Abstract
The default mode network (DMN) is suggested to play a pivotal role in schizophrenia; however, the dissociation pattern of functional connectivity of DMN subsystems remains uncharacterized in this disease. In this study, resting-state fMRI data were acquired from 55 schizophrenic patients and 53 matched healthy controls. DMN connectivity was estimated from time courses of independent components. The lateral DMN exhibited decreased connectivity with the unimodal sensorimotor cortex but increased connectivity with the heteromodal association areas in schizophrenics. The increased connectivity between the lateral DMN and right control network was significantly correlated with negative and anergia factor scores in the schizophrenic patients. The anterior and posterior DMNs exhibited increased and decreased connectivity with the right control and lateral visual networks, respectively, in schizophrenics. The altered DMN connectivity may underlie the hallucinations, delusions, thought disturbances, and negative symptoms involved in schizophrenia. Furthermore, DMN connectivity patterns could be used to differentiate patients from controls with 76.9% accuracy. These findings may shed new light on the distinct role of DMN subsystems in schizophrenia, thereby furthering our understanding of the pathophysiology of schizophrenia. Elucidating key disease-related DMN subsystems is critical for identifying treatment targets and aiding in the clinical diagnosis and development of treatment strategies.
Schizophrenia is a psychotic disorder that impairs multiple cognitive domains, including perception, memory, attention, and executive function, as evidenced by delusions, hallucinations, disorganized speech and thought formation, social withdrawal, gross disorganization, and other negative symptoms1. To date, the causes and mechanisms of schizophrenia remain unclear. However, it has been proposed that the pathophysiology of schizophrenia is associated with the dysfunctional integration of distributed neuronal networks rather than with the breakdown in the function of a single discrete brain region2,3.
Recently, the default mode network (DMN) has attracted increasing attention in the study of psychiatric disorders4, including schizophrenia5,6,7,8. The DMN, which is primarily composed of the medial prefrontal cortex, posterior cingulate cortex/precuneus, bilateral inferior parietal lobule, and temporal cortex9,10, has been suggested to subserve internal mentation11, including autobiographical memory, future planning, theory of mind, self-reference, and affective decision making12,13. Based on resting-state functional magnetic resonance imaging (fMRI), Mingoia et al. reported increased functional connectivity within the DMN in schizophrenics14. Using working memory-related paradigms, several task-related fMRI studies demonstrated that deficits in working memory in schizophrenics may be related to the abnormal activity and connectivity of the DMN7,15,16. Furthermore, investigation of schizophrenics before and after olanzapine treatment demonstrated that this treatment may be associated with the modulation of DMN connectivity17 and that functional abnormalities in the DMN were correlated with structural changes in schizophrenic patients18. In addition to the intra-default connectivity (i.e., within the DMN), inter-default functional connectivity abnormalities were observed between the DMN and ventral attention and frontoparietal control networks in schizophrenic patients relative to healthy controls19,20, suggesting a central role for the DMN in schizophrenia.
The DMN is not as homogenous as previously assumed; it can be divided into several distinct functional subcomponents (or subnetworks) in the human brain21,22,23,24. Previous work supports the hypothesis that anterior DMN is mainly involved in self-referential processing, while the posterior DMN is involved in familiarity and/or autobiographical memory25,26. In particular, several resting-state fMRI studies have demonstrated a dissociation in DMN functional connectivity in psychiatric and neurologic disorders, including depression27 and Alzheimer’s disease28. Damoiseaux and colleagues showed decreased connectivity in the posterior DMN but increased connectivity in the anterior and ventral DMN in patients with Alzheimer’s disease28. Li et al. demonstrated that changes in functional connectivity in the posterior DMN could be normalized with antidepressant treatment, while abnormal functional connectivity persisted within the anterior DMN in depression27. The DMN has been extensively investigated in schizophrenia, but it is unknown whether there is a dissociation pattern within the DMN subnetwork that is correlated with the disorder. In particular, the dissociation of functional connectivity between DMN subsystems and all other intrinsic connectivity networks (ICNs) in schizophrenia remains uncharacterized. Repetitive transcranial magnetic stimulation, as a non-invasive brain stimulation technique, is useful for treating a variety of neuropsychiatric disorders (including schizophrenia29) but lacks target specificity. Separating key disease-related default mode subsystem from other areas in schizophrenics may provide useful information for the identification of treatment targets and the development of treatment strategies, while shedding light on the pathophysiology of the disorder.
In the present study, we used group independent component analysis (ICA) with default parameter settings to extract the ICNs (referred to as “temporal coherent networks” in group ICA) and corresponding time courses from resting-state fMRI data collected from 55 schizophrenic patients and 53 matched healthy controls6. Through this analysis, we identified three DMN subcomponents (anterior, posterior, and lateral ones) and 11 other ICN components relevant in schizophrenia. We then explored intra-default connectivity by estimating the temporal correlation between the ICA component time courses corresponding to DMN subcomponents and the inter-default connectivity by estimating the temporal correlation between DMN subcomponent time courses and the time courses of all other ICNs (i.e., components). Next, group differences in intra- and inter-default connectivity between schizophrenic patients and healthy controls were identified by statistical analysis. In addition, we grouped the ICNs into unimodal sensorimotor cortical networks supporting low-level unimodal processing (i.e., the somatomotor, motor, auditory, and visual cortices) and heteromodal association cortical networks supporting high-level cognitive processing (i.e., the default mode, precuneus, control, and attention networks)24 and performed group-level comparison. Finally, we used multivariate pattern analysis methods combining the linear support vector machine (SVM) with local linear embedding30 to differentiate schizophrenic patients from healthy controls based on DMN functional network connectivity, thus lending support to the hypothesis that there is a dissociation pattern in functional connectivity between DMN subsystems and other ICNs in schizophrenia.
