Abstract
The temporal control of action is a highly conserved and critical mammalian behavior. Here, we investigate the neuronal basis of this process using an interval timing task. In rats and humans, instructional timing cues triggered spectral power across delta and theta bands (2–6 Hz) from the medial frontal cortex (MFC). Humans and rodents with dysfunctional dopamine have impaired interval timing, and we found that both humans with Parkinson's disease (PD) and rodents with local MFC dopamine depletion had attenuated delta and theta activity. In rodents, spectral activity in this range could functionally couple single MFC neurons involved in temporal processing. Without MFC dopamine, these neurons had less functional coupling with delta/theta activity and less temporal processing. Finally, in humans this 2- to 6-Hz activity was correlated with executive function in matched controls but not in PD patients. Collectively, these findings suggest that cue-evoked low-frequency rhythms could be a clinically important biomarker of PD that is translatable to rodent models, facilitating mechanistic inquiry and the development of neurophysiological biomarkers for human disease.
Keywords: medial frontal cortex, dopamine, Parkinson's disease, interval timing
temporal control of action, or guiding movements in time to achieve behavioral goals, is a crucial function of mammalian nervous systems. This process depends on the integrated activity of corticostriatal systems (Buhusi and Meck 2005; Jahanshahi et al. 2010; Matell et al. 2003) and requires intact dopaminergic signaling (Drew et al. 2003). Patients with Parkinson's disease (PD) with depleted dopamine have dramatically impaired temporal control (Malapani et al. 1998). Despite these data, the neural circuitry influenced by dopamine during temporal computations is not understood.
Here we study this issue in PD patients and in animal models by investigating the neural basis of an elementary cognitive task: interval timing. In this task, participants estimate an interval of several seconds as instructed by a cue. In the range of seconds, interval timing requires executive resources, such as working memory and attention to time (Brown 2006; Parker et al. 2013), and is consistently impaired in patients with PD (Buhusi and Meck 2005; Malapani et al. 1998; Merchant et al. 2008). Because this task is highly conserved across mammalian species (Buhusi and Meck 2005; Merchant et al. 2013), it can be rapidly trained in rodent models, facilitating mechanistic hypothesis testing (Drew et al. 2003; Narayanan et al. 2012).
Controlling the timing of action requires the integrated activity of corticostriatal circuits (Hinton and Meck 2004; Matell and Meck 2004) that are dysfunctional in PD patients (Jahanshahi et al. 2010). Recent work has demonstrated that the medial frontal cortex (MFC) is necessary for the temporal control of action. Inactivation of MFC in rodents profoundly impairs interval timing (Kim et al. 2013; Narayanan et al. 2012). Blocking D1-type dopamine receptors impairs both interval timing performance and neural activity correlated with temporal control (Narayanan et al. 2012; Parker et al. 2014). Sustained activity of single MFC neurons appears to be involved in temporal processing across species, including humans, primates, and rodents (Kim et al. 2013; Narayanan and Laubach 2009; Niki and Watanabe 1979; Sheth et al. 2012;). These single MFC neurons are strongly functionally coupled with delta (1–4 Hz) and theta (4–8 Hz) rhythms, which can be attenuated by focal D1 dopamine blockade in the MFC (Parker et al. 2014). Collectively, these findings suggest that functional coupling of D1 neurons underlies the ability to estimate temporal durations.
In humans, EEG events in delta and theta frequencies from MFC can index time-based decision-making processes (van Rijn et al. 2011). Midfrontal low-frequency oscillations are suggested to be a candidate mechanism for cognitive control, underlying a variety of flexible behaviors (Cavanagh et al. 2012; Cavanagh and Frank 2014). These findings motivate the specific hypothesis that low-frequency activity in midfrontal cortex is involved in temporal processing and may be attenuated in PD patients as a consequence of diminished dopamine signaling in MFC neurons. This idea has implications for many basic cognitive operations.
To test this hypothesis, we recorded EEG activity from non-demented PD patients and age- and education-matched controls during performance of an interval timing task. We also recorded local field potentials (LFPs) and single MFC neurons from rodents performing an interval timing task before and after focal dopamine depletion, allowing us to investigate how dopamine influences temporal processing by single MFC neurons. We report four main results: 1) humans and rodents have cue-triggered delta and theta spectral activity during interval timing; 2) this activity was attenuated in PD and in rodent models involving focal MFC dopamine depletion; 3) single MFC neurons were functionally coupled with field potentials at ∼2–6 Hz, and this coupling as well as temporal processing by these neurons was attenuated with dopamine depletion; and 4) in humans, delta/theta activity was correlated with executive function in controls but not PD patients. These data implicate low-frequency activity in MFC as a mechanism of cue-related temporal control requiring intact cortical dopamine.
EXPERIMENTAL PROCEDURES
Human participants.
Informed consent was obtained, and all procedures were approved by the Institutional Review Board at the University of Iowa (no. 201301713). In an initial pilot experiment, data from 11 young undergraduates were collected. In the main study, data from 13 humans with PD and 13 age- and education-matched control participants were enrolled in this study (Table 1). PD patients were recruited from the movement-disorders clinic or a PD patient registry administered by Dr. Ergun Uc at the University of Iowa as part of a study on exercise (Uc et al. 2014). All participants had normal or corrected-to-normal vision and were not demented at the time of evaluation (Montreal Cognitive Assessment or MOCA score ≥ 26). Control and piloting participants had no history of neurological conditions and were free from current psychoactive medication use. Patients with PD did not have other confounding neurological or psychiatric disorders.
Table 1.
Demographics and MOCA scores
| Control | PD | |
|---|---|---|
| Age, yr | 63.4 ± 2.9 | 64.8 ± 3.4 |
| Right handed, % | 92 | 92 |
| Female, % | 58 | 66 |
| Education, yr | 15.8 ± 0.6 | 16 ± 0.5 |
| MOCA | 28.7 ± 0.4 | 27.9 ± 0.4 |
| UPDRS Part III, points | n/a | 9.7 ± 1.8 |
| Levodopa-equivalent dose, mg | n/a | 633 ± 93 |
Values are means ± SE.
PD, Parkinson's disease; MOCA, Montreal Cognitive Assessment; UPDRS, Unified Parkinson's Disease Rating Score.
Procedure.
Eleven PD patients were currently taking levodopa. Two patients were not on levodopa, but their levodopa dose equivalencies for the dopamine agonist pramipexole are included in Table 1 (Tomlinson et al. 2010). PD patients were asked to take medication as usual. Upon arrival, participants provided informed consent in accordance with the Institutional Review Board at the University of Iowa. Electrodes for EEG (described below) and vertical electrooculogram (VEOG) recording were then attached. Participants performed the interval timing task for approximately 1 h. In addition, a battery of neuropsychological tests and a neurological test were administered. All examinations were conducted in the morning. All participants were debriefed at the end of their research participation.
Cognitive and neurological assessment.
Cognitive function was assessed using a battery of neuropsychological tests targeting the executive function domain. Motor status was evaluated by part III of the Unified Parkinson's Disease Rating Score (UPDRS III). All patients were less than Hoehn and Yahr Stage 3 when under medication.
The Trail Making Test (TMT) parts A and B examined the cognitive flexibility in switching attention between two competing tasks, with higher scores indicating worse performance. Difference in time to complete parts A and B was scored (Tombaugh 2004). The Stroop task examined the ability to inhibit dominant responses. Interference scores from the original version (Stroop 1) and revised version (Stroop 2) were used. Higher scores corresponded to better performance (Baldo et al. 2001). Verbal fluency (VF) examines cognitive flexibility. The task consists of three 1-min trials. Participants were asked to generate words that began with F, A, and S in each trial, respectively. The total number of words generated was scored (Baldo et al. 2001). Digit span forward and backward (DFB) examined working memory, where the participants repeat 2–9 digits in either the forward or backward orders. The number of correct repetitions was scored (Anderson et al. 1991). The Wisconsin Card Sort Test (WCST) examined the ability to “learn new rules” and “shift from old to new rules” based on dynamic feedbacks provided by the experimenter. Perseverative error was scored (Robinson et al. 1980).
