Abstract
Background
Evidence for the use of telephone consultation in childhood inflammatory bowel disease (IBD) is lacking. We aimed to assess the effectiveness and cost consequences of telephone consultation compared with the usual out-patient face-to-face consultation for young people with IBD.
Methods
We conducted a randomised-controlled trial in Manchester, UK, between July 12, 2010 and June 30, 2013. Young people (aged 8–16 years) with IBD were randomized to receive telephone consultation or face-to-face consultation for 24 months. The primary outcome measure was the paediatric IBD-specific IMPACT quality of life (QOL) score at 12 months. Secondary outcome measures included patient satisfaction with consultations, disease course, anthropometric measures, proportion of consultations attended, duration of consultations, and costs to the UK National Health Service (NHS). Analysis was by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT02319798.
Findings
Eighty six patients were randomised to receive either telephone consultation (n = 44) or face-to-face consultation (n = 42). Baseline characteristics of the two groups were well balanced. At 12 months, there was no evidence of difference in QOL scores (estimated treatment effect in favour of the telephone consultation group was 5.7 points, 95% CI − 2.9 to 14.3; p = 0.19). Mean consultation times were 9.8 min (IQR 8 to 12.3) for telephone consultation, and 14.3 min (11.6 to 17.0) for face-to-face consultation with an estimated reduction (95% CI) of 4.3 (2.8 to 5.7) min in consultation times (p < 0.001). Telephone consultation had a mean cost of UK£35.41 per patient consultation compared with £51.12 for face-face consultation, difference £15.71 (95% CI 11.8–19.6; P < 0.001).
Interpretation
We found no suggestion of inferiority of telephone consultation compared with face-to-face consultation with regard to improvements in QOL scores, and telephone consultation reduced consultation time and NHS costs. Telephone consultation is a cost-effective alternative to face-to-face consultation for the routine outpatient follow-up of children and adolescents with IBD.
Funding
Research for Patient Benefit Programme, UK National Institute for Health Research.
Keywords: Telephone consultation, Telemedicine, Inflammatory bowel disease, Crohn's disease, Ulcerative colitis
Highlights
-
•
This is the first randomised controlled trial that has formally investigated the role of telephone consultation in paediatric inflammatory bowel disease.
-
•
This study contributes evidence showing that telephone consultation as a substitute for routine outpatient follow-up is feasible and effective.
-
•
There was no suggestion of inferiority of telephone consultation compared with face-to-face consultation with regard to improvements in quality of life scores, and telephone consultation reduced consultation time and NHS costs.
-
•
Telephone consultation is a cost-effective alternative to face-to-face consultation for the routine outpatient follow-up of children and adolescents with inflammatory bowel disease.
1. Introduction
Crohn's disease and ulcerative colitis, collectively known as inflammatory bowel disease (IBD) are chronic relapsing disorders of the gastrointestinal tract. The incidence of childhood IBD has been increasing in the UK (Cosgrove et al., 1996; Gunesh et al., 2008) and is currently estimated to be 5.2 per 100,000 per year in people younger than 16 years old (Sawczenko et al., 2001). There is no medical cure for IBD and the natural history of the disorder is characterised by recurrent relapses alternating with periods of remission. In between periods of ill health, patients can be well for prolonged periods of time when their disease is in remission. At routine outpatient hospital reviews, patients are often well and do not usually require any intervention, highlighting the fact that regular fixed appointments do not necessarily coincide with disease relapses (Gethins et al., 2007).
In the UK, children with IBD are usually managed by paediatric gastroenterologists who are based in a few regional centres. Conventionally, patients are kept under routine face-to face outpatient follow-up. This means that many of them have to be taken out of school and, together with their families, have to travel long distances in order to attend out-patient clinics. For children who are doing well, such routine visits may be unnecessary.
Telephone consultations allow patients to have contact with healthcare professionals without the patient having to travel. For patients, the benefits of telephone consultations may include reduced travel time, reduced costs, and improved satisfaction (Car and Sheikh, 2003). In adults with various chronic diseases, telephone consultations were associated with reduced medical care utilisation without adversely affecting patient health (Wasson et al., 1992). Telephone consultations also cost-effectively increased asthma review rates, enhancing patient confidence with management, with no detriment to asthma morbidity (Pinnock et al., 2007). No study comparing telephone consultations with face-to-face consultations for children with IBD has been reported, but at least two uncontrolled studies have been reported in adults with IBD (Gethins et al., 2007; Miller et al., 2002). Miller et al. showed that a telephone clinic improved the overall quality of follow-up care for adults with IBD (Miller et al., 2002). In another study, Gethins et al. found that telephone clinics significantly reduced non-attendance rates and waiting times for urgent appointments (Gethins et al., 2007). Despite the potential advantages of telephone consultations, this approach has been given little attention in young people with IBD. We, therefore, aimed to provide evidence about the effectiveness and cost consequences of telephone consultations, compared to face-to-face consultations in childhood IBD.