Results
Identification of ICNs
Fourteen functionally relevant ICNs were identified by group ICA analysis according to the previous studies23,24, as shown in Supplementary Fig. 1 (one-sample t-test, P < 0.05, FWE corrected). Three components, or subnetworks, of the DMN were identified: an anterior subnetwork with a correlation (over voxels) of 0.36 (P < 0.001) with the DMN template; a posterior subnetwork with a correlation of 0.19 (P < 0.001); and a lateral subnetwork with a correlation of 0.38 (P < 0.001) (Supplementary Fig. 1). The corresponding time courses of the three subnetworks are shown in Fig. 1. The anterior DMN had the highest amplitude in the medial prefrontal cortex, the posterior DMN had the highest amplitude in the posterior cingulate cortex/precuneus, and the lateral DMN had the highest amplitude in the bilateral temporal cortex. The three networks are spatially independent and their time courses are asynchronous.
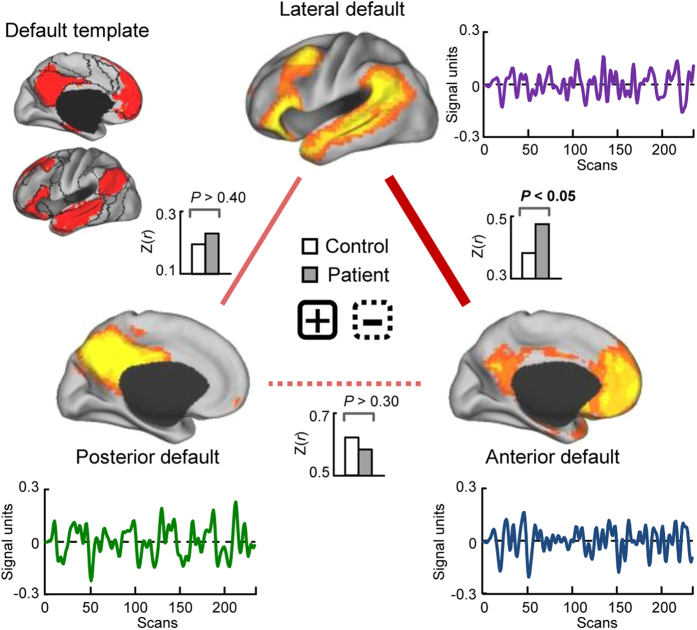
Figure 1. Comparison of intra-default functional connectivity between schizophrenic patients and healthy controls (two-sample t-test, P < 0.05, uncorrected).
The upper left panel presents the default mode network (DMN) template based on prior parcellation of the cerebrum22. The spatial expression and corresponding time courses of the DMN subnetworks for the two groups are also presented (one-sample t-test, P < 0.05, FWE corrected). The anterior DMN mainly consists of the medial prefrontal cortex and portions of the posterior cingulate cortex and bilateral parietal cortex. The posterior DMN consists predominantly of the bilateral precuneus and posterior cingulate cortex, as well as part of the bilateral parietal cortex. The lateral DMN primarily consists of the bilateral parietal cortex, bilateral temporal cortex, and part of the lateral prefrontal cortex. It was observed that positive functional connectivity between the lateral and anterior DMNs was increased in schizophrenic patients relative to healthy controls (P < 0.05, uncorrected), and in non-medicated patients (n = 34) relative to healthy controls (P < 0.05, uncorrected). The red lines represent positive functional connectivity. The solid and dashed lines represent an increase and decrease in schizophrenic patients relative to healthy controls, respectively. CARET software (CARET; http://brainvis.wustl.edu) was used for surface rendering.
Intra-default functional connectivity
The intra-default functional connectivity was calculated for each subject, and the correlation value between each pair of the DMN subnetwork was positive and significant for both control and patient groups (Supplementary Fig. 2; one-sample t-test, P < 0.05, FDR corrected). Two-sample t-tests revealed that the functional connectivity between the lateral and anterior DMNs was significantly increased in schizophrenic patients relative to healthy controls (Fig. 1, P < 0.05, uncorrected) as well as in non-medicated patients (n = 34) relative to healthy controls (P < 0.05, uncorrected). No significant difference was observed in intra-default functional connectivity between non-medicated and medicated patients.
