Abstract
Objective
Leptin modulates food reward via central leptin receptor (LepRb) expressing neurons. Food reward requires stimulation of midbrain dopamine neurons and is modulated by central leptin action, but the exact central mechanisms remain unclear. Stimulatory and inhibitory leptin actions on dopamine neurons have been reported, e.g. by indirect actions on orexin neurons or via direct innervation of dopamine neurons in the ventral tegmental area.
Methods
We showed earlier that LepRb neurons in the lateral hypothalamus (LHA) co-express the inhibitory acting neuropeptide galanin (GAL-LepRb neurons). We studied the involvement of GAL-LepRb neurons to regulate nutrient reward in mice with selective LepRb deletion from galanin neurons (GAL-LepRbKO mice).
Results
We found that the rewarding value and preference for sucrose over fat was increased in GAL-LepRbKO mice compared to controls. LHA GAL-LepRb neurons innervate orexin neurons, but not the VTA. Further, expression of galanin and its receptor GalR1 are decreased in the LHA of GAL-LepRbKO mice, resulting in increased activation of orexin neurons.
Conclusion
We suggest galanin as an important mediator of leptin action to modulate nutrient reward by inhibiting orexin neurons.
Keywords: Sucrose, Intralipid, Incentive runway, Lateral hypothalamus, Locus coeruleus, Two-bottle choice
Highlights
-
•
GAL-LepRbKO shows ↓ galanin and ↓ GalR1 mRNA, ↑ body weight gain.
-
•
GAL-LepRbKO shows ↑ orexin/hypocretin neuronal activation.
-
•
GAL-LepRb neurons innervate local orexin/hypocretin and noradrenergic locus coeruleus neurons.
-
•
Leptin regulates natural reward and body weight via GAL-LepRb neurons.
1. Introduction
Leptin acts centrally via leptin receptor (LepRb) expressing neurons that are found in the hypothalamus and many extra-hypothalamic sites. Recent research has focused on the identification of distinct LepRb populations to understand their contribution to diverse physiological leptin effects, including the ability of leptin to modulate food reward [16,23].
A key component of reward behavior is the mesolimbic dopamine system, which consists of dopaminergic (DA) neurons in the ventral tegmental area (VTA) and their projections to the nucleus accumbens (NAc) as well as other cortico-limbic areas [32,35]. The rewarding value of nutrients, e.g. sucrose or fat, requires activation of midbrain DA neurons and is enhanced by fasting and diminished by leptin [16,18,23], but the precise neuronal mechanisms employed by leptin to reduce nutrient reward are controversial. LepRb is expressed on some midbrain DA neurons, and leptin inhibits these LepRb-DA neurons [30]. Nutrient reward is encoded by NAc dopamine release, so that leptin-inhibited VTA DA neurons could explain a decreased nutrient reward and anorexia. Conversely, NAc DA levels are decreased in severely hyperphagic, leptin deficient ob/ob mice compared to control animals and leptin injections increase NAc DA levels [23]. Thus, NAc DA deficiency may increase motivated behavior, such as food intake, in an attempt to induce DA release and normalize NAc DA levels. This hypothesis is in line with the decreased NAc activity of obese compared to lean humans and shows striking resemblance to the low NAc activity observed in drug addicts [63].
Leptin action in the lateral hypothalamus (LHA) is sufficient to increase NAc DA levels [41] and involves indirect leptin action via orexin neurons [43]. LHA LepRb neurons are distinct from orexin/hypocretin neurons, but they directly innervate orexin/hypocretin neurons [40,43]. Orexin modulates the rewarding value and consumption of sucrose: Central orexin injections promote sucrose intake [24], while genetic or pharmacological blockade of orexin signaling decreases the rewarding value and intake of sucrose [44]. Orexin's effect on reward is mediated via the VTA [27,70], where orexin stimulates DA neurons [47]. Leptin generally inhibits orexin neurons [66], so that leptin inhibition of orexin neurons would be consistent with an inhibitory effect on midbrain DA neurons.
We recently reported that a population of LepRb neurons in the LHA co-expresses the inhibitory acting neuropeptide galanin (GAL-LepRb neurons) and that LHA galanin mRNA (Gal) expression is stimulated by leptin [40]. Central galanin peptide (GAL) injections selectively increase fat intake [33,38], and deficiency in Gal or galanin-receptor-1 (GalR1) results in decreased dietary fat intake [1,34,71]. GAL also modulates reward circuits by inhibition of mesolimbic DA neurotransmission [60], inhibition of noradrenergic LC neurons [54] and counteracts opiate withdrawal behavior [29,52].
We thus hypothesized that GAL-LepRb neurons play a role in nutrient reward and selection, which could lead to changes in body weight. To test this, we studied mice with conditional deletion of LepRb from GAL neurons (GAL-LepRbKO mice). Our data strongly suggest that GAL via GalR1 mediates inhibitory leptin action onto orexin neurons, which could mediate the differential reward modulation of sweet and fatty stimuli.
2. Results
2.1. Generation and validation of GAL-LepRbKO mice
We studied the physiological importance of leptin action in GAL neurons by generating mice with conditional LepRb deletion from GAL neurons (GAL-LepRbKO or KO mice, Figure 1A). Correct cre-expression in GalCre mice was validated in GalYFP reporter mice and compared with galanin mRNA expression from the Allen Brain Atlas (Figure S1) and immunohistochemical staining of galanin peptide (Figure S2). In earlier studies we used transgenic GaltgGFP mice to identify and characterize LHA GAL-LepRb neurons [40]. Thus, we further verified correct reporter gene expression (GFP or YFP) and distribution of GAL-LepRb neurons in GalYFP mice and Gal(-neo)YFP mice compared to GaltgGFP mice. Hypothalamic GalGFP neurons were similarly distributed in all mouse models and as described earlier GAL-LepRb neurons (neurons with GalGFP and leptin-induced phosphorylation of signal-transducer-and-activator-of-transcription-3 (pSTAT3)) were found in the LHA and the nucleus of the solitary tract (NTS; Figure S3A–C). The presence of the neo cassette had no effect on correct GAL-LepRb expression and all following experiments were conducted in GalYFP mice.
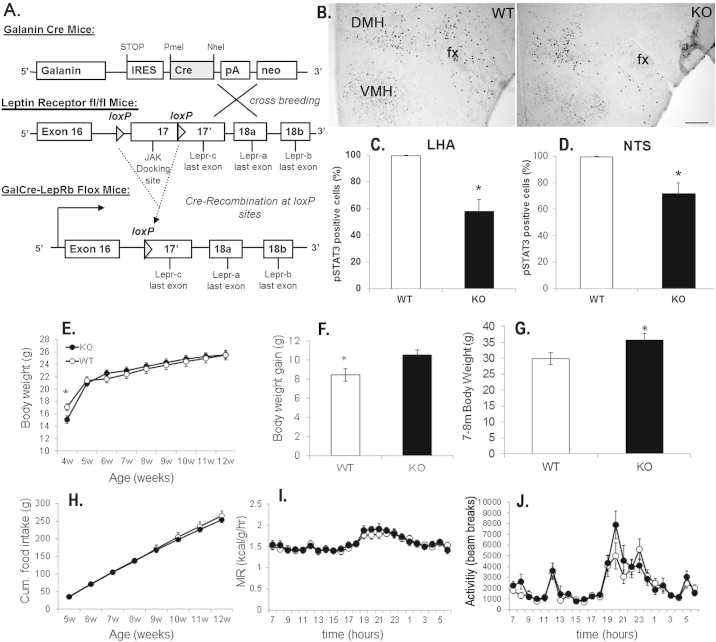
Figure 1.
Increased weight gain and late-onset obesity in KO mice. A. Schematic drawing for the generation of GalCre and KO mice. B–D. Verification of functional LepRb deletion with leptin induced pSTAT3 by immunohistochemistry in the LHA of WT (left) and KO mice (right) and cell counts for pSTAT3 positive nuclei in the LHA (C.) and NTS (D.) (n = 4–8, *pLHA < 0.01, *pNTS < 0.05). Bar size is 200 μm. E. Body weight in WT and KO mice (n = 8–11, *pt-test < 0.05). F. Body weight gain over 8 weeks. G. Increased body weight in aged KO and WT mice (n = 7, pt-test < 0.05). H. Cumulative food intake in WT and KO mice (n = 8–11). I. Metabolic rate in WT and KO mice (n = 8–11). J. Locomotor activity in WT and KO mice (n = 8–11). Gal = galanin; KO = knock out; LepRb = long form leptin receptor; LHA = lateral hypothalamic area; WT = wildtype; pSTAT3 = phosphorylated signal transducer and activator of transcription-3; NTS = nucleus of the solitary tract; fx = fornix.
