Abstract
Background:
Several previous investigations have determined potential risk factors for stress fractures in athletes and military personnel.
Purpose:
To determine factors associated with the development of stress fractures in female athletes.
Study Design:
Case-control study; Level of evidence, 3.
Methods:
A total of 88 female athletes (cross-country, n = 29; soccer, n = 15; swimming, n = 9; track and field, n = 14; volleyball, n = 12; and basketball, n = 9) aged 18 to 24 years were recruited to participate in a longitudinal bone study and had their left distal tibia at the 4%, 20%, and 66% sites scanned by peripheral quantitative computed tomography (pQCT). Patients included 23 athletes who developed stress fractures during the following year (cases). Whole body, hip, and spine scans were obtained using dual-energy x-ray absorptiometry (DXA). Analysis of covariance was used to determine differences in bone parameters between cases and controls after adjusting for height, lower leg length, lean mass, fat mass, and sport.
Results:
No differences were observed between cases and controls in any of the DXA measurements. Cases had significantly greater unadjusted trabecular bone mineral content (BMC), greater polar moment of inertia (PMI) at the 20% site, and greater cortical BMC at the 66% site; however, after adjusting for covariates, the differences became nonsignificant. When analyses were repeated using all individuals who had ever had a stress fracture as cases (n = 31) and after controlling for covariates, periosteal circumference was greater in the cases than the controls (71.1 ± 0.7 vs 69.4 ± 0.5 mm, respectively; P = .04).
Conclusion:
A history of stress fractures is associated with larger bones. These findings are important because larger bones were previously reported to be protective against fractures and stress fractures, but study findings indicate that may not always be true. One explanation could be that individuals who sustain stress fractures have greater loading that results in greater periosteal circumference but also results in the development of stress fractures.
Keywords: stress fractures, female, athletes, moment of inertia, pQCT
Stress fractures are common injuries in athletes competing at all levels. While the causes of stress fractures are not completely understood, theories exist as to possible causes. Participation in athletics appears to be a risk factor for stress fractures, as evidenced by a prospective study that followed 111 track and field athletes for 1 year and reported a stress fracture incidence of 0.70 per 1000 hours of training.7 Stress fractures are also a concern within the military, with 1 study reporting approximately 5% of all personnel sustaining a stress fracture during training1 and another reporting that 5% of males and almost 20% of females sustained a stress fracture within 3 months of the start of training.10 Other risk factors that have been reported include being a female military recruit,8,14,19,28 menstrual irregularity in track and field athletes,5,6 and lower levels of physical fitness prior to the onset of military training.4,32,39 The potential benefit of previous participation in athletics is variable, with 1 study reporting a protective effect of previous sport participation during basic training32 while another reported no effect of previous sport participation on stress fracture incidence in military recruits.39 While some studies in female athletes report that the tibial shaft is the most common site for stress fractures,6,25 others reported that the location of stress fractures are site-specific based on the activities being performed.25,27 Whether low bone mineral density (BMD) is a risk factor is not clear; some studies indicate an increased risk of stress fracture associated with low BMD in track and field athletes6 while others report no association in track and field athletes, cross-country athletes, and military recruits.5,9,11
Increased BMD and larger bone cross-sectional area are thought to be protective against fracture, and physical activity is widely accepted as a method for increasing BMD and bone size in healthy populations.2,21,26,29,30 Our previously published data and data from several other studies have shown that bone characteristics vary among different sports.22–24,34–37,41 Some of the sport-specific differences could potentially increase or decrease an individual’s risk of stress fracture. These findings, such as increased cross-sectional area in high-impact sports, support the Frost15–18 “mechanostat” and the Wolff42,43 law, which state that bone formation occurs based on the magnitude of the load that is placed upon it. These adaptations in bone are thought to create an individualized protection against fracture based on each person’s activity pattern.
Several studies have investigated possible bone measures as predictors of stress fractures.3,11,13,19,20,40 One study of 179 Finnish male military recruits reported that individuals who developed stress fractures were taller and had lower hip areal BMD (aBMD) and bone mineral content (BMC) than recruits who did not develop a stress fracture.40 Additionally, bone geometric properties such as decreased tibia width using dual-energy x-ray absorptiometry (DXA),3 cross-sectional area using DXA and computed tomography (CT),3,11 and section modulus using DXA and CT3,13 have been reported to be negatively associated with stress fracture risk.