Inter-default functional connectivity
Taking each DMN subnetwork as a seed network, the inter-default functional connectivity was calculated between seed networks and other ICNs for each subject. The results of one-sample t-tests for each group can be observed in Supplementary Figs 3–5 (P < 0.05, FDR corrected). Two-sample t-tests revealed that the functional connectivity between the lateral DMN and right frontoparietal control (positive) and dorsal attention networks (negative) was significantly enhanced in schizophrenic patients relative to healthy controls (Fig. 2a, P < 0.05, FDR corrected), while the functional connectivity (positive) between the lateral DMN and somatomotor, motor, lateral visual and auditory cortices was significantly reduced in schizophrenic patients (Fig. 2a, P < 0.05, FDR corrected). Meanwhile, the functional connectivity between the anterior DMN and right frontoparietal control network (positive) was significantly increased in schizophrenic patients (Fig. 2b, P < 0.05, FDR corrected), while the functional connectivity between the posterior DMN and lateral visual cortex (positive) was significantly decreased in schizophrenic patients (Fig. 2c, P < 0.05, FDR corrected). All connections, as shown in Fig. 2, were significantly altered in non-medicated patients (n = 34) relative to healthy controls (P < 0.05, uncorrected). In a comparison between medicated and non-medicated patients, two negative connections were significantly increased in medicated patients relative to non-medicated patients (P < 0.05, FDR corrected): those between the anterior DMN and precuneus and between the anterior DMN and dorsal attention network.
Figure 2. Functional connectivity was significantly altered between the default mode subnetworks and other intrinsic connectivity networks in schizophrenic patients relative to healthy controls (two-sample t-test, P < 0.05, FDR corrected).
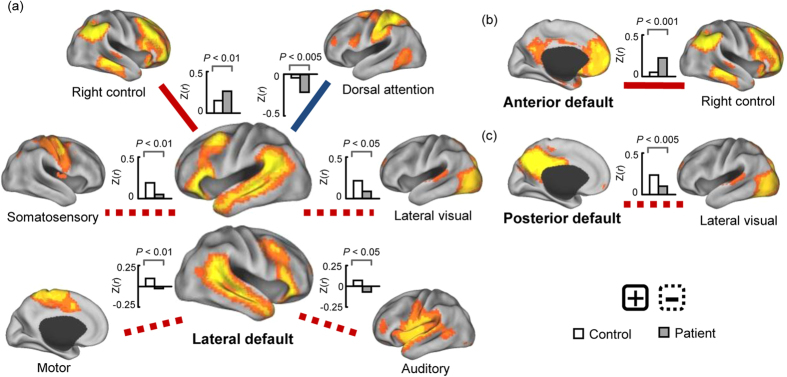
The connections, as shown, were also significantly altered in non-medicated patients (n = 34) relative to healthy controls (two-sample t-test, P < 0.05, uncorrected). The red and blue lines represent positive and negative functional connectivity, respectively. The solid and dashed lines represent an increase and decrease in schizophrenic patients relative to healthy controls, respectively. CARET software (CARET; http://brainvis.wustl.edu) was used for surface rendering.
When the 14 ICNs were grouped into unimodal sensorimotor cortical networks (i.e., somatomotor, motor, auditory, and visual cortices) and heteromodal association cortical networks (i.e., default mode, precuneus, control, and attention networks)24, we observed that functional connectivity was significantly decreased between the lateral DMN and unimodal cortical networks in both non-medicated and medicated patients, while functional connectivity was significantly increased between lateral DMN and heteromodal cortical networks in non-medicated patients only (Fig. 3a, P < 0.05, uncorrected). In addition, functional connectivity between the anterior DMN and heteromodal cortical networks was significantly increased in both non-medicated and medicated patients (Fig. 3b, P < 0.05, uncorrected). No significant functional connectivity difference was detected between the posterior DMN and unimodal and heteromodal cortical networks (Fig. 3c, P < 0.05, uncorrected), and no significant difference in functional connectivity was observed between medicated and non-medicated patients (P < 0.05, uncorrected).
Figure 3. The dissociation pattern of the inter-default functional connectivity within unimodal and heteromodal cortical networks (two-sample t-test, P < 0.05, uncorrected).
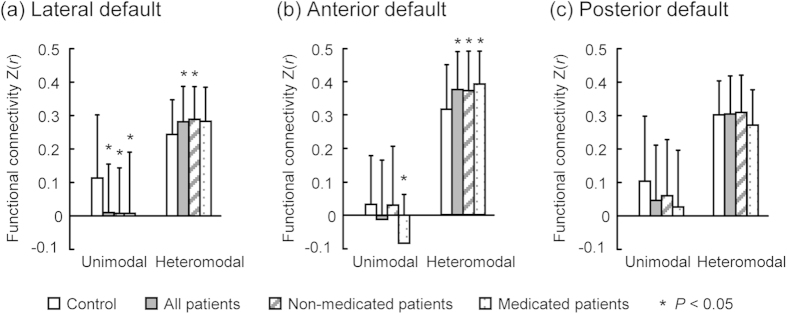
Note that functional connectivity was significantly decreased between the lateral default mode network (DMN) and unimodal cortical networks in both non-medicated and medicated patients, while it was increased between the lateral DMN and heteromodal cortical networks in the non-medicated patients only (a). The functional connectivity between the anterior DMN and heteromodal cortical networks was significantly increased in both non-medicated and medicated patients (b). No significant difference in functional connectivity was detected between the posterior DMN and unimodal and heteromodal cortical networks (c), and no significant difference in the aforementioned functional connectivity was observed between the medicated and non-medicated patients.