2. Methods
2.1. Setting and Participants
We conducted a randomised-controlled trial at the Royal Manchester Children's Hospital, Manchester, UK, a regional Paediatric Gastroenterology referral centre. Children and adolescents with IBD from the North West of England are referred to this centre. Any patient who was aged between 8 and 16 years with a diagnosis of IBD was eligible for entry into the trial.
We identified eligible patients through the hospital's paediatric IBD database. Inclusion criteria were: diagnosis of IBD by established clinical, endoscopic, histological and radiological criteria; clinical remission defined as an abbreviated Paediatric Crohn's Disease Activity Index (aPCDAI) score of ≤ 10 (Shepanski et al., 2004) for patients with Crohn's disease or as a Paediatric Ulcerative Colitis Activity Index (PUCAI) score of < 10 (Turner et al., 2007) for those with ulcerative colitis and indeterminate colitis. Exclusion criteria were: active disease (aPCDAI > 15 or PUCAI ≥ 15), and unwillingness to provide informed consent.
We sent a letter of invitation to participate in the study and a research information sheet outlining the nature of the study to eligible patients and their parents. Those who agreed to take part were interviewed by an investigator who provided full information about the trial and obtained parental and child's written informed consent. We obtained ethics approval from the North West of England research ethics committee.
2.2. Randomization and Masking
By means of a computer-generated randomisation scheme, participants were allocated to telephone consultation or face-to-face consultation. We used randomization with blocks of random sizes, and stratified by type of disease (i.e. Crohn's disease or ulcerative colitis/indeterminate colitis). The assignment schedule was held centrally and allocation was performed by staff of the hospital's pharmacy department independent from the trial team. Masking was not possible because of the nature of the two interventions.
2.3. Procedures
Patients in both groups were offered out-patient appointment dates and times. Those randomized to face-to-face consultation were asked to attend their routine appointments in hospital as usual. Those randomised to telephone consultation were told to expect a call from the gastroenterology doctor at the time of their appointment. The consulting doctor contacted the patient and parents via a telephone number (home or mobile) that the parents and patient had previously supplied as the number they would like to be contacted on. As much as possible, parents and patients were advised to be together at the time of the appointment in order to allow both of them to participate in the consultation as is usual in practice. Up to three attempts within 20 min were made to contact patients by phone. Patients who did not attend an appointment in either group were sent another appointment in accordance with our hospital's policy.
Apart from being randomised to telephone or face-to-face consultation, routine care was the same for patients in both groups. As it is in normal practice, if a participant experienced any symptoms that caused concern at any time during the study, the parent/child contacted the IBD nurse for advice and appropriate arrangements for assessment were made.
2.4. Outcomes
The primary outcome was quality of life (QOL) score at 12 months. QOL scores were assessed using the validated paediatric IBD IMPACT QOL questionnaire (Otley et al., 2002; Loonen et al., 2002; Ogden et al., 2011). Secondary outcome measures were patient and parent satisfaction with consultations (assessed with the Consultation Satisfaction Questionnaire {CSQ}) (Baker, 1990); the number of disease relapses (relapse defined by the aPCDAI or PUCAI); anthropometric measures (body mass index (BMI), height, and weight z-scores); number of hospital admissions; proportion of consultations attended; duration of consultations (measured with a watch); and costs to the UK National Health Service (NHS).
2.5. Statistical Analysis
Our sample size was based on the primary outcome measure and drew on previous studies (Shepanski et al., 2005; Afzal et al., 2004), where the minimum clinically important change in QOL scores was determined to be 10 points (total QOL scores range from 0 to 140 with higher scores representing better QOL). A power calculation indicated the need for 74 participants (37 participants per arm) to have at least 80% power at the 5% significance level to detect a 10 point difference in QOL scores at 12 months.