In KO mice leptin-induced pSTAT3 was significantly decreased in the LHA and NTS compared to control wildtype mice (WT mice) (Figure 1B–D, Figure S4, n = 4–8, pLHA < 0.01, pNTS < 0.05) and confirms the successful deletion of LepRb.
2.2. Increased body weight gain in GAL-LepRbKO mice
The metabolic phenotype of KO mice was assessed from 4 to 12 weeks of age (n = 8–11). At 4 weeks of age, KO mice showed a significantly lower body weight compared to WT mice (Figure 1E, pt-test < 0.05), but at 12 weeks of age body weights were undistinguishable between groups (Figure 1E). However, body weight gain over 8 weeks was significantly increased in KO mice compared to WT mice (Figure 1F, pt-test < 0.02). Furthermore, we found that body weight of KO mice was significantly increased compared to WT mice in a separate cohort of older (7–9 month old) mice (Figure 1G, n = 7, pt-test < 0.05), consistent with an increase in body weight gain. Food intake (Figure 1H), energy expenditure (Figure 1I) or locomotor activity (Figure 1J), measured between 4 and 12 weeks of age, showed no significant difference between groups. At 12 weeks of age we also evaluated fed and fasted blood glucose levels, rectal body temperature and body composition in WT and KO mice with no significant differences between genotypes (Figure S5).
2.3. Decreased LHA galanin signaling is associated with decreased fat intake
Earlier data indicated that leptin induced LHA Gal gene expression [40]. Consistent with this, KO mice showed a 60% decrease in LHA Gal gene expression (Figure 2A, pt-test < 0.01). Furthermore, GalR1 expression, was significantly decreased by 30% (Figure 2B, pt-test < 0.05), while GalR2 and GalR3 expression remained unchanged within the LHA (Figure 2B).
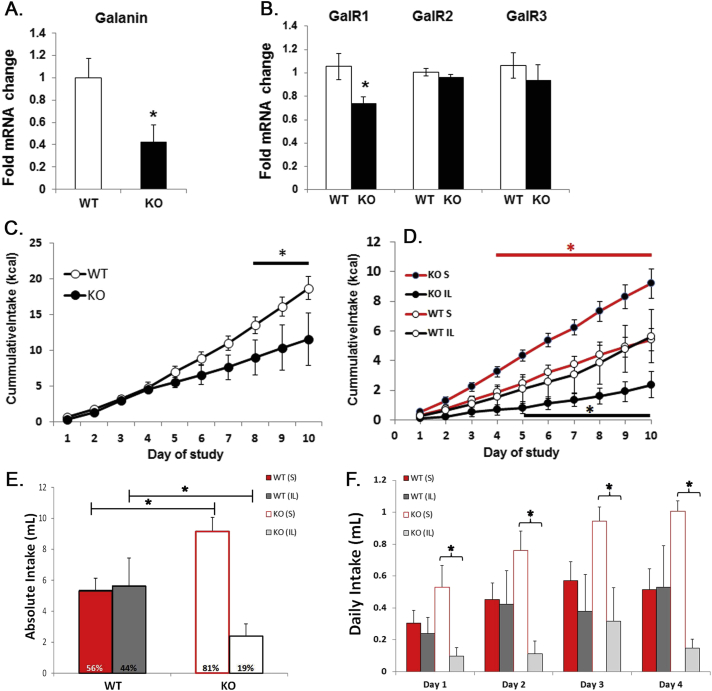
Figure 2.
Altered nutrient reward in KO mice. Gene expression in WT and KO mice (n = 10–13) for LHA galanin (A., *pt-test < 0.01) and LHA galanin receptors (GalR1-3, B., *pt-test < 0.05). C. Cumulative Intralipid (IL) intake in KO and WT mice, 1 h/day access to 10% Intralipid over 10 days (n = 5; pANOVA < 0.001; *pHolm-Sidak < 0.05–0.001). D. Cumulative sucrose (S) and Intralipid intake KO and WT mice, 1 h/day access to a-choice of 10% Intralipid and 25% sucrose solutions (both 1 kcal/ml) over 10 days (n = 5–7; pANOVA < 0.0001; *pHolm-Sidak < 0.05–0.001). E. Total sucrose and Intralipid intake over 10 days in KO mice, while WT mice (n = 5–7, pANOVA < 0.001; * pHolm-Sidak < 0.01). F. Daily intake of sucrose and Intralipid during the first 4 days shows that sucrose preference is prevalent on day 1 in naïve KO mice (pANOVA < 0.05; *pHolm-Sidak < 0.02). WT = wildtype; KO = knock out, LHA = lateral hypothalamic area.
GAL mainly acts as an inhibitory neuropeptide via GalR1 and selectively modulates the ingestion of fat [59], suggesting that decreased Gal and GalR1 mRNA can affect fat intake. To test this, naïve KO and WT mice had access to a 10% Intralipid solution (1 kcal/ml) for 1 h per day over 10 days. Intralipid solution is highly palatable for mice and the amount ingested correlates with the rewarding value of the solution [18,53]. As expected, both WT and KO mice increased their Intralipid consumption over 10 consecutive 1 h sessions. However, over 10 days KO mice consumed significantly less Intralipid solution compared to WT mice (Figure 2C; n = 5; pANOVA < 0.001; *pHolm-Sidak < 0.05–0.001). These data are consistent with the fat intake inducing effect of GAL and indicate a reduced reward value for Intralipid solution in KO mice.
Next we tested whether the altered reward behavior was unique to fat or if other palatable nutrients like sucrose were also less rewarding. In another cohort of naïve mice (n = 5–7) we offered a choice of isocaloric (1 kcal/ml) 25% sucrose and 10% Intralipid solutions over 10 consecutive days. Again, KO mice consumed less Intralipid than WT mice, but strikingly KO mice consumed more sucrose solution compared to WT mice (Figure 2D; n = 5–7; pANOVA < 0.0001; *pHolm-Sidak < 0.05–0.001). In fact, while WT mice ingested equal amounts of Intralipid and sucrose solutions and had no preference for either solution, KO showed a robust and significant preference for sucrose over Intralipid (Figure 2E, n = 5–7, pANOVA < 0.001; *pHolm-Sidak < 0.01). Therefore, lack of LepRb in GAL neurons causes a differential modulation of sucrose and fat preference. The sucrose preference in KO mice was present from the first day of exposure to the solutions (Figure 2F, pANOVA < 0.05; *pHolm-Sidak < 0.02), suggesting that this nutrient selection is not due to a learned behavior.
2.4. GAL-LepRbKO mice work more for sugar rewards
We further tested whether or not the increased sucrose preference affects their motivation to work for food rewards using the incentive runway [51,55]. Mice have to run a straight path with increasing length (up to 75 cm) to obtain a visible food reward.
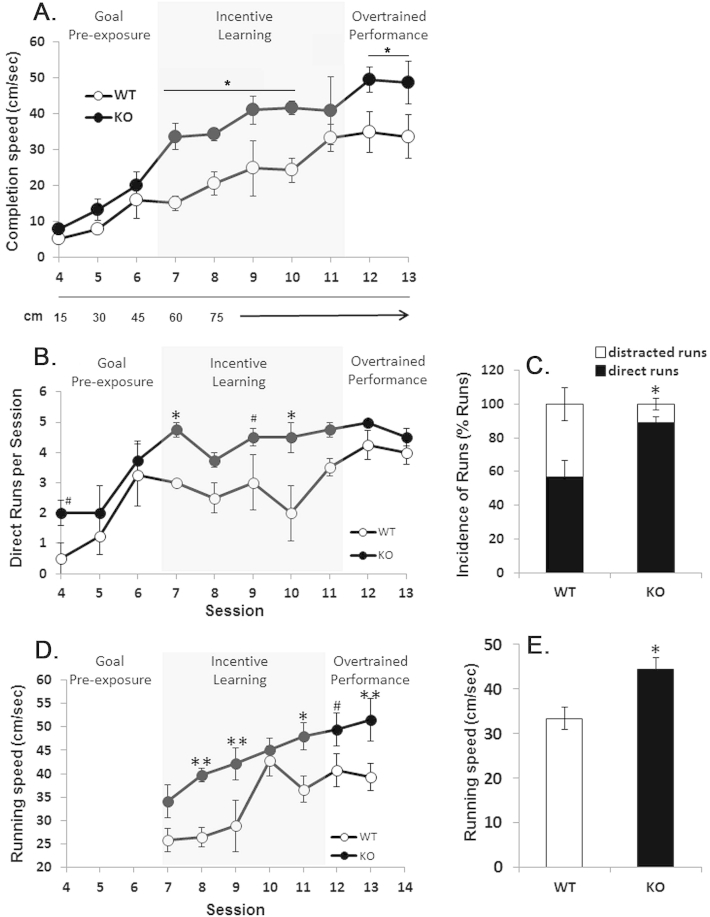
Indeed, KO mice showed an increased completion speed to obtain the sweet (fat free) treat (Figure 3A, n = 4; pANOVA < 0.05; *pHolm-Sidak < 0.05–0.01). Further analysis revealed that KO had more incidences of direct, undistracted runs, while WT mice showed significantly more runs with distractions such as pauses, falters and reversals (Figure 3B, C and videos S1, S2; n = 4; pANOVA < 0.05; pHolm-Sidak < *0.03, # = 0.06; pt-test < 0.03, respectively). In addition, KO also ran faster to obtain the treat compared to WT mice (Figure 3D, E and videos S1, S2; n = 4; pANOVA < 0.03; pHolm-Sidak < *0.03, ** < 0.02, # = 0.08; pt-test < 0.03, respectively).