Using DXA and peripheral quantitative computed tomography (pQCT) technology, along with participant information on previously identified potential stress fracture risks, could add valuable knowledge to the field. A 3-site pQCT protocol for imaging at the 4%, 20%, and 66% distal tibia site allows investigation of trabecular bone at the 4% site, cortical bone at the 20% site, and muscle cross-sectional area for muscle-bone relationships at the 66% site. Analysis of pQCT images allow us to investigate different regions within the cortical shell to determine whether the distribution of cortical bone throughout the shaft is related to stress fracture occurrence. The purpose of this study was to determine how factors such as bone mass and geometry, distribution of mass throughout the cortical shell, and body composition influence the risk of stress fractures in collegiate female athletes. Based on previous literature, we hypothesized that participants who developed stress fractures (cases) within 6 months of the bone measurements would have smaller bones than those participants who did not fracture (controls).
Materials and Methods
Subjects included 88 National Collegiate Athletic Association (NCAA) Division 1 female athletes from various teams at South Dakota State University. All visits were completed within 1 month of the beginning of each sport’s competitive season. Control subjects were defined as athletes with no history of stress fractures and who did not develop a stress fracture during the study (n = 57). Cases in the primary analyses were athletes who had developed any stress fracture during the study period (n = 23) or only tibial stress fractures (n = 10). A secondary analysis was performed comparing individuals who had suffered a stress fracture previously or developed a stress fracture during the study as cases (n = 31).
Height was measured to the nearest 0.5 cm using a portable stadiometer (Seca Model 225), and weight was measured to the nearest 0.1 kg using a digital scale (Seca Model 770). All participants completed a questionnaire to obtain information about menstrual status, family history of osteoporosis, medication and supplement use, sleeping habits, and activities of daily living. Participants also kept a 72-hour diet record to determine vitamin D, calcium, and macronutrient intakes. Diets were analyzed using The Food Processor Software (v 10.2; ESHA Research).
Peripheral Quantitative Computed Tomography
Tibia length was measured using a segmometer (Rosscraft) as the total distance between the medial tibial plateau and the medial malleolus of the tibia. A scout view was used to mark the endplate of the bone, and slice images were obtained at 4%, 20%, and 66% of the tibia length from the distal end using the XCT 3000 (Orthometrix Inc). Voxel size was set to 0.5 mm and a scan speed of 20 mm/s to obtain the images. Slice images were analyzed using the manufacturer’s software (version 6.0B). Contour mode 2, pPeel mode 2, and a threshold of 400 mg/cm3 were settings for 4% trabecular bone analysis. Trabecular outcomes were trabecular area, BMC, and volumetric bone mineral density (vBMD). Cort mode 1 with a threshold of 280 mg/cm3 to identify the bone edge for strength strain index (SSI) and 710 mg/cm3 to identify the bone edge for other cortical bone measures were used for cortical bone analysis. At the 20% and 66% slices, cortical area, thickness, BMC, and vBMD along with polar moment of inertia (PMI), polar strength strain index (pSSI), and periosteal and endosteal circumferences were measured. Additionally, muscle cross-sectional area was measured at the 66% slice. The manufacturer recommends a 2-step process for calculating muscle area at the 66% site. Step 1 utilized contour mode 3 with a threshold of 40 mg/cm3 and peel mode 1 at 100%, with smoothing filter F03F05 selected. This step removes subcutaneous fat and determines the cross-sectional area of muscle (including the bone area) as the total area. Step 2 of the analysis used contour mode 1 with a threshold of 280 mg/cm3, peel mode 1 at 100%, and smoothing filter F03F05 to obtain the bone area of the tibia and fibula, given as the total area outcome. The total area determined in step 2 is subtracted from that in step 1 to measure muscle cross-sectional area of the 66% site. BoneJ12 and ImageJ38 software, utilizing the same threshold parameters, were also used to analyze the bone images. Additional variables obtained using BoneJ and ImageJ included radial (endocortical, midcortical, and pericortical) and polar (anterior, posterior, medial, lateral) vBMD measurements. Coefficients of variation for these measurements at our site using the Stratec software are 0.5% to 2.8% for trabecular (4% distal site) and 0.5% to 1.2% for cortical measurements (20% distal site).