All data were analysed on an intention-to-treat (ITT) basis. For the primary and secondary quantitative/summary score outcomes (QOL score, anthropometric measures, CSQ scores and duration of consultations) analysis of covariance was used. For secondary count outcomes (number of disease relapses and number of hospital admissions for IBD), Poisson regression models were used. For the proportion of out-patient consultations attended throughout the duration of the study, a binomial logistic model was fitted. For all models, the baseline outcome in question, and disease type were included as covariates. The mean difference, the rate ratio, and the odds ratio on the treatment allocation factor were estimated as appropriate to evaluate the intervention effect. All tests were conducted at a significance level of 5%.
Missing baseline data were imputed as a ‘missing’ category for discrete covariates or using simple conditional mean imputation (White and Thompson, 2005) for continuous covariates. Sensitivity analyses for the primary outcome included multiple imputation under a pattern mixture model (Little, 1993) to explore informatively missing outcome data. This involved performing standard multiple imputation on the QOL data but modelling a conditional difference (delta) between these missing and observed outcomes. By varying delta, we assessed how robust our estimates and inferences are to the missing data. Baseline QOL scores, age at randomisation, gender, disease type and consultation group were used in the imputation model. The number of imputed datasets was set to be greater than the percentage of missing data in both groups (White et al., 2011).
Efficacy of treatment was evaluated by a complier-adjusted causal effect analysis using an instrumental variable approach (Nagelkerke et al., 2000; Dunn and Bentall, 2007) with minimal participation/non-participation in the allocated consultation type by 12 month follow-up included as a mediator, and allocated consultation type as an instrumental variable. A treatment by disease-type interaction was also modelled to assess differential effects in the Crohn's disease and ulcerative colitis/indeterminate colitis subgroups. This trial is registered with ClinicalTrials.gov, number NCT02319798.
2.6. Economic Evaluation
The economic evaluation compared costs incurred in each group from the point of randomisation to the end of the 2-year follow-up period from the perspective of the NHS. We estimated the costs to provide telephone consultations and face-to-face consultations by combining the time needed to do the consultations and matched them with the unit cost of health and social care (Curtis, 2010). The costs used included medical consultant level wages/salary (since all the consultations in this study were conducted by consultants), national insurance, overheads, training, and capital overheads. We originally also intended to collect data on costs from the perspective of the patient/parents using a patient diary, which was distributed to patients at the start of randomisation. Unfortunately, the completion rate of the diaries was very poor and we did not obtain adequate data to allow the assessment of these costs.