Human interval timing task.
The interval timing task consisted of 4 blocks of 40 trials (160 trials in total). In each trial, participants were asked to estimate a time period of 3 or 12 s (Int3 or Int12, respectively) (Fig. 1A). Trials were presented in pseudorandom order. All trials began when a numerical cue stimulus appeared on the center of the screen indicating the temporal interval the participants were instructed to estimate (3 or 12 s). Participants made responses by pressing the space bar on a keyboard using their dominant hand when they estimated the temporal interval had elapsed. Participants received feedback about their response time at the end of each trial. There was a 3- to 6-s interval between response and feedback. After feedback, participants moved to the next trial by pressing the space bar again. The task was self-paced, and the participants were asked not to count in their head during the task. Participants performed four practice trials prior to the real task. For pilot experiments in young subjects, there was a 1-s pause between pressing space bar (to start a trial) and the appearance of the next numerical cue stimulus (3 or 12 s).
Fig. 1.
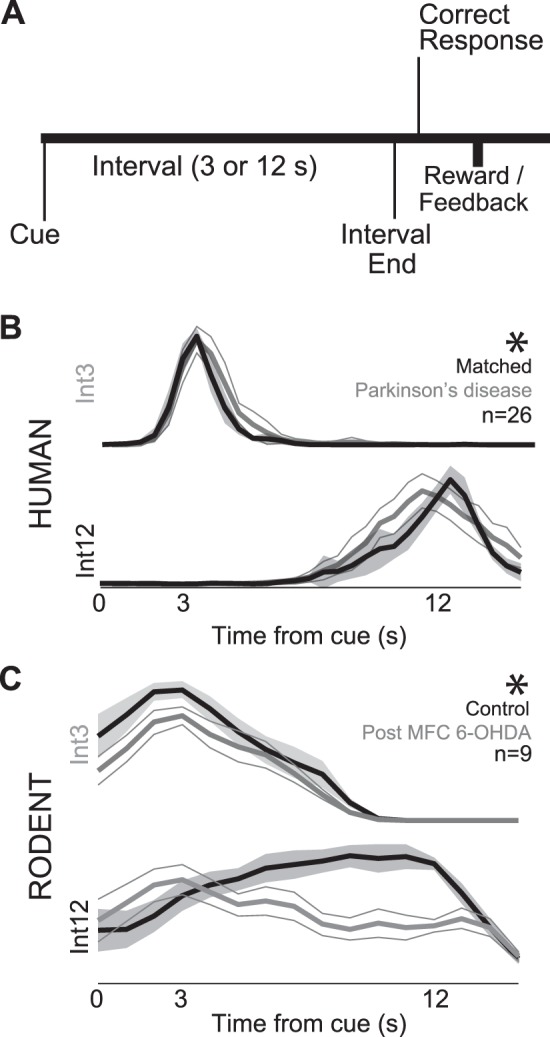
Interval timing task. A: participants estimate 3-s (Int3) and 12-s intervals (Int12) starting with the onset of an instructional cue by making a motor response. Rodents received liquid rewards for the first response after interval end, whereas humans received visual feedback of their response time. B: average response-timing curves indicate that patients with Parkinson's disease (PD; gray traces) had flatter response curves at Int12 than age-matched controls (black traces). C: although rodents were earlier and much more variable (control, prior to dopamine depletion, black traces), medial frontal dopamine depletion [medial frontal cortex (MFC) 6-hydroxydopamine (6-OHDA), gray traces], unilateral medial frontal dopamine depletion flattened response-timing curves, as in humans with PD. *Significant interaction between interval and dopamine depletion (via ANOVA).
EEG recording and preprocessing.
EEG was recorded on a Nihon Kohden system with a sampling rate of 500 Hz. EEG was recorded from 21 channels based on the 10–20 system (Fz, Cz, Pz, FP1/2, F3/4, C3/4, P3/4, F7/8, T3/4, T5/6, O1/2, M1/2), as well as left-eye VEOG and ground (forehead). Impedance of all electrodes was below 5 kΩ. Continuous data were parsed into 16-s epochs (−2 to 14 s following the cue) and re-referenced to the mathematical average of the two mastoid channels, yielding a total of 19 scalp EEG channels. Bad channels were interpolated (11 participants had one interpolated electrode, 1 had two interpolated, 14 had none; midfrontal leads were never interpolated), and bad epochs were rejected (median number of rejected epochs were eight for patients and seven for controls). Eye blinks and horizontal eye movements were removed following independent component analysis from EEGLab (Delorme and Makeig 2004). For cue-related activity, our exploratory analyses suggested that cue-related activity on Int3 and Int12 trials were not significantly different for human or rodents; all subsequent analyses of EEG and field potentials collapsed across Int3 and Int12 trials. For boxplots, outliers were defined as those 1.5 interquartile ranges outside of quartiles 1 and 3 (Tukey 1977).
Rodent operant behavior.
Eleven Long Evans rats (aged 2 mo; 200–225 g) were trained to perform interval timing tasks using standard operant procedures and by motivation through regulated access to water, while food was available ad libitum. The Animal Care and Use Committee at the University of Iowa approved all procedures. Rats were motivated by regulated access to water. Rats consumed 10–15 ml of water during each behavioral session, and additional water (5–10 ml) was provided 1–3 h after each behavioral session in the home cage. Single housing and a 12:12-h light-dark cycle was used; all experiments took place during the light cycle. Rats were maintained at ∼90% of their free-access body weight during the course of these experiments and received 1 day of free access to water per week.
To learn interval timing tasks, animals first learned to make operant lever presses to receive liquid rewards (Narayanan et al. 2006). After fixed-ratio training, animals were trained in a 12-s fixed-interval timing task in which rewards were delivered for responses after a 12-s interval (Fig. 1A). Rewarded presses were signaled by a click and an “off” house light. Each rewarded trial was followed by a 6-, 8-, 10- or 12-s pseudorandom intertrial interval which concluded with an “on” house light signaling the beginning of the next trial. All behavioral devices were extinguished during the intertrial interval. For some animals in some sessions, the intertrial interval began 6 s after interval end if the animal did not respond. Early responses occurring before interval end were not reinforced. After animals learned the 12-s interval as indicated by a peak in their time-response histograms, a secondary delay of 3 s was added (either before or after implantation). The short interval was signaled with an additional light on the left side of the drinking tube. All behavior took place in operant chambers (MedAssociates, St. Albans, VT), which were equipped with a lever and a drinking tube. Behavioral arenas were housed in sound-attenuating chambers (MedAssociates). Water rewards were delivered via a pump (MedAssociates) connected to a standard metal drinking tube (AnCare) via Tygon tubing.
Rodent surgical procedures: surgical, infusion, and perfusion procedures.
Rats trained in the interval timing procedure were implanted with a 33-gauge infusion cannula (Plastics One; n = 11) in the MFC according to procedures described previously (Narayanan et al. 2006; Parker et al. 2014). Eight of these animals also had microwire arrays implanted into MFC. Briefly, animals were anesthetized using ketamine (100 mg/kg) and xylazine (10 mg/kg). A surgical level of anesthesia was maintained with hourly (or as needed) ketamine supplements (10 mg/kg). Under aseptic surgical conditions, the scalp was retracted, and the skull was leveled between bregma and lambda. A single craniotomy over the area above the left MFC and four holes for skull screws were drilled. A microelectrode array configured in 4 × 4 (n = 1) or 2 × 8 (n = 7) arrays of 50-μm stainless steel wires (250 μm between wires and rows; impedance measured in vitro at 400–600 kΩ; Plexon, Dallas, TX) were implanted in eight animals (coordinates from bregma: anterior-posterior +3.2, medial-lateral ± 1.2, dorsal-ventral −3.5 at 12° in the lateral plane). Electrode ground wire was wrapped around the skull screws. The electrode array was inserted while concurrently recording neuronal activity to verify implantation in layer II/III of the MFC. The infusion cannula was then lowered at an angle to target the neurons being recorded (coordinates from bregma: anterior-posterior +0.6, medial-lateral ± 1.0, dorsal-ventral −4.6 at 40° in the lateral plane). The craniotomy was sealed with cyanoacrylate (“SloZap”, Pacer Technologies, Rancho Cucamonga, CA) accelerated by “ZipKicker” (Pacer Technologies), and methyl methacrylate (i.e., dental cement; AM Systems, Port Angeles, WA). Following implantation, animals recovered for 1 wk before being reacclimatized to behavioral and recording procedures. Animals were lightly anesthetized with isoflorane via a nosecone for 5 min, recording cables were attached, and the animal was allowed to recover for 30 min before being tested in the interval timing task. After full recovery, neuronal activity was recorded during a normal preinjection period, followed by medial frontal dopamine depletion using a focal infusion of 3 μl of 1 μg/μl 6-hydroxydopamine (6-OHDA) into MFC, according to procedures described previously (Narayanan et al. 2006).