Figure 3.
KO mice work more for sugar rewards. Incentive runway testing of WT and KO mice (n = 4 each genotype) for completion speed (cm/sec = runway length [cm]/time from leaving start box till entering goal box [sec]) (A. pANOVA < 0.05; *pHolm-Sidak < 0.05–0.01), the number of direct runs (in contrast to distracted runs) (B., C.; pANOVA < 0.05; pHolm-Sidak<*0.03, # = 0.06; pt-test < 0.03, respectively) and running speed (mean completion speed from all direct runs) (D., E.; pANOVA < 0.03; pHolm-Sidak<*0.03, ** < 0.02, # = 0.08; pt-test < 0.03, respectively). Each session consisted of 5 trials and sessions that were held every other day. WT = wildtype; KO = knock out.
The following are the supplementary data related to this article:
WT mouse in an incentive runway trial at session 9.
KO mouse in an incentive runway trial at session 9.
2.5. GAL-LepRb neurons innervate orexin neurons
We further aimed to identify the anatomical sites and neurons that were innervated by both LHA GAL and LepRb neurons in order to understand how GAL-LepRb neurons may regulate food reward.
Projections of LepRb and GAL neurons were identified by injecting the adenoviral construct Ad-iZ/EGFPf [41] into the LHA of LepRbCre (n = 4) or GalCre mice (n = 3), which resulted in a cre-specific expression of a farnesylated enhanced green fluorescent protein (EGFPf) and a non-cre dependent β-galactosidase expression from all adenovirus infected cells (Figure 4A–F). The farnesylation anchors EGFPF to the cell membrane and enhances the visualization of thin axonal processes; the β-galactosidase expression is used to track the spread of overall virally infected cells. Correctly targeted Ad-iZ/EGFPf injection into the LHA of LepRbCre (Figure 4A) or GalCre mice (Figure 4D) was verified by confined EGFPf (green) and β-galactosidase (red) expression to LHA. EGFPf positive processes from LHA LepRb and GAL neurons were found locally within the LHA (Figure 4A, D, respectively). We further confirmed that LHA LepRb neurons, strongly innervate the VTA (Figure 4B, H) [41,42], but LHA GAL neurons rarely projected to the VTA (Figure 4E, K), suggesting that GAL-LepRb neurons do not innervate the VTA. Instead, both – LHA LepRb and GAL neurons – strongly innervated the locus coeruleus (LC) (Figure 4C, F, respectively), a site that had not yet been associated with leptin function.
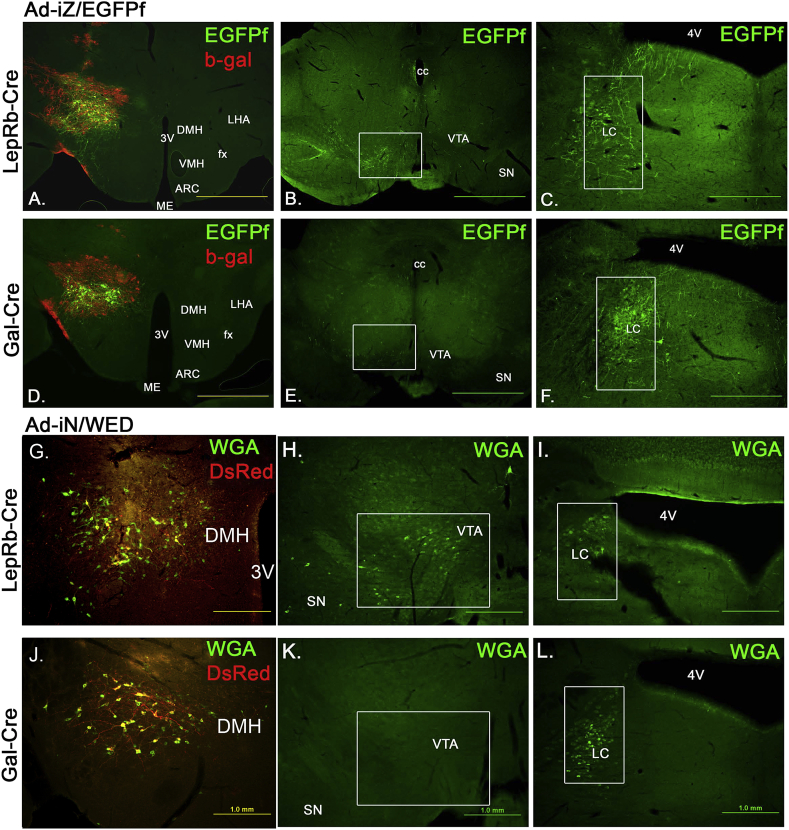
Figure 4.
GAL-LepRb neurons project to the LC, but not the VTA. A. Overview of LHA Ad-iZ/EGFPf injections into the LHA of LepRbCre mice, the spread of infected neurons is shown in red (β-gal expression), virally infected neurons with cre-recombination are shown in green (representing LepRb neurons). B/C. EGFPf projections from LHA LepRb-cre neuron to the VTA (B.) or LC (C.). D. Overview of LHA Ad-iZ/EGFPf injections into the LHA of GalCre mice, the spread of infected neurons is shown in red (β-gal expression), virally infected neurons with cre-recombination are shown in green (representing GAL neurons). E./F. LHA GAL neurons do not project into the VTA (E.), but strongly innervate the LC (F.). G. Injection of Ad-iN/WED into the LHA of LepRbCre mice (n = 7) with infected first order neurons in yellow, co-localization of DsRed (red) and wheat germ agglutinin (WGA, green) and second order neurons in green.(WGA). H./I. Second order neurons with single WGA labeling (green) in the VTA (H.) and LC (I). J–L. Injection of Ad-iN/WED into the LHA of GalCre mice (n = 6) shows local first order neurons (yellow) and second order neurons (green) in the LHA (J.) and second order neurons in the LC (L.), but not the VTA (K.). Areas of interest are highlighted with white boxes. Gal = galanin; LepRb = long form leptin receptor; LC = locus coeruleus; VTA = ventral tegmental area; LHA = lateral hypothalamic area; Ad = adenovirus; EGFPf = farnesylated enhanced green fluorescent protein; β-gal = β-galactosidase.
In a separate cohort of LepRbCre (n = 7) or GalCre (n = 4) mice, we injected another adenoviral construct, Ad-iN/WED [43], that resulted in cre-dependent expression of wheat germ agglutinin (WGA) and the fluorescent protein DsRed. WGA can be transported anterogradely and transsynaptically into 2nd order neurons, while DsRed remains in 1st order neurons (here LepRb or GAL neurons) (Figure 4G–L).
LHA Ad-iN/WED injections in LepRbCre or GalCre mice, resulted in WGA labeling within the LHA, where 1st order neurons are identified by WGA/DsRed co-expression (= yellow neurons) and 2nd order neurons by single WGA labeling (= green neurons) (Figure 4G, J). Several 2nd order neurons were found in the LHA indicating that GAL-LepRb neurons project locally onto LHA neurons.
We further observed many 2nd order neurons in the LC labeled from LHA GAL neurons (Figure 4L), while 2nd order labeling from LHA LepRb neurons (Figure 4I) was less strong and not observed in all cases of injected animals. LC neurons do not express LepRb as shown in a LepRbGFP reporter mouse with prominent GFP labeling in the hypothalamus, but complete absence of GFP labeling within the LC of the same animal (Figure S6A, B, respectively). We further confirmed the projections of GAL-LepRb neurons to the LC with injections of the retrograde tracer fluorogold (FG) into the LC of GalYFP mice. GAL is densely co-expressed with noradrenergic LC neurons as indicated by co-labeling of GalGFP with tyrosine hydroxylase (TH) (Figure S6C), so that the GalGFP signal (green label) served as an excellent visual guide for LC neurons to verify the accuracy of FG injections (red label) (Figure S6A). We found many triple labeled FG/pSTAT3/GalGFP neurons in the LHA surrounding the fornix (Figure S6B), which showed that many GAL-LepRb neurons innervate the LC. Furthermore, GAL neurons derived WGA labeled LC neurons indeed represent noradrenergic TH-positive neurons (Figure 5A).