Dual-Energy X-Ray Absorptiometry
Whole-body, lumbar spine, and hip images were obtained using DXA (v 3.2; Hologic Apex). Whole-body images were analyzed for lean mass, fat mass, BMC, and percentage body fat (Apex v 3.2; non–National Health and Nutrition Examination Survey reference base). Bone area, BMC, and aBMD of the spine, femoral neck, and total hip were measured. Our institution’s coefficients of variation for these measurements range from 0.8% to 1.3% for whole body, 0.9% to 1.5% for the spine, and 1.7% to 2.8% for the hip.
Subjects
All procedures and questionnaires used in this study were reviewed and approved by the human subjects review committee, and written informed consent was obtained from all participants.
Statistical Analysis
All analyses were performed in STATA release 11 (STATA Corp). Analyses were performed using the groups described above (developed any stress fracture or a tibial stress fracture during the study period or ever had a stress fracture vs controls). In all analyses, the controls never had or developed a stress fracture. Anthropometric and muscle and bone measurements were compared between cases and controls using independent t tests. Potential covariates considered were anthropometric measurements (height, lower leg length, weight, lean and fat mass), age, sport (cross-country, n = 29; soccer, n = 15; swimming, n = 9; track and field, n = 14; volleyball, n = 12; basketball, n = 9), and dietary intakes of calcium, vitamin D, protein, carbohydrates, fat, and total caloric intake. All variables found to differ between cases and controls or associated with a bone outcome were entered into a general linear regression model, and a backward stepwise approach was used to determine each variable’s contribution to stress fracture risk. Analysis of covariance (ANCOVA) was used to determine differences in bone parameters between cases and controls after adjusting for height, lower leg length, lean mass, fat mass, and sport.
Results
Population Characteristics
Table 1 gives the participant characteristics of the controls, cases that developed any stress fracture or a tibial stress fracture during the study period, and cases who ever had or developed a stress fracture. Cases and controls did not differ in age, height, lower leg length, mass, fat mass, body mass index (BMI), total body BMC, sport, time spent in weightbearing activity, or dietary intakes of calcium, vitamin D, protein, fat, and total calories. The only differences observed were a lower intake of carbohydrates in cases with any stress fracture and lower protein and vitamin D intake in tibial stress fractures compared with controls. A total of 23 athletes sustained stress fractures during the study period; there were 10 stress fractures of the tibia (6 cross-county, 1 track and field, 1 soccer, 2 volleyball, 1 basketball), 14 of the metatarsal (3 cross-country, 1 soccer, 2 basketball, 1 volleyball, 1 swimming, 6 track and field), 1 femur (swimming), and 1 fibula (basketball). Six participants sustained more than 1 stress fracture during the study (1 cross-county [3 tibia], 1 soccer [1 metatarsal and 1 tibia], 3 track and field [1 vertebra, 2 tibias, and 3 metatarsals], and 1 volleyball [1 metatarsal and 1 tibia]).
TABLE 1.
Participant Characteristicsa
| Controls (n = 57) | Developed Any SF (n = 23) | Developed Tibia SF (n = 10) | Ever Had Any SF (n = 31) | |
|---|---|---|---|---|
| Age, y | 20.1 ± 1.3 | 20.5 ± 1.4 | 19.9 ± 1.1 | 20.4 ± 1.6 |
| Height, cm | 167 ± 15 | 172 ± 7 | 172 ± 8 | 170 ± 8 |
| Lower leg length, mm | 377 ± 29 | 383 ± 38 | 377 ± 54 | 378 ± 35 |
| Total body lean mass, kg | 52 ± 7 | 52 ± 7 | 53 ± 6 | 53 ± 7 |
| Total body fat mass, kg | 15 ± 5 | 15 ± 6 | 15 ± 4 | 15 ± 5 |
| Total body BMC, kg | 2.5 ± 0.4 | 2.6 ± 0.4 | 2.7 ± 0.4 | 2.6 ± 0.4 |
| BMI, kg/m2 | 25 ± 16 | 23 ± 4 | 23 ± 1 | 23 ± 3 |
| Sport (XC/SC/SW/T&F/VB/BB), n | 18/11/7/7/9/5 | 6/2/2/6/3/4 | 4/0/0/2/3/1 | 11/4/2/7/3/4 |
| Dietary intake | ||||
| Kilocalories, kcal | 2505 ± 623 | 2477 ± 1218 | 2661 ± 401 | 2522 ± 1135 |
| Protein, g/d | 87 ± 26 | 94 ± 56 | 72 ± 19b | 89 ± 49 |
| Fat, g/d | 89 ± 36 | 98 ± 48 | 95 ± 27 | 90 ± 42 |
| Carbohydrates, g/d | 347 ± 96 | 304 ± 137b | 354 ± 148 | 343 ± 185 |
| Calcium, mg/d | 976 ± 389 | 948 ± 322 | 1129 ± 238 | 1005 ± 353 |
| Vitamin D, IU/d | 125 ± 136 | 111 ± 73 | 76 ± 85b | 145 ± 127 |
| Weightbearing exercise, min/d | 65 ± 26 | 70 ± 23 | 78 ± 6 | 71 ± 20 |
aData are expressed as mean ± SD. BB, basketball; BMC = bone mineral content; BMI = body mass index; SC = soccer, SF, stress fracture; SW = swimming, T&F = track and field; VB = volleyball; XC = cross-country.