2.7. Role of the Funding Source
This study was funded by the Research for Patient Benefit Programme, UK National Institute for Health Research. The funding source had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
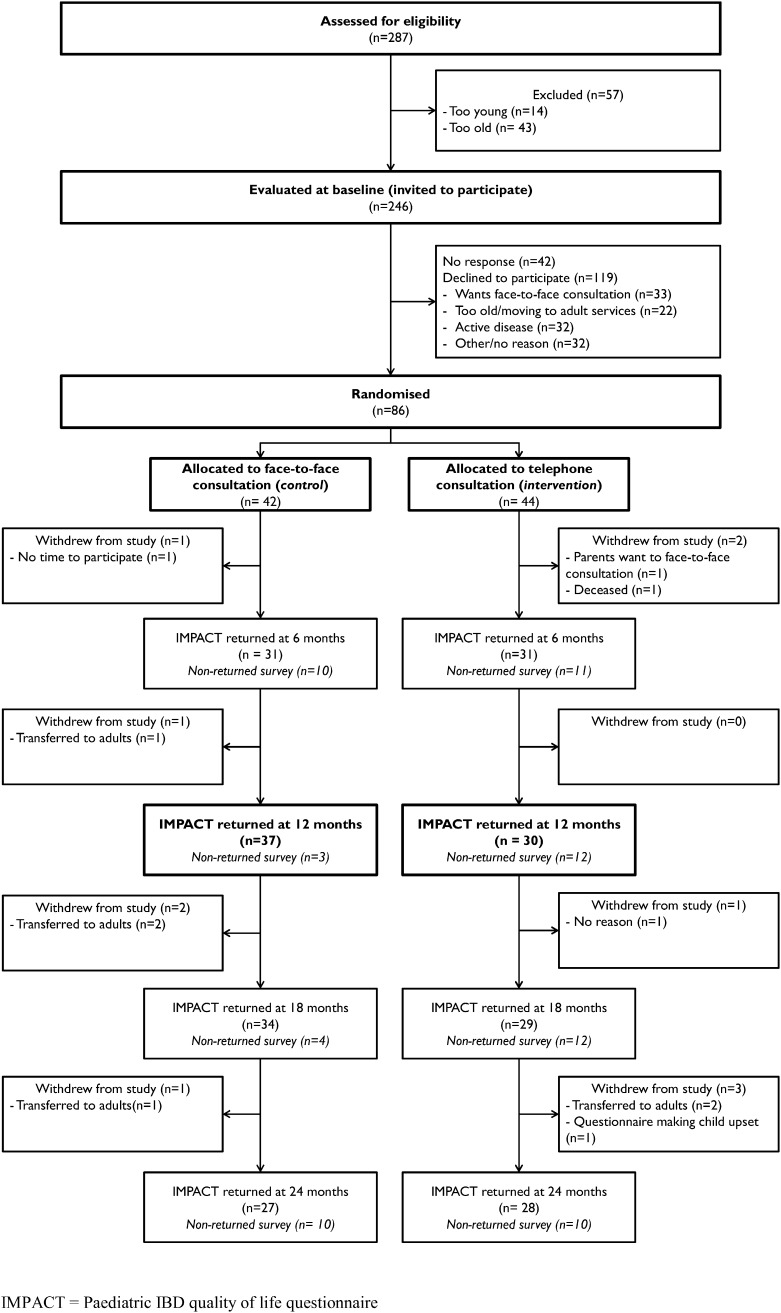
Recruitment ran between July 12, 2010 and June 30, 2011, with follow-up completed on June 30, 2013. Fig. 1 shows the reasons for ineligibility and the flow of participants through the trial. Of 287 patients assessed for eligibility, 86 were randomly assigned: 44 to the telephone group and 42 to the face-to-face group. Characteristics at enrolment were reasonably well balanced between the two groups (Table 1).
Fig. 1.
Trial profile.
IMPACT = Paediatric IBD quality of life questionnaire.
Table 1.
Baseline characteristics.
| Characteristic | Control “face-to-face” | Intervention “telephone” |
|---|---|---|
| Disease type | ||
| Crohn's disease (%) | 35 (83) |
36 (82) |
| Ulcerative or indeterminate colitis (%) | 7 (17) |
8 (18) |
| Gender | ||
| Male (%) | 24 (57) |
30 (68) |
| Female (%) | 18 (43) |
14 (32) |
| Age, years | 13.8 (11.2, 15.3) |
13.9 (12.1, 15.9) |
| IMPACT QOL score | 110 (96, 121) [1] |
112 (100, 120) [1] |
| BMI, kg/m2 | 20.2 (17.2, 22.1) [1] |
20.1 (18.2, 23.1) [0] |
| Weight, kg | 48.5 (37.6, 59.5) |
52.5 (43.7, 58.4) |
| Height, cm | 155 (145 164) [1] |
157 (149, 165) [0] |
| aPCDAI, N = 71 | ||
| Score = 0 | 20 (57) |
16 (44) |
| Score = 5 | 9 (26) |
16 (44) |
| Score = 10 | 6 (17) |
4 (11) |
| PUCAI, N = 15 | ||
| Score = 0 | 4 (50) |
7 (78) |
| Score = 5 | 4 (50) |
2 (22) |
Values shown are n (%) or median (IQR) with number of missing values [m] where appropriate. IQR = interquartile range; BMI = body mass index; aPCDAI = abbreviated Crohn's disease activity index; PUCAI = paediatric ulcerative colitis activity index.
At baseline and 12 months, 98% and 78% of participants had returned a QOL questionnaire and were included in the primary analysis (98% and 88% for the face-to-face and 97% and 68% for the telephone consultation groups respectively). A total of 13 (15%) participants had withdrawn from the study by the completion of the trial, 8 (19%) from the face-to-face group and 5 (11%) from the telephone group. Any available follow-up data for withdrawn patients e.g. a returned questionnaire was used in analysis as per the ITT principle. One participant allocated to telephone consultation group reverted to face-to-face consultations. In the face-to-face consultation group 40 (95%) participants had at least one consultation, as allocated before the 12 month follow-up. This figure was 36 (82%) for the telephone consultation group. For the period participants remained in the study, the median (IQR) number of consultations scheduled by the hospital for each patient which were not then cancelled by the hospital was 5 (3 to 6) for the face-to-face group and 4.5 (3 to 5.3) for the telephone group. The median (IQR) number of consultation attended per patient was 3 (2 to 4) for the face-to-face group and 4 (3 to 4) for the telephone group.