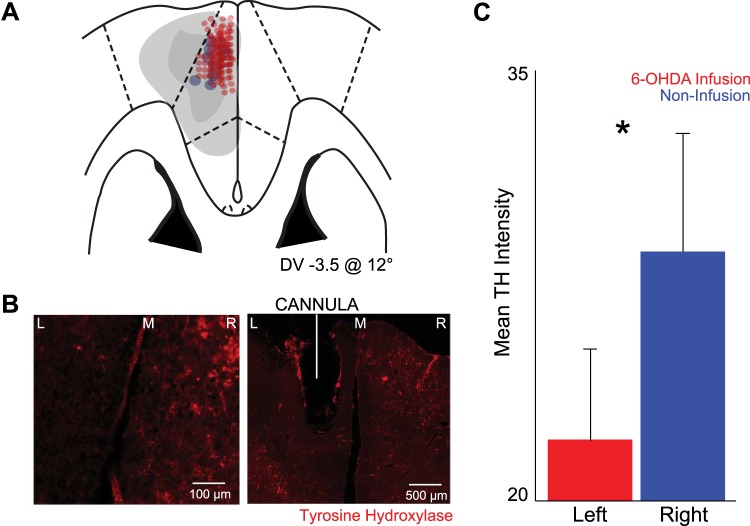
Following completion of recording experiments, rats were anesthetized, killed by injections of 100 mg/kg sodium pentobarbital, and transcardially perfused with 4% formalin. Brains were postfixed in a solution of 4% formalin and 20% sucrose before being sectioned on a freezing microtome. Brain slices were mounted on gelatin-subbed slides and stained for immunofluorescence using tyrosine hydroxylase (TH; Rabbit anti-TH; Millipore; 1:500), with secondary antibody (Alexa Fluor 568 goat anti-rabbit IgG, 1:400, Invitrogen, Grand Island, NY), and stained for cell bodies using DAPI. Histological reconstruction was completed by analyzing electrode and cannula placements in the MFC (Fig. 2A). Fluorescent microscopy revealed the intensity of TH staining in dopaminergic axons in the MFC between the injected (left) and noninjected (right) control side (Fig. 2B). Mean intensity was measured using the Photoshop histogram feature (Fig. 2C). Higher intensity indicated the presence of more TH immunofluorescent axons.
Fig. 2.
Infusions of 6-OHDA in rodent MFC causes dopamine depletion. A: histology from brain slices from 8 animals revealed approximate electrode placement in the MFC (red dots). Cannula from all 11 animals located within MFC and within recording distance of the electrode tips (blue dots) are shown. Light gray patch, approximate maximum region of dopamine depletion; dark gray path, approximate minimum region of dopamine depletion. DV, dorsal-ventral. B: low and medium power coronal sections stained with tyrosine hydroxylase (TH; red); MFC 6-OHDA was injected on the left side. M, midline; R, right; L, left. C: there was less TH+ staining in sections ipsilateral to MFC 6-OHDA infusion (left) compared with control, contralateral sides without infusion (right). Values are means ± SE. *Significance at P < 0.05 via paired t-test.
Neurophysiological recordings.
Neuronal activity was recorded immediately before, and 5–7 days after MFC 6-OHDA infusion. Neuronal ensemble recordings in the MFC were made using a multielectrode recording system (Plexon, Dallas, TX). Putative single neuronal units were identified on-line using an oscilloscope and audio monitor. The Plexon Off-Line Sorter program was used to analyze the signals after the experiment completion to remove artifacts. Spike activity was analyzed for all cells that fired at rates above 0.1 Hz. Statistical summaries were based on all recorded neurons. No subpopulations were selected or filtered out of the neuron database. LFP was recorded using wide-band boards with band-pass filters between 0.07 and 8,000 Hz. Principal component analysis and waveform shape were used for spike sorting. Single units were identified as having 1) consistent waveform shape; 2) separable clusters in principal component analysis space; 3) average amplitude estimated at least three times larger than background activity; and 4) a consistent refractory period of at least 0.002 s in interspike interval histograms. All LFPs were averaged over the recording array and compared with single-neuron activity on each electrode. Analysis of neuronal activity and quantitative analysis of basic firing properties were carried out using NeuroExplorer (Nex Technologies, Littleton, MA), and with custom routines for MATLAB. Peri-event rasters and average histograms were constructed around light on, lever release, lever press, and lick.
Time-frequency and statistical analysis.
Time-frequency measures were computed by multiplying the fast Fourier transformed (FFT) power spectrum of single-trial EEG data with the FFT power spectrum of a set of complex Morlet wavelets, defined as a Gaussian-windowed complex sine wave: ei2πtf e−t2/(2xσ2), where t is time, f is frequency (which increased from 1 to 50 Hz in 50 logarithmically-spaced steps), and σ defines the width (or “cycles”) of each frequency band, set according to 4/(2πf), and taking the inverse FFT. The end result of this process is identical to time-domain signal convolution, and it resulted in estimates of instantaneous power (the magnitude of the analytic signal). Each epoch was then cut in length (−0.5 to +2 s). Power was normalized by conversion to a decibel scale [10 × log10(powert/powerbaseline)], allowing a direct comparison of effects across frequency bands (Cohen 2014). The baseline for each frequency consisted of the average power from −0.5 to −0.3 s prior to the onset of the cues.
Human time-frequency plots and event-related potentials (ERPs) are displayed from the Cz electrode. Statistical significance against the baseline was computed via a paired t-test and is indicated by contours in the time-frequency plots, with a minimum threshold of a 500-pixel cluster size. Correction for multiple comparisons were not run because the low N does not facilitate effective permutation of participants between groups, thus inflating the range of critical values to a degree that precludes data-driven correction in a special population. To ameliorate this problem, a similarly powered pilot study was run to identify regions of interest (ROI), obviating the need for data-driven correction since most of the time-frequency activity was not identified to be of interest. This pilot study identified a low-frequency (∼2–6 Hz) burst of activity immediately following the cue that was similar to ROIs previously studied in humans and rats (Narayanan et al. 2013a).
To examine the time-frequency component of interactions between individual spikes and the field potential, we applied spike-field coherence analysis using the Neurospec toolbox (Rosenberg et al. 1989), in which multivariate Fourier analysis was used to extract phase-locking among spike trains and LFPs. Coherence and phase between spikes and fields was calculated using type “2” analysis with the Neurospec function sp2a_m1.m. Theta coherence was calculated with sliding windows 1.024 s (1,024 points at 1 kHz), shifting by increments of 100 ms, resulting in ∼0.9 Hz frequency resolution. A hanning window with full cosine taper and line frequency suppression was used. Phase-locking coherence values varied from 0 to 1, where 0 indicates no coherence, and 1 indicates perfect coherence. For coherence, an average of LFP across the array was compared with individual neuronal activity. Spike-field coherence was normalized to 95% confidence intervals of coherence to compare across neurons, animals, and sessions. Confidence intervals were verified by bootstrapping time-shuffled data.
RESULTS
Interval timing and dopamine in humans and rodents.
