Abstract
Surround inhibition (SI) is a feature of motor control in which activation of task-related muscles is associated with inhibition of neighboring, nonprotagonist muscles, allowing selective motor control. The physiological basis for SI still remains unknown. In all previous studies, SI in the motor system was measured during movement initiation by using transcranial magnetic stimulation (TMS) to deliver a posteroanterior current at a single suprathreshold intensity. To expand our understanding of SI, we explored this phenomenon at a wide range of intensities and by stimulating motor cortex with currents along anteroposterior and lateromedial directions. Fifteen healthy volunteers performed a brief isometric index finger flexion on hearing a tone. Electromyography was recorded from the synergist and surround finger muscles. Single-pulse TMS was applied to stimulate the surround muscle at different intensities at rest or movement initiation. The motor evoked potential (MEP) amplitudes were then plotted against stimulation intensities to obtain the MEP recruitment curves for the rest and movement initiation conditions and for the three current directions for every subject. From the recruitment curves, we found that surround inhibition could be elicited only by the posteroanterior current. Hence, we postulate that surround inhibition is mediated by intracortical circuits in the motor cortex. Also, for the first time, we observed surround facilitation when the motor cortex was stimulated with anteroposterior current. Further studies are needed to investigate the mechanisms underlying both these phenomena individually in healthy subjects and patients with dystonia and other movement disorders.
Keywords: transcranial magnetic stimulation, premotor-motor interaction, surround inhibition, MEP recruitment curve
surround inhibition (SI) is a neurophysiological mechanism wherein the excitability of the periphery of an activated neural network is suppressed, thereby enhancing the contrast between the center and the surround. SI in the sensory system has been known for several decades (Blakemore et al. 1970). However, the phenomenon was proposed (Mink 1996) and demonstrated (Sohn and Hallett 2004b) for the human motor cortex only much later. SI is postulated to be associated with selectivity of motor activity in fine motor tasks (Sohn and Hallett 2004b) and would therefore be an important feature of motor control. However, the physiology underlying the phenomenon is still unknown (Beck and Hallett 2011). SI is reduced in patients with focal hand dystonia (Beck et al. 2008; Hallett 2004; Sohn and Hallett 2004a) and may partially explain some of its clinical manifestations. SI is also known to be deficient in Parkinson's disease patients even before clinical manifestations (Shin et al. 2007). Hence, it is clinically relevant to study SI in more detail.
Transcranial magnetic stimulation (TMS) has proved to be a useful tool in elucidating the mechanisms of SI in the primary motor cortex (M1) and has been used extensively to study the phenomenon in the superficial hand muscles (Beck and Hallett 2010; Kassavetis et al. 2012; Shin et al. 2012; Sohn and Hallett 2004b; Stinear and Byblow 2004). These studies demonstrated SI as a reduction in the amplitude of motor evoked potentials (MEP) in the surround muscles when stimulated by TMS during the movement initiation phase. However, SI has been elicited only at a single suprathreshold intensity and by stimulating M1 with brain current flowing only in the posteroanterior direction, even though the importance of coil direction and stimulation intensity has been very well studied in the TMS literature (Brasil-Neto et al. 1992; Mills et al. 1992). SI has been demonstrated at either 1-mV stimulation intensity (Kassavetis et al. 2012) or at 140% of resting motor threshold (Beck et al. 2008, 2009b; Shin et al. 2012; Sohn and Hallett 2004b). It is believed that both posteroanterior (P-A) and anteroposterior (A-P) currents in M1 stimulate the corticospinal tract predominantly transsynaptically, whereas lateromedial (L-M) current predominantly stimulates it directly (Di Lazzaro et al. 2012; Lazzaro et al. 2008). The evidence comes from epidural recordings in conscious humans that showed long-latency indirect waves (I waves) from P-A and A-P stimulation and short-latency direct waves (D waves) from L-M stimulation (Di Lazzaro et al. 1998). At higher stimulation intensities, however, D waves can also be generated by P-A and A-P currents. Hence, stimulating M1 at low or medium intensities along different directions and intensities probably activates different neuronal circuits (Di Lazzaro et al. 2012; Lazzaro et al. 2008). Thus the evaluation of SI by positioning the coil along different directions may offer additional insight into the underlying mechanism and the cortical circuitry involved.
In the current observational study, our objective was to explore the physiology underlying SI in greater detail. Because different current directions are known to activate different neuronal circuits, we expected that SI would be elicited only by current along a specific direction, thereby indicating which circuits could be mediating the phenomenon. We stimulated M1 over the motor hotspot of the surround muscle with P-A, A-P, and L-M directed brain currents using a wide range of both subthreshold and suprathreshold stimulation intensities and examined the input-output recruitment curves at rest and during movement initiation.