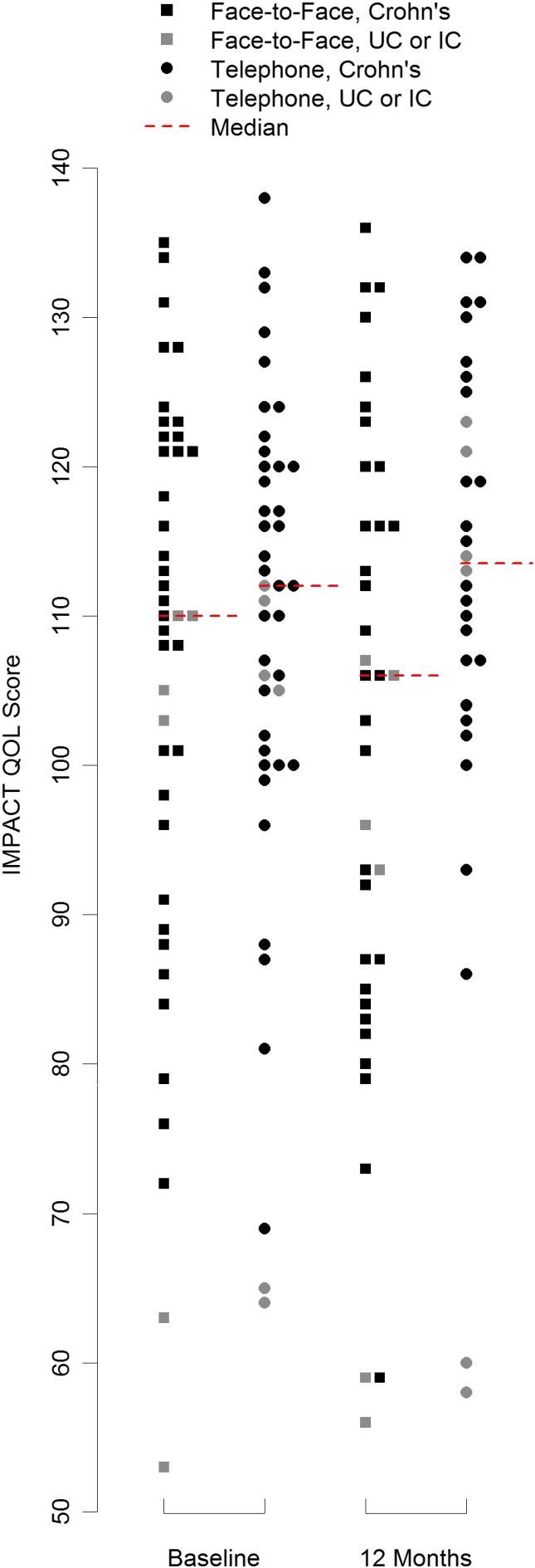
A summary of all observed outcomes and the treatment effect estimates is given in Table 2. The median (IQR) of the QOL score in the face-to-face group was 106 (95, 116) and 113 (105, 125) in the telephone group. Fig. 2 illustrates all observed QOL scores at baseline and 12 month follow-up. The specified ANCOVA model estimated a mean (95% CI) QOL score of 102.5 (96.5, 108.4) in the face-to-face group and 108.2 (101.6, 114.7) in the telephone group. The treatment effect estimate (95% CI) was then 5.7 (− 2.9, 14.3) units on the IMPACT QOL scale (P = 0.19) in favour of the telephone consultation group.
Table 2.
Summary of outcomes, number of participants or median values given for summary.