Clinical correlation analysis
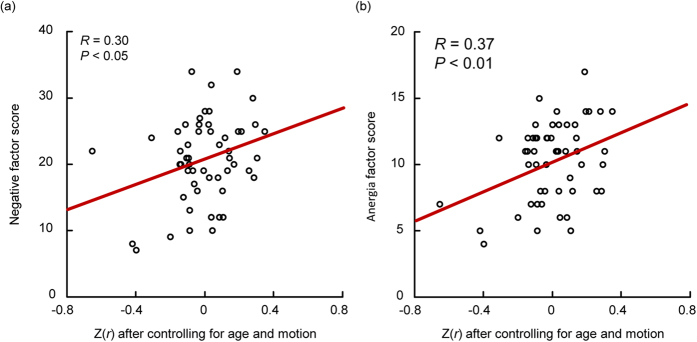
The functional connectivity between lateral DMN and right frontoparietal control network was positively correlated with Positive and Negative Syndrome Scale (PANSS) negative factor scores (R = 0.30, P < 0.05, uncorrected) and anergia factor scores (R = 0.37, P < 0.01, uncorrected), after controlling for age and head motion (Fig. 4) and when thepoints corresponding to the patient with largest PANSS total score (208≫ mean score) and lowest PANSS positive factor score (7≪ mean score) were treated as ‘outliers’ and were removed.
Figure 4. The functional connectivity between the lateral default mode and right frontoparietal control networks is significantly correlated with PANSS negative factor scores (a, R = 0.30, P < 0.05) and anergia factor scores (b, R = 0.37, P < 0.01) in schizophrenic patients.
Classification results
Classification results demonstrated that anterior, posterior, and lateral DMN functional network connectivity, respectively, could be used to differentiate schizophrenic patients from healthy controls with peak accuracies of 71.3% (69.1% for patients and 73.6% for controls; P < 0.0001), 63.9% (61.8% for patients and 66.0% for controls; P < 0.005), and 73.2% (74.6% for patients and 71.7% for controls; P < 0.0001) via leave-one-out cross-validation; the corresponding averaged classification rates of the training datasets were 78.6%, 67.3%, and 77.0%, respectively. When the functional network connectivity of all the three subnetworks was taken together as classification features, an accuracy of 76.9% (80.0% for patients and 73.6% for controls; P < 0.0001) was achieved, and the corresponding averaged classification rates of the training datasets were 78.6%.
Discussion
In this study, we investigated the functional network connectivity of DMN subnetworks in schizophrenic patients using group ICA of resting-state fMRI scans. Three components, or subnetworks, associated with the DMN were identified: the anterior, posterior and lateral DMNs. We observed that the intra-default functional connectivity between the anterior and lateral DMNs was significantly increased in schizophrenic patients relative to healthy controls. The calculation of inter-default functional correlation revealed that the lateral DMN exhibited decreased functional connectivity with unimodal cortical networks and increased functional connectivity with heteromodal cortical networks in the patient group. The increased functional connectivity between the lateral DMN and right control network was significantly correlated with PANSS negative and anergia factor scores in schizophrenic patients. Furthermore, the functional connectivity between the anterior DMN and right control network and between the posterior DMN and lateral visual network were significantly increased and decreased in schizophrenics, respectively. In addition, DMN functional network connectivity could be used to differentiate patients from controls with an accuracy of 76.9%. These findings provide new insight into the distinct roles of DMN subsystems in schizophrenia, thereby developing our understanding of the pathophysiology of schizophrenia. Differentiating key disease-related DMN subsystems from those not involved is critical for identifying treatment targets and the developing treatment strategies for schizophrenia.
The DMN has been suggested to underlie external environment monitoring, spontaneous cognition, and autobiographical thinking12,13. General symptoms of schizophrenia include delusion, autistic thinking, and the splitting of autobiographical thought, therefore suggesting that dysfunction of the DMN may play a role in the disorder. In the current study, the DMN was separated into the lateral, anterior, and posterior subsystems, which exhibit distinct, abnormal inter-default functional network connectivity patterns in schizophrenic patients relative to healthy controls. Among the three subsystems, the functional connectivity between the anterior and lateral DMN was enhanced in schizophrenic patients, consistent with the majority of previous studies7,14. Similar results were obtained for the non-medicated patient group alone. As the anterior hub of the DMN, the medial prefrontal cortex is critical in self-referential processing. Abnormalities in this region have been suggested to underlie the impairment in reality monitoring characteristic of schizophrenia31,32. During rest, increased activity in the medial prefrontal cortex is correlated with the severity of positive symptoms in schizophrenia6; while performing passive tasks, this increase was negatively correlated with cognitive test scores in schizophrenic patients33. Furthermore, Whitfield-Gabrieli et al. observed that schizophrenic patients exhibited hyperactivity in the medial prefrontal cortex during a working memory task and increased connectivity between the medial prefrontal cortex and other DMN regions, both during rest and a task7. A failure in the deactivation of the anterior DMN has also been reported by Pomarol-Clotet and colleagues15. Accordingly, our observations may confirm the previous findings, and altered intra-default functional connectivity may contribute to disturbances of thought as well as self- and source-monitoring in schizophrenia.