bStatistically significant difference compared with the control group (P < .05).
Development of Any Stress Fracture
Univariate and multivariate results for bone mass and geometry are given in Table 2. No differences were observed for any of the DXA measurements between cases and controls. Prior to adjusting for covariates, cases had greater PMI at the 20% and 66% sites and greater cortical BMC at the 66% site. Additionally, no significant differences in polar or radial vBMD were observed (Figure 1). These bone differences became nonsignificant after controlling for height, lower leg length, lean and fat mass, and sport.
TABLE 2.
Bone Measurements in Cases and Controlsa
| Measurement | Controls (n = 57) | Developed Any SF (n = 23) | Developed Tibia SF (n = 10) | Ever Had Any SF (n = 31) |
|---|---|---|---|---|
| Hip DXA | ||||
| Neck area, cm2 | 5.0 ± 0.4 | 5.0 ± 0.4 | 5.2 ± 0.2 | 5.2 ± 0.3 |
| Neck BMC, g | 5.0 ± 0. | 5.0 ± 0.9 | 5.6 ± 0.9 b | 5.3 ± 1.0 |
| Neck aBMD, g/cm2 | 0.981 ± 0.143 | 1.030 ± 0.170 | 1.070 ± 0.159 | 1.018 ± 0.173 |
| Total area, cm2 | 34.5 ± 4.0 | 34.5 ± 4.0 | 33.5 ± 3.1 | 34.5 ± 3.0 |
| Total BMC, g | 37.3 ± 7.7 | 37.3 ± 7.7 | 39.6 ± 7.8 | 38.8 ± 7.2 |
| Total aBMD, g/cm2 | 1.134 ± 0.172 | 1.074 ± 0.138 | 1.177 ± 0.146 b | 1.122 ± 0.166 |
| Spine DXA | ||||
| Total area, cm2 | 62.9 ± 6.2 | 63.6 ± 5.0 | 63.9 ± 7.2 | 63.6 ± 6.0 |
| Total BMC, g2 | 70.3 ± 14.7 | 74.0 ± 15 | 73.8 ± 13.6 | 71.9 ± 15.0 |
| Total aBMD, g/cm2 | 1.110 ± 0.158 | 1.133 ± 0.162 | 1.150 ± 0.126 | 1.123 ± 0.168 |
| 4% pQCT | ||||
| Trabecular area, mm2 | 816 ± 145 | 869 ± 159 | 903 ± 170 | 848 ± 156 |
| Trabecular BMC, mg | 226 ± 39 | 244 ± 44 | 256 ± 44 | 237 ± 40 |
| Trabecular vBMD, mg/cm3 | 278 ± 25 | 279 ± 26 | 284 ± 12 | 281 ± 24 |
| 20% pQCT | ||||
| Cortical area, mm2 | 217 ± 31 | 227 ± 27 | 227 ± 27 | 225 ± 27 |
| Cortical BMC, mg | 250 ± 35 | 261 ± 32 | 259 ± 31 | 259 ± 32 |
| Cortical vBMD, mg/cm3 | 1150 ± 22 | 1148 ± 21 | 1143 ± 24 | 1150 ± 19 |
| Cortical thickness, mm | 3.8 ± 0.4 | 3.8 ± 0.6 | 3.8 ± 0.6 | 3.8 ± 0.5 |
| PMI, mm4 | 20.5 ± 5.5 | 23.5 ± 6.3b | 23.7 ± 7.7 | 23.0 ± 4.6b |
| pSSI, mm3 | 14.9 ± 3.1 | 16.4 ± 3.4 | 16.3 ± 3.9 | 16.2 ± 3.1 |
| Periosteal circumference (mm) | 69.1 ± 5.1 | 71.8 ± 5.9 | 72.1 ± 7.5 | 71.5 ± 5.2 b |
| Endosteal circumference, mm | 45.2 ± 5.5 | 47.7 ± 8.0 | 48.1 ± 10.2 | 47.6 ± 7.1 |
| Endocortical vBMD, mg/cm3 | 1169 ± 31 | 1168 ± 34 | 1162 ± 38 | 1172 ± 30 |
| Midcortical vBMD, mg/cm3 | 1250 ± 20 | 1249 ± 17 | 1142 ± 18 | 1250 ± 16 |
