Abstract
Objective
Melanocortin-4 receptors (MC4Rs) are highly expressed by dopamine-secreting neurons of the mesolimbic tract, but their functional role has not been fully resolved. Voluntary wheel running (VWR) induces adaptations in the mesolimbic dopamine system and has a myriad of long-term beneficial effects on health. In the present experiments we asked whether MC4R function regulates the effects of VWR, and whether VWR ameliorates MC4R-associated symptoms of the metabolic syndrome.
Methods
Electrically evoked dopamine release was measured in slice preparations from sedentary wild-type and MC4R-deficient Mc4rK314X (HOM) rats. VWR was assessed in wild-type and HOM rats, and in MC4R-deficient loxTBMc4r mice, wild-type mice body weight-matched to loxTBMc4r mice, and wild-type mice with intracerebroventricular administration of the MC4R antagonist SHU9119. Mesolimbic dopamine system function (gene/protein expression) and metabolic parameters were examined in wheel-running and sedentary wild-type and HOM rats.
Results
Sedentary obese HOM rats had increased electrically evoked dopamine release in several ventral tegmental area (VTA) projection sites compared to wild-type controls. MC4R loss-of-function decreased VWR, and this was partially independent of body weight. HOM wheel-runners had attenuated markers of intracellular D1-type dopamine receptor signaling despite increased dopamine flux in the VTA. VWR increased and decreased ΔFosB levels in the nucleus accumbens (NAc) of wild-type and HOM runners, respectively. VWR improved metabolic parameters in wild-type wheel-runners. Finally, moderate voluntary exercise corrected many aspects of the metabolic syndrome in HOM runners.
Conclusions
Central dopamine dysregulation during VWR reinforces the link between MC4R function and molecular and behavioral responding to rewards. The data also suggest that exercise can be a successful lifestyle intervention in MC4R-haploinsufficient individuals despite reduced positive reinforcement during exercise training.
Keywords: MC4R, Nucleus accumbens, Voluntary wheel running, Dopamine, Obesity, Diabetes, Food intake
Highlights
-
•
MC4R-deficiency causes metabolic syndrome.
-
•
Loss of MC4R signaling decreases voluntary wheel running (VWR).
-
•
Despite moderate amounts of VWR, MC4R-associated metabolic syndrome is severely attenuated.
-
•
MC4R-deficiency is associated with mesolimbic dopamine dysregulation during VWR.
1. Introduction
Central nervous system (CNS) melanocortin-4 receptors (MC4Rs) are critical for normal regulation of energy homeostasis [1,2]. MC4R-deficiency in humans and rodents induces a metabolic syndrome characterized by hyperphagia, severe obesity, decreased energy expenditure, and glucose intolerance [3–6]. In fact, MC4R loss-of-function mutations are the most commonly observed monogenetic associations with the metabolic syndrome in humans [1,7]. Although several anti-obesity drugs have been developed, they display limited efficacy. Bariatric surgery does improve metabolic health, and its efficacy is independent of MC4R-deficiency in both humans and rats [8,9]; but it is expensive and invasive and not a viable choice for all obese patients. Thus, the development of more sophisticated pharmacological, surgical, or behavioral interventions is an important goal.
Like bariatric surgery, regular exercise improves metabolic health through system-wide influences, including alterations in the CNS regulation of energy metabolism [10–13]. This prompted our interest to determine the efficacy of a lifestyle intervention (i.e. exercise training) as a suitable strategy to ameliorate established MC4R-associated metabolic complications. Voluntary wheel running (VWR) is a physical activity displayed by rodents that mimics regular physical activity in humans. Laboratory as well as wild rodents run voluntarily [14], lever-press for access to running wheels [15], and develop a conditioned place preference to an environment paired with VWR [16]. Thus, similar to regular exercise in many humans, VWR serves as a natural reinforcer to rodents.
MC4Rs are highly expressed in the mesolimbic dopaminergic (DAergic) system [17], where they modulate dopamine (DA) release [18], food intake [19], motivation for sucrose reward [20], grooming [21], procedural learning [22], synaptic plasticity [23,24], and molecular and behavioral responding to cocaine [19,25,26]. Loss of MC4R signaling in rodents has negative effects on locomotion [4,27], palatable food preference [28], cocaine reward value [25], and cocaine-induced hyperactivity [19,25,26]. Importantly, MC4Rs and D1-type dopamine receptors (D1Rs) are co-expressed and share post-receptor signaling pathways that converge on dopamine- and cAMP-regulated phosphoprotein, Mr 32 kDa (DARPP32), a key regulator protein of neuronal function [22,29,30]. Collectively, these findings imply that melanocortinergic signaling within DAergic circuits influences molecular and behavioral responses to natural reinforcers and drugs of abuse.
Ay mice and neuronal-specific POMC-deficient mice, which have impaired MC4R signaling and develop a MC4R-deficiency-associated metabolic syndrome, engage in less VWR compared to wild-type controls [31,32]. In contrast, studies comparing VWR behavior of young Mc4r null mice, which had not yet fully developed the MC4R-associated metabolic syndrome, and wild-type controls, revealed no statistical differences, although the amounts of VWR tended to be lower in the Mc4r null mice [33,34]. Furthermore, these studies did not investigate if MC4R loss-of-function during VWR induced adaptations to central circuits regulating metabolism and reward-related behavior. It thus remains to be determined if MC4R signaling regulates central adaptations and behavioral responding to VWR.
We hypothesized that MC4Rs interact with DAergic neurons to promote VWR. To this end, we first measured VWR and various aspects of mesolimbic DAergic function in wild-type rats and rats homozygous for a loss-of-function (K314X) mutation in Mc4r [4]. LoxTBMc4r mice, a murine model of MC4R deficiency [5], were used to perform species-specific comparisons with previous VWR studies in Mc4r null mice [33,34]. Intracerebroventricular (ICV) administration of SHU9119, a non-specific MC4R antagonist [35], was used to evaluate the acute effects of MC4R blockade on VWR in wild-type mice. Finally, we also sought to determine whether exercise intervention could overcome the metabolic perturbations induced by the MC4R mutations via CNS mechanisms.
2. Materials and methods
2.1. Housing and diet
The University of Cincinnati and the Tufts University School of Medicine Institutional Animal Care and Use Committees approved all animal procedures. Animals were maintained at the AAALAC-accredited animal facilities of the Metabolic Diseases Institute, University of Cincinnati, with ad libitum access to water and chow (LM-485 #7012, percentage of energy from protein:25%, carbohydrate:58%, and fat:17%, 3.1 kcal/g, Harlan Teklad) on a 12:12-h light:dark cycle.
2.2. Animals
Two different rodent models of MC4R-deficiency were used. Male wild-type (Mc4r+/+; WT) and homozygous (Mc4rK314X/K314X; HOM) littermate rats were derived from in-house heterozygous (Mc4r+/K314X) breeding pairs on a >8 generation Wistar:Crl background [4]. DNA isolation and genotyping of experimental rats were performed as previously described [4]. In short, DNA was processed from ear punches and genotyped for the ENU-induced single nucleotide polymorphism (SNP) in Mc4r (K314X) using the KASPar SNP genotyping system (KBiosciences; Hoddesdon, UK). We also used a mouse model of MC4R-deficiency that was created by inserting a loxP-flanked transcriptional blocking (loxTB) sequence between the transcriptional start site and the ATG of the Mc4r gene [5]. These loxTBMc4r mice were obtained from the Jackson Laboratory (JAX strain 006414) and outcrossed to WT C57Bl/6J mice (JAX strain 000664) for at least 8 generations. In-house bred heterozygous mice were subsequently used to generate experimental male WT and homozygous loxTBMc4r littermate mice. For the ICV SHU9119 experiment, WT C57BL/6J mice were obtained from the Jackson Laboratory (JAX strain 000664). For body weight-matched controls to loxTBMc4r mice (WTBWM mice) twenty-four wk-old WT C57BL/6J mice (JAX strain 000664) were used. An alternative approach would have been to use age-matched WT mice matched for obesity by high fat diet (HFD)-feeding, but as HFD feeding itself induces ΔFosB in the NAc [36], we opted for older weight-matched chow-fed controls.
2.3. Carbon fiber amperometry
To examine ex vivo electrically-evoked DA release we performed amperometric recordings of coronal slices or dissociated chromaffin cells as previously described [37,38]. Briefly, a carbon fiber electrode inserted into acute coronal slices of dorsal striatum, nucleus accumbens (NAc) shell, or medial prefrontal cortex (mPFC) was used to collect amperometric recordings from DAergic axons arising from the ventral tegmental area (VTA). An adjacent bipolar electrode delivered single pulses to trigger the release of synaptic DA. The current caused by the electrochemical oxidation of the extracellular DA serves as a measure of DA release kinetics in real time. A 0.5-mA electrical pulse lasting 2 ms was used as a stimulant with an interstimulus interval of 5 min to allow full recovery. Data were recorded at 50 kHz and filtered at 1 kHz. Amperometric peaks were identified as events greater than 3.5 times the root-mean-square noise of the baseline. The event width was the duration between (i) the baseline intercept of the maximal incline from the baseline to the first point that exceeded the cut off, and (ii) the first data point following the maximal amplitude that registered a value of ≤0 pA. The maximum amplitude (imax) of the event was the highest value within the event.