In this study, the lateral DMN exhibited significantly decreased functional connectivity with unimodal systems, including the somatomotor, motor, lateral visual and auditory networks, in schizophrenic patients, supporting the hypothesis that functional disconnectivity underlies schizophrenia. Similar results were obtained for the non-medicated patients alone. The unimodal sensorimotor cortex is involved in creating representations that convey the visual, auditory and somatomotor information necessary for real-world perception. Note that the lateral DMN overlaps the language network, mainly the bilateral superior medial and inferior frontal cortex (including Broca’s area), the lateral temporal cortex (including Wernicke’s area and the superior temporal sulcus), and the temporal parietal junction. The previous voxel-based morphometry studies reported that gray matter volume reduction in the superior temporal gyrus may be related to the positive symptoms, such as hallucinations and thought disturbances, in schizophrenia34. Functional anomalies of the language network were also demonstrated in schizophrenia35. Reduced coupling between the lateral DMN and unimodal sensorimotor cortex was not found to be significantly correlated with positive symptoms in the current study; thus, further studies are needed to confirm whether the abnormal modulation of unimodal sensorimotor cortex by the lateral DN underlies the somatomotor, visual, auditory-verbal and multimodality hallucinations characteristic of schizophrenia33 that create a vague boundary between internal and external real-world perception.
Previous studies have demonstrated dynamic cooperation and competition between the DMN and other ICNs in the human brain36,37, perhaps underlying the shift in focus from internal state to external environment. In the current study, the lateral DMN exhibited increased functional connectivity with the right frontoparietal control and dorsal attention networks in schizophrenia, and functional connectivity between the anterior DMN and right control network was enhanced in schizophrenic patients, consistent with the results of previous studies20. Similar results were obtained for non-medicated patients alone. Furthermore, the increased functional connectivity between the lateral DMN and right control network was significantly correlated with PANSS negative and anergia factor scores in schizophrenic patients. The frontoparietal control network is suggested to play a central role in cognitive control and information integration from the default mode and dorsal attention systems38,39. The abnormal coupling between the DMN and control/attention networks implies that schizophrenic brains do not successfully balance the shift of activation and deactivation between these networks (especially for anti-correlated networks). Thus, these anomalies in network coordination may lead to an inability to cycle out of internal thought and into response to external stimuli, resulting in hallucinations, emotional disturbance, social and attention deficits, disrupted corollary and judgment (such as persecutory delusions), and a split between internal mentation and external environment.
We also tested the discriminative power of the functional network connectivity among the anterior, posterior, and lateral DMNs between the patient and control groups, yielding accuracies of 71.3% (P < 0.0001), 63.9% (P < 0.005), and 73.2% (P < 0.0001), respectively. When taking all the three subnetworks together, an accuracy of 76.9% (P < 0.0001) was achieved. According to the classification results, the two populations are likely to exhibit DMN connectivity patterns40. Recently, several fMRI studies have attempted to distinguish schizophrenic patients from healthy controls41,42. Our findings suggest that DMN connectivity may also be a potential biomarker for the clinical diagnosis of schizophrenia.
The current study posed several limitations. First, group ICA does not always divide the DMN into three components for all datasets or populations under default parameter settings, but three components can be achieved with a larger component number parameter. Nevertheless, the current study provides preliminary evidence for the functional connectivity dissociation of DMN subnetworks in schizophrenia. Second, the increased functional connectivity between the lateral and anterior DMNs as well as the patterns of functional connectivity between unimodal and heteromodal cortical networks were uncorrected, so these results should be interpreted with caution. Third, IQ was not assessed in the current study, and it remains unclear whether IQ was matched between the patient and control groups. This potential confounding factor should be considered in future studies. Fourth, we estimated static functional connectivity alterations in schizophrenia in this study; dynamic functional connectivity and effective connectivity should be investigated to shed light on the abnormal functional interactions between DMN subnetworks in schizophrenia. Fifth, Salvador et al. demonstrated cortical-subcortical functional connectivity abnormalities in schizophrenia43. However, due to noise in our data, subcortical systems were not included in the current analyses, and functional connectivity between DMN subsystems and subcortical areas should be examined in the future. Though it remains unclear how these results could be used in clinical practice, differentiating the key disease-related default mode subsystem from others may prove useful in the identification of treatment targets and the development of treatment strategies for schizophrenia, although the DMN subnetwork may not, in practice, be a suitable treatment target in schizophrenia. Finally, due to inter-subject brain differences and scanner variability, it is important to confirm the results with a larger sample size and multicenter imaging data. An expansion of the medicated sample group is ongoing. It is also important to study the influence of antipsychotic medication in schizophrenia in future work.