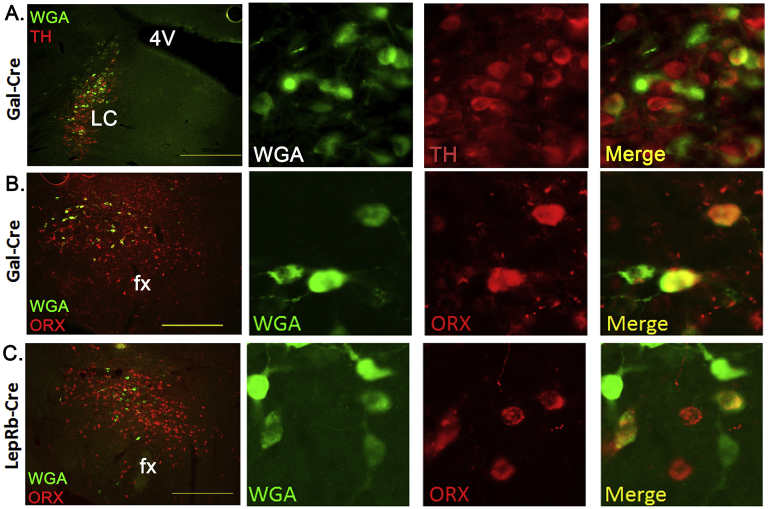
Figure 5.
GAL-LepRb neurons innervate orexin neurons and noradrenergic LC neurons. A/B. GalCre mice with LHA Ad-iN/WED injections show that within the LC WGA neurons co-express tyrosine hydroxylase (TH), depicting noradrenergic neurons (A) and within the LHA many WGA neurons are co-expressed with orexin (B). C. LepRbCre mice with LHA Ad-iN/WED injections show that many LHA WGA neurons are co-expressed with orexin. Bar size is 1 mm (Figure 5B and C) and 500 μm (Figure 5A). Gal = galanin; LepRb = long form leptin receptor; LC = locus coeruleus; LHA = lateral hypothalamic area; Ad = adenovirus; WGA = wheat germ agglutinin; ORX = orexin.
LepRb neurons in the LHA innervate local orexin/hypocretin neurons [43]. Similarly, we found that WGA labeled 2nd order neurons from LHA GAL and LepRb neurons co-labeled with orexin/hypocretin (Figure 5B, C, respectively). Importantly, neither LepRb nor GAL neurons co-localize with orexin/hypocretin [40], thus further supporting that GAL-LepRb neurons innervate orexin/hypocretin neurons.
2.6. Galanin mediated inhibition of orexin neurons
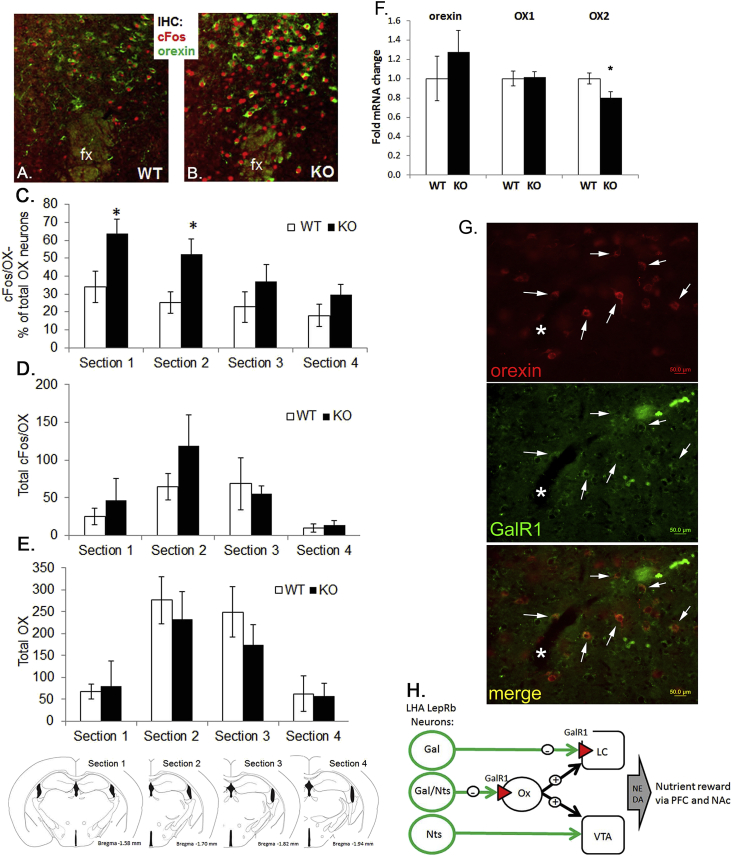
LHA Gal and GalR1 expression was decreased in KO mice (Figure 2A, B) and GAL acts via GalR1 to potently inhibit neuronal excitation in many neuronal systems, including the hypothalamus [4,19,20]. We hypothesized that GAL may mediate the inhibitory actions of leptin on orexin neurons. Therefore, we predicted an increased activation of orexin neurons in KO mice. We analyzed KO and WT brains for basal levels of cFos (as a surrogate for neuronal activation) in orexin neurons and indeed found increased cFos/orexin co-localization, which was most prominent in the rostral portion of orexin/hypocretin neurons (Figure 6A–E, n = 5, pANOVA < 0.003,*pHolm-Sidak < 0.05). This was due to an increased number of cFos/ox neurons (Figure 6D), while the total number of orexin neurons was unchanged between groups (Figure 6E). Enhanced cFos expression was restricted to the LHA, while other adjacent sites, e.g. the DMH, showed similar cFos expression (Figure S8). Further, orexin gene expression was similar in KO and WT mice (Figure 6F). However, the gene expression of LHA orexin receptor Ox2, but not Ox1, was significantly decreased (Figure 6F, *pt-test < 0.02), indicating that within the LHA Ox1 signaling would be preferentially enhanced. We further determined that orexin neurons were co-labeled with GalR1 (Figure 6G), demonstrating that orexin neurons have the molecular capability to respond to GAL (Figure 6H).
Figure 6.
Increased activation of orexin neurons in KO mice. A–B. Immunohistochemical staining for cFos (red) and orexin (green). The fornix (fx) is shown as a landmark for the location within the LHA. C. Percentage of orexin neurons that co-express cFos, in comparison to the total number of cFos/orexin neurons (D.) and total number of orexin neurons (E.). F. Quantification of LHA orexin mRNA and its receptors OX1 and OX2 (n = 10–13; *pt-test < 0.02). G. Immunohistochemical staining for orexin (red) and GalR1 (green). H. Schematic summary of distinct LHA LepRb neurons and their projections. KO = knock out; GalR1 = galanin receptor 1; LHA = lateral hypothalamic area; OX1 and OX2 = orexin receptor 1 and 2.
3. Discussion
Our data demonstrate that leptin action via GAL neurons regulates nutrient reward. Dysregulation of this system by deleting LepRb from GAL neurons caused increased reward value and consumption for sucrose, while fat consumption was decreased.
Our data uncover a novel neuronal circuit where LHA GAL-LepRb neurons directly innervate orexin neurons as well as noradrenergic locus coeruleus neurons. In contrast to LHA LepRb neurons, LHA galanin neurons and therefore GAL-LepRb neurons do not innervate the VTA directly.
Leptin stimulates LHA Gal gene expression, while lack of LepRb in GAL neurons decreased LHA Gal gene expression. GAL is an inhibitory neuropeptide and we further speculate that leptin inhibits orexin neurons via GAL→GalR1 signaling to modulate nutrient reward and body weight.
3.1. Leptin and regulation of VTA DA neurons
Central leptin controls nutrient reward via interactions with VTA dopaminergic neurons [18,22,23], but the exact circuits involved are unclear. There is an ongoing controversy regarding the stimulatory or inhibitory effect of leptin on DA neurons. Some VTA dopamine neurons express LepRb and leptin inhibits these DA-LepRb neurons [30], suggesting that leptin decreases food reward to mediate its anorexigenic effects.
In contrast, the lack of leptin in ob/ob mice results in decreased DA content within the NAc. Peripheral, central and intra-LHA leptin injections increase NAc DA content and increases tyrosine-hydroxylase expression in DA neurons, indicating a stimulatory leptin effect on DA neurons [23,41]. This is consistent with the DA deficiency theory, which argues that a low DA content would enhance behaviors like feeding, aimed at increasing NAc DA content and avoiding potentially unpleasant effects of low NAc DA levels [8]. Thus, inhibitory and stimulatory leptin effects on DA neurons are plausible and may both contribute to the modulation of food reward.