2.4. VWR
Eight-week old WT and HOM littermate rats and 9-week old WT and loxTBMc4r littermate mice were divided into body weight-matched sedentary and wheel-running groups. All rodents were housed individually and acclimated to the VWR cages for 4 d before collection of baseline parameters. Wheel-running rodents had access to an unlocked running wheel (rats: model 80859, 14″ diameter; 1.10 m/revolution; mice: model 80820, 5″ diameter; 0.40 m/revolution; Lafayette Instrument Co., Lafayette, IN) or to a locked running wheel (sedentary animals). VWR parameters were recorded as previously described [36] for indicated periods: HOM rats, 5 wk; loxTBMc4r mice, 12 wk; WTBMW mice, 12 wk; ICV SHU9119, 7 d. Rat VWR data of the 5th wk were not included in the data analysis due to possible behavioral effects of the glucose tolerance test on VWR behavior. Running wheel occupancy was calculated as follows: if a positive value for running wheel activity was observed during a 10-min interval, a value of 1 was assigned, if not, a value of 0 was assigned. All rodents had a similar diurnal cycle of running behavior and conducted the majority of running activity (more than 90% of daily activity) during the dark period (data not shown).
2.5. ICV surgery and SHU9119 administration
ICV (3rd ventricular) surgery was performed as previously described [36]. Mice were equipped with brain-infusion kits and osmotic mini-pumps (Alzet) for ICV delivery of saline or the non-selective MC3/4R antagonist SHU9119 [35] (dissolved in saline) at a rate of 5 ng/d.
2.6. Tissue collection
Running wheels were blocked and food was removed at the onset of the light phase after 5 wk of VWR. Because rats conducted the majority of running activity (more than 90% of daily activity) during the dark period (data not shown), blockade of the wheels during the light phase had very minimal effects on their behavior. Six h later, rats were euthanized by CO2 asphyxiation and rapid decapitation to collect plasma, brains, liver, subcutaneous white adipose tissue (scWAT), and front paw triceps muscle. All tissues were immediately frozen in dry ice-chilled isopentane. Blood was cold-centrifuged for plasma collection. For immunoblotting and qPCR analysis, the mediobasal hypothalamus (MBH), ventral tegmental area (VTA), and NAc were micro-dissected from frozen brains.
2.7. Immunoblotting
Muscle and NAc samples were pulverized and homogenized as described previously [39], and protein concentrations were determined via the Bradford protein assay. Proteins (50 μg) were resolved by SDS-PAGE for Western blot analysis [40]. Antibody-bound proteins were visualized on film using chemiluminescence detection reagents (Perkin–Elmer Life Sciences). Protein bands were scanned, and quantitated by densitometry (ImageJ, National Institute of Health). Commercially available primary antibodies were against DARPP32 (AB-10518); pThr34-DARPP32 (AB-9206); D1R (ABN20; all Millipore, Temecula, CA); TH (2792), pThr75-DARPP32 (2301), ΔFosB (9890; all Cell Signaling Technology, Danvers, MA); D2-type dopamine receptor (D2R; ab85367; Abcam, Cambridge, MA); and glucose transporter 4 (GLUT4; sc-7938), hexokinase 2 (HXK2; sc-6521; all Santa Cruz Biotechnology, Dallas, TX). Loading was controlled using glyceraldehyde 3-phosphate dehydrogenase (GAPDH; sc-25337; Santa Cruz Biotechnology) or β-tubulin (05–661; Millipore, Temecula, CA).
2.8. RNA isolation and quantitative PCR
RNA isolation, quantitative PCR (qPCR), and data analysis were performed as previously described [41]. Primers for all indicated genes were designed using Vector NTI (Life Technologies) and Primer-BLAST (NCBI; primer sequences are listed in Table S1 in Supplement 1).
2.9. Energy balance parameters
Body weight and food intake were measured weekly. Body composition (fat- and lean mass) was determined using nuclear magnetic resonance spectroscopy (EchoMRI; Echo Medical Systems, Houston, TX).
2.10. Glucose tolerance
After 4.5 weeks of VWR, rats were fasted for 4 h and an intraperitoneal (i.p.; 1.25 g/kg body weight) glucose tolerance test (ipGTT) was performed, and blood samples were collected for insulin determination as previously described [8]. HOMA-IR was calculated using the following formula: fasting glucose (mg/dL) × fasting insulin (μU/mL)/405.
2.11. Plasma and liver analyses
Plasma leptin and insulin were assayed by ELISA as previously described [8]. Circulating and hepatic triglycerides, and circulating cholesterol levels were measured in plasma and liver samples using specific colorimetric assay reagents (Pointe Scientific, Inc., Canton, MI), following the manufacturer's instructions.
2.12. Statistical analyses
Data were analyzed by unpaired Student's t-test or two-way analysis of variance (ANOVA), with repeated measures where applicable. Post hoc Tukey's HSD tests were performed where significant interactions were observed in ANOVAs. Significance was accepted at p < 0.05, with data reported as mean ± SEM. Statistical analyses were made using Statistica 10 (StatSoft, Tulsa, OK, USA). Details regarding statistical analyses are presented in Table S2 in Supplement 1.
3. Results
3.1. Sedentary, obese MC4R-deficient rats have increased evoked DA release in brain regions that receive projections from midbrain DA neurons
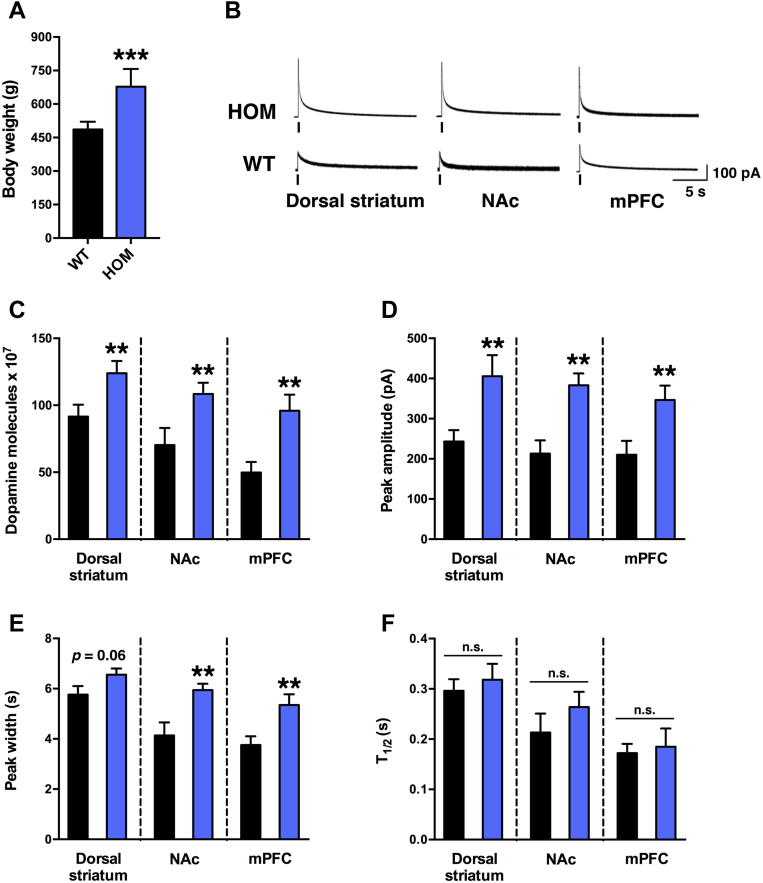
Based on the evidence that melanocortinergic and DAergic circuitries functionally interact to regulate molecular and behavioral responses to natural reinforcers and drugs of abuse [4,17–21,25–28,31–35,42], we hypothesized that compromised MC4R function alters DAergic neurotransmission. To test this hypothesis we directly assessed DA release by examining ex vivo electrically-evoked DA release in acute coronal slices of sedentary WT and HOM rats. At the time of the amperometry studies, HOM rats were significantly heavier than WT controls (Figure 1A). The mean number of molecules released per stimulation, the mean evoked DA event amplitude, and the mean DA-event peak width from the dorsal striatum, NAc and mPFC of HOM rats were increased compared to brain slices from WT littermates (Figure 1B–E). In contrast, T1/2 (duration of the signal at 50% of its amplitude) did not differ between genotypes in any of the tested brain regions, suggesting normal DA reuptake dynamics (Figure 1F). Finally, catecholamine quantal release from the adrenal glands of sedentary HOM rats was also higher than in WT rats (Figure S1A–C in Supplement 1). Taken together, these data reveal for the first time that MC4R deficiency results in significant aberrations in catecholamine release and dysregulation of the mesolimbic DA system characterized by high levels of evoked DA release and normal DA reuptake.
Figure 1.