Methods
Participants
Fifty-five patients diagnosed with schizophrenia and fifty-five healthy controls were involved in the study, all of whom were right-handed Chinese speakers. All patients were recruited from the Department of Psychiatry, Xijing Hospital of the Fourth Military Medical University, Xi’an, China. All patients were evaluated with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Patient Edition (SCID-I/P)44 and were required to meet the DSM-IV1 diagnostic criteria for schizophrenia. The diagnosis of schizophrenia was confirmed after at least six months. No patients had a history of neurological disorders, severe medical disorders, substance abuse, or electroconvulsive therapy. First-episode patients were included in the current study and 34 of the patients were drug-naïve, while the remainder were receiving antipsychotic medications at the time of image acquisition [risperidone (n = 10, 2–6 mg/day), clozapine (n = 5, 200–350 mg/day), quetiapine (n = 4, 400–600 mg/day), and sulpiride (n = 2, 200 mg/day)]. Patients were considered to be antipsychotic drug-naïve if the following criteria were met: 1) the patient was not receiving antipsychotics at the time of the study; 2) the patient had no history of receiving antipsychotics, based on information provided by two or more family members or care providers; and 3) the patient had no history of antipsychotic prescriptions, based on information provision forms from medical institutions that the patient had visited or a history of prescriptions from pharmacies. The ages of the non-medicated patients were 27.38 ± 5.77 years, and the duration of illness in the non-medicated patients was 11.74 ± 15.04 months, similar to those reported in previous studies45. The healthy controls were recruited via local recruitment advertisements in the hospital and in the communities neighboring. All participants provided written informed consent, and the study was approved by the Ethics Committee of the Xijing Hospital of the Fourth Military Medical University. The methods were carried out in accordance to the approved guidelines.
Two healthy controls were discarded due to excessive head motion during scanning acquisition (>2.5 mm translation and/or >2.5° rotation), resulting in the inclusion of 55 schizophrenic patients and 53 healthy controls in follow-up analyses. No significant difference in mean motion was observed between the two groups (patient: 0.066 ± 0.038, control: 0.055 ± 0.024 mm, P > 0.05)46,47. The information collected from the remaining subjects is presented in Table 1, including their sex, age, PANSS, and other factor scores48.
Table 1. Demographic and clinical profiles of the participants in this study.
| Variable | Schizophrenic patient (mean ± s.d.) | Healthy control (mean ± s.d.) | p-value |
|---|---|---|---|
| Sample size | 55 | 53 | |
| Gender (female/male) | 23/32 | 25/28 | >0.50a |
| Age (year) | 27.27 ± 5.51 | 28.08 ± 4.68 | >0.40b |
| Duration (month) | 30.15 ± 34.66 | ||
| PANSS total | 91.76 ± 21.95 | ||
| PANSS positive | 22.34 ± 4.49 | ||
| PANSS negative | 21.07 ± 6.57 | ||
| PANSS general | 44.45 ± 7.85 | ||
| Anergia | 10.64 ± 3.43 | ||
| Thought disturbance | 11.58 ± 3.45 | ||
| Activation | 6.35 ± 2.35 | ||
| Paranoid/Belligerence | 9.25 ± 3.20 | ||
| Depression | 9.78 ± 3.20 | ||
| Supplementary items (Attack) | 9.55 ± 5.04 |
aPearson Chi-square test.
bTwo-tailed two-sample t-test; PANSS = Positive and Negative Syndrome Scale. The following clinical variables were evaluated according to PANSS factors: a) “Anergia” = N1+N2+G7+G10; b) “Thought disturbance” = P2+P3+P5+G9; c) “Activation” = P4+G4+G5; d) “Paranoid/belligerence” = P6+P7+G8; and e) “Supplementary” = P4+P7+G6+S1+S2+S3. P2, Conceptual disorganization; P3, Hallucinations; P4, Hyperactivity; P5, Grandiosity; P6, Suspiciousness/persecution; P7, Hostility; N1, Blunted affect; N2, Emotional Withdrawal; G4, Tension; G5, Mannerisms and posturing; G6, Depression; G7, Motor retardation; G8, Uncooperativeness; G9, Unusual thought content; G10, Disorientation; S1, Angry; S2, Inability to delay gratification; S3, Affective lability.
Image acquisition and preprocessing
All data were collected on a 3T Tim Trio scanner (Siemens, Erlangen, Germany) using a 12-channel phased-array head coil. Images were acquired using a gradient-echo echo-planar pulse sequence sensitive to blood oxygenation level-dependent (BOLD) contrast [repetition time (TR)/echo time (TE) = 2000/30 ms; flip angle (FA) = 90°; matrix = 64 × 64; field of view (FOV) = 220; thickness/gap = 4/0.6 mm; slices = 33]. Each resting-state fMRI run lasted 8 min, and each subject underwent two runs. Structural data made use of a high-resolution multi-echo T1-weighted magnetization-prepared gradient-echo image [TR = 2530 ms; Time Inversion (TI) = 1100 ms; TE = 3.5 ms; FA = 7°; 1-mm isotropic voxels; FOV = 256]. Subjects were instructed to stay awake, keep their eyes closed, and minimize head movement; no other task instruction was provided. After each session, the subjects were asked whether they were awake and relaxed in the previous session, and all of the subjects confirmed that they were.
There were no artifacts or structural abnormalities in any of the structural MRI images, as determined by visual inspection. Data were preprocessed using a statistical parametric mapping software package (SPM8, Welcome Department of Cognitive Neurology, Institute of Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm). The first five volumes of each run were discarded to allow for T1-equilibration effects. The following steps were performed: 1) slice timing correction; 2) rigid body correction for head motion; 3) atlas registration with an EPI template in the Montreal Neurological Institute (MNI) atlas space, resampling to 3-mm isotropic voxels and spatially smoothing using a 6-mm full-width half-maximum (FWHM) Gaussian kernel; 4) normalization for global mean signal intensity across runs; and 5) linear detrend and band-pass temporal filtering (0.01–0.08 Hz).