The presented data demonstrate that sucrose preference further translates into enhanced work for a sweet treat in KO mice. KO mice demonstrate increased reward value for sucrose and sugar, indicated by increased sucrose consumption and incentive runway performance. Orexin action is well known to modulate the rewarding value of food, by inducing NAc DA release via activation of VTA DA neurons [61,62]. This enhances the motivation to work for sucrose reward, which is blocked by orexin receptor inhibitors [5,12,27,44]. Thus, the increased sucrose reward value in KO mice can be explained by the observed increase in baseline activation of orexin neurons.
Leptin inhibits orexin neurons, possibly via inhibitory GABAergic LHA LepRb neurons [43]. In addition, our data strongly support a role of GAL to mediate inhibitory leptin effects onto orexin neurons: 1) LHA GAL and LepRb neurons innervate local orexin neurons 2) Leptin regulates LHA Gal gene expression, with leptin being sufficient to increase LHA Gal mRNA [40] and LepRb expression on GAL neurons being necessary to maintain normal LHA Gal mRNA expression. 3) GalR1 mRNA, the predominant receptor responsible for inhibitory GAL actions [4,20], is decreased in the LHA of KO mice. 4) GalR1 is co-expressed with orexin neurons. 5) KO mice show an increased percentage of activated orexin neurons. Indeed, a recent study confirmed in slice preparations that GAL inhibits orexin neurons [26]. Thus, our study is the first to highlight a direct interaction of LHA galanin neurons with orexin neurons and we speculate that the inhibitory acting neuropeptide GAL could modulate food reward by inhibiting orexin neurons.
LHA LepRb neurons also express the inhibitory neurotransmitter GABA and innervate the VTA and local orexin neurons [41,43]. Our study confirms LHA LepRb → VTA projections, but LHA GAL neurons do not innervate the VTA. Therefore, we conclude that LHA GAL-LepRb neurons are distinct from LHA LepRb → VTA projecting neurons.
A subset of LHA LepRb neurons and LHA GAL neurons co-express the neuropeptide neurotensin (Nts) [40]. LHA neurotensin neurons, but not LHA GAL neurons, project directly to the VTA, thus we conclude that LHA Nts-LepRb → VTA projecting neurons are distinct from GAL-LepRb neurons.
LHA Nts-LepRb neurons [42] and GAL-LepRb neurons both innervate orexin neurons, further suggesting that LHA LepRb→orexin projecting neurons may indeed co-express GAL and neurotensin. However, orexin neurons do not express neurotensin receptors [49], while we found GalR1co-expressed with orexin neurons and GalR1 mRNA down-regulation in KO mice. Thus, we further suggest that GAL, not neurotensin, regulates orexin neurons and that this involves inhibitory GAL actions via GalR1.
We found a strong projection of GAL-LepRb neurons to the LC. LHA Nts neurons do not project to the LC [42]; and personal communication with Dr. MG Myers); thus, we further conclude that GAL-LepRb → LC projecting neurons are distinct from LHA Nts neurons. We have further clarified this classification of LHA LepRb populations in Figure 6H.
3.2. Fat intake and galanin
Selective deletion of LepRb in GAL neurons caused a decrease in fat intake, which cannot be explained by anorexigenic leptin action. The concept that GAL controls macronutrient selection was first introduced by Leibowitz and colleagues, who found that central GAL injections selectively increased fat intake, while carbohydrate and protein intake remained unchanged [59]. The reported effects of GAL on macronutrient selection were small and varied dependent on the initial nutrient preferences [56,57]. More recently, GAL's effect on fat intake was further supported in GAL deficient mice, which ate less of a high fat diet compared to WT mice [1,34]. Similarly, GalR1 null mice consume less high fat diet [71], therefore low Gal and GalR1 mRNA expression in the LHA of GAL-LepRbKO mice could sufficiently explain the observed decrease in fat consumption. Furthermore, in a macronutrient choice paradigm Gal-KO mice preferred carbohydrates over fat [1]. This effect is very similar to the observed sucrose preference over Intralipid in GAL-LepRbKO mice and strongly suggests that GAL indeed mediates the differential effects on sucrose and fat intake in GAL-LepRbKO mice.
Similar to sucrose, fat rich solutions like Intralipid are highly rewarding for rodents and similarly involve reward circuits that are associated with DA release [36,58]. Thus, a differential regulation of sucrose and fat reward value cannot be explained exclusively by a regulation of DA neurons and suggests that other reward circuits are modulated in KO mice.
Nutrient reward is also modulated by the opioid system, a pathway that interacts with the dopaminergic system as well as other central sites. Importantly, activation or inhibition of μ-opioid receptors (MOR) in the NAc of rats robustly modulates fat intake, while chow intake remains unchanged and sucrose intake is only minimally affected [64,65,67,68]. Indeed, several studies found that fat intake stimulates the endogenous opioid enkephalin and Gal expression simultaneously [6], and GAL induced fat intake is prevented by selective blockade of MOR [7]. Thus, it may be possible that sucrose intake can be regulated by the enhanced orexin release in the VTA, while fat intake remains suppressed due to the blunted GAL action on MOR. Further studies will need to address these distinct aspects of nutrient reward.
3.3. Leptin and galanin interaction with the locus coeruleus
GAL-LepRb neurons strongly innervate noradrenergic neurons in the LC. The LC is the major projection sites of orexin neurons and orexin and LC neurons both control arousal and reward [9–11]. The LC is the sole NE source for many central sites (e.g. prefrontal cortex, PFC), while other sites (e.g. hypothalamus) receive additional NE input from the brainstem (NTS). Noradrenergic LC neurons do not express LepRb, and whether leptin regulates LC function has not been investigated to our knowledge. However, leptin injections decrease and leptin deficiency increases hypothalamic NE levels (even though the source of NE was unclear in these studies), while central blockade of NE signaling substantially decreases hyperphagia in leptin deficient mice [13–15,37].
In line with this, chemical deletion of noradrenergic LC fibers, but not noradrenergic NTS fibers, results in decreased food and sucrose consumption [2] as well as impaired incentive runway behavior [3], further suggesting that LC neurons could at least contribute to the observed sucrose preference and rewarding value of sweet treats in KO mice. The functional connectivity of orexin and LC neurons has been convincingly shown for arousal behavior and may be reflected in the improved runway behavior, where KO mice are less distracted during trials.
The LC is clearly an important mediator of opioid signaling and best known for its role in physical dependence and opiate withdrawal behavior (recently reviewed in [45]). Importantly, orexin and GAL have reciprocal effects on opiate withdrawal, which further supports an inhibitory role of GAL for orexin neurons. GAL decreases opiate withdrawal behavior via GalR1 [28,29], while orexin increases opiate withdrawal behavior [25]. Thus, future experiments will have to further depict if the LC indeed plays a role in GAL-LepRb mediated nutrient reward.
In summary, our data highlight an important role of GAL-LepRb neurons in nutrient reward. We show evidence that galanin mediates the inhibitory actions of leptin on orexin neurons via GalR1 and suggest that this contributes to the differential regulation in the rewarding value of sucrose and Intralipid.
4. Material and methods
4.1. Animals
Mice were bred and housed at 22 °C on a 12-hour light/dark cycle. Food and water were available ad libitum unless otherwise specified. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center. Hemizygous BAC transgenic GalTgGFP mice with green fluorescent protein (GFP) expression under the control of the Gal promoter (GalTgGFP, Stock Tg(Gal-EGFP)109Gsat, #0163420UCD) were obtained from the Mutant Mouse Regional Resource Center (MMRRC, http://www.mmrrc.org), a NCRR-NIH funded strain repository, and was donated to the MMRRC by the NINDS funded GENSAT BAC transgenic project (http://www.gensat.org).
4.2. Generation of GalCre mice
An IRES-cre sequence was inserted into the murine Gal gene between the Stop codon and polyadenylation site; an frt-flanked neo cassette was inserted downstream of the polyadenylation sites. The construct was electroporated into mouse ES cells (albino C57/B6 background), screened for construct insertion and injection into blastocysts to generate chimeras. Chimeras were bred to C57/B6 and germ line transmission was determined by coat color. Correct genotypes and 5’-recombination was confirmed by PCR with transgene spanning amplicons (fwd: 5′-TTG AAA CCT GCC CTG ACT CTC AGC A, reverse: 5′-AGG GAA ACC GTT GTG GTC TGA CTA) and 3′-recombianation (fwd: 5′- CCA TCA GAA GCT GAC TCT AGC GAA, rev: 5′-CTT GCT AGC TCT TCC CCA ACT CTA).