Increased electrically-stimulated DA release in VTA projection targets during MC4R-deficiency. (A) Body weight of sedentary WT and HOM rats at time of amperometry studies. (B) Representative amperometric traces of electrical stimulation-evoked DA release in the dorsal striatum, nucleus accumbens shell (NAc) and medial prefrontal cortex (mPFC) from coronal slices of sedentary HOM rats (top) and WT littermate controls (bottom; electrical stimulation is indicated by vertical line). (C) Mean number of molecules released per stimulation, (D) mean evoked DA-event amplitude, and (E) DA-event peak width from the dorsal striatum, NAc and mPFC were significantly higher in brain slices from HOM rats (n = 34, 28 and 18 slices for each region, respectively, from 6 animals) than from WT littermate controls (n = 33, 25, and 22 slices for each region, respectively, from 6 animals). Peak width in the dorsal striatum of HOM rats trended to be higher (p = 0.06). (F) T1/2 (duration of the signal at 50% of its amplitude) did not differ among genotypes in any of the tested brain regions. *p < 0.05, **p < 0.01, ***p < 0.0005, WT versus HOM, n.s., not significant.
3.2. Obese MC4R-deficient rodents have decreased VWR
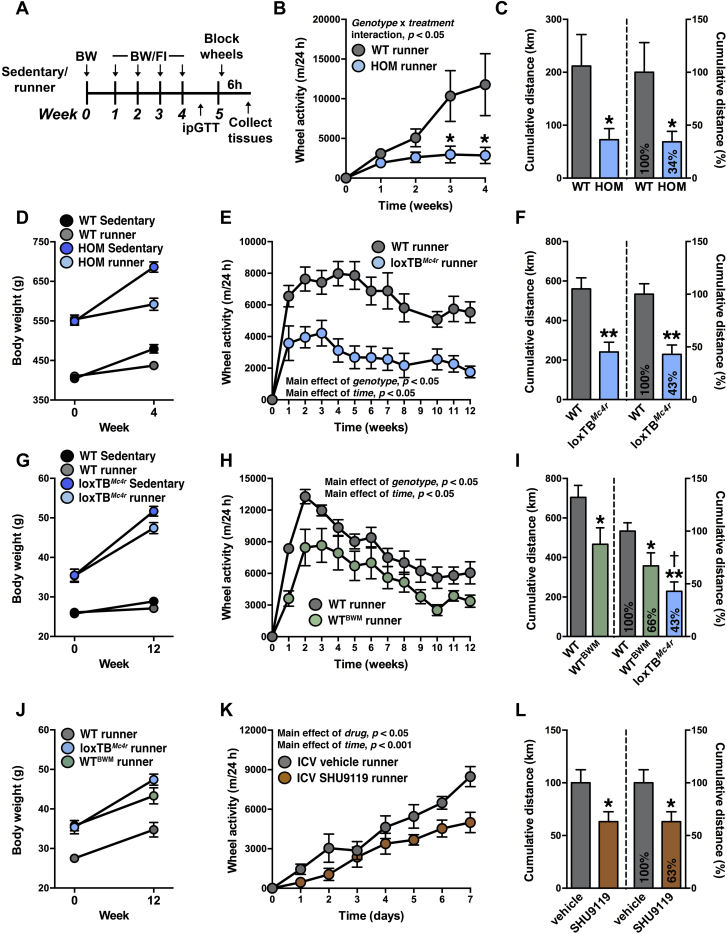
Exercise increases DA levels in the NAc and dorsal striatum of mice [43], is a self-reinforcing behavior [14–16,44], and induces molecular adaptations in the mesolimbic reward pathway [36,44,45]. Because sedentary HOM rats have increased ex vivo electrically evoked DA release, we predicted that HOM rats would have decreased behavioral responding to VWR. To test this, we assessed VWR in WT and HOM rats during 5-wk voluntary access to freely moving running wheels (Figure 2A). Sedentary controls had access to blocked running wheels. WT runners increased their daily running distance to a greater extent than HOM wheel-runners (Figure 2B), which resulted in 66% less cumulative running distance traveled by HOM wheel-runners compared with WT wheel-runners (Figure 2C). Dark-phase running wheel occupancy was similar between genotypes during week 1, but was lower in HOM than in WT wheel-runners during week 4 (Figure S2A in Supplement 1). At the onset of VWR, HOM rats were 35% heavier than WT rats (552 ± 2 g vs. 407 ± 4 g; Figure 2D). VWR blunted body weight gain in both WT and HOM wheel-runners (Figure 2D). Our observations in MC4R-deficient rats contrast with previous reports that VWR is similar in WT and Mc4r null mice, although VWR trended to be lower in the Mc4r null mice [33,34]. Thus, to exclude species-specific differences, we also analyzed VWR parameters in WT and loxTBMc4r mice during 12-wk voluntary access to freely moving or blocked running wheels. Compared to WT wheel-runners, loxTBMc4r wheel-runners had smaller daily running distances (Figure 2E), had smaller cumulative running distances (57% reduction; Figure 2F), and were 41% heavier at the onset of VWR (36.4 ± 1.1 g vs. 25.9 ± 0.4 g; Figure 2G). VWR blunted body weight gain in both WT and loxTBMc4r wheel-runners (Figure 2G). Dark-phase running wheel occupancy was lower in loxTBMc4r runners compared to WT wheel-runners during week 1 and week 11 (Figure S2B in Supplement 1). Thus, obese MC4R-deficient mice as well as rats demonstrate decreased VWR.
Figure 2.
MC4R loss-of-function decreases wheel running independent of body weight. (A) Experimental timeline of VWR in WT and HOM rats, with tissues collected 6 h after final night of VWR. (B) Mean daily running distance, (C) cumulative running distance, and (D) body weights at week 0 and 4 of WT and HOM littermate rats without (sedentary) or with (runner) 4-wk free access to running wheels (n = 14/group). (E) Mean daily running distance, (F) cumulative running distance, and (G) body weights at week 0 and 12 of WT and loxTBMc4r littermate mice without (sedentary) or with (runner) 12-wk free access to running wheels (n = 6–7/group). (H) Mean daily running distance, (I) cumulative running distance, and (J) body weights at week 0 and 12 of lean WT and WTBWM mice (body-weight matched to loxTBMc4r mice; values for loxTBMc4r mice are depicted in I and J for comparison) with 12-wk free access to running wheels (n = 6–7/group). (K) Mean daily running distance, and (L) cumulative running distance of WT mice with ICV administration of vehicle or SHU9119 (5 ng/day) during 7-d free access to running wheels (n = 5–6/group). *p < 0.05, **P < 0.001 versus WT wheel-runner/vehicle; †p < 0.05 versus WTBWM wheel-runner; Different letters indicate significant difference as following: a,bp < 0.05, main effect of genotype.
3.3. MC4Rs regulate VWR independent of body weight
The fact that MC4R-deficient rats and mice were heavier than controls may have influenced their ability to utilize the running wheels. Therefore, to determine if MC4R loss-of-function has body weight-independent effects on VWR, we compared VWR parameters in lean WT mice versus 24 wk-old WT mice that were body-weight matched to loxTBMc4r mice (WTBWM mice). WTBWM wheel-runners were 37% heavier than WT non-runners at the onset of VWR (37.1 ± 1.3 g vs. 27.1 ± 0.5 g; Figure 2J). Mice were allowed 12-wk voluntary access to freely moving or blocked running wheels. Similar to loxTBMc4r wheel-runners, WTBWM wheel-runners had smaller daily running distances, resulting in smaller cumulative running distances (33% reduction; Figure 2H). However, the relative decrease in cumulative VWR compared to lean WT control wheel-runners was significantly less profound in WTBWM wheel-runners than in loxTBMc4r wheel-runners (33 ± 1% reduction and 57 ± 9% reduction, respectively; P < 0.05; Figure 2I), indicating body weight-independent effects of MC4R signaling on VWR. Dark-phase running wheel occupancy was lower in WTBWM wheel-runners compared to WT wheel-runners during week 1 and week 11 (Figure S2C in Supplement 1). To confirm that blockade of MC4R signaling regulates VWR in a body weight-independent manner, we next used ICV administration of SHU9119, a non-selective MC3/4R antagonist [35], in wild-type mice. SHU9119 (5 ng/day) or saline was administered ICV for 7 d using Alzet osmotic mini-pumps in mice that were body weight-matched at the onset of VWR (Figure S3A in Supplement 1). This dose of SHU9119 increased body weight gain and food intake, independent of treatment (Figure S3B, C in Supplement 1). SHU9119-treated mice had decreased VWR compared to saline-treated controls, resulting in smaller cumulative running distances (37% reduction; Figure 2K, L). Together these data indicate that MC4Rs regulate VWR independently of body weight.