Group independent component analysis
All preprocessed fMRI data were analyzed using GIFT software (http://mialab.mrn.org/software/gift/). Group ICA was performed to decompose the resting-state images into spatially independent components49,50. For each subject, 20 spatially independent components were obtained with default parameters. The ICNs (14; the remainder were noise components) were identified from the 20 independent components, as reported in previous studies22,23,24. In particular, DMN components (3) were identified using a DMN template based on the prior parcellation of the human brain22,51,52. Operationally, a multiple regression was performed over voxels, and components (i.e., subnetworks) that best fit the DMN template were selected.
Intra- and inter-default functional connectivity analysis
Taking each DMN subnetwork as a seed network, the intra-default functional correlations between the seed network and other DMN subnetworks as well as inter-default functional correlations between the seed network and other ICNs were estimated from the corresponding component time courses by Pearson correlation coefficient, then were converted to z-scores by applying the Fisher r-to-z transformation53. Two-tailed one-sample t-tests were used to test whether the functional connectivity between each pair of ICNs was significant for each group (P < 0.05, FDR corrected). Finally, two-tailed two-sample t-tests were used to compare the intra- and inter-default functional connectivity between the patient and control groups with a threshold of P < 0.05 (FDR corrected).
Clinical correlation analysis
Spearman’s rank correlation analysis was performed to assess the correlation between DMN connectivity with significant group differences and clinical variables, as shown in Table 1. Age and mean motion were included as confounding covariates47. Two-tailed two-sample significance levels were set at P < 0.05 and were uncorrected for multiple comparisons in the correlation analysis.
Multivariate pattern analysis
Linear SVMs (http://www.isis.ecs.soton.ac.uk/resources/svminfo/), together with local linear embedding, were used to test the discriminative power of DMN connectivity between the patient and control groups42. The algorithms were reported with the best parameter settings. Due to the limited sample size of our study, the leave-one-out cross-validation strategy was used to estimate the generalization ability of the classification. Permutation tests were implemented to assess the performance of the classifier40, and the tests were repeated 10,000 times as described previously54.
Additional Information
How to cite this article: Wang, H. et al. Evidence of a dissociation pattern in default mode subnetwork functional connectivity in schizophrenia. Sci. Rep. 5, 14655; doi: 10.1038/srep14655 (2015).
Supplementary Material
Acknowledgments
This work was supported by the National Basic Research Program of China (2013CB329401), National High-tech Program of China (2012AA011601), and National Science Foundation of China (61503397, 61420106001, 81371478, and 81201041).
Footnotes
Author Contributions Q.T., D.H., H.W. and L.-L.Z. designed research, H.W., Y.C. and H.Y. performed research; H.W., L.-L.Z. and Q.T. contributed new reagents/analytic tools; L.-L.Z., H.W. and D.H. analyzed data, and L.-L.Z. and H.W. wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders (4th edition) (American Psychiatric Press, Washington, DC, 2000).
- Friston K. J. & Frith C. D. Schizophrenia: a disconnection syndrome? Clin Neurosci 3, 89–97 (1995). [PubMed] [Google Scholar]
- Andreasen N. C. et al. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry 46, 908–920 (1999). [DOI] [PubMed] [Google Scholar]
- Fox M. D. & Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci 4, 19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R. L. et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull 33, 1004–1012 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity A. G. et al. Aberrant “Default Mode” Functional Connectivity in Schizophrenia. Am J Psychiatry 164, 450–457 (2007). [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S. et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA 106, 1279–1284 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson N. et al. Lateral Differences in the Default Mode Network in Healthy Controls and Patients With Schizophrenia. Hum Brain Mapp 32, 654–664 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E. A default mode of brain function. Proc Natl Acad Sci USA 98, 676–682 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. D., Krasnow B., Reiss A. L. & Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100, 253–258 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R. The brain’s default network and its adaptive role in internal mentation. Neuroscientist 18, 251–270 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R. L., Andrews-Hanna J. R. & Schacter D. L. The brain’s default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci 1124, 1–38 (2008). [DOI] [PubMed] [Google Scholar]
- Spreng R. N., Mar R. A. & Kim A. S. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci 21, 489–510 (2009). [DOI] [PubMed] [Google Scholar]
- Mingoia G. et al. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr Res 138, 143–149 (2012). [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E. et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med 38, 1185–1193 (2008). [DOI] [PubMed] [Google Scholar]
- Kim D. I. et al. Dysregulation of Working Memory and Default-Mode Networks in Schizophrenia Using Independent Component Analysis, an fBIRN and MCIC Study. Hum Brain Mapp 30, 3795–3811 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F. et al. Treatment with Olanzapine is Associated with Modulation of the Default Mode Network in Patients with Schizophrenia. Neuropsychopharmacology 35, 904–912 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado-Pined P. et al. Correlated structural and functional brain abnormalities in the default mode network in schizophrenia patients. Schizophr Res 125, 101–109 (2011). [DOI] [PubMed] [Google Scholar]