| Pericortical vBMD, mg/cm3 | 1247 ± 21 | 1242 ± 22 | 1237 ± 18 | 1243 ± 21 |
| 66% pQCT | ||||
| Cortical area, mm2 | 329 ± 47 | 352 ± 52 | 353 ± 55 | 345 ± 52 |
| Cortical BMC, mg | 369 ± 52 | 395 ± 56b | 394 ± 57 | 387 ± 56 |
| Cortical vBMD, mg/cm3 | 1119 ± 25 | 1124 ± 24 | 1117 ± 30 | 1125 ± 23 |
| Cortical thickness, mm | 4.7 ± 0.6 | 4.9 ± 0.7 | 5.0 ± 0.8 | 4.8 ± 0.7 |
| PMI, mm4 | 45.1 ± 1.2 | 51.0 ± 1.4b | 50.1 ± 16.3 | 49.4 ± 13.0 |
| pSSI, mm3 | 24.4 ± 4.6 | 26.8 ± 5.6 | 26.6 ± 6.7 | 26.2 ± 5.2 |
| Periosteal circumference, mm | 84.7 ± 6.1 | 86.9 ± 6.5 | 86.6 ± 7.6 | 86.5 ± 6.0 |
| Endosteal circumference, mm | 54.9 ± 6.8 | 55.8 ± 7.6 | 55.0 ± 9.6 | 56.0 ± 7.2 |
| Muscle area, mm2 | 71.3 ± 1.1 | 63 ± 3 | 68.3 ± 1.5 | 73.8 ± 1.5 |
aValues are expressed as mean ± SD. Entries in boldface indicate statistically significant difference compared with controls after adjusting for height, lower leg length, lean mass, fat mass, and sport. Polar moment of inertia (PMI) denoted as 103; polar strength strain index (pSSI) denoted as 102; muscle area denoted as 102. aBMD, areal bone mineral density; BMC, bone mineral content; BMD, bone mineral density; DXA, dual-energy x-ray absorptiometry; pQCT, peripheral quantitative computed tomography; SF, stress fracture; vBMD, volumetric bone mineral density.
bStatistically significant difference compared with the control group (P < .05).
Figure 1.
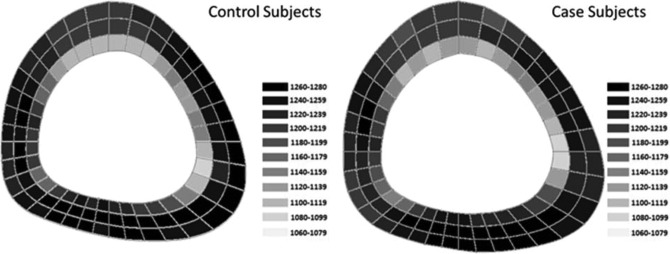
Polar and radial distribution of cortical volumetric bone mineral density (vBMD) in cases who developed a stress fracture during the study and controls at the 20% tibial slice. No differences in cortical distribution were observed; however, the cases did have larger bones than controls. Drawings are to scale.
Tibia Stress Fracture
An additional analysis was performed using all individuals who developed a tibial stress fracture as cases (n = 10) and individuals who never had a stress fracture as controls. In this analysis, unadjusted femoral neck BMC and total hip BMD were greater in cases than controls (Table 2), and these differences remained significant after adjusting for height, lower leg length, lean mass, fat mass, and sport.
History of Stress Fracture
Unadjusted periosteal circumference and PMI at the 20% slice were greater in cases than controls (Table 2). After controlling for the covariates described previously, periosteal circumference remained greater in cases than controls (71.1 ± 0.7 and 69.4 ± 0.5 mm, respectively; P = .04), while PMI became nonsignificant.