3.4. Indices of increased DAergic regulators in the VTA of obese MC4R-deficient wheel-runners
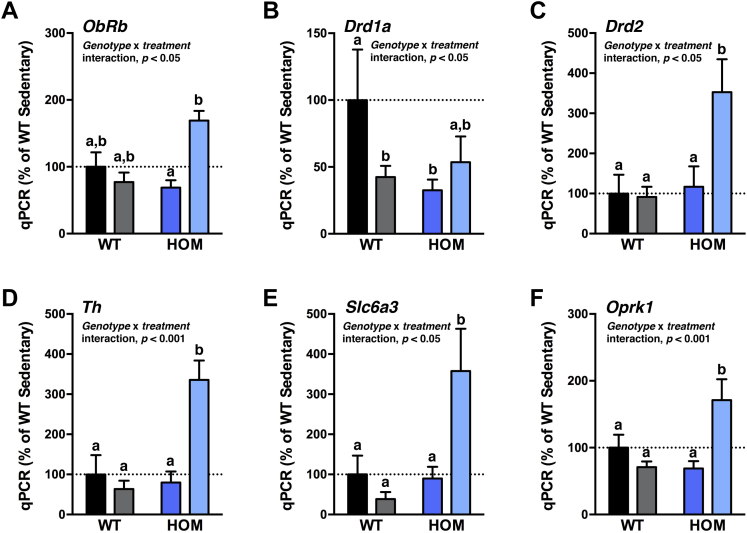
Within the mesolimbic DA system, the VTA innervates the NAc, among other regions, and VWR can induce neuronal plasticity in the VTA [44]. Therefore, we measured expression of genes capable of modulating DAergic neurotransmission in the VTA after 5 wk of VWR (Figure 3A–F). Leptin receptor (ObRb) expression was higher in HOM wheel-runners than sedentary HOM rats, but did not differ among the other groups. Drd1a (D1R) mRNA expression was lower in WT wheel-runners and sedentary HOM rats compared to sedentary WT rats but did not differ significantly from HOM wheel-runners. Drd2 (D2R), Th (tyrosine hydroxylase), Slc6a3 (dopamine transporter; DAT), and Oprk1 (κ-opioid receptor) expression were each elevated in HOM wheel-runners by 2–4 fold compared to all other experimental groups. Presynaptic D2Rs, TH, and DAT exert counter-regulatory roles for DA release. Thus, these data indirectly indicate that HOM wheel-runners may have increased DAergic flux originating from the VTA compared to controls. Bdnf (brain-derived neurotrophic factor), Oprm1 (μ-opioid receptor 1), and Ox1r (orexin receptor type 1) expression in the VTA did not differ among any of the experimental groups (Figure S4A–C in Supplement 1).
Figure 3.
Indices of increased dopaminergic regulators in the VTA of HOM wheel-runners. (A) ObRb, (B) Drd1a, (C) Drd2, (D) Th, (E), Slc6a3, and (F) Oprk1 gene expression in the ventral tegmental area of WT and HOM littermate rats without (sedentary) or with (runner) free access to running wheels for 5 wk (n = 5–6/group). All data are represented relative to WT sedentary controls. Different letters indicate significant difference as following: a,bp < 0.05, genotype × treatment interaction.
3.5. Obese MC4R-deficient wheel-runners have dysregulated intracellular signaling in NAc MSNs
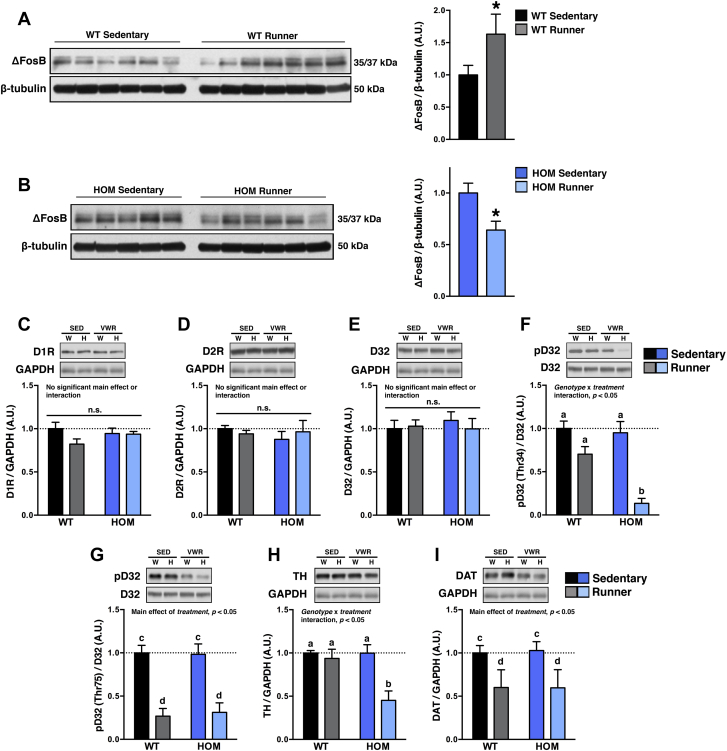
VWR also induces adaptations in the NAc [44]. Notably, the NAc is a key brain reward region that regulates the motivation to obtain natural reinforcers (e.g., palatable food, sexual behavior) as well as drugs of abuse [46]. The vast majority of cells in the NAc are medium spiny neurons (MSNs), which are broadly divided into two equally sized clusters based on expression of D1Rs (i.e. D1-type MSNs) or D2Rs (i.e. D2-type MSNs) [47]. In the context of physical activity, activation of D1-type MSNs promotes motor function whereas activation of D2-type MSNs attenuates movement [48]. ΔFosB, a very stable Fos-family transcription factor involved in MSN functional plasticity [49], is induced in the NAc following 4–6 wk of VWR [36,44,45], an effect regulated by D1Rs [45]. MC4Rs and D1Rs are co-expressed on MSNs in the NAc and share post-receptor signaling pathways that converge on DARPP32, a key regulator protein of neuronal function [22,29,30]. Therefore we hypothesized that the higher evoked DA release in sedentary HOM rats is a functional compensation for attenuated intracellular signaling in predominantly D1-type MSNs. VWR increased ΔFosB levels in NAc tissue punches of WT wheel-runners relative to what occurred in sedentary controls (Figure 4A). In contrast, VWR unexpectedly decreased ΔFosB levels in HOM wheel-runners compared to sedentary controls (Figure 4B). DAergic signaling can modulate intracellular pathways that promote the accumulation of ΔFosB. Therefore, we next measured biochemical markers of DAergic signaling in the NAc using protein quantification. D1R, D2R, and total DARPP-32 protein levels were not altered by genotype or VWR (Figure 4C–E). However, VWR decreased phosphorylation of DARPP-32 at Thr34 [pDARPP-32 (Thr34)] in HOM wheel-runners but not in WT wheel-runners, compared to sedentary controls (Figure 4F). Phosphorylation of DARPP-32 at Thr75 [pDARPP-32 (Thr75)] was similarly decreased in both WT and HOM wheel-runners (Figure 4G). TH levels were decreased in HOM wheel-runners compared to sedentary controls (Figure 4H). Finally, DAT levels were lower during VWR, independent of genotype (Figure 4I). None of the measured proteins were different between sedentary WT and HOM rats (Figure 4A–I; P > 0.05). Unchanged DAT levels in the NAc of sedentary HOM rats are consistent with normal DA reuptake dynamics in the NAc (Figure 1F). Induction of ΔFosB [50,51] or pDARPP-32 (Thr34) [29] requires activation of D1Rs, whereas cAMP-mediated activation of cAMP response element-binding (CREB) increases TH levels [52]. Collectively these data indicate that HOM rats have dysregulated intracellular signaling in NAc MSNs, which becomes evident during stimulated conditions such as VWR (this study) and is similar to what happens during chronic cocaine administration [19].
Figure 4.
Markers of dysregulated intracellular MSN signaling in the NAc of HOM wheel-runners. Representative images (left) and immunoblot analyses (right) of ΔFosB in NAc samples of (A) WT and (B) HOM rats without (sedentary) or with (runner) free access to running wheels for 5 wk (n = 5–7/group). Representative images (top) and immunoblot analyses (bottom) of (C) D1R, (D) D2R, (E) total DARPP-32 (D32), (F) pD32-(Thr34), (G) pD32-(Thr75), (H) TH, and (I) DAT in NAc samples of WT (W) and HOM (H) rats without (sedentary; SED) or with (runner; VWR) free access to running wheels for 5 wk (n = 4–7/group; data are represented relative to sedentary WT controls). Protein loading was controlled using β-tubulin or GAPDH. *p < 0.05; different letters indicate significant difference as following: a,bp < 0.05, genotype × treatment interaction; c,dp < 0.05, effect of treatment; n.s., not significant.