4.3. Experimental mice
GalCre mice were crossed with LepRbfl/fl mice ([46] and kindly provided by Dr. Streamson Chua) to generate Leprfl/fl mice (referred to as WT mice) and Galcre/+; Leprfl/fl mice (referred to as GAL-LepRbKO or KO mice). GalYFP reporter mice were generated by breeding with B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J (stock#006148) and compared to transgenic GaltgGFP mice, which we used earlier to identify and characterize GAL-LepRb neurons in the LHA [40]. In some mice the frt-flanked neo cassette was removed from the genome by crossing GalYFP mice with 129S4/SvJaeSor-Gt(ROSA)26Sortm1(FLP1)Dym/J mice (stock#003946) (Gal(-neo)YFP reporter mice) to prove that reporter expression pattern was not compromised by the presence of the neo cassette.
Genotyping of experimental animals was performed by PCR: GalCre (cre primers: 5′-CCT CTC CCA AGC GGC CGG AGA ACC (fwd), 5′- CCG GCT CCG TTC TTT GGT GGC CCC TTC GCG (rev); wt-cre primers: 5′-TCC TGA GAC CAT GTC CAC TG (fwd), 5′-CTG CCA CTC CTG TGA TCT GA (rev)); Leprfl (mLepr106: 5′-GTC TGA TTT GAT AGA TGG TCT T (fwd), mLepr105: 5′-ACA GGC TTG AGA ACA TGA ACA (fwd), mLepr65A: 5′-AGA ATG AAA AAG TTG TTT TGG (rev)). We used the latter primers also to screen for potential germline Lepr excision as described elsewhere [39]; such animals were excluded from breeding and experiments.
4.4. Metabolic phenotyping
Male KO and WT littermates (n = 11–15) were individually housed at 4 weeks of age; food intake and body weight was measured weekly until twelve weeks. At 12 weeks of age body composition was determined by NMR (minispec-mq series Bruker Billerica), fed and over-night fasted blood glucose was evaluated with a glucometer (One Touch Ultra Mini). After that mice were acclimated to the Comprehensive Laboratory Animal Monitoring System (CLAMS, Columbus Instruments) for 3 days and energy expenditure and locomotor activity was recorded continuously over 4 days. In another cohort of 7–9 month old, male KO and WT littermates (n = 7) body weight was again evaluated. Differences between groups were statistically analyzed with a repeated measure 2-way ANOVA or a Student's t-test.
4.5. Microdissection and qPCR
Microdissection was performed as described earlier [21] and visual landmarks, such as the mammillothalamic tract, fornix and the third ventricle were used to strategically dissect the LHA. RNA was prepared following manufacturers recommendations (ToTALLY RNA kit, Ambion) and 350 ng RNA was converted to cDNA (RETROscript Kit, Ambion). For qPCR 10.5 ng cDNA was used in triplicates to analyze multiple TaqMan assays (Applied Biosystems): Gapdh (housekeeping gene; Mm99999915_g1), Galanin (Mm01236508_m1), GalR1 (Mm00433515_m1), GalR2 (Mm00726392_s1), and GalR3 (Mm00443617_m1), orexin (Mm04210469_m1), OX1 (Mm01185776_m1), OX2 (Mm01179312_m1). Reactions were pipetted by a robotic liquid handling system (Perkin Elmer Multiprobe II EX) and run on the 7500 HT Fast Real-Time PCR System (Applied Biosystems). Fold induction of gene expression was calculated using the ΔΔCT method as recommended for TaqMan assays.
4.6. Nutrient preference and reward
To test the consumption of palatable fat we provided a fat emulsion (10% Intralipid, Baxter) in a sipper bottle for 1 h per day over 10 days to naïve WT and KO mice (n = 4–5) and compare their cumulative fat intake using a repeated measure 2-way ANOVA. Similarly, we tested their preferences for isocaloric solutions (1 kcal/ml) of sucrose (25% sucrose) and Intralipid (10%) in a two-bottle-choice test in naïve WT and KO mice (n = 5–9), again with 1 h daily access over 10 days; bottle positions were alternated every other day to prevent side preferences. Statistical differences were analyzed via 3-way repeated-measure ANOVA and post-hoc analysis.
WT and KO mice were further tested in an incentive runway [51]. Briefly, mice (n = 4) were food restricted (ad lib food restricted from 12:00–4:00 p.m.) and allowed overnight access to the sugar treat (Froot Loops®, Kellogg's) to avoid neophobic responses. Incentive runway training was performed every other day for a total of 13 training sessions. Each session consisted of 5 runway trials (between 8:00a.m. and 12:00p.m.), sessions 1–3 were strictly acclimation trials with access to the goal box + treat. Session 4 included a 15 cm runway distance from the start to the goal box. With each subsequent session the runway length was increased in 15 cm increments until reaching a maximal distance of 75 cm and mice could consume the treat for 30 s after fulfilling their task. Session were recorded and analyzed in slow motion to determine completion speed (time from leaving the start box to reaching the goal box [sec]/runway length [cm]), running speed (=completion speed of direct runs in seconds/cm), number of direct and distracted runs (distractions = pauses, falters and reversals). Differences between groups were analyzed by repeated measure 3-way ANOVA (completion speed over time) or Student's t-test for two-group comparison.
4.7. Viral tracing studies
Stereotaxic surgeries were performed as reported earlier [40]. The LHA was targeted at coordinates: X −0.9 mm, Y −1.3 mm, Z −5.2 mm relative to Bregma according to the Paxinos Mouse Brain Atlas [50]. Two adenoviral constructs (Ad-IZ/EGFPf and Ad-iN/WED; described in detail earlier [41,43]) were acutely injected into the LHA of GalCre or LepRbCre mice. Ad-iZ/EGFPf results in cre-inducible expression of farnesylated EGFP and visualizes cell bodies and processes of cre-expressing neurons, the viral injection spread can be monitored by β-galactosidase expression in non-cre expressing neurons. Ad-iN/WED results in cre-inducible expression of DsRed and wheat germ agglutinin (WGA). WGA is anterogradely and transsynaptically transported into second order neurons, while DsRed remain in first order cre-expressing neurons. Deeply anesthetized mice received 250–350 nl adenovirus (10 nl/20 s, 2–6 × 1012 PFU/ml) unilaterally. After 7–10 days incubation time mice were perfused and brains extracted for histological analysis.
Leptin-induced phosphorylation of signal-tranducer-and-activator-of-transcription-3 (pSTAT3):
The histochemical visualization of LepRb expressing neurons was done by detection of leptin-induced pSTAT3, which is a signaling target of LepRb and has been shown by us and others as an excellent marker of functional LepRb neurons in the hypothalamus [17,21,31,48]. Mice received a bolus injection of leptin (5 mg/kg i.p.) to induce phosphorylation of STAT3, a downstream signaling target of LepRb. Mice were perfused 1 h post-injection and brains were extracted for further histological analysis as described earlier [69].
4.8. Immunohistochemistry
Free-floating immunohistochemistry was done after pretreatment (1% peroxide in ice cold methanol for 20 min, 0.3% glycine in phosphate buffered saline (PBS) for 10 min and 0.03% SDS in PBS for 10 min) and blocking (3% normal donkey serum in PBS/0.25% Triton X-100 for 1–3 h). All primary and secondary antibodies were diluted in blocking solutions. Primary antibodies were incubated at 4 °C at least over night or for 48 h and secondary antibodies were incubated at room temperature for 2 h. For rabbit anti-pSTAT3 (1:1000, Cell Signaling) and rabbit anti-cFos (1:5000, Millipore/Calbiochem) staining was developed with ABC/3,3′ diaminobenzidine tetrahydrochloride (Pierce DAB Substrate Kit, ThermoScientific) as described earlier [69]. Double labeling was done with either DsRed (1:1000, rabbit anti-DsRed, Santa Cruz), WGA (1:1000, goat anti-WGA, Vector Laboratories), GFP (1:000, chicken anti-GFP; AbCAM), or orexin/hypocretin-A (1:1000, goat anti-orexin/hypocretin-A, Santa Cruz) was visualized with fluorophor-labeled secondary antibodies (1:200 Alexa 568 or 488, Invitorgen or 1:400 Dylite, Jackson ImmunoResearch). Sections were mounted onto gelatin-subbed slides and cover slipped with ProLong Anti-fade mounting medium (Invitrogen) for further microscopy analysis.