Discussion
While previous studies have reported that hip and spine aBMD were lower in individuals who developed any stress fractures throughout the body,6,33,40 our study indicated no difference in these measurements between cases and controls in our primary analysis. We suggest that previous studies did not include athletes from a wide variety of sports, leading to less variability in the bone measurements and increased power to detect a difference between their cases and controls.
Contrary to our hypothesis, participants who developed stress fractures had greater unadjusted PMI at the 20% site than controls. Our findings are somewhat contrary to previous studies that have reported narrower tibias,3,20 smaller cross-sectional areas,3,11 and lower section modulus in stress fracture cases.3,13 Since bone size and cortical area plays a large role in the calculation of PMI (∑ (d2 * A), where A is the area of each voxel [0.25 mm2] and d is the distance of each voxel from the center of gravity), we would have expected individuals who developed stress fractures to have lower PMI as a result of having smaller bones. Cortical thickness and periosteal circumference are the 2 variables that have the greatest influence on PMI. However, means for cortical thickness were similar among groups, while mean periosteal circumference was larger in all stress fracture groups than controls. Based on these data, we postulate that individuals experiencing greater loads may be undergoing greater amounts of remodeling, resulting in periosteal expansion that results in a greater PMI. However, the greater PMI is not enough to compensate for the loads demanded and stress fractures occur. An additional explanation could be that the individuals with the largest bones are the most active and therefore are at greater risk of stress fracture.
After adjusting for covariates, the differences in PMI between cases and controls became nonsignificant. This is due to cases being slightly, but not significantly, taller. In addition to being taller, the lower leg measurements of cases were also slightly longer than those of controls. This difference could also explain why these individuals developed stress fractures. We postulate that individuals with longer tibias may experience greater tibia bending during activity, leading to increased tension and compression forces that may result in the development of a stress fracture.
When individuals who had a stress fracture at any point in their life were compared with controls, both periosteal circumference and PMI at the 20% slice were greater in cases than controls. The difference in periosteal circumference remained significant after adjusting for covariates. These findings support our explanation that individuals who develop stress fractures may be more active than individuals who do not develop stress fractures, and therefore are subjected to more bone loading. Additionally, if these individuals are more active, they are likely spending more time exercising while fatigued. When muscles are fatigued during training or competition, the loads placed on the skeleton increase,31 and load-bearing bones may adapt by increasing periosteal circumference. If the bone cannot adapt sufficiently, a stress fracture may occur.15
Another intriguing finding from our study was that the differences in periosteal circumference and PMI between cases and controls were not observed when comparing cases of tibial stress fractures to controls. However, the femoral neck BMC and aBMD at the hip were both greater in cases than controls. Previous studies have reported lower aBMD in people with stress fractures than controls,6,33,40 but this does not appear to be the case for tibial stress fractures in our study. We feel that our findings for tibial stress fractures may be more indicative of overall bone health affecting the development of stress fractures rather than a mechanistic relationship. While no definitive explanation for this difference exists, it is worth exploring in future studies with a larger number of tibia stress fractures and may be related to differences in levels of physical activity among individual athletes.
Our findings allow us to speculate that stress fractures may not be solely influenced by bone size and density, but rather by physiological and genetic factors as well as fatigue. As the results from Milgrom et al31 indicate, a stress fracture is very unlikely to occur if an individual does not experience muscle fatigue. If stress fractures were purely an anatomical condition related to bone health, the body would adapt after injury occurred, and recurrence would not be a problem. However, this does not appear to be the case, with more than 25% of the stress fracture cases in our study and 20% of stress fracture cases in a previous study27 experiencing recurrent stress fractures.