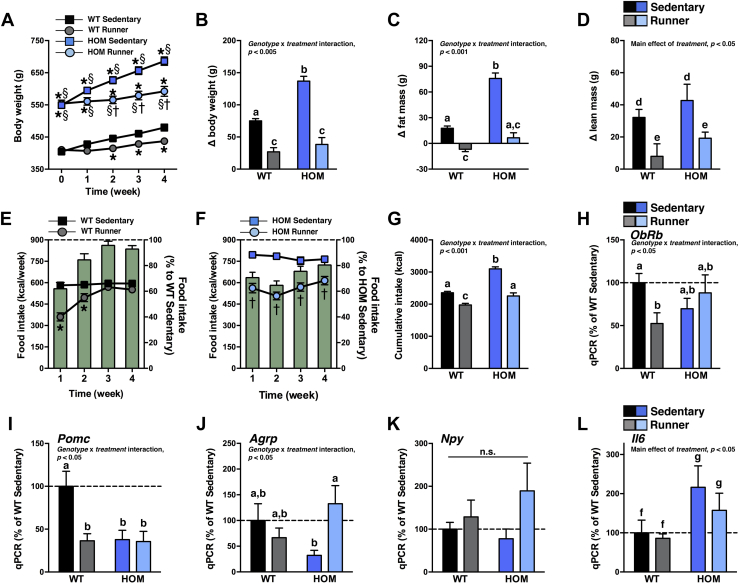
3.6. Obese MC4R-deficient wheel-runners have normalized body weight growth and food intake
VWR can delay the development of late-onset obesity in Mc4r null mice [33,34]. Here we asked whether, despite the dysregulation of the mesolimbic DA system and moderate amounts of exercise, VWR can ameliorate MC4R-associated metabolic complications after it has already developed. Although HOM wheel-runners were still heavier than sedentary WT rats at the end of the 5-wk study (Figure 5A), the moderate amounts of VWR by HOM runners nonetheless normalized body weight gain (Figure 5B), fat mass gain (Figure 5C), and caloric intake to sedentary WT levels (Figure 5E–G). To elucidate potential molecular pathways that regulate energy metabolism during VWR, we measured mRNA expression in the MBH after 5 wk of VWR. In general, WT wheel-runners had a decreased anorexigenic tone (lower ObRb and Pomc expression compared to sedentary WT controls), sedentary HOM rats had a decreased melanocortinergic tone (lower Pomc and Agrp expression compared to sedentary WT controls), and despite their reduced food intake, HOM wheel-runners had an orexigenic tone (lower Pomc, normal Agrp and Npy expression compared to sedentary WT controls; Figure 5H–K). Inflammatory signaling in the hypothalamus has been proposed to link the beneficial physiological effects of (forced swimming) exercise to the central effects of insulin and leptin [53]. However, interleukin-6 expression was higher in HOM rats, independent of treatment (Figure 5L).
Figure 5.
Moderate voluntary exercise normalizes body weight growth and hyperphagia in HOM rats. (A) Body weight, cumulative change in (B) body weight, (C) fat mass, and (D) lean mass, and weekly (E) WT and (F) HOM food intake (kcal, left axis; % to sedentary controls, right axis), and (G) cumulative food intake of WT and HOM littermate rats without (sedentary) or with (runner) free access to running wheels for 4 wk (n = 14/group). (H) ObRb, (I) Pomc, (J) Agrp, (K) Npy, and (L) Il6 gene expression in mediobasal hypothalamus of WT and HOM littermate rats without (sedentary) or with (runner) free access to running wheels for 5 wk (n = 5–7/group). qPCR data are represented relative to WT sedentary controls. *p < 0.05, vs. WT sedentary, †p < 0.05, vs. HOM sedentary, §p < 0.05, vs. HOM wheel-runner. Different letters indicate significant difference as following: a,b,cp < 0.05, genotype × treatment interaction; d,ep < 0.05, effect of treatment; f,gp < 0.05, effect of genotype; n.s., not significant.
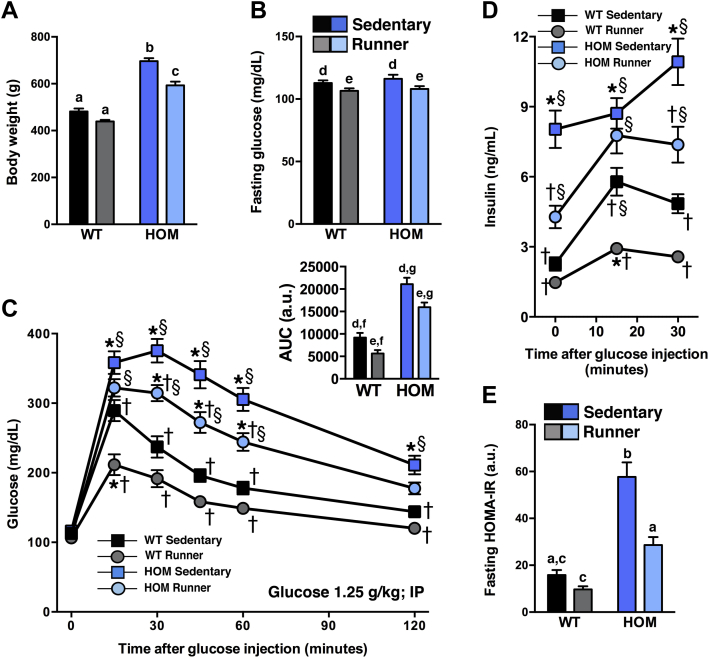
3.7. Obese MC4R-deficient wheel-runners have improved glucose tolerance and insulin sensitivity
To assess if VWR ameliorates glucose intolerance during MC4R deficiency, we performed an ipGTT after 4.5 wk of VWR. VWR lowered fasting glucose in both WT and HOM rats (Figure 6B). HOM wheel-runners were still heavier then sedentary WT controls (Figure 6A), but had substantially improved glucose tolerance and reduced hyperinsulinemia (Figure 6C, D). Lastly, HOMA-IR was significantly improved by VWR in both groups and in HOM wheel-runners even reached a level that was equivalent to that of the WT sedentary controls (Figure 6E). Thus, the moderate amounts of VWR were able to induce notable improvements in glucose tolerance during MC4R deficiency.
Figure 6.
Moderate voluntary exercise improves glucose tolerance and insulin sensitivity in HOM rats. (A) Body weight during ipGTT, (B) fasting blood glucose, (C) blood glucose before (0) and following (15, 30, 45, 60, and 120 min) an i.p. injection of 1.25 g/kg glucose, (C, insert) blood glucose as area under the curve (AUC), (D) plasma insulin before (0) and following (15, 30 min) glucose bolus injection, and (E) fasting HOMA-IR values of WT and HOM littermate rats without (sedentary) or with (runner) free access to running wheels for 4.5 wk (n = 12–14/group). Different letters indicate significant difference as following: a,b,cp < 0.05, genotype × treatment interaction; d,ep < 0.05, effect of treatment; f,gp < 0.05, effect of genotype. *p < 0.05, vs. WT sedentary, †p < 0.05, vs. HOM sedentary, §p < 0.05, vs. WT wheel-runner.
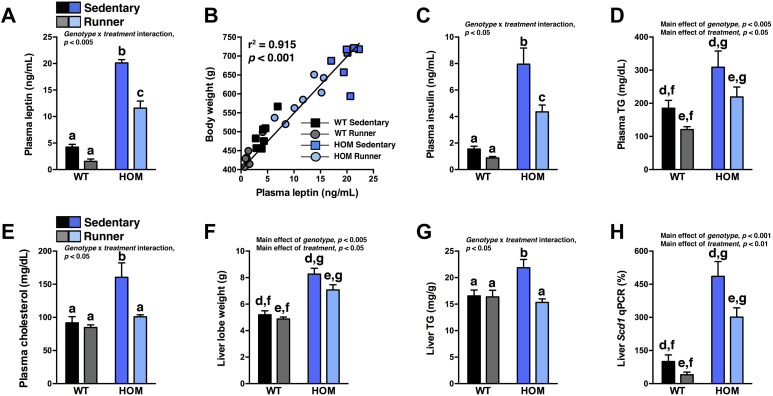
3.8. Moderate exercise lowers hyperleptinemia and normalizes hypercholesterolemia and hepatic steatosis in obese MC4R-deficient rats
Finally, we also investigated several characteristic MC4R-associated metabolic complications after 5 wk of VWR. The moderate amounts of VWR drastically lowered plasma leptin and insulin levels in HOM runners compared to sedentary HOM controls, normalized plasma TG and cholesterol levels, and reduced liver TG levels to those of sedentary WT controls (Figure 7A–G). VWR decreased liver Scd1 expression in HOM wheel-runners compared to sedentary HOM controls (Figure 7H). The central melanocortin system regulates leptin-induced skeletal muscle AMPK activation and GLUT4 translocation [54,55], resulting in decreased skeletal muscle glucose uptake during MC4R deficiency [56]. Because impaired substrate utilization can decrease exercise capacity, we measured protein levels of GLUT4 and HXK2, important regulators of increased muscle glucose utilization during exercise [57], in triceps muscle after 5 wk of VWR. VWR induction of muscle GLUT4 was severely blunted in HOM wheel-runners, whereas VWR induction of HXK2 was similar in WT and HOM wheel-runners (Figure S5A–C in Supplement 1).
Figure 7.
Moderate voluntary exercise decreases hyperleptinemia and normalizes hypercholesterolemia and hepatic steatosis in HOM rats. (A) Plasma leptin, (B) correlation analysis of final body weight and plasma leptin, (C) plasma insulin, (D) plasma triglycerides (TG), (E) plasma cholesterol, (F) liver lobe weight, (G) hepatic TG levels (n = 7/group), and hepatic (H) Scd1 mRNA expression (n = 12–14/group) of WT and HOM rats without (sedentary) or with (runner) free access to running wheels for 5 wk. Different letters indicate significant difference as following: a,b,cp < 0.05, genotype × treatment interaction; d,ep < 0.05, effect of treatment; f,gp < 0.05, effect of genotype.
4. Discussion
Treatment of compromised MC4R functioning and the associated metabolic syndrome requires identification of downstream neurobiological effects and adaptations. Here we used genetic, pharmacological and behavioral approaches to demonstrate that MC4R-deficiency results in reduced VWR, an effect that is at least partially independent of body weight. Specifically, we observed a dysregulation of the mesolimbic DA system in HOM rats during basal as well as VWR conditions. Moderate amounts of VWR by HOM rats normalized several characteristic phenotypes of MC4R deficiency, including body weight gain, hyperphagia, insulin insensitivity, hypercholesterolemia, and hepatic steatosis, relative to sedentary WT levels. Overall, these data indicate that MC4R plays a permissive role in the regulation of VWR-induced improvements in metabolic health. The implications of these findings are discussed below.
