauquier F., Leotoing L., Coxam V., Guicheux J., Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009;15:468–477. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Kular J., Tickner J., Chim S.M., Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin. Biochem. 2012;45:863–873. doi: 10.1016/j.clinbiochem.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Manolagas S.C. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietschmann P., Rauner M., Sipos W., Kerschan-Schindl K. Osteoporosis: an age-related and gender-specific disease – a mini-review. Gerontology. 2009;55:3–12. doi: 10.1159/000166209. [DOI] [PubMed] [Google Scholar]
- 5.Almeida M., Han L., Martin-Millan M., Plotkin L.I., Stewart S.A., Roberson P.K., Kousteni S., O’Brien C.A., Bellido T., Parfitt A.M., Weinstein R.S., Jilka R.L., Manolagas S.C. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lean J.M., Davies J.T., Fuller K., Jagger C.J., Kirstein B., Partington G.A., Urry Z.L., Chambers T.J. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Investig. 2003;112:915–923. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue B., Zhao Y., Johnson A.K., Hay M. Central estrogen inhibition of angiotensin II-induced hypertension in male mice and the role of reactive oxygen species. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1025–H1032. doi: 10.1152/ajpheart.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha B.J. Oxidative stress in ovariectomy menopause and role of chondroitin sulfate. Arch. Pharm. Res. 2004;27:867–872. doi: 10.1007/BF02980181. [DOI] [PubMed] [Google Scholar]
- 9.Muthusami S., Ramachandran I., Muthusamy B., Vasudevan G., Prabhu V., Subramaniam V., Jagadeesan A., Narasimhan S. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin. Chim. Acta. 2005;360:81–86. doi: 10.1016/j.cccn.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Lean J.M., Jagger C.J., Kirstein B., Fuller K., Chambers T.J. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology. 2005;146:728–735. doi: 10.1210/en.2004-1021. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Grueso R., Gambini J., Abdelaziz K.M., Monleon D., Diaz A., El Alami M., Bonet-Costa V., Borras C., Vina J. Early, but not late onset estrogen replacement therapy prevents oxidative stress and metabolic alterations caused by ovariectomy. Antioxid. Redox Signal. 2014;20:236–246. doi: 10.1089/ars.2012.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y., Morse L.R., da Silva R.A., Odgren P.R., Sasaki H., Stashenko P., Battaglino R.A. PAMM: a redox regulatory protein that modulates osteoclast differentiation. Antioxid. Redox Signal. 2010;13:27–37. doi: 10.1089/ars.2009.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wymann M.P., Bulgarelli-Leva G., Zvelebil M.J., Pirola L., Vanhaesebroeck B., Waterfield M.D., Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol. Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryningen A., Reikvam H., Nepstad I., Paulsen Rye K., Bruserud O. Inhibition of Mammalian target of rapamycin in human acute myeloid leukemia cells has diverse effects that depend on the environmental in vitro stress. Bone Marrow Res. 2012;2012:329061. doi: 10.1155/2012/329061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huh Y.J., Kim J.M., Kim H., Song H., So H., Lee S.Y., Kwon S.B., Kim H.J., Kim H.H., Lee S.H., Choi Y., Chung S.C., Jeong D.W., Min B.M. Regulation of osteoclast differentiation by the redox-dependent modulation of nuclear import of transcription factors. Cell Death Differ. 2006;13:1138–1146. doi: 10.1038/sj.cdd.4401793. [DOI] [PubMed] [Google Scholar]
- 16.Glantschnig H., Fisher J.E., Wesolowski G., Rodan G.A., Reszka A.A. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10:1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- 17.Sobotta M.C., Barata A.G., Schmidt U., Mueller S., Millonig G., Dick T.P. Exposing cells to H2O2: a quantitative comparison between continuous low-dose and one-time high-dose treatments. Free Radic. Biol. Med. 2013;60:325–335. doi: 10.1016/j.freeradbiomed.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Nindl G., Peterson N.R., Hughes E.F., Waite L.R., Johnson M.T. Effect of hydrogen peroxide on proliferation, apoptosis and interleukin-2 production of Jurkat T cells. Biomed. Sci. Instrum. 2004;40:123–128. [PubMed] [Google Scholar]
- 19.Hyeon S., Lee H., Yang Y., Jeong W. Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic. Biol. Med. 2013;65:789–799. doi: 10.1016/j.freeradbiomed.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Sugatani T., Hruska K.A. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J. Biol. Chem. 2005;280:3583–3589. doi: 10.1074/jbc.M410480200. [DOI] [PubMed] [Google Scholar]
- 21.Lee N.K., Choi Y.G., Baik J.Y., Han S.Y., Jeong D.W., Bae Y.S., Kim N., Lee S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 22.Xu X., Li H., Hou X., Li D., He S., Wan C., Yin P., Liu M., Liu F., Xu J. Punicalagin induces Nrf2/HO-1 expression via upregulation of PI3K/AKT pathway and inhibits LPS-induced oxidative stress in RAW264.7 macrophages. Mediat. Inflamm. 2015;2015:380218. doi: 10.1155/2015/380218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin D., Rojo A.I., Salinas M., Diaz R., Gallardo G., Alam J., De Galarreta C.M., Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 24.Kawamura N., Kugimiya F., Oshima Y., Ohba S., Ikeda T., Saito T., Shinoda Y., Kawasaki Y., Ogata N., Hoshi K., Akiyama T., Chen W.S., Hay N., Tobe K., Kadowaki T., Azuma Y., Tanaka S., Nakamura K., Chung U.I., Kawaguchi H. Akt1 in osteoblasts and osteoclasts controls bone remodeling. PLoS One. 2007;2:e1058. doi: 10.1371/journal.pone.0001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwatake M., Okamoto K., Tanaka T., Tsukuba T. Punicalagin attenuates osteoclast differentiation by impairing NFATc1 expression and blocking Akt- and JNK-dependent pathways. Mol. Cell. Biochem. 2015 doi: 10.1007/s11010-015-2466-3. [DOI] [PubMed] [Google Scholar]
- 26.Hemmings B.A., Restuccia D.F. PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hambright H.G., Meng P., Kumar A.P., Ghosh R. Inhibition of PI3K/AKT/mTOR axis disrupts oxidative stress-mediated survival of melanoma cells. Oncotarget. 2015;6:7195–7208. doi: 10.18632/oncotarget.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girasole G., Jilka R.L., Passeri G., Boswell S., Boder G., Williams D.C., Manolagas S.C. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J. Clin. Investig. 1992;89:883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shevde N.K., Pike J.W. Estrogen modulates the recruitment of myelopoietic cell progenitors in rat through a stromal cell-independent mechanism involving apoptosis. Blood. 1996;87:2683–2692. [PubMed] [Google Scholar]
- 30.Erben R.G., Raith S., Eberle J., Stangassinger M. Ovariectomy augments B lymphopoiesis and generation of monocyte-macrophage precursors in rat bone marrow. Am. J. Physiol. 1998;274:E476–E483. doi: 10.1152/ajpendo.1998.274.3.e476. [DOI] [PubMed] [Google Scholar]