We collected EEG data from 13 PD patients and 13 age- and education-matched controls. There were no significant differences of age, education, or MOCA scores between groups (MOCA mean ± SE: 28.6 ± 0.4 vs. 27.9 ± 0.4; Table 1). PD patients had 633 ± 93 mg dose of levodopa, including the levodopa equivalencies of Pramipexole for two of the patients (Table 1).
Consistent with previous reports (Buhusi and Meck 2005; Malapani et al. 1998; Merchant et al. 2008), patients with PD were impaired on the interval timing task with 3- and 12-s intervals (Int3 and Int12, respectively; Fig. 1B; Table 2). A mixed ANOVA revealed that, across participants, there was a main effect of both PD and interval, as well as a significant interaction term (F = 100.8, P << 0.05). We measured interval timing performance using a curvature index that increases as responses are guided by time during interval timing tasks (Fry et al. 1960; Narayanan et al. 2012; Parker et al. 2014). Around 12-s peaks, humans with PD response-time distributions were less curved compared with controls [curvature index 0.17 ± 0.6 vs. 0.0 ± 0.04; t(1,24) = 2.16, P < 0.04], while around 3-s peaks, curvature was not significantly different [curvature index −0.47 ± 0.08 vs. −0.41 ± 0.04; t(1,24) = 1.5, P < 0.14]. These data are consistent with prior work suggesting that patients with PD have impaired interval timing due to “temporal migration”, responding faster at long delays (Malapani et al. 1998).
Table 2.
Response time medians and means
| Species | Condition | Interval Length | Median | Mean | SE |
|---|---|---|---|---|---|
| Human | Young | 3 | 3.0 | 3.1 | 0.1 |
| 12 | 12.4 | 12.4 | 0.1 | ||
| Control | 3 | 3.1 | 3.1 | 0.1 | |
| 12 | 12.3 | 12.5 | 0.1 | ||
| PD | 3 | 3.4 | 3.4 | 0.1 | |
| 12 | 12.1 | 12.2 | 0.1 | ||
| Rodent | Control | 3 | 3.7 | 4.0 | 0.3 |
| 12 | 9.1 | 9.1 | 0.5 | ||
| MFC 6-OHDA | 3 | 3.9 | 4.2 | 0.2 | |
| 12 | 7.1 | 7.1 | 0.9 |
All values are in seconds. Statistics are given in the text.
MFC, medial frontal cortex; 6-OHDA, 6-hydroxydopamine.
Rodent behavior was significantly more variable than humans, possibly as a result of distinct behavioral strategies [greater SEs for humans compared with rats: t(1,81) = 9.1, P < <0.05; Table 2; Fig. 1C]. The variability of rodent behavior observed in this study was in the range of previous studies of interval timing in rodents (Kim et al. 2013; Meck 2006; Narayanan et al. 2012; Parker et al. 2014; Xu et al. 2014). Nonetheless, a repeated-measures ANOVA revealed that, across rodents, there was a main effect of both MFC 6-OHDA and interval, as well as a significant interaction term (Table 2; F = 7.7, P < 0.005). In sessions after unilateral MFC dopamine depletion (Figs. 1C and 2), animals pressed the lever less often overall [147 ± 49 vs. 49 ± 7; t(1,8) = 2.4, P < 0.05]. Rodent dopamine depletion also decreased curvature indexes for 12-s trials only [Int3: −0.36 ± 0.04 vs. −0.32 ± 0.06; Int12 0.0 ± 0.05 vs. −0.32 ± 0.08; t(1,8) = 2.9, P < 0.02; Fig. 1C]. Consistent with our prior work, these data suggest that unilateral rodent MFC dopamine depletion modeled aspects of PD in humans (Narayanan et al. 2012; Parker et al. 2014). Despite this, rodents still had different response times on Int3 and Int12 trials in control and dopamine-depleted session [control: t(1,8) = 11.3, P < <0.05; 6-OHDA: t(1,8) = 3.2, P < 0.01]. These data suggest that disrupting dopamine impaired interval timing performance in both species by attenuating response-timing curves on Int12 trials.
Interval timing involves MFC delta and theta activity in humans and rodents.
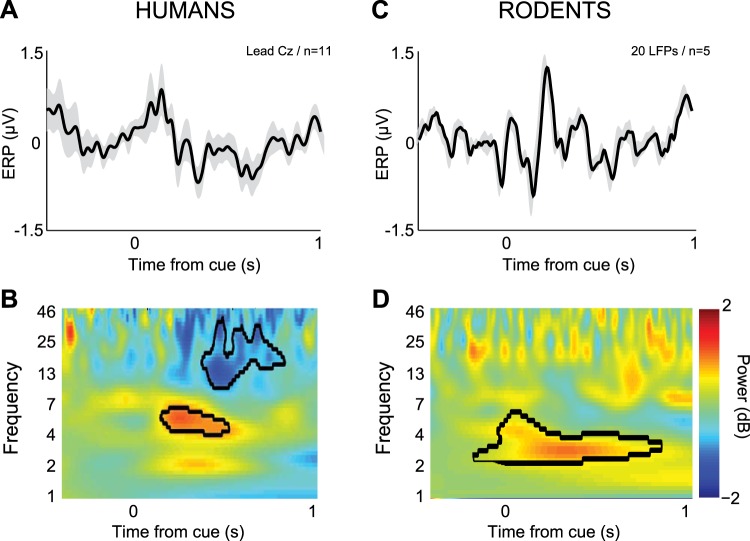
To explore the neurophysiological basis of interval timing, we recorded EEG data from 11 young participants during an interval timing task with 3- and 12-s delays (see Table 2 for behavioral data). We found consistent cue-related potentials (Fig. 3A) and delta/theta spectral activity occurring ∼0–0.5 s after instructional cue (∼4–7 Hz; Fig. 3B). Our laboratory's previous work has suggested that humans and rodents had similar patterns of activity in this range at critical moments during behavior (Narayanan et al. 2013a). To test this idea, we recorded LFPs from the MFC of five rodents. This signal represents the integrated synaptic activity of a brain region and has some similarities to EEG in humans (Murthy and Fetz 1996). We found that in these rodents, ERPs and 2- to 6-Hz bursts of activity were triggered by the cue during interval timing tasks (Fig. 3, C and D). Activity in this band is a well-described mechanism of cognitive control (Cavanagh and Frank 2014; Harmony 2013). For the remainder of the paper, we focus on and test hypotheses about these cue-related delta/theta oscillations.
Fig. 3.
Cue-related delta/theta activity (2–6 Hz) during timing tasks in humans and rodents. A: in 11 young participants, normalized EEG event-related potential (ERP) revealed peaks ∼0.2 s from the cue over midfrontal lead Cz on 12-s trials. B: time-frequency analysis of signals from lead Cz revealed a prominent burst of delta and theta activity (∼3–6 Hz). C: in 5 rodents, 20 local field potentials (LFPs) were recorded from MFC and also revealed an ERP. D: a burst of delta and theta activity was triggered by the cue during interval timing tasks in rodents (B) from ∼3–6 Hz. This band was subsequently studied in PD patients, age-matched controls, and rodents with and without MFC dopamine.
MFC delta/theta activity in humans and rodents requires intact dopamine circuits.