MATERIALS AND METHODS
Study subjects.
Fifteen healthy volunteers (age range 23–46 yr, mean 33.9 ± 6.9 yr; 6 women) participated in the study after giving written informed consent. Handedness was assessed using the Edinburgh handedness inventory (Oldfield 1971). All subjects were right-handed (mean laterality index = 88; range = 53–100) and underwent general physical and neurological examination at the National Institutes of Health (NIH) within the past year. None of the subjects had history of seizures, head injury, or any other neurological or psychiatric illness. They did not have any metal implants in the body. They did not take any medication regularly or within a week before the experiment. None of them abused alcohol and/or drugs in the past 6 mo. Acute/chronic medical illnesses and pregnancy were excluded by oral interview before the experiment. The study was approved by the Neuroscience Institutional Review Board of the NIH and conformed to the Declaration of Helsinki.
EMG recording.
The subjects were seated in a comfortable chair with their hands resting on a pillow to ensure maximum relaxation of the arm. Disposable Ag-AgCl surface EMG electrodes were placed in a belly-tendon montage over the right abductor pollicis brevis (APB) and the right first dorsal interosseus (FDI) muscles. The electrode impedance was reduced to <20 kΩ. The EMG was recorded using a conventional EMG acquisition system (Nihon Kohden Neuropack MEB 2200, v. 08.15), amplified, bandpass filtered (20-2,000 Hz), and digitized at 5 kHz (CED Micro1401; Cambridge Electronic Design, Cambridge, UK). The digitized output was viewed on a computer screen in real time as well as stored for offline analysis using Signal version 4.09 (Cambridge Electronic Design).
Motor task.
The task used in the current study is known to demonstrate SI in the APB muscle (Beck et al. 2008). It is a simple acoustic response task consisting of isometric right index finger flexion in response to a brief tone. The auditory stimulus (500 ms, 1,000 Hz) was delivered to the subjects approximately once every 5 s with the use of specially designed earplugs with auditory ports (E-A-RLINK 3A auditory system; Aearo Technologies, Indianapolis, IN). The loudness of the tone was adjusted such that it did not induce a startle response such as contraction of the sternocleidomastoid muscle in the subjects. The subjects placed their right hand flat on a table beside them, with the tip of the right index finger resting on a force transducer (model S215 load cell; Strain Measurement Devices, Meriden, CT). The subject's wrist was supported by a towel roll to relax the hand muscles, if required. The output of the force transducer was digitized at 5 kHz (CED Micro1401; Cambridge Electronic Design) and displayed on a screen in front of the subject for visual feedback. The maximum force exerted by isolated right index finger flexion (Fmax) was assessed. A horizontal line corresponding to the subject's 10% Fmax was displayed on the screen, and the subject was asked to press just strongly enough to hit the target line as quickly as possible on hearing the auditory tone for the duration of the tone. The subjects practiced the task before the experiment to achieve consistency in their motor response. Approximate reaction time was computed from the practice data set as the mean duration from the start of the tone to the onset of EMG activity in the FDI muscle. The period before the tone was considered the “rest” phase. The period after the tone and ∼50 ms before the onset of muscle activity on EMG was considered the “premotor” phase or the movement initiation (MI) phase, which is the time when SI is expected to be observed (Beck et al. 2008).
Transcranial magnetic stimulation.
TMS was applied by using a figure-of-eight coil (outer diameter 70 mm) connected to a Magstim 200 stimulator (Magstim, Dyfed, UK) that delivers monophasic pulses. The motor “hotspot” for the right APB muscle (surround muscle) was identified by positioning the coil tangentially on the scalp at an angle of 45° to the midline with the handle pointing posteriorly and laterally. This induced a current along the P-A direction in the brain perpendicular to the central sulcus (Brasil-Neto et al. 1992; Mills et al. 1992).