| Outcome | Control “face-to-face” | Missing, n (%) | Intervention “telephone” | Missing, n (%) | Adjusted treatment effect (95% CI) |
P |
|---|---|---|---|---|---|---|
| IMPACT QOL score at 12 months | 106 (95, 116) |
6 (14) |
113 (105, 125) |
13 (30) |
5.7 (− 2.9, 14.3) |
0.19 |
| Disease relapses over 24 months | 4 (10) |
1 (2) |
0.20 | |||
| BMI z-score at 12 months | 0.020 (− 0.85, 1.02) |
11 (26) |
0.329 (− 0.63, 0.57) |
19 (44) |
0.34 (− 0.02, 0.69) |
0.06 |
| Weight z-score at 12 months | 0.39 (− 0.86, 0.96) |
11 (26) |
0.548 (− 0.35, 1.04) |
19 (44) |
0.08 (− 0.22, 0.37) |
0.59 |
| Height z-score at 12 months | − 0.13 (− 0.85, 1.07) |
11 (26) |
0.21 (− 0.53, 1.50) |
19 (44) |
− 0.04 (− 0.32, 0.24) |
0.79 |
| CSQ-child (modified) by 24 months | 46 (44, 51) |
2 (5) |
48 (45, 51) |
8 (18) |
0.59 (− 2.05, 3.24) |
0.65 |
| CSQ-parent (modified) by 24 months | 48 (44, 52) |
2 (5) |
48 (45, 51) |
8 (18) |
0.44 (− 2.01, 2.89) |
0.72 |
| Proportion of out-patient consultations attended over 24 months, % | 71 (50, 80) |
0 (0) |
67 (50, 75) |
1 (2) |
1.06 (0.784, 1.43) |
0.71 |
| Duration of consultations over 24 months, mins | 14.3 (11.6, 17.0) |
1 (2) |
9.8 (8, 12.3) |
3 (7) |
4.3 (2.8, 5.7) |
< 0.001 |
Fig. 2.
Dot-plot of all observed IMPACT QOL scores at baseline and 12 month follow-up with medians in each group at each time-point.
Sensitivity analysis confirmed that the conclusion of non-inferiority implied by this confidence interval was robust to a wide range of plausible reasons for missing outcome data. The complier-adjusted analysis of QOL scores showed similar conclusions when there was at least minimal consultation participation (as defined previously); the efficacy-based treatment effect estimate (95% CI) was 4.8 (− 4.1 to 13.6) points; p = 0.285. There was no evidence that the treatment effect differed between Crohn's disease and ulcerative colitis/indeterminate colitis subgroups (p = 0.15).
For the anthropometric scores, there was no evidence of a difference between telephone consultation and face-to-face consultation. Disease relapses defined by a single or multiple PUCAI or aPCDAI scores greater than 15 over the 24 month follow-up period were so rare (4 [10%] in the face-to-face arm and 1 [2%] in the telephone arm) as to prevent a multi-factorial analysis, so Fisher's exact test was used. There were no statistically significant differences between the control and intervention arm of CSQ scores at the last available observation as completed by either the participating children or their parents.
There was a statistically significant lower mean consultation time for telephone consultations across all available follow-up compared to face-to-face consultation. Mean consultation times were 9.8 min (IQR 8 to 12.3) for telephone consultation, and 14.3 min (11.6 to 17.0) for face-to-face consultation with an estimated reduction (95% CI) of 4.3 (2.8 to 5.7) min in consultation times (p < 0.001). One participant in each consultation group had one or more hospital admissions due to IBD. There was no evidence for a difference in the proportion of out-patient appointments attended between trial arms by binomial logistic regression.
Estimates of NHS costs for the intervention (including staff costs and telephone costs) showed that telephone consultation had a mean cost of UK£35.41 per patient consultation compared with £51.12 for face–face consultation, difference £15.71 (95% CI 11.8–19.6; P < 0.001).
No adverse events were noted for either intervention. One patient in the telephone group died during follow-up but the cause of death was neither related to the disease nor the intervention.
4. Discussion
To the best of our knowledge, this is the first randomised controlled trial that has formally investigated the role of telephone consultation in paediatric IBD. Telephone consultation was shown not to be inferior to face-to-face consultation with regard to QOL scores, and did reduce consultation time and NHS costs. Whilst the study was, a priori, a superiority trial, the design, analysis, and results of the trial do not preclude post-hoc conclusions about the non-inferiority of telephone consultation.
Non-trivial proportions of missing data in some of the secondary outcomes may present a considerable departure from the ITT principle in our analysis and treatment effect estimates may be subject to bias.
With an equivalence or non-inferiority conclusion regarding a treatment effect, a potential source of bias towards no difference in the finding may be due to the proportion of participants in the intervention arm of the study who revert to the control treatment or those participants in both arms who do not receive any treatment at all. In this study, these are indicated by the 18% of participants in the telephone group and 5% of participants in the face-to-face group without any consultations before the 12 month follow-up assessment. These can be thought of as non-compliers, not engaging in the consultation process. That these were higher in the telephone group may indicate either a reduced need for consultation, or a reduced engagement with the consultation process. The estimate of the differences between compliers between groups of 4.8, with a 95% CI of − 4.1 to 13.6, was itself not statistically significant in terms of improved efficacy and similar in magnitude to that of the main analysis. This also supports our finding that participants receiving telephone consultations compared to face-to-face do not have worse QOL outcomes.