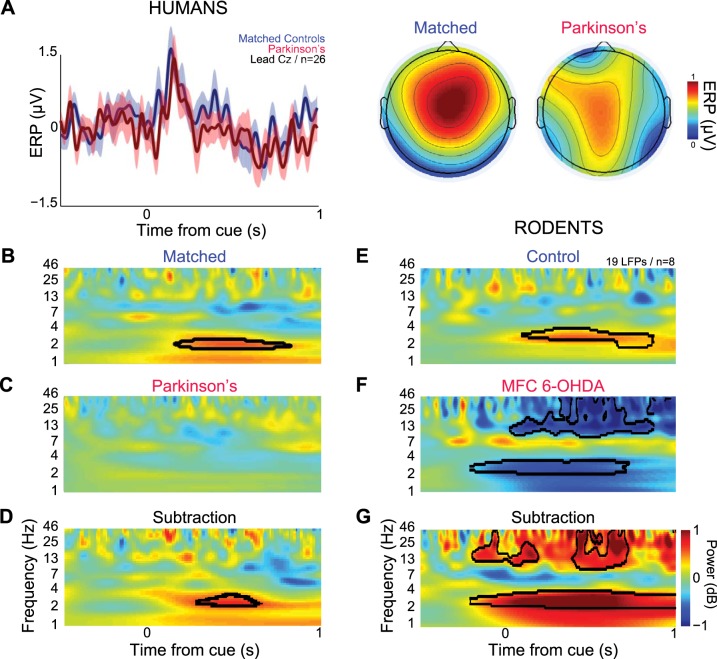
We hypothesized that delta/theta activity in MFC would be attenuated in patients with PD and in PD animal models with dysfunctional dopamine signaling (allowing for some temporal slowing and frequency decline in these ROI boundaries due to the advanced age of the PD and control participants, c.f., Kok 2000; Polich 1997; Picton et al. 1984). To directly test this idea, we recorded EEG data from 13 patients with PD and 13 age- and education-matched controls using a standard clinical 19-lead montage. We found obligatory cue-locked ERPs in PD and in matched controls from midfrontal lead Cz, with peaks ∼0.2 s from the onset of the cue (Fig. 4A, left; data from 3-s and 12-s trials were combined). Voltage topography was focused over MFC in matched controls as determined within the limits of a 19-lead clinical montage (0.1–0.2 s after cue; Fig. 4A, right). Both matched control and PD groups had a vertex/midfrontal orientation of event-related activity to the cue.
Fig. 4.
Medial frontal delta and theta rhythms depend on dopamine. A, left: normalized EEG ERP from 13 matched control participants (blue) and PD patients (red) revealed peaks ∼0.2 s from the onset of the cue on midfrontal lead Cz. At right, the topographic distribution of ERPs over MFC are plotted (0.1–0.2 s after cue) in controls (left) and in PD patients (right). Time-frequency analysis of signals from lead Cz revealed a prominent burst of delta and theta activity (∼2–6 Hz) in matched controls (B), while patients with PD had deactivations 0.5–1.0 s following the cue (C). D: the between-group subtraction revealed that PD patients had significantly less low-frequency activity (∼2–4 Hz) than the matched controls. E: rodents had delta and theta cue-triggered activity (∼2–4 Hz; note that these data are similar to Fig. 3D in a different set of rodents) over MFC. F: with MFC dopamine depletion, deactivation was seen in this band. G: the between-group subtraction revealed that depleting dopamine in the MFC significantly minimizes the low-frequency activity (∼2–5 Hz) compared with control animals. Black lines indicate increases in power relative to baseline or matched controls in the subtraction condition at P < 0.05 via a t-test.
Consistent with previous findings and with our initial experiments in young humans (Fig. 3), significant activity in delta and theta was observed in matched controls (Fig. 4B; compared with the − 0.5- to −0.3-s baseline). This oscillation was attenuated in humans with PD, who had significant deactivation in this range (Fig. 4C). A direct comparison of these signals revealed significantly less ∼2- to 5-Hz activity 0.3–0.5 s after the cue in PD patients compared with age-matched controls (Fig. 4D). These data support the hypothesis that MFC delta/theta rhythms are diminished in PD.
Our laboratory's recent work established that, in rodents, MFC delta/theta rhythms are decreased with D1 dopamine receptor blockade (Parker et al. 2014). These data predict that depletion of MFC dopamine will attenuate delta/theta rhythms. Consistent with our laboratory's prior work, we observed spectral activity from ∼2–5 Hz in MFC LFPs (Fig. 4E) (Narayanan et al. 2013a; Parker et al. 2014). This activity significantly attenuated in rodents after focal MFC dopamine depletion using the toxin 6-OHDA (Fig. 4, F and G).
We examined the distribution of delta/theta activity in PD and in animal models. Both human and rodents had normal distributions of cue-triggered delta/theta activity (0.3–0.5 s after cue onset, 2–5 Hz; Jarque-Bera test for normality; P < 0.42 for humans; P < 0.13 for rats). Both control humans and rats had similar strengths of cue-triggered delta/theta activity [t(1,30) = 0.1, P < 0.92]. PD patients had significantly less cue-triggered delta/theta activity compared with matched controls [t(1,24) = 2.4, P < 0.03; Fig. 5A]. A similar effect was also observed in rodents [paired t(1,18) = 2.7, P < 0.02; Fig. 5B]. Taken together, these data indicate that in both human PD and in animal models, delta/theta activity depends on intact dopaminergic circuitry. While activation and deactivation in other bands were observed, we restricted our interpretation to cue-related delta/theta activity as predicted by our pilot experiment and past work. These data provide evidence that delta/theta activity triggered by the cue requires medial frontal dopamine in humans and rodents.
Fig. 5.
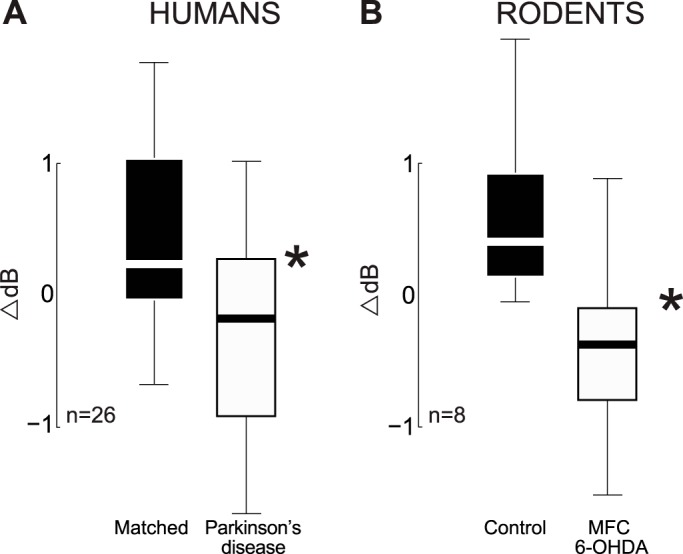
Boxplots of cue-triggered delta/theta bursts in PD and in animal models. A: in humans, 2- to 5-Hz activity 0.3–0.5 s after cue onset was attenuated in PD patients compared with age/education-matched controls. B: in rodents, 2- to 5-Hz activity 0.3–0.5 s after cue onset was attenuated in rodents with unilateral MFC dopamine depletion. The top of the box is the third quartile, the white/black line is the second quartile, and bottom of the box is the first quartile, and the whiskers range from the smallest to the largest nonoutlier. *Significance via a t-test.
MFC neurons are functionally coupled to delta/theta activity.
Next, we examined MFC single-neuron activity in detail. These neurons are intimately involved in temporal processing (Ito et al. 2003; Kim et al. 2013; Niki and Watanabe 1979; Parker et al. 2014; Sheth et al. 2012; Xu et al. 2014). Here, we recorded MFC neurons from the same neurons with which we recorded MFC LFPs, facilitating analysis of how cue-related MFC LFP delta/theta activity was related to single MFC neurons during interval timing.
We recorded MFC neurons 1 wk after MFC dopamine depletion (109 vs. 101 in MFC 6-OHDA sessions). Among these neurons, dopamine depletion did not change overall firing rate [2.6 ± 0.2 vs. 2.2 ± 0.3; t(1,208) = 0.7, P > 0.5] or modulation around behavioral events such as house light (7 vs. 6 neurons in MFC 6-OHDA sessions) or lever press (12 vs. 13 neurons in MFC 6-OHDA sessions).