Conclusion
Stress fractures continue to be a significant concern for sports medicine professionals. Our findings are important because to our knowledge, this is the first time individuals with stress fractures have been reported to have larger bones and higher measures of bone strength. The current study attempted to identify lifestyle factors and bone parameters that were associated with stress fractures; however, a larger prospective study utilizing plasma markers of bone remodeling will be important in determining at what point stress fractures occur and what factors, such as muscle fatigue, may contribute to the development of stress fractures.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
References
- 1. Armstrong DW, 3rd, Rue JP, Wilckens JH, Frassica FJ. Stress fracture injury in young military men and women. Bone. 2004;35:806–816. [DOI] [PubMed] [Google Scholar]
- 2. Augat P, Reeb H, Claes LE. Prediction of fracture load at different skeletal sites by geometric properties of the cortical shell. J Bone Miner Res. 1996;11:1356–1363. [DOI] [PubMed] [Google Scholar]
- 3. Beck TJ, Ruff CB, Mourtada FA, et al. Dual-energy X-ray absorptiometry derived structural geometry for stress fracture prediction in male U.S. Marine Corps recruits. J Bone Miner Res. 1996;11:645–653. [DOI] [PubMed] [Google Scholar]
- 4. Beck TJ, Ruff CB, Shaffer RA, Betsinger K, Trone DW, Brodine SK. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone. 2000;27:437–444. [DOI] [PubMed] [Google Scholar]
- 5. Bennell KL, Malcolm SA, Thomas SA, et al. Risk factors for stress fractures in female track-and-field athletes: a retrospective analysis. Clin J Sports Med. 1995;5:229–235. [DOI] [PubMed] [Google Scholar]
- 6. Bennell KL, Malcolm SA, Thomas SA, et al. Risk factors for stress fractures in track and field athletes. A twelve-month prospective study. Am J Sports Med. 1996;24:810–818. [DOI] [PubMed] [Google Scholar]
- 7. Bennell KL, Malcolm SA, Thomas SA, Wark JD, Brukner PD. The incidence and distribution of stress fractures in competitive track and field athletes. A twelve-month prospective study. Am J Sports Med. 1996;24:211–217. [DOI] [PubMed] [Google Scholar]
- 8. Brudvig TJ, Gudger TD, Obermeyer L. Stress fractures in 295 trainees: a one-year study of incidence as related to age, sex, and race. Mil Med. 1983;148:666–667. [PubMed] [Google Scholar]
- 9. Cline AD, Jansen GR, Melby CL. Stress fractures in female army recruits: implications of bone density, calcium intake, and exercise. J Am Coll Nutr. 1998;17:128–135. [DOI] [PubMed] [Google Scholar]
- 10. Cosman F, Ruffing J, Zion M, et al. Determinants of stress fracture risk in United States Military Academy cadets. Bone. 2013;55:359–366. [DOI] [PubMed] [Google Scholar]
- 11. Crossley K, Bennell KL, Wrigley T, Oakes BW. Ground reaction forces, bone characteristics, and tibial stress fracture in male runners. Med Sci Sports Exerc. 1999;31:1088–1093. [DOI] [PubMed] [Google Scholar]
- 12. Doube M, Klosowski MM, Arganda-Carreras I, et al. BoneJ: free and extensible bone image analysis in ImageJ. Bone. 2010;47:1076–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franklyn M, Oakes B, Field B, Wells P, Morgan D. Section modulus is the optimum geometric predictor for stress fractures and medial tibial stress syndrome in both male and female athletes. Am J Sports Med. 2008;36:1179–1189. [DOI] [PubMed] [Google Scholar]
- 14. Friedl KE, Nuovo JA, Patience TH, Dettori JR. Factors associated with stress fracture in young army women: indications for further research. Mil Med. 1992;157:334–338. [PubMed] [Google Scholar]
- 15. Frost HM. Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1081–1101. [DOI] [PubMed] [Google Scholar]
- 16. Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1–9. [DOI] [PubMed] [Google Scholar]
- 17. Frost HM. The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner. 1987;2:73–85. [PubMed] [Google Scholar]
- 18. Frost HM. Perspectives: a proposed general model of the “mechanostat” (suggestions from a new skeletal-biologic paradigm). Anat Rec. 1996;244:139–147. [DOI] [PubMed] [Google Scholar]
- 19. Giladi M, Milgrom C, Simkin A, Danon Y. Stress fractures. Identifiable risk factors. Am J Sports Med. 1991;19:647–652. [DOI] [PubMed] [Google Scholar]
- 20. Giladi M, Milgrom C, Simkin A, et al. Stress fractures and tibial bone width. A risk factor. J Bone Joint Surg Br. 1987;69:326–329. [DOI] [PubMed] [Google Scholar]