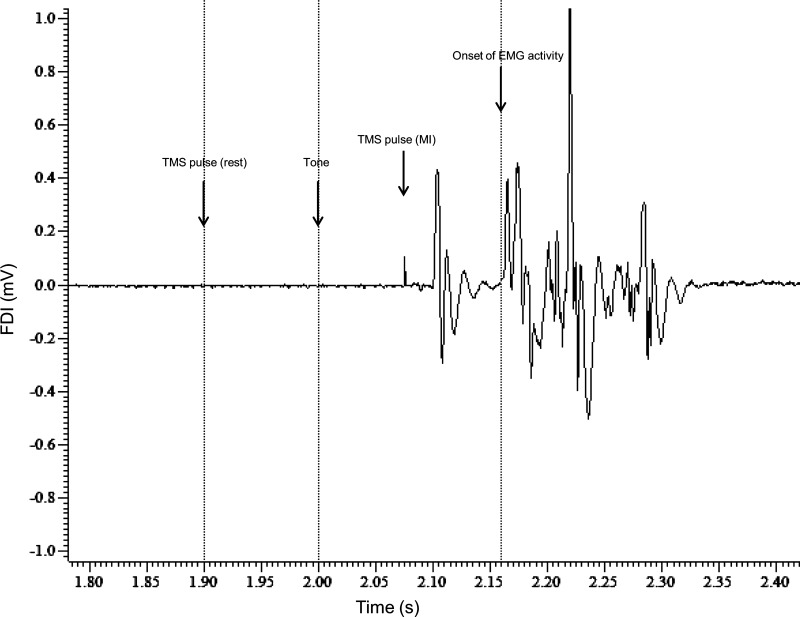
The experiment consisted of three blocks. The subjects received TMS in different coil positions during each block. The coil orientation was either P-A, A-P, or L-M, with each orientation indicating the direction of flow of induced current in the brain. The P-A orientation is described above. For the A-P orientation, the coil was rotated 180° from the coil orientation in the P-A position in the plane tangential to the skull. For the L-M orientation, the coil was rotated 90° from the P-A position along the same plane and the coil handle pointed laterally. The order of the P-A, A-P, and L-M blocks was randomized across subjects. For each coil orientation, single-pulse TMS was applied at a frequency of 0.2 Hz at intensities ranging from 20% to 100% of maximum stimulator output at 5% intervals (20, 25, 30, . . ., 100). The TMS pulses were delivered either before the tone (rest) or after the tone and around 50 ms before expected onset of muscle activity (MI). Three pulses were delivered at each of the 17 intensities for each condition (rest and MI), resulting in a total of 102 pulses per block (Kukke et al. 2014). The rest and MI conditions, and also the intensities, were all randomized within every block. Figure 1 shows the EMG trace from the synergist muscle of a subject, illustrating the time course of a single trial of the experiment.
Fig. 1.
Time course of a single trial of the experiment. A single frame of the EMG recording from the right first dorsal interosseous (FDI) muscle of a subject during movement initiation (MI) is shown. The tone was always delivered at 2 s. A transcranial magnetic stimulation (TMS) pulse was delivered during MI ∼50 ms before onset of EMG activity (indicated by arrow at 2.075 ms). For the rest condition, the TMS pulse would be delivered 100 ms before the tone (indicated by arrow at 1.9 s).
Data analysis.
Peak-to-peak MEP amplitude was calculated for each trial. Trials with background EMG activity of >20 μV over the 200 ms preceding MEP onset were discarded. Input-output recruitment curves (intensity vs. MEP amplitude) were plotted individually for each condition (rest or MI) and coil direction (P-A, A-P, and L-M). Thus each subject had six recruitment curves. The data were fitted to the Boltzmann sigmoid function using the Levenberg-Marquardt nonlinear least-mean-squares algorithm in IGOR Pro 6.22 (WaveMetrics). The Boltzmann function is given by
where MEPmax is the MEP amplitude at plateau, X50 is the stimulation intensity required to obtain a response of 50% of the maximum, X is the stimulation intensity (independent variable), and k is Boltzmann's slope parameter, the inverse of which is directly proportional to the maximum steepness function at X50 (Devanne et al. 1997).
The recruitment curves that did not reach the plateau even at 100% maximum stimulator output (the MEP amplitudes did not reach a saturation point) were discarded from further analysis because the parameters of the Boltzmann sigmoid function could not be estimated. Fifteen curves for P-A at rest, 12 for P-A at MI, 10 for A-P at rest, 9 for A-P at MI, 8 each for L-M at rest, and L-M at MI remained. Because the threshold for L-M stimulation was higher than for other current directions, MEPmax was not achievable in many subjects. Using the regression parameters, we estimated the motor threshold (MT) using the intercept method (Devanne et al. 1997). MEP amplitudes for stimulation intensities ranging from 100% to 200% MT were calculated from the individual curve equations. The MEP amplitudes obtained were normalized to the individual subject's MEPmax. Table 1 shows the mean MEPmax values for the different conditions and coil directions. The normalized recruitment curves were compared across subjects and conditions. Statistical analysis was performed on this data set.
Table 1.