To formally quantify relationships between MFC neural activity and LFPs, we used spike-field coherence (Rosenberg et al. 1989). Across 109 neurons, we found significant average spike-field coherence (Fig. 6A, top; spike-field coherence plotted relative to 95% confidence interval for coherence) in delta/theta ranges around the time of the imperative stimuli. This coherence was also observed in our laboratory's prior work (Parker et al. 2014). With MFC 6-OHDA, this pattern of average coherence across 101 MFC neurons appeared to be distinct (Fig. 6A, middle). A direct comparison of coherence between control and MFC revealed that there was significantly more coherence in control compared with MFC 6-OHDA sessions [spike-field coherence 2–6 Hz 0–2 s after cue; t(1,208) = 2.6, P < 0.001; Fig. 6A, bottom].
Fig. 6.
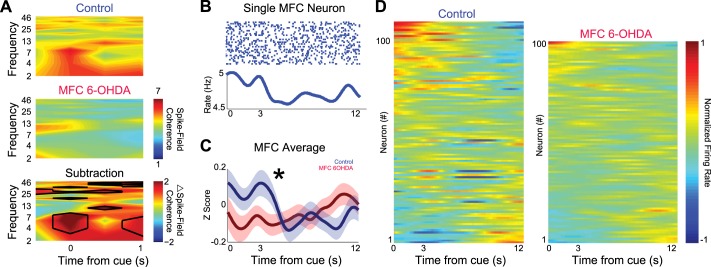
Focal MFC dopamine depletion impairs delta/theta coupling of single MFC neurons with MFC field potentials. A, top: spike-field coherence from control sessions revealed marked coherence in delta and theta range around the cue. Spike-field coherence was normalized to 95% confidence intervals of coherence to compare across neurons, animals, and sessions. Middle: in sessions with MFC 6-OHDA, a markedly different pattern was observed in the same rodents from the same MFC neuronal ensembles. Bottom: subtraction of control and MFC 6-OHDA sessions revealed significantly more spike-field coherence between 2 and 6 Hz around the cue during timing tasks. Black lines indicate significance via a t-test. B: a peri-event raster plot from a single MFC neuron that had increased firing earlier in the interval on Int12 trials only. C: on average, firing rates were significantly higher immediately after the cue in control sessions compared with sessions with MFC 6-OHDA (109 vs. 101 neurons; 0–2 s after the cue). *P < 0.05 via a t-test. D: all neuronal activity sorted by principal component analysis in control (left) and MFC 6-OHDA (right) sessions; much less modulation was observed in MFC 6-OHDA sessions.
To investigate how MFC neurons involved in temporal processing were influenced by dopamine, we examined neuronal activity in MFC after dopamine depletion. While there are diverse patterns of temporal processing within MFC, one pattern is “ramping” activity that consistently changes over the temporal interval, integrating temporal evidence during timing tasks (Durstewitz 2003; Parker et al. 2014). Single neurons could decrease firing over the interval on Int12 trials, with an inflection point after 3 s (the timing that reward would be available on Int3 trials; Fig. 6B). On average, the firing rate in MFC 6-OHDA sessions was significantly less immediately after the cue [0–2 s after cue; t(1,208) = 2.2, P < 0.03; Fig. 6, C and D]. Taken together, these data indicate that, after MFC 6-OHDA, single MFC neurons tended to be less coherent with MFC fields at delta/theta bands and less active immediately after the cue during interval timing tasks.
Delta/theta activity correlates with executive function.
The preceding sections establish that low-frequency spectral activity in MFC is attenuated in PD patients and attenuates temporal processing by single MFC neurons in rodent models. If this activity was related to cognitive performance during interval timing, then it should correlate with executive functions. In our patient populations, we performed a battery of standard neuropsychological tests focusing on this domain, including Stroop tests, TMT A and B, DFB, VF, and WCST. We focused only on tests in which PD patients and controls did not differ (Stroop, TMT, WCST). A composite cognitive index was generated by averaging test scores using rectified z-score, where higher z-scores correspond to better executive functions. We correlated cognitive performance with spectral activity that was significantly distinct between control and PD patients (2–4 Hz; 0.3–0.5 s after the cue; Fig. 4, B and C).
In controls, cue-related spectral activity was significantly correlated with composite executive indexes (R = 0.75, P < 0.004). This was not true in PD patients. This finding was replicated in both the Int3 and Int12 trials (Int3: R = 0.61, P < 0.03; Int12 R = 0.70, P < 0.01). A Fisher's R-to-Z transformation indicated that this relationship was significantly different between PD patients and controls (T = 2.6, P < 0.007; Fig. 7). These data indicate that MFC low-frequency spectral activity in MFC correlates with executive function during interval timing, and this relationship is aberrant in PD.
Fig. 7.
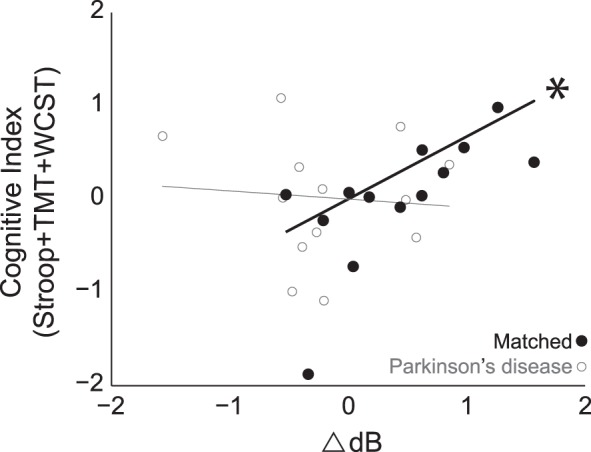
Low-frequency activity was correlated with cognitive function in control but not PD patients. In matched controls, cue-related spectral activity (2–4 Hz, 0.3–0.5 s after cue) was strongly related to cognitive function (R = 0.75, *P < 0.004). This was not observed in PD patients. Executive function was indexed by neuropsychological tests that did not differ in PD vs. controls [Trail Making Test (TMT) + Stroop + Wisconsin Card Sort Test (WCST); Table 3.] A Fisher's R-to-Z transformation indicated that this relationship was significantly stronger for controls compared with PD patients.
Table 3.
Neuropsychological tests
| Task | Matched | PD | P Value |
|---|---|---|---|
| TMT | 32.6 ± 6.1 | 44.7 ± 10.0 | 0.16 |
| Stroop 1 | −0.2 ± 3.3 | 0.0 ± 1.7 | 0.48 |
| Stroop 2 | 30.1 ± 3.1 | 27.5 ± 2.5 | 0.26 |
| Verbal fluency | 50.0 ± 4.1 | 38.4 ± 2.4 | 0.01* |
| Digit total | 19.5 ± 1.0 | 17.0 ± 0.9 | 0.03* |
| WCST | 12.0 ± 3.1 | 10.0 ± 2.1 | 0.30 |
Values are means ± SE.
TMT, Trail Making Test; WCST, Wisconsin Card Sort Test.
Statistically significant.
DISCUSSION
We studied the neurophysiological basis of interval timing in humans and rodents, as well as how neural activity is affected by disruption in dopamine signaling in PD and in animal models of PD. We found four major results. First, humans and rodents had cue-triggered delta/theta band MFC activity during interval timing. Second, this activity was attenuated in patients with PD and in rodent models with focal MFC dopamine depletion. Third, single MFC neurons involved in temporal processing were functionally coupled with delta/theta rhythms and attenuated by MFC dopamine depletion. Finally, cue-related delta/theta activity correlated with executive performance in controls but not PD, suggesting a disease-specific (and not treatment-specific) aberration in basic mechanisms that contribute to elementary cognitive processes.
Neuronal oscillations in low frequencies are a consistent correlate of cognitive control in adaptation, error, uncertainty, conflict, and surprise (Cavanagh et al. 2009; Cavanagh and Shackman 2015; Harmony 2013). Low frequencies have been observed in rodent MFC (Narayanan et al. 2013a; Parker et al. 2014; Warren et al. 2015) and are observed again here triggered by the cue. It is likely that these signals are not unique to timing tasks, as they may represent an alerting or orienting response signifying a generic need for cognitive control (Cavanagh and Frank 2014). Our data do not indicate that these signals are directly predictive of timing behavior. Rather, they are present at the time of cue, altered in humans and rodents with dysfunctional medial frontal dopamine, and can be coherent with medial frontal neurons that encode temporal signals (Bekolay et al. 2014; Durstewitz 2003; Matell and Meck 2004; Niki and Watanabe 1979). This paper and prior work indicate that delta/theta oscillations could be involved in synchronizing neurons involved in temporal processing at the time of cue (Parker et al. 2014). Directly testing this hypothesis would involve manipulating these oscillations independent of medial frontal neurons, which would require understanding the source of delta/theta activity in the cortex.