4.9. Estimate of cell counts
For quantification of pSTAT3 in the LHA and NTS from leptin treated WT and KO mice (n = 4–8 per genotype) 2 sections per anatomical location were imaged using the Olympus BX51 brightfield microscope and Olympus DP30BW digital camera. Signals were amplified by identical changes in brightness and contrast for all analyzed brain sections. The number of pSTAT3 positive cell nuclei was quantified and compared between groups with a Student's t-test.
WT and KO mice (n = 5 per genotype) we analyzed for baseline cFos in LHA orexin neurons. Four sections containing the entire population of LHA orexin neurons were identified for each animal, organized anatomically in a rostral to caudal manner and the total number of orexin neurons and the number of cFos expressing orexin neurons were counted (similar as described previously [40]). Differences between groups were evaluated with a 2-way ANOVA and posthoc-test.
4.10. Statistic
Statistical analysis was done with SPSS Statistics (repeated measure 3-way ANOVA) (IBM, Armonk, NY) or SigmaPlot 11.2 (all other statistics) (Systat Sofware Inc, San Jose, CA). The individual tests used are noted with the according experiments. Significant differences were accepted at a p-value < 0.05.
Acknowledgments
This work was supported by AHA053298N, P/F DK020572-30, P20 RR02195, P/F NORC #2P30-DK072476-06 (HM) and 2T32 DK064584 (EQC). This work utilized the facilities of the Cell Biology and Bioimaging Core, supported in part by COBRE (NIH P20-RR021945) and CNRU (NIH 1P30-DK072476) center grants from the National Institutes of Health. Partial support was provided through the Animal Phenotyping Core supported through NIDDK NORC Center Grant #s2P30 DK072476 entitled ‘Nutritional Programming: Environmental and Molecular Interactions’ at the Pennington Biomedical Research Center, and the Islet Cell Biology Core of the DRTC at the University of Chicago (DK020595) for generation of adenoviral vectors.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2015.07.002.
Conflict of interest
All authors declare no conflict of interest.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Adams A.C., Clapham J.C., Wynick D., Speakman J.R. Feeding behaviour in galanin knockout mice supports a role of galanin in fat intake and preference. Journal of Neuroendocrinology. 2008;20:199–206. doi: 10.1111/j.1365-2826.2007.01638.x. [DOI] [PubMed] [Google Scholar]
- 2.Ammar A.A., Sodersten P., Johnson A.E. Locus coeruleus noradrenergic lesions attenuate intraoral intake. Neuroreport. 2001;12:3095–3099. doi: 10.1097/00001756-200110080-00023. [DOI] [PubMed] [Google Scholar]
- 3.Anlezark G.M., Crow T.J., Greenway A.P. Impaired learning and decreased cortical norepinephrine after bilateral locus coeruleus lesions. Science. 1973;181:682–684. doi: 10.1126/science.181.4100.682. [DOI] [PubMed] [Google Scholar]
- 4.Anselmi L., Stella S.L., Jr., Brecha N.C., Sternini C. Galanin inhibition of voltage-dependent Ca(2+) influx in rat cultured myenteric neurons is mediated by galanin receptor 1. Journal of Neuroscience Research. 2009;87:1107–1114. doi: 10.1002/jnr.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baird J.P., Choe A., Loveland J.L., Beck J., Mahoney C.E., Lord J.S. Orexin-A hyperphagia: hindbrain participation in consummatory feeding responses. Endocrinology. 2009;150:1202–1216. doi: 10.1210/en.2008-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barson J.R., Chang G.Q., Poon K., Morganstern I., Leibowitz S.F. Galanin and the orexin 2 receptor as possible regulators of enkephalin in the paraventricular nucleus of the hypothalamus: relation to dietary fat. Neuroscience. 2011;193:10–20. doi: 10.1016/j.neuroscience.2011.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton C., York D.A., Bray G.A. Opioid receptor subtype control of galanin-induced feeding. Peptides. 1996;17:237–240. doi: 10.1016/0196-9781(95)02103-5. [DOI] [PubMed] [Google Scholar]
- 8.Berthoud H.R. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Current Opinion in Neurobiology. 2011;21:888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourgin P., Huitron-Resendiz S., Spier A.D., Fabre V., Morte B., Criado J.R. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. Journal of Neuroscience. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter M.E., Brill J., Bonnavion P., Huguenard J.R., Huerta R., de L.L. Mechanism for hypocretin-mediated sleep-to-wake transitions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2635–E2644. doi: 10.1073/pnas.1202526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter M.E., de L.L., Adamantidis A. Functional wiring of hypocretin and LC-NE neurons: implications for arousal. Frontiers in Behaviour Neuroscience. 2013;7:43. doi: 10.3389/fnbeh.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cason A.M., Aston-Jones G. Attenuation of saccharin-seeking in rats by orexin/hypocretin receptor 1 antagonist. Psychopharmacology (Berl) 2013;228:499–507. doi: 10.1007/s00213-013-3051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currie P.J., Wilson L.M. Bidirectional effects of clonidine on carbohydrate intake in genetically obese (ob/ob) mice. Pharmacology Biochemistry and Behavior. 1991;38:177–184. doi: 10.1016/0091-3057(91)90607-4. [DOI] [PubMed] [Google Scholar]
- 14.Currie P.J., Wilson L.M. Yohimbine attenuates clonidine-induced feeding and macronutrient selection in genetically obese (ob/ob) mice. Pharmacology Biochemistry and Behavior. 1992;43:1039–1046. doi: 10.1016/0091-3057(92)90478-x. [DOI] [PubMed] [Google Scholar]
- 15.Currie P.J., Wilson L.M. Potentiation of dark onset feeding in obese mice (genotype ob/ob) following central injection of norepinephrine and clonidine. European Journal of Pharmacology. 1993;232:227–234. doi: 10.1016/0014-2999(93)90778-g. [DOI] [PubMed] [Google Scholar]
- 16.Davis J.F., Choi D.L., Schurdak J.D., Fitzgerald M.F., Clegg D.J., Lipton J.W. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biological Psychiatry. 2011;69:668–674. doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhillon H., Zigman J.M., Ye C., Lee C.E., McGovern R.A., Tang V. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Domingos A.I., Vaynshteyn J., Voss H.U., Ren X., Gradinaru V., Zang F. Leptin regulates the reward value of nutrient. Nature Neuroscience. 2011;14:1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Y., Tyszkiewicz J.P., Fong T.M. Galanin and galanin-like peptide differentially modulate neuronal activities in rat arcuate nucleus neurons. Journal of Neurophysiology. 2006;95:3228–3234. doi: 10.1152/jn.01117.2005. [DOI] [PubMed] [Google Scholar]
- 20.Endoh T., Sato D., Wada Y., Shibukawa Y., Ishihara K., Hashimoto S. Galanin inhibits calcium channels via Galpha(i)-protein mediated by GalR1 in rat nucleus tractus solitarius. Brain Research. 2008;1229:37–46. doi: 10.1016/j.brainres.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 21.Faouzi M., Leshan R., Bjornholm M., Hennessey T., Jones J., Munzberg H. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology. 2007;148:5414–5423. doi: 10.1210/en.2007-0655. [DOI] [PubMed] [Google Scholar]
- 22.Figlewicz D.P., Bennett J.L., Naleid A.M., Davis C., Grimm J.W. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiology & Behavior. 2006;89:611–616. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Fulton S., Pissios P., Manchon R.P., Stiles L., Frank L., Pothos E.N. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Furudono Y., Ando C., Yamamoto C., Kobashi M., Yamamoto T. Involvement of specific orexigenic neuropeptides in sweetener-induced overconsumption in rats. Behaviournal Brain Research. 2006;175:241–248. doi: 10.1016/j.bbr.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Georgescu D., Zachariou V., Barrot M., Mieda M., Willie J.T., Eisch A.J. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. Journal of Neuroscience. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goforth P.B., Leinninger G.M., Patterson C.M., Satin L.S., Myers M.G., Jr. Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA-independent mechanisms. Journal of Neuroscience. 2014;34:11405–11415. doi: 10.1523/JNEUROSCI.5167-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris G.C., Wimmer M., Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 28.Hawes J.J., Narasimhaiah R., Picciotto M.R. Galanin attenuates cyclic AMP regulatory element-binding protein (CREB) phosphorylation induced by chronic morphine and naloxone challenge in Cath.a cells and primary striatal cultures. Journal of Neurochemistry. 2006;96:1160–1168. doi: 10.1111/j.1471-4159.2005.03613.x. [DOI] [PubMed] [Google Scholar]
- 29.Holmes F.E., Armenaki A., Iismaa T.P., Einstein E.B., Shine J., Picciotto M.R. Galanin negatively modulates opiate withdrawal via galanin receptor 1. Psychopharmacology (Berl) 2012;220:619–625. doi: 10.1007/s00213-011-2515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hommel J.D., Trinko R., Sears R.M., Georgescu D., Liu Z.W., Gao X.B. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 31.Hosoi T., Kawagishi T., Okuma Y., Tanaka J., Nomura Y. Brain stem is a direct target for leptin's action in the central nervous system. Endocrinology. 2002;143:3498–3504. doi: 10.1210/en.2002-220077. [DOI] [PubMed] [Google Scholar]