Using different rodent models we demonstrated that MC4R signaling regulates VWR. First, MC4R-deficient rats and mice with characteristic obesity have significantly reduced VWR. Previous studies reported no significant differences in VWR between WT and Mc4r null mice [33,34], although it has to be noted that in those studies the Mc4r null mice did in fact run less and a failure to observe statistical differences may have been due to low statistical power and/or the genetic background of the mouse models used. Previously studied Mc4r null mice were generated on a 129 background [6]. Details about the genetic composition of the Mc4r null mice used in those VWR studies were, however, not reported [33,34]. The loxTBMc4r mice used in our study were also generated on a 129 background [5], but they and their experimental littermates had been backcrossed to a C57BL/6J background for at least 8 generations. C57BL/6J mice have a high preference whereas 129 mice have very little preference for VWR [58], thus likely limiting the ability to discover statistical differences in VWR behavior on a (partial) 129 background. Finally, using both body weight-matched WT controls and ICV SHU9119 administration, we demonstrated that loss of MC4R signaling has body weight-independent effects on VWR. The latter observations are in line with the body weight-independent effects of MC4R signaling on spontaneous ambulatory activity [59].
In terms of mesolimbic function, our carbon-fiber amperometry studies revealed that sedentary HOM rats have greater evoked dopamine release in all VTA target sites tested compared to lean WT controls. Furthermore, quantal catecholamine release from the adrenal glands was also higher in HOM rats than WT littermate controls. These purported increases in evoked DA release may be linked to presynaptic mechanisms involved in the regulation of catecholamine exocytosis from large dense-core granules in the periphery and small clear synaptic vesicles in the brain. The exact nature of the compensational adaptations in the mesolimbic DA system of MC4R-haploinsufficient humans is not completely understood. Our preclinical data are the first to indicate that mesolimbic function could be dysregulated in the MC4R-insufficient condition. Interestingly, data from separate models of metabolic dysfunction, including leptin-deficient (ob/ob) and leptin resistant (db/db or diet-induced obese) mice, are associated with reduced evoked DA release in the NAc [60–62]. These opposing effects on evoked DA release observed in the context of leptin- or MC4R-deficiency suggest that orexigenic and anorexigenic signals interact within the mesolimbic DA system in complex and potentially different ways to regulate body weight gain and physical activity.
It has been clearly established that central MC4Rs are critical for the normal regulation of energy and glucose homeostasis [1,2]. Furthermore, several studies have demonstrated that MC4Rs in the mesolimbic DA system regulate many reward-related behaviors. For example, palatable high-fat diets and cocaine each have decreased reward value in MC4R-deficient mice [25,28]. Home-cage and cocaine-induced locomotion are decreased in MC4R-deficient rats and mice [4,19,25–27]. Because VWR is a self-reinforcing behavior, it utilizes both the DAergic and melanocortinergic circuitries such that they necessarily functionally interact, and we found that HOM rats have greater evoked DA release in all VTA target sites tested. Further, our behavioral observations suggest that altered DAergic neurotransmission might decrease preference for VWR in HOM rats. To assess this possibility, we first measured gene expression of several regulators of DA flux from the VTA to projection sites, including the NAc. ObRb, Drd2, Th, Slc6a3 (DAT), and Oprk1 expression were all upregulated approximately 3 fold in the VTA of HOM wheel-runners. Increased TH, DAT, and D2R levels on presynaptic axons projecting from the VTA positively correlate with DA output. Our data thus suggest an increase in DA flux towards VTA projection sites that occurred uniquely in HOM wheel-runners. Furthermore, presynaptic DATs and D2Rs work together to limit extracellular DA levels [63–65], whereas κ-opioid receptor agonists administered in the NAc also decrease DA release [66]. The VTA of HOM wheel-runners thus exhibit a hypersensitive response to VWR, with a gene expression profile indicative of increased DA release as well as compensatory mechanisms that attempt to limit DA release.
These changes in reward-related gene expression in the VTA were only observed in HOM wheel-runners, and surprisingly not in WT wheel-runners or sedentary HOM rats. An attractive candidate regulating these unique changes is leptin. For one thing, MC4R-deficiency is associated with hyperleptinemia and resistance to the effects of exogenous administration of leptin [67]. Second, VWR improves leptin signaling in the VTA but not in hypothalamic nuclei or the substantia nigra of lean and obese rats [68,69]. Third, leptin signaling in the VTA regulates feeding behavior, locomotor activity, and sensitivity to and motivation for highly palatable food via modulation of DA neuron activity [60,70,71]. Thus, we speculate that VWR ameliorates the central leptin resistance that occurs during MC4R-deficiency. Although this hypothesis remains to be corroborated experimentally, HOM wheel-runners had significant reductions in plasma leptin levels (∼50% decrease) compared to levels in sedentary HOM rats, higher VTA ObRb expression compared to all other groups, and a remarkable normalization of their characteristic hyperphagia. In addition, our data are consistent with that of Shapiro et al., who demonstrated that leptin therapy in combination with seemingly negligible amounts of VWR prevented progression of dietary obesity in leptin-resistant rats [72], and Bi et al., who found that VWR strongly reduced hyperleptinemia and normalized hyperphagia in OLETF rats [73].
Because of its centrality to reward circuits, we focused on the NAc, measuring protein expression of several genes downstream of DA receptor signaling in MSNs. Although WT wheel-runners had the expected increases in ΔFosB in the NAc, HOM wheel-runners had unexpectedly decreased ΔFosB levels in the NAc. Induction of ΔFosB in the NAc requires D1Rs [50,51] and appears specific for D1-type MSNs [45]; overexpression of ΔFosB in D1-type MSNs increases VWR during the third week of wheel-running, likely through its effects on neuronal plasticity [45,49]. Because NAc D1R levels were unaltered during VWR or MC4R deficiency, we next investigated signaling downstream of D1Rs and found that pDARPP-32 (Thr34) levels in the NAc were significantly lower in HOM wheel-runners relative to other groups. Of note, loxTBMc4r mice also had decreased pDARPP-32 (Thr34) levels in the NAc compared to WT controls following chronic cocaine administration, and re-expression of Mc4r on D1-type MSNs fully normalized pDARPP-32 (Thr34) levels to WT levels [19]. MC4Rs are preferentially expressed on D1-type MSNs [25], and like D1Rs, MC4Rs activate the cAMP/PKA/DARPP-32 pathway by binding to Gs/olf proteins [22,30]. The data we present here provide novel insights regarding how melanocortin neurons can directly modulate intracellular signaling and ΔFosB levels in D1-type MSNs, and how MC4R-deficiency dysregulates this process resulting in decreased behavioral responding to rewarding stimuli.
VWR and palatable HFDs, both natural rewards, similarly activate ΔFosB immunoreactivity in the NAc [36]. Interestingly, C57Bl/6J mice that were given simultaneous free access to a HFD and running wheels showed normal VWR behavior and no additive effect on ΔFosB activation [36], suggesting that these treatments compete for activation of the mesolimbic DA system. In contrast, mice that were bred for high levels of VWR demonstrate DAergic dysregulation and higher amounts of VWR in response to HFD consumption [74]. These findings indicate a complex interplay between genetic predisposition and natural rewards that regulate DAergic function and subsequent behavior. Given that HOM rats display substantial DAergic dysregulation, additional studies will investigate their VWR behavior during simultaneous free access to a HFD and running wheels.
Despite the many beneficial effects on general health, VWR is also considered to be a physiological stressor [75]. VWR is associated with increased circulating levels of glucocorticoids, including corticosterone (CORT), an effect that appears to be transient and levels off after 3–4 weeks of VWR [76]. Stress can increase food intake but mainly decreases intake of non-palatable diets in animal models [77]. Although CORT levels were not measured in the current study, it is tempting to speculate that transient alterations in CORT levels during VWR are related to the transient reductions in food intake in WT runners. In contrast, HOM runners demonstrated consistently reduced caloric intake, suggesting hyperactivation of the axis. However, HOM rats have an attenuated activation of the HPA axis following psychological stress [78]. Collectively, these findings suggest that the normalization of food intake in HOM wheel runners is not stress-related, although a role for the HPA axis cannot be excluded based on the current study and this possibility will be explored in later studies.
In this study, MC4R-deficiency blunted VWR induction of GLUT4 levels in muscle and negatively impacted VWR. Despite this, greater body weight gain, hyperphagia, insulin insensitivity, hypercholesterolemia, and hepatic steatosis, all characteristic metabolic phenotypes of MC4R-deficiency, were substantially improved and, in some cases, even normalized to sedentary WT levels. Furthermore, the moderate levels of VWR achieved substantially lowered insulin and leptin levels in HOM wheel-runners. These observations are consistent with and extend previous studies [33,34] and confirm that exercise training could be a powerful lifestyle intervention to improve metabolic health in MC4R-haploinsufficient humans. Although exercise studies in MC4R-haploinsufficient human subjects are scarce, Reinehr and colleagues observed that a lifestyle intervention promoted weight loss similarly in children with reduced MC4R function and control subjects. However, children with reduced MC4R function had much greater difficulties maintaining this weight loss [79].