- 21. Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone. 2000;27:351–357. [DOI] [PubMed] [Google Scholar]
- 22. Heinonen A, Oja P, Kannus P, et al. Bone mineral density in female athletes representing sports with different loading characteristics of the skeleton. Bone. 1995;17:197–203. [DOI] [PubMed] [Google Scholar]
- 23. Heinonen A, Oja P, Kannus P, Sievanen H, Manttari A, Vuori I. Bone mineral density of female athletes in different sports. Bone Miner. 1993;23:1–14. [DOI] [PubMed] [Google Scholar]
- 24. Heinonen A, Sievanen H, Kannus P, Oja P, Vuori I. Site-specific skeletal response to long-term weight training seems to be attributable to principal loading modality: a pQCT study of female weightlifters. Calcif Tissue Int. 2002;70:469–474. [DOI] [PubMed] [Google Scholar]
- 25. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8:273–278. [DOI] [PubMed] [Google Scholar]
- 26. Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res. 2003;18:352–359. [DOI] [PubMed] [Google Scholar]
- 27. Korpelainen R, Orava S, Karpakka J, Siira P, Hulkko A. Risk factors for recurrent stress fractures in athletes. Am J Sports Med. 2001;29:304–310. [DOI] [PubMed] [Google Scholar]
- 28. Kowal DM. Nature and causes of injuries in women resulting from an endurance training program. Am J Sports Med. 1980;8:265–269. [DOI] [PubMed] [Google Scholar]
- 29. Lai YM, Qin L, Hung VW, Chan KM. Regional differences in cortical bone mineral density in the weight-bearing long bone shaft—a pQCT study. Bone. 2005;36:465–471. [DOI] [PubMed] [Google Scholar]
- 30. Liu L, Maruno R, Mashimo T, et al. Effects of physical training on cortical bone at midtibia assessed by peripheral QCT. J Appl Physiol. 2003;95:219–224. [DOI] [PubMed] [Google Scholar]
- 31. Milgrom C, Radeva-Petrova DR, Finestone A, et al. The effect of muscle fatigue on in vivo tibial strains. J Biomech. 2007;40:845–850. [DOI] [PubMed] [Google Scholar]
- 32. Milgrom C, Simkin A, Eldad A, Nyska M, Finestone A. Using bone’s adaptation ability to lower the incidence of stress fractures. Am J Sports Med. 2000;28:245–251. [DOI] [PubMed] [Google Scholar]
- 33. Myburgh KH, Hutchins J, Fataar AB, Hough SF, Noakes TD. Low bone density is an etiologic factor for stress fractures in athletes. Ann Intern Med. 1990;113:754–759. [DOI] [PubMed] [Google Scholar]
- 34. Nikander R, Sievanen H, Heinonen A, Kannus P. Femoral neck structure in adult female athletes subjected to different loading modalities. J Bone Miner Res. 2005;20:520–528. [DOI] [PubMed] [Google Scholar]
- 35. Nikander R, Sievanen H, Heinonen A, Karstila T, Kannus P. Load-specific differences in the structure of femoral neck and tibia between world-class moguls skiers and slalom skiers. Scand J Med Sci Sports. 2008;18:145–153. [DOI] [PubMed] [Google Scholar]
- 36. Rantalainen T, Nikander R, Daly RM, Heinonen A, Sievanen H. Exercise loading and cortical bone distribution at the tibial shaft. Bone. 2011;48:786–791. [DOI] [PubMed] [Google Scholar]
- 37. Rantalainen T, Nikander R, Heinonen A, Suominen H, Sievanen H. Direction-specific diaphyseal geometry and mineral mass distribution of tibia and fibula: a pQCT study of female athletes representing different exercise loading types. Calcif Tissue Int. 2010;86:447–454. [DOI] [PubMed] [Google Scholar]
- 38. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swissa A, Milgrom C, Giladi M, et al. The effect of pretraining sports activity on the incidence of stress fractures among military recruits. A prospective study. Clin Orthop Relat Res. 1989;(245):256–260. [PubMed] [Google Scholar]
- 40. Välimäki VV, Alfthan H, Lehmuskallio E, et al. Risk factors for clinical stress fractures in male military recruits: a prospective cohort study. Bone. 2005;37:267–273. [DOI] [PubMed] [Google Scholar]
- 41. Weidauer LA, Eilers MM, Binkley TL, Vukovich MD, Specker BL. Effect of different collegiate sports on cortical bone in the tibia. J Musculoskel Neuronal Interact. 2012;12:68–73. [PubMed] [Google Scholar]
- 42. Wolff J. Das gesetz der transformation der knochen. DMW–Deutsch Med Wochenschr. 1892;19:1222–1224. [Google Scholar]
- 43. Wolff J. The Law of Bone Remodeling [translated from the 1892 original, Das Gesetz der Transformation der Knochen, by P. Maquet and R. Furlong] Berlin, Germany: Springer Verlag; 1986. [Google Scholar]