- 32.Ikemoto S., Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Research. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 33.Karatayev O., Baylan J., Leibowitz S.F. Increased intake of ethanol and dietary fat in galanin overexpressing mice. Alcohol. 2009;43:571–580. doi: 10.1016/j.alcohol.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karatayev O., Baylan J., Weed V., Chang S., Wynick D., Leibowitz S.F. Galanin knockout mice show disturbances in ethanol consumption and expression of hypothalamic peptides that stimulate ethanol intake. Alcoholism, Clinical and Experimental Research. 2010;34:72–80. doi: 10.1111/j.1530-0277.2009.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelley A.E., Baldo B.A., Pratt W.E., Will M.J. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiology & Behavior. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 36.Kraft T.T., Yakubov Y., Huang D., Fitzgerald G., Acosta V., Natanova E. Dopamine D1 and opioid receptor antagonism effects on the acquisition and expression of fat-conditioned flavor preferences in BALB/c and SWR mice. Pharmacology, Biochemistry and Behavior. 2013;110:127–136. doi: 10.1016/j.pbb.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Kutlu S., Aydin M., Alcin E., Ozcan M., Bakos J., Jezova D. Leptin modulates noradrenaline release in the paraventricular nucleus and plasma oxytocin levels in female rats: a microdialysis study. Brain Research. 2010;1317:87–91. doi: 10.1016/j.brainres.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 38.Kyrkouli S.E., Stanley B.G., Seirafi R.D., Leibowitz S.F. Stimulation of feeding by galanin: anatomical localization and behavioral specificity of this peptide's effects in the brain. Peptides. 1990;11:995–1001. doi: 10.1016/0196-9781(90)90023-x. [DOI] [PubMed] [Google Scholar]
- 39.Lam D.D., Leinninger G.M., Louis G.W., Garfield A.S., Marston O.J., Leshan R.L. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metabolism. 2011;13:584–591. doi: 10.1016/j.cmet.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laque A., Zhang Y., Gettys S., Nguyen T.A., Bui K., Morrison C.D. Leptin receptor neurons in the mouse hypothalamus are co-localized with the neuropeptide galanin and mediate anorexigenic leptin action. American Journal of Physiology, Endocrinology and Metabolism. 2013 doi: 10.1152/ajpendo.00643.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leinninger G.M., Jo Y.H., Leshan R.L., Louis G.W., Yang H., Barrera J.G. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metabolism. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leinninger G.M., Opland D.M., Jo Y.H., Faouzi M., Christensen L., Cappellucci L.A. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metabolism. 2011;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louis G.W., Leinninger G.M., Rhodes C.J., Myers M.G., Jr. Direct innervation and modulation of orexin neurons by lateral hypothalamic LepRb neurons. Journal of Neuroscience. 2010;30:11278–11287. doi: 10.1523/JNEUROSCI.1340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuo E., Mochizuki A., Nakayama K., Nakamura S., Yamamoto T., Shioda S. Decreased intake of sucrose solutions in orexin knockout mice. Journal of Molecular Neuroscience: MN. 2011;43:217–224. doi: 10.1007/s12031-010-9475-1. [DOI] [PubMed] [Google Scholar]
- 45.Mazei-Robison M.S., Nestler E.J. Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harbor Perspectives in Medicine. 2012;2:a012070. doi: 10.1101/cshperspect.a012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMinn J.E., Liu S.M., Dragatsis I., Dietrich P., Ludwig T., Eiden S. An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mammalian Genome: Official Journal of the International Mammalian Genome Society. 2004;15:677–685. doi: 10.1007/s00335-004-2340-1. [DOI] [PubMed] [Google Scholar]
- 47.Moorman D.E., Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. Journal of Neuroscience. 2010;30:15585–15599. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munzberg H., Huo L., Nillni E.A., Hollenberg A.N., Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- 49.Opland D., Sutton A., Woodworth H., Brown J., Bugescu R., Garcia A. Loss of neurotensin receptor-1 disrupts the control of the mesolimbic dopamine system by leptin and promotes hedonic feeding and obesity. Molecular Metabolism. 2013;2:423–434. doi: 10.1016/j.molmet.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paxinos G., Franklin K.B.J. Elsevier Science; USA: 2004. The mouse brain in stereotaxic coordinates. [Google Scholar]
- 51.Pecina S., Cagniard B., Berridge K.C., Aldridge J.W., Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. Journal of Neuroscience. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Picciotto M.R., Hawes J.J., Brunzell D.H., Zachariou V. Galanin can attenuate opiate reinforcement and withdrawal. Neuropeptides. 2005;39:313–315. doi: 10.1016/j.npep.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Sclafani A. Fat and sugar flavor preference and acceptance in C57BL/6J and 129 mice: experience attenuates strain differences. Physiology & Behavior. 2007;90:602–611. doi: 10.1016/j.physbeh.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Seutin V., Verbanck P., Massotte L., Dresse A. Galanin decreases the activity of locus coeruleus neurons in vitro. European Journal of Pharmacology. 1989;164:373–376. doi: 10.1016/0014-2999(89)90481-0. [DOI] [PubMed] [Google Scholar]
- 55.Shin A.C., Townsend R.L., Patterson L.M., Berthoud H.R. “Liking” and “wanting” of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2011;301:R1267–R1280. doi: 10.1152/ajpregu.00314.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith B.K., Berthoud H.R., York D.A., Bray G.A. Differential effects of baseline macronutrient preferences on macronutrient selection after galanin, NPY, and an overnight fast. Peptides. 1997;18:207–211. doi: 10.1016/s0196-9781(96)00318-x. [DOI] [PubMed] [Google Scholar]
- 57.Smith B.K., York D.A., Bray G.A. Effects of dietary preference and galanin administration in the paraventricular or amygdaloid nucleus on diet self-selection. Brain Research Bulletin. 1996;39:149–154. doi: 10.1016/0361-9230(95)02086-1. [DOI] [PubMed] [Google Scholar]
- 58.Tellez L.A., Medina S., Han W., Ferreira J.G., Licona-Limon P., Ren X. A gut lipid messenger links excess dietary fat to dopamine deficiency. Science. 2013;341:800–802. doi: 10.1126/science.1239275. [DOI] [PubMed] [Google Scholar]
- 59.Tempel D.L., Leibowitz K.J., Leibowitz S.F. Effects of PVN galanin on macronutrient selection. Peptides. 1988;9:309–314. doi: 10.1016/0196-9781(88)90265-3. [DOI] [PubMed] [Google Scholar]
- 60.Tsuda K., Tsuda S., Nishio I., Masuyama Y., Goldstein M. Effects of galanin on dopamine release in the central nervous system of normotensive and spontaneously hypertensive rats. American Journal of Hypertension. 1998;11:1475–1479. doi: 10.1016/s0895-7061(98)00168-x. [DOI] [PubMed] [Google Scholar]
- 61.Vittoz N.M., Berridge C.W. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- 62.Vittoz N.M., Schmeichel B., Berridge C.W. Hypocretin/orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. European Journal of Neuroscience. 2008;28:1629–1640. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang G.J., Volkow N.D., Thanos P.K., Fowler J.S. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. Journal of Addictive Diseases. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- 64.Will M.J., Franzblau E.B., Kelley A.E. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. Journal of Neuroscience. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Will M.J., Pratt W.E., Kelley A.E. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiology & Behavior. 2006;89:226–234. doi: 10.1016/j.physbeh.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 66.Yamanaka A., Beuckmann C.T., Willie J.T., Hara J., Tsujino N., Mieda M. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 67.Zhang M., Gosnell B.A., Kelley A.E. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. Journal of Pharmacology and Experimental Therapeutics. 1998;285:908–914. [PubMed] [Google Scholar]
- 68.Zhang M., Kelley A.E. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y., Kerman I.A., Laque A., Nguyen P., Faouzi M., Louis G.W. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. Journal of Neuroscience. 2011;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng H., Patterson L.M., Berthoud H.R. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. Journal of Neuroscience. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zorrilla E.P., Brennan M., Sabino V., Lu X., Bartfai T. Galanin type 1 receptor knockout mice show altered responses to high-fat diet and glucose challenge. Physiology & Behavior. 2007;91:479–485. doi: 10.1016/j.physbeh.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
WT mouse in an incentive runway trial at session 9.
KO mouse in an incentive runway trial at session 9.