Differences in the number of non-returned QOL questionnaires at 12 month follow-up (32% telephone group vs. 12% face-to-face group) mean that the main findings are subject to non-testable assumptions about missing QOL data from these “non-responders”. Essentially we assume in our main analysis that the QOL of a given participant who did not return the survey is no different to that of a similar participant who did return the survey — termed “ignorable missing data”. In order to investigate the robustness of our conclusions to departures from this assumption we performed a sensitivity analysis under a plausible range of systematic differences between observed and missing data. These analyses indicate that the treatment effect estimate of the primary outcome, its inferences and ultimately our conclusions are robust to departures from the assumptions of ignorable missing data.
We analysed the QOL scores as a conditionally normally distributed continuous outcome and this would seem sensible given the 140 point scale produced by this instrument. Given the mean values in each arm reported in this study, there were not likely to be any ‘floor’ or ‘ceiling’ effects on our estimate. Previous validation studies have assumed a normal distribution for the QOL score (Otley et al., 2002; Loonen et al., 2002; Ogden et al., 2011) but there are no previously published detailed characterisations of this score's distribution in any sample of patients that we are aware of.
The findings of this study are consistent with the results of recent adult IBD studies where telemonitoring and teleconsulting using various information and communication technologies such as a web-based system, and a home telemanagement system were found to be safe and feasible (Aguas Peris et al., 2015).
As telephone consultation is not inferior to face-to-face consultation with regard to QOL scores and actually costs less, by principle of extended dominance, it can be concluded that telephone consultation is a cost-effective alternative to face-to-face consultation for the routine out-patient follow-up of children and adolescents with IBD. In routine clinical practice, children and adolescents with IBD could be offered the choice of either telephone consultation or face-to-face consultation for their routine out-patient follow-up. Those who are doing well would not have to make unnecessary journeys to hospitals. By freeing up out-patient clinic spaces, access for patients in relapse and for newly referred patients could be improved, thus reducing waiting times for appointments. The findings of this study could also be applicable to the routine follow-up of children with other chronic diseases.
Contributors
AKA was principal investigator; AV, DW, and AGT were coapplicants for funding and contributed to the design of the original study protocol; AKA was responsible for obtaining research ethics and governance approvals; DW led the economic evaluation; NB, AF, AGT, and AKA collected data; NB managed the data; and NO and AV analysed the data. All authors contributed to interpretation of results and drafting of the final article, including critique for important intellectual content.
Declaration of Interests
The authors report grants from Research for Patient Benefit Programme, UK National Institute for Health Research, during the conduct of the study.
Acknowledgements
The project was funded by Research for Patient Benefit Programme, UK National Institute for Health Research (grant number PB-PG-0408-16218). The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Research for Patient Benefit Programme or the National Institute for Health Research.
References
- Afzal N.A., Van Der Zaag-Loonen H.J., Arnaud-Battandier F., Davies S., Murch S., Derkx B., Heuschkel R., Fell J.M. Improvement in quality of life of children with acute Crohn's disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Aliment. Pharmacol. Ther. 2004;20:167–172. doi: 10.1111/j.1365-2036.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- Aguas Peris M., Del Hoyo J., Bebia P., Faubel R., Barrios A., Bastida G. Telemedicine in inflammatory bowel disease: opportunities and approaches. Inflamm. Bowel Dis. 2015;21:392–399. doi: 10.1097/MIB.0000000000000241. [DOI] [PubMed] [Google Scholar]
- Baker R. Development of a questionnaire to assess patient's satisfaction with consultations in general practice. Br. J. Gen. Pract. 1990;40:487–490. [PMC free article] [PubMed] [Google Scholar]