Comparison of means of recruitment curve parameters in different experimental conditions for surround muscle (APB)
| P-A_Rest | P-A_MI | A-P_Rest | A-P_MI | L-M_Rest | L-M_MI | |
|---|---|---|---|---|---|---|
| N | 15 | 12 | 10 | 9 | 8 | 8 |
| MEPmax | 2.8 ± 1.5 | 3.0 ± 1.5 | 2.9 ± 2.1 | 2.9 ± 2.1 | 2.5 ± 1.6 | 2.6 ± 1.9 |
| k | 6.6 ± 0.6 | 7.4 ± 0.9 | 6.7 ± 0.8 | 6.1 ± 0.9 | 5.2 ± 0.7 | 5.7 ± 1.3 |
| Threshold, %MSO | 41 ± 2.5 | 36 ± 4.1 | 48 ± 2.3 | 46 ± 3.8 | 58 ± 3.0 | 52 ± 6.3 |
Values are means ± SD (N = sample size) for parameters at rest and during movement initiation (MI) in posteroanterior (P-A), lateromedial (L-M) and anteroposterior (A-P) direction of induced current in the brain for the surround muscle abductor pollicis brevis (APB). MEPmax, is the motor evoked potential (MEP) amplitude at plateau, k is the Boltzmann slope parameter, and threshold is expressed as a percentage of maximum stimulator output.
Statistical analysis.
Because individual MEP distributions for all measured intensities in each coil direction group showed a skew toward the larger MEP sizes, all single MEP amplitudes were transformed using a natural log function before statistical testing. Multivariate analysis of variance (MANOVA) was conducted to compare three coil directions by using both coil direction and intensity as fixed factors while considering all measurements from a particular subject to be correlated by assuming compound symmetric covariance structure. Furthermore, post hoc comparisons adjusted for multiple comparisons using Bonferroni correction were performed to determine which coil orientations showed significantly different effects. Similarly, a second MANOVA was conducted to compare rest vs. MI conditions. Analyses were conducted using PROC Mixed (Littell et al. 2006) in SAS.
RESULTS
All subjects tolerated the experiment well, and none of them complained of any adverse effects related to the stimulation.
Impact of coil orientation on the recruitment curve of surround muscle.
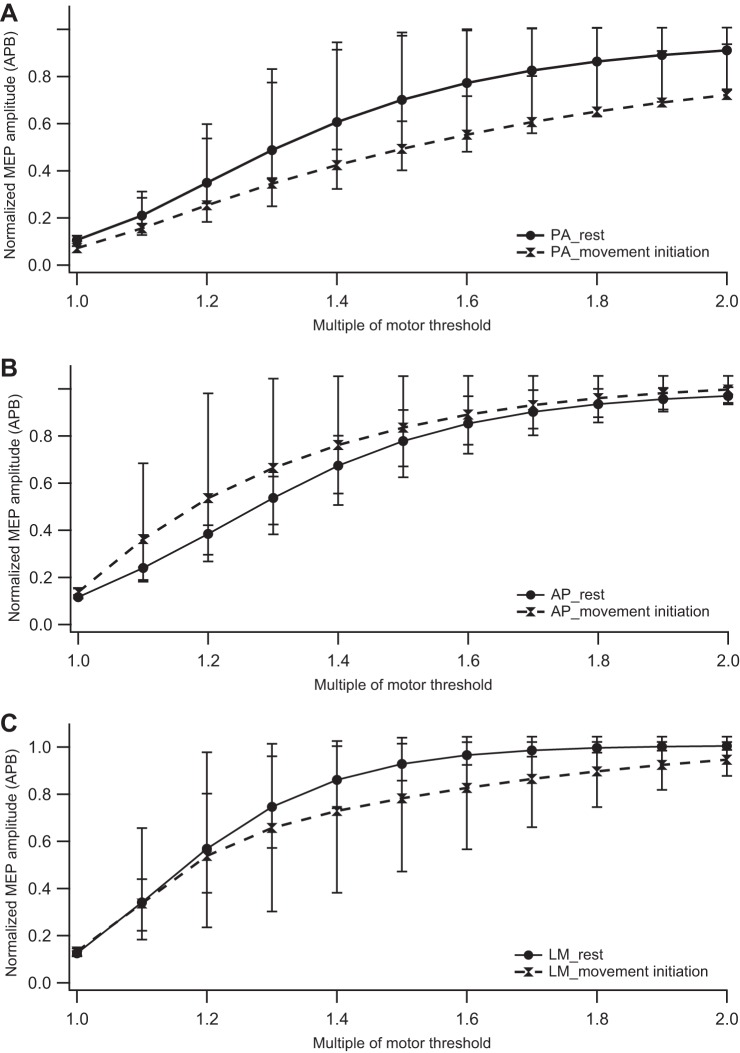
At rest, the normalized MEP amplitude across suprathreshold intensities differed significantly with coil direction (F2,15 = 29.94, P < 0.01). Post hoc analysis (Table 2) revealed significant differences for A-P vs. L-M and L-M vs. P-A comparisons; that is, the L-M curve was steeper than the A-P and P-A curves. Notably, the A-P vs. P-A comparison did not show any significant difference.