Based on the present data, we propose that the combination of MC4R-deficiency and VWR unbalances postsynaptic signaling in the NAc, predominantly in D1-type MSNs. This translates to decreased levels of key neuronal regulator proteins such as ΔFosB and DARPP-32. Not only is this disequilibrium evident during VWR (present study), but MC4R-deficiency also decreases molecular and behavioral responding to other rewarding stimuli such as drugs and HFDs [19,25,26,28]. Our data thus reinforce the link between MC4R function and molecular and behavioral responding to natural reinforcers and drugs of abuse. Collectively, our preclinical data provide evidence that individuals with MC4R-haploinsufficiency may likely experience reduced positive reinforcement during exercise training although small increases in physical activity will improve their overall metabolic health.
Disclosures
This work was funded by a pilot and feasibility grant from the Cincinnati Diabetes and Obesity Center to S.O., and by NIH grant DK065872 and Tufts Center for Neuroscience Research grant P30 NS047243 to E.N.P.S.C.W. is supported by DK017884. J.D.M. is supported by an American Diabetes Association mentor-based fellowship grant 7-12-MN-22 awarded to L.J.G. J.D.M. has received a lecture fee from Taconic.
Acknowledgments
We thank Sonia Lipp for assistance with colorimetric assays, members of the Seeley and Obici labs for helpful discussions, and Dr. Susanne la Fleur for her insightful comments on the manuscript.
Footnotes
Prior presentation: Parts of this study were presented at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014, and at the 97th Annual Meeting of the Endocrine Society, San Diego, CA, 5–8 March 2015.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2015.07.003.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Tao Y.X. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocrine Reviews. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cone R.D. Anatomy and regulation of the central melanocortin system. Nature Neuroscience. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 3.Farooqi I.S., Keogh J.M., Yeo G.S., Lank E.J., Cheetham T., O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. The New England Journal of Medicine. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 4.Mul J.D., van Boxtel R., Bergen D.J., Brans M.A., Brakkee J.H., Toonen P.W. Melanocortin receptor 4 deficiency affects body weight regulation, grooming behavior, and substrate preference in the rat. Obesity (Silver Spring) 2012;20:612–621. doi: 10.1038/oby.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balthasar N., Dalgaard L.T., Lee C.E., Yu J., Funahashi H., Williams T. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Huszar D., Lynch C.A., Fairchild-Huntress V., Dunmore J.H., Fang Q., Berkemeier L.R. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 7.Vaisse C., Clement K., Durand E., Hercberg S., Guy-Grand B., Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. The Journal of Clinical Investigation. 2000;106:253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mul J.D., Begg D.P., Alsters S.I., Haaften G., Duran K.J., D'Alessio D.A. Effect of vertical sleeve gastrectomy in melanocortin receptor 4-deficient rats. American Journal of Physiology. Endocrinology and Metabolism. 2012;303:E103–E110. doi: 10.1152/ajpendo.00159.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valette M., Poitou C., Le Beyec J., Bouillot J.L., Clement K., Czernichow S. Melanocortin-4 receptor mutations and polymorphisms do not affect weight loss after bariatric surgery. PLoS One. 2012;7:e48221. doi: 10.1371/journal.pone.0048221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colberg S.R., Sigal R.J., Fernhall B., Regensteiner J.G., Blissmer B.J., Rubin R.R. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33:2692–2696. doi: 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson C.M., Levin B.E. Role of exercise in the central regulation of energy homeostasis and in the prevention of obesity. Neuroendocrinology. 2008;87:65–70. doi: 10.1159/000100982. [DOI] [PubMed] [Google Scholar]
- 12.Rowe G.C., Safdar A., Arany Z. Running forward: new frontiers in endurance exercise biology. Circulation. 2014;129:798–810. doi: 10.1161/CIRCULATIONAHA.113.001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Praag H., Fleshner M., Schwartz M.W., Mattson M.P. Exercise, energy intake, glucose homeostasis, and the brain. The Journal of Neuroscience. 2014;34:15139–15149. doi: 10.1523/JNEUROSCI.2814-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meijer J.H., Robbers Y. Wheel running in the wild. Proceedings Biological Sciences/The Royal Society. 2014;281 doi: 10.1098/rspb.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iversen I.H. Techniques for establishing schedules with wheel running as reinforcement in rats. Journal of the Experimental Analysis of Behavior. 1993;60:219–238. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lett B.T., Grant V.L., Byrne M.J., Koh M.T. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34:87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- 17.Kishi T., Aschkenasi C.J., Lee C.E., Mountjoy K.G., Saper C.B., Elmquist J.K. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. The Journal of Comparative Neurology. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 18.Lindblom J., Opmane B., Mutulis F., Mutule I., Petrovska R., Klusa V. The MC4 receptor mediates alpha-MSH induced release of nucleus accumbens dopamine. Neuroreport. 2001;12:2155–2158. doi: 10.1097/00001756-200107200-00022. [DOI] [PubMed] [Google Scholar]
- 19.Cui H., Lutter M. The expression of MC4Rs in D1R neurons regulates food intake and locomotor sensitization to cocaine. Genes, Brain, and Behavior. 2013 doi: 10.1111/gbb.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandit R., van der Zwaal E.M., Luijendijk M.C., Brans M.A., van Rozen A.J., Oude Ophuis R.J. Central melanocortins regulate the motivation for sucrose reward. PLoS One. 2015;10:e0121768. doi: 10.1371/journal.pone.0121768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mul J.D., Spruijt B.M., Brakkee J.H., Adan R.A. Melanocortin MC(4) receptor-mediated feeding and grooming in rodents. European Journal of Pharmacology. 2013;719:192–201. doi: 10.1016/j.ejphar.2013.04.060. [DOI] [PubMed] [Google Scholar]
- 22.Cui H., Mason B.L., Lee C., Nishi A., Elmquist J.K., Lutter M. Melanocortin 4 receptor signaling in dopamine 1 receptor neurons is required for procedural memory learning. Physiology & Behavior. 2012;106:201–210. doi: 10.1016/j.physbeh.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caruso V., Lagerstrom M.C., Olszewski P.K., Fredriksson R., Schioth H.B. Synaptic changes induced by melanocortin signalling. Nature Reviews. Neuroscience. 2014;15:98–110. doi: 10.1038/nrn3657. [DOI] [PubMed] [Google Scholar]
- 24.Lim B.K., Huang K.W., Grueter B.A., Rothwell P.E., Malenka R.C. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu R., Taylor J.R., Newton S.S., Alvaro J.D., Haile C., Han G. Blockade of melanocortin transmission inhibits cocaine reward. The European Journal of Neuroscience. 2005;21:2233–2242. doi: 10.1111/j.1460-9568.2005.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvaro J.D., Taylor J.R., Duman R.S. Molecular and behavioral interactions between central melanocortins and cocaine. The Journal of Pharmacology and Experimental Therapy. 2003;304:391–399. doi: 10.1124/jpet.102.040311. [DOI] [PubMed] [Google Scholar]
- 27.Chen A.S., Metzger J.M., Trumbauer M.E., Guan X.M., Yu H., Frazier E.G. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Research. 2000;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 28.Panaro B.L., Cone R.D. Melanocortin-4 receptor mutations paradoxically reduce preference for palatable foods. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7050–7055. doi: 10.1073/pnas.1304707110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svenningsson P., Nishi A., Fisone G., Girault J.A., Nairn A.C., Greengard P. DARPP-32: an integrator of neurotransmission. Annual Review of Pharmacology and Toxicology. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- 30.Gantz I., Miwa H., Konda Y., Shimoto Y., Tashiro T., Watson S.J. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. The Journal of Biological Chemistry. 1993;268:15174–15179. [PubMed] [Google Scholar]
- 31.Chiu S., Fisler J.S., Espinal G.M., Havel P.J., Stern J.S., Warden C.H. The yellow agouti mutation alters some but not all responses to diet and exercise. Obesity Research. 2004;12:1243–1255. doi: 10.1038/oby.2004.158. [DOI] [PubMed] [Google Scholar]
- 32.Richard C.D., Tolle V., Low M.J. Meal pattern analysis in neural-specific proopiomelanocortin-deficient mice. European Journal of Pharmacology. 2011;660:131–138. doi: 10.1016/j.ejphar.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irani B.G., Xiang Z., Moore M.C., Mandel R.J., Haskell-Luevano C. Voluntary exercise delays monogenetic obesity and overcomes reproductive dysfunction of the melanocortin-4 receptor knockout mouse. Biochemical and Biophysical Research Communications. 2005;326:638–644. doi: 10.1016/j.bbrc.2004.11.084. [DOI] [PubMed] [Google Scholar]