Although it is clear that delta and theta activity is generated in the MFC networks, the exact origin is still unclear. MFC has been shown to generate theta in humans and nonhuman primates (Wang et al. 2005; Tsujimoto et al. 2010; Womelsdorf et al. 2010), but it is not known how much of the scalp-recorded signal is comprised of MFC-generated activity. Our laboratory's prior work (Parker et al. 2014) and data in this paper demonstrate that disrupting dopamine signaling in the MFC attenuates interval timing behavior, cue-related delta/theta activity and theta/delta coupling with single neurons. However, with our current approach we cannot infer causal relationships between cortical dopamine, temporal processing, and spectral activity. Our methods also do not indicate if our spikes are coherent with delta/theta activity, or if both phenomenon are coherent with some other process, such as behavior or ascending neuromodulatory activity (Aru et al. 2015). We observe decreased cue-related field potential activity in the delta/theta range, spike-field coherence in delta/theta range, as well as decreased temporal modulation by MFC neurons with dopamine depletion. This decrease in spike-field coherence may not be independent from the field-potential results. We cannot determine how these phenomena are related, as decreased spike-field coupling that we observe in MFC 6-OHDA sessions could simply be a related correlate of decreased field potential power in this range, and the physiological significance of this loss of coupling is difficult to determine. Optogenetic techniques that can independently manipulate spiking activity may provide insight into the relationship between these phenomena.
Our data are among the first to report alterations in these frequencies in patients and animal models of PD in MFC. In previous studies, it was found that temporal processing in PD appears to markedly affect beta and alpha rhythms in PD in motor cortex, although previous investigators did not focus on lower frequencies (Praamstra and Pope 2007). Recent work has implicated decreased theta in addition to alpha and beta frequencies in diagnosis of PD, in distinct brain regions (Benz et al. 2014; Gu et al. 2014 Han et al. 2013; Praamstra and Pope 2007). Our work extends this line of research by focusing on MFC and linking spectral activity in PD patients with mechanistic theories (Cavanagh and Frank 2014) and single neuron activity that is consistently involved in temporal processing (Bekolay et al. 2014; Kim et al. 2013; Matell and Meck 2004; Narayanan and Laubach 2009; Niki and Watanabe 1979; Parker et al. 2014; Xu et al. 2014).
The findings reported here suggest that cortical dopamine is a significant contributor to temporal processing by medial frontal brain networks (Narayanan et al. 2013b). In MFC, cortical dopamine appears to act via D1 dopamine receptors to achieve temporal control. Focal MFC administration of D1 but not D2 antagonists selectively impairs interval timing (Narayanan et al. 2012) and temporal processing in reaction-time tasks (Parker et al. 2013). Moreover, optogenetic inhibition of MFC neurons expressing D1 dopamine receptors impairs interval timing (Narayanan et al. 2012). Finally, MFC D1 blockade selectively attenuated 4-Hz activity and ramping activity of MFC neuronal ensembles (Parker et al. 2014). D1-type dopamine receptors in prefrontal regions are intimately involved in a range of executive processes (Abi-Dargham et al. 2002; Goldman-Rakic et al. 2000). Our data indicate that patients with PD no longer had correlations between low-frequency spectral activity and executive functions, suggesting that activity ∼2–6 Hz may contribute to this cognitive processing.
Of note, PD is a heterogeneous disease with massive loss of dopaminergic signaling in corticostriatal circuits, and with broad changes in neurotransmitters such as acetylcholine, norepinephrine, serotonin, as well as corresponding changes in cortical networks (Narayanan et al. 2013b). Our data imply that, in rodents, unilateral MFC 6-OHDA is sufficient to attenuate delta/theta cue-related activity in MFC, modeling deficits in PD patients. 6-OHDA depletes not only dopamine but related catecholamines; therefore, we could not rule out the possibility that the observed effect in rodent is caused by disruption of catecholamines. However, in PD patients, the unique laterality and complexity of human cortex may be relevant. Because PD often begins unilaterally, future studies could study how delta/theta rhythms are influenced by laterality in PD and study disease progression (Djaldetti et al. 2006). These studies could also investigate the contribution of the diverse neurotransmitter systems involved in PD, as well as synuclein overexpression on elementary cognitive function (Eberling et al. 2013).
These findings are limited by low-density clinical recording, obviating any ability to estimate generative sources. In addition, our ERPs were low amplitude, in part due to the age of our population. Time-frequency findings were robust even with this highly limited clinical montage, suggesting that potential future biomarker assays could utilize clinical setups that are common in most major hospitals and clinics. However, a more near-term goal remains to address these definitive limitations using high-density research arrays, magnetoencephalography, or by using intraoperative recordings. We also find significant activations in other frequencies, such as beta rhythms, particularly in rodents. We are attempting to compare rodent intracranial field-potentials with human extrascalp EEG. Despite vast differences in methods, species, and behavioral strategies, delta/theta activity was observed after the instructional stimulus from medial frontal regions of humans and rodents. This activity was attenuated with dopamine disruption. Future work should explore this issue with human intracranial recordings from MFC as well as in nonhuman primates may help explore the generalizability of our work. Finally, rodents and humans may adopt vastly distinct behavioral strategies during this elementary task. Both human and rodent impairments could be due to a similar degradation in a low-level mechanism that contributes to a wealth of cognitive states, which in turn may be differentially utilized on this task (e.g., working memory in humans, yet sustained attention in rats). Future work could explore this issue by comparing the performance of PD patients and animal models on tasks that test these possibilities (Donnelly et al. 2015).
In summary, our data show that both humans and rodents have ∼4-Hz MFC activity triggered by the instructional cue during timing tasks, and that this activity is attenuated when dopamine signaling is dysfunctional. Our data contribute to the neurophysiological basis of interval timing and provide insight into how medial frontal brain networks are dysfunctional during elementary executive processing in PD. These findings could be helpful in developing novel EEG-based biomarkers for PD and other diseases involving MFC dysfunction.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants R01 NS089470 and K08 NS078100, National Alliance for Research on Schizophrenia and Depression Young Investigator Awards to K. L. Parker and N. S. Narayanan, and Nellie Ball Research Trust to K. L. Parker.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.L.P., K.-H.C., J.F.C., and N.S.N. conception and design of research; K.L.P., K.-H.C., and J.R.K. performed experiments; K.L.P., J.R.K., J.F.C., and N.S.N. analyzed data; K.L.P., K.-H.C., J.R.K., and N.S.N. interpreted results of experiments; K.L.P., J.F.C., and N.S.N. prepared figures; K.L.P. and N.S.N. drafted manuscript; K.L.P., K.-H.C., J.R.K., J.F.C., and N.S.N. edited and revised manuscript; K.L.P., J.F.C., and N.S.N. approved final version of manuscript.
ACKNOWLEDGEMENTS
We acknowledge Ergun Uc for advice on subject recruitment, Steven W. Anderson for advice on cognitive testing, Deanne Tadlock for technical consult advice, and Tracy Lukasiewicz for data entry.