Table 2.
Results of post hoc analysis comparing effects of different coil directions for surround muscle (APB)
| Rest |
MI |
|||||
|---|---|---|---|---|---|---|
| Coil Directions | df | t value | Adjusted P (Bonferroni) | df | t value | Adjusted P (Bonferroni) |
| A-P vs. L-M | 15 | −5.54 | <0.01* | 10 | 0.87 | 1 |
| A-P vs. P-A | 15 | 1.64 | 0.36 | 10 | 6 | <0.01* |
| L-M vs. P-A | 15 | 7.63 | <0.01* | 10 | 4.14 | <0.01* |
Results are degrees of freedom (df), t values, and P values adjusted with Bonferroni correction for comparisons indicated.
P < 0.05.
During movement initiation, the effect of coil direction on MEP amplitude was significant for the entire range of suprathreshold intensities (F2,10 = 19.08, P < 0.01). Post hoc analysis (Table 2) across all intensities revealed significant differences in MEP amplitudes for A-P vs. P-A and L-M vs. P-A comparisons but not for the A-P vs. L-M comparison; that is, both A-P and L-M curves were steeper than the P-A curve. Figure 2, A and B, show the recruitment curves for all three coil directions at rest and MI conditions, respectively.
Fig. 2.
Impact of movement initiation on the input-output recruitment curve of the surround muscle (abductor pollicis brevis, APB). Normalized motor evoked potential (MEP) amplitudes are plotted against stimulation intensity (represented as a percentage of motor threshold) at rest and during movement initiation for posteroanterior (PA), anteroposterior (AP), and lateromedial (LM) coil directions. A: recruitment curves at rest and during movement initiation for P-A direction. B: recruitment curves at rest and during movement initiation for A-P direction. C: recruitment curves at rest and during movement initiation for L-M direction. Symbols represent median values, and error bars indicate 25th and 75th interquartile range.
Impact of movement initiation on the recruitment curve of surround muscle.
The normalized MEP amplitudes were significantly different between the MI and the rest conditions across all intensities for all coil orientations (Table 3). Also, there was a significant main effect of stimulation intensity for all three coil directions. Figure 2 compares the recruitment curves at rest vs. MI for each of the three coil directions, showing inhibition with P-A and L-M and facilitation with A-P direction during MI.
Table 3.
Results of MANOVA comparing MEP amplitudes of surround muscle (APB) at rest versus movement initiation
| Coil Direction | Factor | F Statistic | P Value |
|---|---|---|---|
| P-A | Condition | F1,11 = 69.30 | <0.01* |
| Intensity | F10,140 = 149.58 | <0.01* | |
| L-M | Condition | F1,4 = 31.96 | <0.01* |
| Intensity | F10,90 = 116.76 | <0.01* | |
| A-P | Condition | F1,6 = 10.80 | 0.017* |
| Intensity | F10,90 = 104.58 | <0.01* |
Results are F statistics and P values for multivariate analysis of variance (MANOVA) comparisons indicated.
P < 0.05.
Impact of coil direction and movement initiation on synergist muscle.
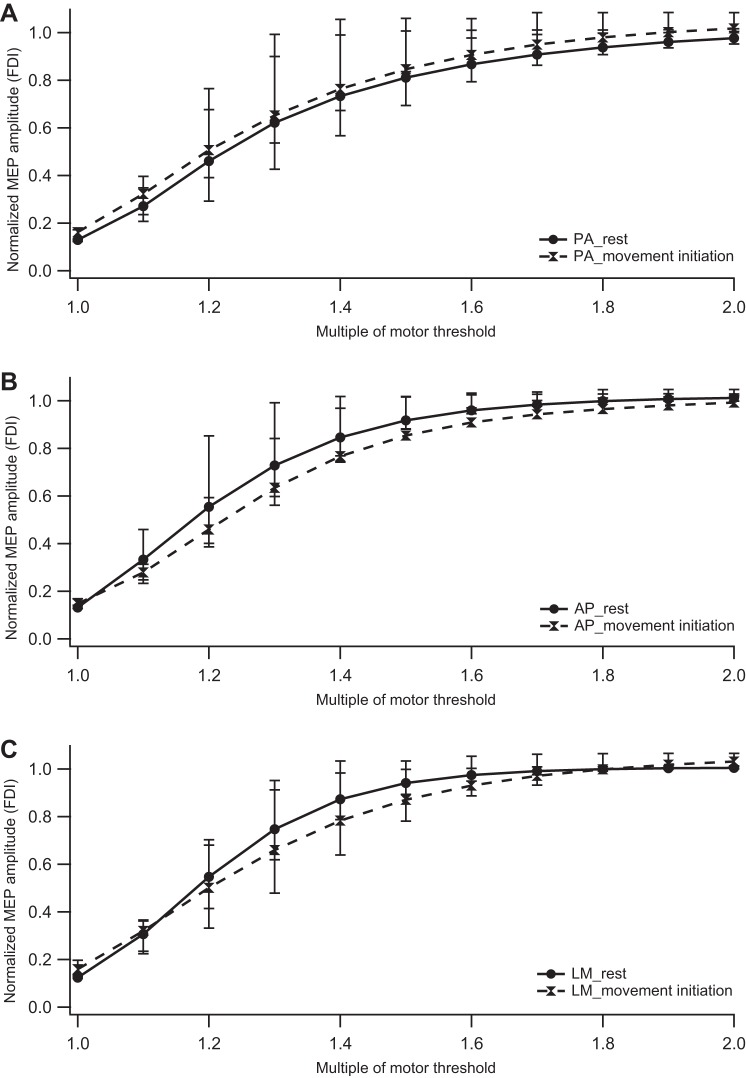
MANOVA for the synergist muscle (FDI) also showed a statistically significant difference in MEP amplitudes for coil directions across all intensities at rest (F2,22 = 17.02; P < 0.01). Post hoc comparisons revealed the P-A direction to be significantly different from A-P and L-M at rest (P-A Vs. A-P, P < 0.01; P-A vs. L-M, P < 0.01). During MI, coil direction did not show a significant difference (P = 0.69).
The rest and MI conditions were significantly different only for A-P and P-A directions. As opposed to the surround muscle, mild facilitation was observed with P-A stimulation (F1,12 = 7.75; P = 0.02) and inhibition with A-P stimulation (F1,12 = 16.18; P < 0.01) during MI (Fig. 3).