- 34.Haskell-Luevano C., Schaub J.W., Andreasen A., Haskell K.R., Moore M.C., Koerper L.M. Voluntary exercise prevents the obese and diabetic metabolic syndrome of the melanocortin-4 receptor knockout mouse. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2009;23:642–655. doi: 10.1096/fj.08-109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan W., Boston B.A., Kesterson R.A., Hruby V.J., Cone R.D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 36.Krawczewski Carhuatanta K.A., Demuro G., Tschop M.H., Pfluger P.T., Benoit S.C., Obici S. Voluntary exercise improves high-fat diet-induced leptin resistance independent of adiposity. Endocrinology. 2011;152:2655–2664. doi: 10.1210/en.2010-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinnayah P., Jobst E.E., Rathner J.A., Caldera-Siu A.D., Tonelli-Lemos L., Eusterbrock A.J. Feeding induced by cannabinoids is mediated independently of the melanocortin system. PLoS One. 2008;3:e2202. doi: 10.1371/journal.pone.0002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pothos E.N., Mosharov E., Liu K.P., Setlik W., Haburcak M., Baldini G. Stimulation-dependent regulation of the pH, volume and quantal size of bovine and rodent secretory vesicles. The Journal of Physiology. 2002;542:453–476. doi: 10.1113/jphysiol.2002.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakamoto K., Hirshman M.F., Aschenbach W.G., Goodyear L.J. Contraction regulation of Akt in rat skeletal muscle. The Journal of Biological Chemistry. 2002;277:11910–11917. doi: 10.1074/jbc.M112410200. [DOI] [PubMed] [Google Scholar]
- 40.Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mul J.D., Yi C.X., van den Berg S.A., Ruiter M., Toonen P.W., van der Elst M.C. Pmch expression during early development is critical for normal energy homeostasis. American Journal of Physiology. Endocrinology and Metabolism. 2010;298:E477–E488. doi: 10.1152/ajpendo.00154.2009. [DOI] [PubMed] [Google Scholar]
- 42.Butler A.A., Marks D.L., Fan W., Kuhn C.M., Bartolome M., Cone R.D. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nature Neuroscience. 2001;4:605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- 43.Sutoo D.E., Akiyama K. The mechanism by which exercise modifies brain function. Physiology & behavior. 1996;60:177–181. doi: 10.1016/0031-9384(96)00011-x. [DOI] [PubMed] [Google Scholar]
- 44.Greenwood B.N., Foley T.E., Le T.V., Strong P.V., Loughridge A.B., Day H.E. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behavioural Brain Research. 2011;217:354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werme M., Messer C., Olson L., Gilden L., Thoren P., Nestler E.J. Delta FosB regulates wheel running. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelley A.E., Berridge K.C. The neuroscience of natural rewards: relevance to addictive drugs. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gangarossa G., Espallergues J., de Kerchove d'Exaerde A., El Mestikawy S., Gerfen C.R., Herve D. Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Frontiers in Neural Circuits. 2013;7:22. doi: 10.3389/fncir.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kravitz A.V., Freeze B.S., Parker P.R., Kay K., Thwin M.T., Deisseroth K. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nestler E.J. Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang D., Zhang L., Lou D.W., Nakabeppu Y., Zhang J., Xu M. The dopamine D1 receptor is a critical mediator for cocaine-induced gene expression. Journal of Neurochemistry. 2002;82:1453–1464. doi: 10.1046/j.1471-4159.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- 51.Pitchers K.K., Vialou V., Nestler E.J., Laviolette S.R., Lehman M.N., Coolen L.M. Natural and drug rewards act on common neural plasticity mechanisms with DeltaFosB as a key mediator. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2013;33:3434–3442. doi: 10.1523/JNEUROSCI.4881-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis E.J., Harrington C.A., Chikaraishi D.M. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ropelle E.R., Flores M.B., Cintra D.E., Rocha G.Z., Pauli J.R., Morari J. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS Biology. 2010;8 doi: 10.1371/journal.pbio.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Tanaka T., Masuzaki H., Yasue S., Ebihara K., Shiuchi T., Ishii T. Central melanocortin signaling restores skeletal muscle AMP-activated protein kinase phosphorylation in mice fed a high-fat diet. Cell Metabolism. 2007;5:395–402. doi: 10.1016/j.cmet.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Hardie D.G. Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism. The Proceedings of the Nutrition Society. 2011;70:92–99. doi: 10.1017/S0029665110003915. [DOI] [PubMed] [Google Scholar]
- 56.Berglund E.D., Liu T., Kong X., Sohn J.W., Vong L., Deng Z. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nature Neuroscience. 2014 doi: 10.1038/nn.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richter E.A., Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiological Reviews. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 58.Brene S., Bjornebekk A., Aberg E., Mathe A.A., Olson L., Werme M. Running is rewarding and antidepressive. Physiology & behavior. 2007;92:136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adage T., Scheurink A.J., de Boer S.F., de Vries K., Konsman J.P., Kuipers F. Hypothalamic, metabolic, and behavioral responses to pharmacological inhibition of CNS melanocortin signaling in rats. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2001;21:3639–3645. doi: 10.1523/JNEUROSCI.21-10-03639.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fulton S., Pissios P., Manchon R.P., Stiles L., Frank L., Pothos E.N. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Geiger B.M., Haburcak M., Avena N.M., Moyer M.C., Hoebel B.G., Pothos E.N. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–1199. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwartz M.W., Seeley R.J., Woods S.C., Weigle D.S., Campfield L.A., Burn P. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 63.Gilliam P.E., Spirduso W.W., Martin T.P., Walters T.J., Wilcox R.E., Farrar R.P. The effects of exercise training on [3H]-spiperone binding in rat striatum. Pharmacology, Biochemistry, and Behaviour. 1984;20:863–867. doi: 10.1016/0091-3057(84)90008-x. [DOI] [PubMed] [Google Scholar]
- 64.MacRae P.G., Spirduso W.W., Cartee G.D., Farrar R.P., Wilcox R.E. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolite levels. Neuroscience letters. 1987;79:138–144. doi: 10.1016/0304-3940(87)90686-0. [DOI] [PubMed] [Google Scholar]
- 65.Kennedy R.T., Jones S.R., Wightman R.M. Dynamic observation of dopamine autoreceptor effects in rat striatal slices. Journal of Neurochemistry. 1992;59:449–455. doi: 10.1111/j.1471-4159.1992.tb09391.x. [DOI] [PubMed] [Google Scholar]
- 66.Spanagel R., Herz A., Shippenberg T.S. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marsh D.J., Hollopeter G., Huszar D., Laufer R., Yagaloff K.A., Fisher S.L. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nature Genetics. 1999;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 68.Scarpace P.J., Matheny M., Zhang Y. Wheel running eliminates high-fat preference and enhances leptin signaling in the ventral tegmental area. Physiology & behavior. 2010;100:173–179. doi: 10.1016/j.physbeh.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shapiro A., Cheng K.Y., Gao Y., Seo D.O., Anton S., Carter C.S. The act of voluntary wheel running reverses dietary hyperphagia and increases leptin signaling in ventral tegmental area of aged obese rats. Gerontology. 2011;57:335–342. doi: 10.1159/000321343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hommel J.D., Trinko R., Sears R.M., Georgescu D., Liu Z.W., Gao X.B. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 71.Davis J.F., Choi D.L., Schurdak J.D., Fitzgerald M.F., Clegg D.J., Lipton J.W. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biological Psychiatry. 2011;69:668–674. doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shapiro A., Matheny M., Zhang Y., Tumer N., Cheng K.Y., Rogrigues E. Synergy between leptin therapy and a seemingly negligible amount of voluntary wheel running prevents progression of dietary obesity in leptin-resistant rats. Diabetes. 2008;57:614–622. doi: 10.2337/db07-0863. [DOI] [PubMed] [Google Scholar]
- 73.Bi S., Scott K.A., Hyun J., Ladenheim E.E., Moran T.H. Running wheel activity prevents hyperphagia and obesity in Otsuka long-evans Tokushima fatty rats: role of hypothalamic signaling. Endocrinology. 2005;146:1676–1685. doi: 10.1210/en.2004-1441. [DOI] [PubMed] [Google Scholar]
- 74.Meek T.H., Eisenmann J.C., Garland T., Jr. Western diet increases wheel running in mice selectively bred for high voluntary wheel running. International Journal of Obesity (Lond) 2010;34:960–969. doi: 10.1038/ijo.2010.25. [DOI] [PubMed] [Google Scholar]
- 75.Tharp G.D. The role of glucocorticoids in exercise. Medicine and Science in Sports. 1975;7:6–11. [PubMed] [Google Scholar]
- 76.Fediuc S., Campbell J.E., Riddell M.C. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. Journal of Applied Physiology (Bethesda, Md.: 1985) 2006;100:1867–1875. doi: 10.1152/japplphysiol.01416.2005. [DOI] [PubMed] [Google Scholar]
- 77.Adam T.C., Epel E.S. Stress, eating and the reward system. Physiology & Behavior. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 78.Ryan K.K., Mul J.D., Clemmensen C., Egan A.E., Begg D.P., Halcomb K. Loss of melanocortin-4 receptor function attenuates HPA responses to psychological stress. Psychoneuroendocrinology. 2014;42:98–105. doi: 10.1016/j.psyneuen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reinehr T., Hebebrand J., Friedel S., Toschke A.M., Brumm H., Biebermann H. Lifestyle intervention in obese children with variations in the melanocortin 4 receptor gene. Obesity (Silver Spring) 2009;17:382–389. doi: 10.1038/oby.2008.422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.