REFERENCES
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 22: 3708–3719, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SW, Damasio H, Jones RD, Tranel D. Wisconsin card sorting test performance as a measure of frontal lobe damage. J Clin Exp Neuropsychol 13: 909–922, 1991. [DOI] [PubMed] [Google Scholar]
- Aru J, Aru J, Priesemann V, Wibral M, Lana L, Pipa G, Singer W, Vicente R. Untangling cross-frequency coupling in neuroscience. Curr Opin Neurobiol 31: 51–61, 2015. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP, Delis DC, Kramer J, Kaplan E. Verbal and design fluency in patients with frontal lobe lesions. J Int Neuropsychol Soc 7: 586–596, 2001. [DOI] [PubMed] [Google Scholar]
- Bekolay T, Laubach M, Eliasmith C. A spiking neural integrator model of the adaptive control of action by the medial prefrontal cortex. J Neurosci 34: 1892–1902, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz N, Hatz F, Bousleiman H, Ehrensperger MM, Gschwandtner U, Hardmeier M, Ruegg S, Schindler C, Zimmermann R, Monsch AU, Fuhr P. Slowing of EEG background activity in Parkinson's and Alzheimer's disease with early cognitive dysfunction. Front Aging Neurosci 6: 314, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SW. Timing and executive function: bidirectional interference between concurrent temporal production and randomization tasks. Mem Cognit 34: 1464–1471, 2006. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 6: 755–765, 2005. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJB. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci 29: 98–105, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci 18: 414–421, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. J Physiol Paris 109: 3–15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJB. Theta lingua franca: a common mid-frontal substrate for action monitoring processes. Psychophysiology 49: 220–238, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. A neural microcircuit for cognitive conflict detection and signaling. Trends Neurosci 37: 480–490, 2014. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. [DOI] [PubMed] [Google Scholar]
- Djaldetti R, Ziv I, Melamed E. The mystery of motor asymmetry in Parkinson's disease. Lancet Neurol 5: 796–802, 2006. [DOI] [PubMed] [Google Scholar]
- Donnelly NA, Paulsen O, Robbins TW, Dalley JW. Ramping single unit activity in the medial prefrontal cortex and ventral striatum reflects the onset of waiting but not imminent impulsive actions. Eur J Neurosci 41: 1524–1537, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Fairhurst S, Malapani C, Horvitz JC, Balsam PD. Effects of dopamine antagonists on the timing of two intervals. Pharmacol Biochem Behav 75: 9–15, 2003. [DOI] [PubMed] [Google Scholar]
- Durstewitz D. Self-organizing neural integrator predicts interval times through climbing activity. J Neurosci 23: 5342–5353, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Dave KD, Frasier MA. α-Synuclein imaging: a critical need for Parkinson's disease research. J Parkinsons Dis 3: 565–567, 2013. [DOI] [PubMed] [Google Scholar]
- Fry W, Kelleher RT, Cook L. A mathematical index of performance on fixed-interval schedules of reinforcement. J Exp Anal Behav 3: 193–199, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev 31: 295–301, 2000. [DOI] [PubMed] [Google Scholar]
- Gu Y, Chen J, Lu Y, Pan S. Integrative frequency power of EEG correlates with progression of mild cognitive impairment to dementia in Parkinson's disease. Clin EEG Neurosci 1550059414543796, 2014. [DOI] [PubMed] [Google Scholar]
- Han CX, Wang J, Yi GS, Che YQ. Investigation of EEG abnormalities in the early stage of Parkinson's disease. Cogn Neurodyn 7: 351–359, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmony T. The functional significance of delta oscillations in cognitive processing. Front Integr Neurosci 7: 83, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton SC, Meck WH. Frontal-striatal circuitry activated by human peak-interval timing in the supra-seconds range. Brain Res Cogn Brain Res 21: 171–182, 2004. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science 302: 120–122, 2003. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jones CRG, Zijlmans J, Katzenschlager R, Lee L, Quinn N, Frith CD, Lees AJ. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson's disease. Brain 133: 727–745, 2010. [DOI] [PubMed] [Google Scholar]
- Kim J, Ghim JW, Lee JH, Jung MW. Neural correlates of interval timing in rodent prefrontal cortex. J Neurosci 33: 13834–13847, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP). Biol Psychol 54: 107–143, 2000. [DOI] [PubMed] [Google Scholar]
- Malapani C, Rakitin B, Levy R, Meck WH, Deweer B, Dubois B, Gibbon J. Coupled temporal memories in Parkinson's disease: a dopamine-related dysfunction. J Cogn Neurosci 10: 316–331, 1998. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain Res Cogn Brain Res 21: 139–170, 2004. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH, Nicolelis MAL. Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behav Neurosci 117: 760–773, 2003. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuroanatomical localization of an internal clock: a functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res 1109: 93–107, 2006. [DOI] [PubMed] [Google Scholar]
- Merchant H, Harrington DL, Meck WH. Neural basis of the perception and estimation of time. Annu Rev Neurosci 36: 313–336, 2013. [DOI] [PubMed] [Google Scholar]
- Merchant H, Luciana M, Hooper C, Majestic S, Tuite P. Interval timing and Parkinson's disease: heterogeneity in temporal performance. Exp Brain Res 184: 233–248, 2008. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Synchronization of neurons during local field potential oscillations in sensorimotor cortex of awake monkeys. J Neurophysiol 76: 3968–3982, 1996. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Cavanagh JF, Frank MJ, Laubach M. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat Neurosci 16: 1888–1897, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience 139: 865–876, 2006. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ. Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci U S A 109: 20726–20731, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Methods for studying functional interactions among neuronal populations. Methods Mol Biol 489: 135–165, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Rodnitzky RL, Uc EY. Prefrontal dopamine signaling and cognitive symptoms of Parkinson's disease. Rev Neurosci 24: 267–278, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki H, Watanabe M. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res 171: 213–224, 1979. [DOI] [PubMed] [Google Scholar]
- Parker KL, Chen KH, Kingyon JR, Cavanagh JF, Narayanan NS. D1-dependent 4 Hz oscillations and ramping activity in rodent medial frontal cortex during interval timing. J Neurosci 34: 16774–16783, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Lamichhane D, Caetano MS, Narayanan NS. Executive dysfunction in Parkinson's disease and timing deficits. Front Integr Neurosci 7: 75, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Champagne SC, Nelson RF. The effects of age on human event-related potentials. Psychophysiology 21: 312–325, 1984. [DOI] [PubMed] [Google Scholar]
- Polich J. EEG and ERP assessment of normal aging. Electroencephalogr Clin Neurophysiol 104: 244–256, 1997. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Pope P. Slow brain potential and oscillatory EEG manifestations of impaired temporal preparation in Parkinson's disease. J Neurophysiol 98: 2848–2857, 2007. [DOI] [PubMed] [Google Scholar]
- Robinson AL, Heaton RK, Lehman RA, Stilson DW. The utility of the Wisconsin Card Sorting Test in detecting and localizing frontal lobe lesions. J Consult Clin Psychol 48: 605–614, 1980. [DOI] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53: 1–31, 1989. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 488: 218–221, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 19: 203–214, 2004. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 25: 2649–2653, 2010. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y, Sasaki K. Theta oscillations in primate prefrontal and anterior cingulate cortices in forewarned reaction time tasks. J Neurophysiol 103: 827–843, 2010. [DOI] [PubMed] [Google Scholar]
- Tukey J. Exploratory Data Analysis. Reading, MA: Addison-Wesley, 1977. [Google Scholar]
- Uc EY, Doerschug KC, Magnotta V, Dawson JD, Thomsen TR, Kline JN, Rizzo M, Newman SR, Mehta S, Grabowski TJ, Bruss J, Blanchette DR, Anderson SW, Voss MW, Kramer AF, Darling WG. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology 83: 413–425, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rijn H, Kononowicz TW, Meck WH, Ng KK, Penney TB. Contingent negative variation and its relation to time estimation: a theoretical evaluation. Front Integr Neurosci 5: 91, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci 25: 604–613, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CM, Hyman JM, Seamans JK, Holroyd CB. Feedback-related negativity observed in rodent anterior cingulate cortex. J Physiol Paris 109: 87–94, 2015. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Johnston K, Vinck M, Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc Natl Acad Sci U S A 107: 5248–5253, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Zhang S, Dan Y, Poo M. Representation of interval timing by temporally scalable firing patterns in rat prefrontal cortex. Proc Natl Acad Sci U S A 111: 480–485, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]