Fig. 3.
Impact of movement initiation on the input-output recruitment curve of the synergist muscle (FDI). Normalized MEP amplitudes are plotted against stimulation intensity (represented as a percentage of motor threshold) at rest and during movement initiation for PA, AP, and LM coil directions. A: recruitment curves at rest and during movement initiation for P-A direction. B: recruitment curves at rest and during movement initiation for A-P direction. C: recruitment curves at rest and during movement initiation for L-M direction. Symbols represent median values, and error bars indicate 25th and 75th interquartile range.
DISCUSSION
The results of our study showed that the recruitment curves of the surround muscle were significantly different for the three coil directions (A-P, P-A, and L-M) both at rest and during movement initiation. However, for the synergist muscle, the curves were different only at rest, and not during movement initiation. The recruitment curve for the surround muscle during movement initiation was flatter than at rest with P-A and L-M stimulation but significantly steeper with A-P stimulation. This implies smaller MEP amplitudes during movement initiation with P-A and L-M stimulation (surround inhibition) and higher MEP amplitudes with A-P stimulation (surround facilitation) for a certain range of suprathreshold intensities. For the synergist muscle, there was little or no change in the slope of the curves for the P-A and L-M direction, whereas the A-P curve flattened. Hence, contrary to results in the surround muscle, the MEP amplitudes increased during movement initiation in the synergist muscle when stimulated in the P-A and L-M direction but decreased with A-P stimulation. Our findings substantiate that different neuronal circuits are activated depending on the direction of the current induced in the brain. We have specifically shown that surround inhibition can be elicited only by a P-A current and that A-P current elicits surround facilitation.
Different sets of neuronal elements are activated by changing current direction.
Stimulating the motor cortex along different directions is known to activate different neuronal circuits (Lazzaro et al. 2008; Ziemann and Rothwell 2000). We know from previous studies that stimulation of M1 along the P-A direction results in descending corticospinal volleys that are predominantly I1 waves, especially at intensities below 180% active motor threshold. Beyond this intensity, D waves and later I waves also appear (Di Lazzaro et al. 1998). I1 waves most likely come from the intracortical excitatory interneurons from the layer 2/3 of the motor cortex that are afferent on the layer 5 corticospinal tract neuron. The later I waves from P-A stimulation arise from activation of reciprocal inhibitory connections within this intracortical circuit (Di Lazzaro et al. 2012). A-P stimulation predominantly generates later I waves, mainly the I3 wave. It is believed that these later I waves arise from cortico-cortical inputs to the layer 5 corticospinal neuron (Di Lazzaro et al. 2012). Few studies have suggested that the corticocortical input could be from the premotor cortex (Groppa et al. 2012a, 2012b; Shimazu et al. 2004). L-M stimulation generates D and I1 waves (Sakai et al. 1997) by directly stimulating the corticospinal tract neuron. Thus different I waves are generated from different sets of presynaptic neural elements, and therefore it is not surprising that the recruitment curves we obtained were different for the three coil orientations.