- Jafri M. J., Pearlson G. D., Stevens M. & Calhoun V. D. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. NeuroImage 39, 1666–1681 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A. et al. Aberrant Dependence of Default Mode/Central Executive Network Interactions on Anterior Insular Salience Network Activity in Schizophrenia. Schizophr Bull 40, 428–437 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R., Reidler J. S., Sepulcre J., Poulin R. & Buckner R. L. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron 65, 550–562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B. T. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106, 1125–1165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M. et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA 106, 13040–13045 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A. R. et al. Behavioral Interpretations of Intrinsic Connectivity Networks. J Cogn Neurosci 23, 4022–4037 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P. et al. Dissociation between anterior and posterior cortical regions during self-specificity and familiarity: a combined fMRI-meta-analytic study. Hum Brain Mapp 33, 154–164 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S. et al. Associations and dissociations between default and self-reference networks in the human brain. NeuroImage 55, 225–232 (2011). [DOI] [PubMed] [Google Scholar]
- Li B. J. et al. A treatment-resistant default mode subnetwork in major depression. Biol Psychiatry 74, 48–54 (2013). [DOI] [PubMed] [Google Scholar]
- Damoiseaux J. S., Prater K. E., Miller B. L. & Greicius M. D. Functional connectivity tracks clinical deterioration in Alzheimer’s disease. Neurobiol Aging 33, 828.e819–e830 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraldsson H. M., Ferrarelli F., Kalin N. H. & Tononi G. Transcranial Magnetic Stimulation in the investigation and treatment of schizophrenia: a review. Schizophr Res 71, 1–16 (2004). [DOI] [PubMed] [Google Scholar]
- Roweis S. T. & Saul L. K. Nonlinear dimensionality reduction by locally linear embedding. Science 290, 2323–2326 (2000). [DOI] [PubMed] [Google Scholar]
- Vinogradov S., Luks T. L., Schulman B. J. & Simpson G. V. Deficit in a neural correlate of reality monitoring in schizophrenia patients. Cereb Cortex 18, 2532–2539 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. F., Welsh R. C., Chen A. C., Velander A. J. & Liberzon I. Medial frontal hyperactivity in reality distortion. Biol Psychiatry 61, 1171–1178 (2007). [DOI] [PubMed] [Google Scholar]
- Harrison B. J., Yucel M., Pujol J. & Pantelies C. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr Res 91, 82–86 (2007). [DOI] [PubMed] [Google Scholar]
- Sun J., Maller J. J., Guo L. & Fitzgerald P. B. Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain Res Rev 61, 14–32 (2009). [DOI] [PubMed] [Google Scholar]
- Liemburg E. J. et al. Abnormal connectivity between attention, language and auditory networks in schizophrenia. Schizophr Res 135, 15–22 (2012). [DOI] [PubMed] [Google Scholar]
- Fornito A., Harrison B. J., Zalesky A. & Simons J. S. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc Natl Acad Sci USA 109, 12788–12793 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102, 9673–9678 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J. L., Kahn I., Snyder A. Z., Raichle M. E. & Buckner R. L. Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. J Neurophysiol 100, 3328–3342 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto T. P. & Gazzaley A. Fronto-parietal network: flexible hub of cognitive control. Trends Cogn Sci 17, 602–603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golland P. & Fischl B. Permutation tests for classification: Towards statistical significance in image-based studies. Inf Process Med Imaging 2732, 330–341 (2003). [DOI] [PubMed] [Google Scholar]
- Liu M., Zeng L.-L., Shen H., Liu Z. & Hu D. Potential risk for healthy siblings to develop schizophrenia: evidence from pattern classification with whole-brain connectivity. NeuroReport 23, 265–269 (2012). [DOI] [PubMed] [Google Scholar]
- Shen H., Wang L., Liu Y. & Hu D. Discriminative analysis of resting-state functional connectivity patterns of schizophrenia using low dimensional embedding of fMRI. NeuroImage 49, 3110–3121 (2010). [DOI] [PubMed] [Google Scholar]
- Salvador R. et al. Overall brain connectivity maps show cortico-subcortical abnormalities in schizophrenia. Hum Brain Mapp 31, 2003–2014 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M. & Williams J. B. structured clinical interview for DSM-IV axis I disorder-patients edition (SCID-I/P) (Biometrics Research Department, New York State Psychiatric Institute, New York, 1996). [Google Scholar]
- Enez Darcin A., Yalcin Cavus S., Dilbaz N., Kaya H. & Dogan E. Metabolic syndrome in drug-naïve and drug-free patients with schizophrenia and in their siblings. Schizophr Res. In press (2015). [DOI] [PubMed] [Google Scholar]
- Van Dijk K. R. A., Sabuncu M. R. & Buckner R. L. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 59, 431–438 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L.-L. et al. Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci USA 111, 6058–6062 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley R. K., Abraham F. & Lewis A. O. The Posotive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr Bull 13, 261–278 (1987). [DOI] [PubMed] [Google Scholar]
- Calhoun V. D., Kiehl K. A. & Pearlson G. D. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp 29, 828–838 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M. J. et al. Spatially independent activity patterns in functional MRI data during the stroop color-naming task. Proc Natl Acad Sci USA 95, 803–810 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E. Y., Yeo B. T. T. & Buckner R. L. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol 108, 2242–2263 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R. L., Krienen F. M., Castellanos A., Diaz J. C. & Yeo B. T. T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106, 2322–2345 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar J. H. Biostatistical Analysis (Prentice Hall, Upper Saddle River, New Jersey, 1996). [Google Scholar]
- Zeng L.-L. et al. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain 135, 1498–1507 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.