- Car J., Sheikh A. Telephone consultations. BMJ. 2003;326:966–969. doi: 10.1136/bmj.326.7396.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove M., Al-Atia R.F., Jenkins H.R. The epidemiology of paediatric inflammatory bowel disease. Arch. Dis. Child. 1996;74:460–461. doi: 10.1136/adc.74.5.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis L. University of Kent at Canterbury: Personal Social Services Research Unit. 2010. Unit costs of health and social care 2010. [Google Scholar]
- Dunn G., Bentall R. Modelling treatment-effect heterogeneity in randomised controlled trials of complex interventions (psychological treatments) Stat. Med. 2007;26:4719–4745. doi: 10.1002/sim.2891. [DOI] [PubMed] [Google Scholar]
- Gethins S., Robinson R., de Caestecker J., Stewart J. Impact of a nurse-led telephone clinic on quality of IBD care. Gastrointest. Nurs. 2007;1:34–39. [Google Scholar]
- Gunesh S., Thomas G.A., Williams G.T., Roberts A., Hawthorne A.B. The incidence of Crohn's disease in Cardiff over the last 75 years: an update for 1996–2005. Aliment. Pharmacol. Ther. 2008;27:211–219. doi: 10.1111/j.1365-2036.2007.03576.x. [DOI] [PubMed] [Google Scholar]
- Little R.J.A. Pattern-mixture models for multivariate incomplete data. J. Am. Stat. Assoc. 1993;88:125–134. [Google Scholar]
- Loonen H.J., Grootenhuis M.A., Last B.F., de Haan R.J., Bouquet J., Derkx B.H. Measuring quality of life in children with inflammatory bowel disease: the impact-II (NL) Qual. Life Res. 2002;1:47–56. doi: 10.1023/a:1014455702807. [DOI] [PubMed] [Google Scholar]
- Miller L., Caton S., Lynch D. Telephone clinic improved quality of follow-up care for chronic bowel disease. Nurs. Times. 2002;98:36–38. [PubMed] [Google Scholar]
- Nagelkerke N., Fidler V., Bernsen R., Borgdorff M. Estimating treatment effects in randomised clinical trials in the presence of noncompliance. Stat. Med. 2000;19:1849–1864. doi: 10.1002/1097-0258(20000730)19:14<1849::aid-sim506>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ogden C.A., Akobeng A.K., Abbott J., Aggett P., Sood M.R., Thomas A.G. Validation of an instrument to measure quality of life in British children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2011;53:280–286. doi: 10.1097/MPG.0b013e3182165d10. [DOI] [PubMed] [Google Scholar]
- Otley A., Smith C., Nicholas D., Munk M., Avolio J., Sherman P.M., Griffiths A.M. The IMPACT questionnaire: a valid measure of health-related quality of life in pediatric inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2002;35:557–563. doi: 10.1097/00005176-200210000-00018. [DOI] [PubMed] [Google Scholar]
- Pinnock H., Adlem L., Gaskin S., Harris J., Snellgrove C., Sheikh A. Accessibility, clinical effectiveness, and practice costs of providing a telephone option for routine asthma reviews: phase IV controlled implementation study. Br. J. Gen. Pract. 2007;57:714–722. [PMC free article] [PubMed] [Google Scholar]
- Sawczenko A., Sandhu B.K., Logan R.F., Jenkins H., Taylor C.J., Mian S., Lynn R. Prospective survey of childhood inflammatory bowel disease in the British Isles. Lancet. 2001;357:1093–1094. doi: 10.1016/s0140-6736(00)04309-9. [DOI] [PubMed] [Google Scholar]
- Shepanski M.A., Markowitz J.E., Mamula P., Hurd L.B., Baldassano R.N. Is an abbreviated Pediatric Crohn's Disease Activity Index better than the original? J. Pediatr. Gastroenterol. Nutr. 2004;39:68–72. doi: 10.1097/00005176-200407000-00014. [DOI] [PubMed] [Google Scholar]
- Shepanski M.A., Hurd L.B., Culton K., Markowitz J.E., Mamula P., Baldassano R.N. Health-related quality of life improves in children and adolescents with inflammatory bowel disease after attending a camp sponsored by the Crohn's and Colitis Foundation of America. Inflamm. Bowel Dis. 2005;11:164–170. doi: 10.1097/00054725-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Turner D., Otley A.R., Mack D., Hyams J., de Bruijne J., Uusoue K. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423–432. doi: 10.1053/j.gastro.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Wasson J., Gaudette C., Whaley F., Sauvigne A., Baribeau P., Welch H.G. Telephone care as a substitute for routine clinic follow-up. JAMA. 1992;267:1788–1793. [PubMed] [Google Scholar]
- White I.R., Thompson S.G. Adjusting for partially missing baseline measurements in randomized trials. Stat. Med. 2005;24:993–1007. doi: 10.1002/sim.1981. [DOI] [PubMed] [Google Scholar]
- White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat. Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]