Surround inhibition is mediated by intracortical interneuronal circuits.
The motor cortex recruits the appropriate synergist muscles for performing the specific task while simultaneously inhibiting the surround muscles. Past studies have shown that while the synergist muscle is facilitated during movement initiation, the surround muscle is inhibited. This is only observed as early as 50 ms preceding movement onset (Houdayer et al. 2012). In our study, we observed steeper recruitment curve (implying higher MEP amplitudes) on stimulating the synergist muscle with P-A current during movement initiation. The recruitment curve of the surround muscle, however, was flatter, indicating less recruitment of corticospinal neurons for the same stimulation intensity during movement initiation than at rest, demonstrating surround inhibition. This phenomenon was specific to P-A stimulation, indicating that intracortical interneurons are primarily involved in surround inhibition. Short-latency intracortical inhibition (SICI), a well-known intracortical inhibitory phenomenon, behaves differently from surround inhibition. Hanajima et al. (1998) studied the influence of current direction on SICI. They showed that SICI could be elicited by both A-P and P-A currents and that SICI affected only the later I waves and not the I1 wave, irrespective of the current direction. On this basis, they suggested that SICI is not mediated by modifying the excitatory inputs to layer 5 pyramidal neurons, but by the enhancement of GABAergic transmission in the presynaptic neurons that affect subsequent suprathreshold depolarization (Di Lazzaro et al. 2012). Therefore, it appears that SICI and surround inhibition are mediated by different intracortical mechanisms within the motor cortex. To further support our speculation, activation of cortico-cortical circuits, such as dorsal premotor-motor (Beck et al. 2009a), ventral premotor-motor (Houdayer et al. 2012), or interhemispheric (Beck and Hallett 2011), has not been able to modulate surround inhibition. Hence, it is conceivable that intracortical but not cortico-cortical networks mediate surround inhibition.
We also observed little surround inhibition with L-M stimulation, which can be explained by the activation of I1 waves in addition to D waves (Lazzaro et al. 2008), but unlike P-A current, L-M stimulation did not show significant MEP facilitation for the synergist muscle. This is probably because a maximal number of corticospinal neurons would have been recruited at that intensity by L-M stimulation through direct activation (D waves).
Surround facilitation.
In the present study, we also have shown that A-P stimulation, which activates the cortico-cortical (probably premotor-motor) circuit, is not involved in surround inhibition. This is in agreement with past studies that have demonstrated that activation of the premotor-motor circuit does not influence surround inhibition (Beck et al. 2009a; Houdayer et al. 2012). Twin-coil TMS studies have shown that a conditioning pulse to the premotor cortex along the A-P direction inhibits motor output (Davare et al. 2008; Houdayer et al. 2012; Koch et al. 2007). Along similar lines, we also have observed inhibition of the synergist muscle with A-P current, indicating that A-P current probably activates premotor inputs to the corticospinal tract. We speculate that this inhibition of the synergist muscle could be necessary to prevent premature onset of a planned movement. More detailed experiments are needed to study the time course of this inhibition relative to movement onset. Davare et al. (2008) showed that the ventral premotor-motor inhibition at rest is either lost or converted to facilitation during power or precision grip. Additionally, we have demonstrated the phenomenon of surround facilitation for the first time in the human motor system. We think that cortico-cortical circuits, by inhibiting the synergist muscle, could disinhibit the surround muscle resulting in surround facilitation.
Limitations.
In this study, we have demonstrated surround facilitation only from the MEP recruitment curves. We used only three pulses per stimulation intensity that generated 51 points, sufficient enough to plot a recruitment curve. The phenomenon needs to be further validated by hypothesis-testing studies measuring absolute MEP amplitudes with A-P stimulation. Also, we were not able to show differences in the MEP latencies for the different coil directions. The difference in MEP latencies are best observed around active motor threshold (Di Lazzaro et al. 1998). In our study, the subjects were at rest and MEPs around the active motor threshold intensity could not be elicited to estimate the latencies.
Conclusion.
The recruitment curves that we obtained for the three current directions further support that the different coil directions stimulate different sets of neuronal elements that are afferent to the corticospinal tract. Our results suggest that surround inhibition is mediated by intracortical interneuronal circuits. We also have demonstrated surround facilitation for the first time in addition to surround inhibition during movement initiation in healthy subjects by changing the current direction and probably activating premotor inputs to the corticospinal tract. Further studies are needed to study the functional significance of surround inhibition/facilitation in motor control and their role in the pathophysiology of focal hand dystonia and other movement disorders.
GRANTS
This study was supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.T. and M.H. conception and design of research; N.T., R.K., and H.W. performed experiments; N.T. analyzed data; N.T., S.N.K., and M.H. interpreted results of experiments; N.T. prepared figures; N.T. drafted manuscript; N.T., R.K., H.W., S.N.K., and M.H. edited and revised manuscript; N.T., R.K., H.W., S.N.K., and M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Sungyoung Auh for statistical support and Nguyet Dang for technical assistance.
Present address of R. Khera: Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, IA 52242.
Present address of S. N. Kukke: Department of Biomedical Engineering, The Catholic University of America, Washington, DC 20064.
REFERENCES
- Beck S, Hallett M. Surround inhibition in the motor system. Exp Brain Res 210: 165–172, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Hallett M. Surround inhibition is modulated by task difficulty. Clin Neurophysiol 121: 98–103, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Houdayer E, Richardson SP, Hallett M. The role of inhibition from the left dorsal premotor cortex in right-sided focal hand dystonia. Brain Stimul 2: 208–214, 2009a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Richardson SP, Shamim EA, Dang N, Schubert M, Hallett M. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J Neurosci 28: 10363–10369, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Schubert M, Richardson SP, Hallett M. Surround inhibition depends on the force exerted and is abnormal in focal hand dystonia. J Appl Physiol 107: 1513–1518, 2009b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C, Carpenter RH, Georgeson MA. Lateral inhibition between orientation detectors in the human visual system. Nature 228: 37–39, 1970. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol 9: 132–136, 1992. [PubMed] [Google Scholar]
- Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol 586: 2735–2742, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114: 329–338, 1997. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol 109: 397–401, 1998. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A, Pilato F. I-wave origin and modulation. Brain Stimul 5: 512–525, 2012. [DOI] [PubMed] [Google Scholar]
- Groppa S, Schlaak BH, Munchau A, Werner-Petroll N, Dunnweber J, Baumer T, van Nuenen BF, Siebner HR. The human dorsal premotor cortex facilitates the excitability of ipsilateral primary motor cortex via a short latency cortico-cortical route. Hum Brain Mapp 33: 419–430, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppa S, Werner-Petroll N, Münchau A, Deuschl G, Ruschworth MFS, Siebner HR. A novel dual-site transcranial magnetic stimulation paradigm to probe fast facilitatory inputs from ipsilateral dorsal premotor cortex to primary motor cortex. Neuroimage 62: 500–509, 2012b. [DOI] [PubMed] [Google Scholar]
- Hallett M. Dystonia: abnormal movements result from loss of inhibition. Adv Neurol 94: 1–9, 2004. [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol 2: 607–618, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdayer E, Beck S, Karabanov A, Poston B, Hallett M. The differential modulation of the ventral premotor-motor interaction during movement initiation is deficient in patients with focal hand dystonia. Eur J Neurosci 35: 478–485, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis P, Saifee T, Sadnicka A, Pareés I, Kojovic M, Rothwell J, Edwards M. Adaptation of surround inhibition in the human motor system. Exp Brain Res 222: 211–217, 2012. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Mochizuki H, Marconi B, Caltagirone C, Rothwell JC. Interactions between pairs of transcranial magnetic stimuli over the human left dorsal premotor cortex differ from those seen in primary motor cortex. J Physiol 578: 551–562, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukke SN, Paine RW, Chao CC, de Campos AC, Hallett M. Efficient and reliable characterization of the corticospinal system using transcranial magnetic stimulation. J Clin Neurophysiol 31: 246–252, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro VD, Ziemann U, Lemon RN. State of the art: Physiology of transcranial motor cortex stimulation. Brain Stimul 1: 345–362, 2008. [DOI] [PubMed] [Google Scholar]
- Littell R, Milliken G, Stroup W, Wolfinger R, Schabenberger O. SAS for Mixed Models. Cary, NC: SAS Publishing, 2006. [Google Scholar]
- Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol 85: 17–21, 1992. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425, 1996. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res 113: 24–32, 1997. [DOI] [PubMed] [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J Neurosci 24: 1200–1211, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HW, Kang SY, Hallett M, Sohn YH. Reduced surround inhibition in musicians. Exp Brain Res 219: 403–408, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HW, Kang SY, Sohn YH. Disturbed surround inhibition in preclinical parkinsonism. Clin Neurophysiol 118: 2176–2179, 2007. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann Neurol 56: 595–599, 2004a. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Surround inhibition in human motor system. Exp Brain Res 158: 397–404, 2004b. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Impaired Modulation of Intracortical Inhibition in Focal Hand Dystonia. Cereb Cortex 14: 555–561, 2004. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. I-waves in motor cortex. J Clin Neurophysiol 17: 397–405, 2000. [DOI] [PubMed] [Google Scholar]