Abstract
Background:
Osgood-Schlatter disease (OSD) is a traction apophysitis of the tibial tuberosity. Ultrasonography (US) is able to detect pathologic changes, such as cartilage swelling and fragmentation of the tibial tubercle ossification center.
Purpose:
To compare the US stages of tibial tuberosity development and the physical features and prevalence of OSD in this patient cohort.
Study Design:
Cross-sectional study; Level of evidence, 3.
Methods:
Subjects included 238 males (n = 476 joints) with a mean age of 11.4 ± 1.6 years (range, 7-14 years). The tibial tuberosity development on US was divided into 3 stages: the cartilaginous stage (stage C), apophyseal stage (stage A), and epiphyseal stage (stage E). It was then investigated whether the subjects had pain in the tibial tuberosity on application of pressure. Age, height, body weight, body mass index (BMI), heel-buttock distance (HBD, cm), and straight-leg raise angle (SLRA) were evaluated. To confirm the diagnosis of OSD, the participant had to fulfill the following clinical criteria: pain with direct pressure on the tibial apophysis, fragmentation of the bone, and irregularity of the ossification center detected by US.
Results:
The tibial tuberosity was stage C in 195 knees, stage A in 105 knees, and stage E in 176 knees. The subjects’ heights, weights, and BMIs significantly increased with advancing development of the tibial tuberosity. The HBD increased in stage E (P < .01). The SLRA was not significantly different among groups. There was fragmentation of the bone and irregularity of the ossification center in 32 knees (6.8%): 0 in stage C, 21 (4.3%) in stage A, and 11 (2.3%) in stage E. Fragmentation of the bone and irregularity were observed significantly more often in stage A (P < .01). On the other hand, there were 10 joints with OSD (2.1%): 0 in stage C, 3 (0.6%) in stage A, and 7 (1.5%) in stage E. OSD was observed significantly more often in stage E than in the other stages (P < .05).
Conclusion:
The present study showed that the HBD increased from stage A to stage E. The prevalence of OSD was highest in stage E.
Keywords: Osgood-Schlatter disease, tibial tuberosity development, ultrasonography, preadolescent males
Osgood-Schlatter disease (OSD) is a traction apophysitis of the tibial tuberosity that is thought to occur due to repetitive strain from the quadriceps muscle and chronic avulsion of the tibia.20,25–27 Repeated tensile extension forces from the quadriceps are applied to the weak apophyseal cartilage of the tibial tuberosity, resulting in avulsion of segments of the anterior cartilage and anterior bone.12,27 Clinically, it is characterized by pain, swelling, and enlargement of the proximal tibia at the site of the patellar tendon’s insertion. The pain is exacerbated with physical activity that involves running, jumping, and kneeling.5–7 It has been claimed that, in the majority of adolescents, symptoms may completely resolve after conservative treatment consisting of activity modification, application of ice, use of anti-inflammatory agents, and physical therapy with stretching.4
Radiographic examination of the lateral view of the knee shows fragmentation of the tibial tubercle ossification center, which has diagnostic value only when associated with soft tissue swelling, partial obliteration of the retrotendinous fat pad, and patellar tendon thickening.14 Ultrasonography (US) has been used to detect pathological features and monitor the course of OSD, including cartilage swelling, fragmentation of the tibial tubercle ossification center, patellar tendon lesions, and reactive bursitis of the deep or superficial tibial patellar bursae.3,9,11,17,22,29 Ehrenborg13 evaluated the tibial tuberosity radiographically and classified its development into 4 stages: the cartilaginous stage, apophyseal stage, epiphyseal stage, and bony stage. Nakase et al24 evaluated the stages of the tibial tuberosity development and physical features using ultrasound according to this classification. However, they did not evaluate the prevalence of OSD and in which stage(s) it occurred.
The aim of this study was to compare the ultrasonography stages of tibial tuberosity development to the physical features and determine the prevalence of OSD in this population using ultrasound.
Materials and Methods
Preparticipation physical examinations were conducted on preadolescent baseball players. The subjects were 238 males (n = 476 joints) who were baseball players, with a mean age of 11.4 years (range, 7-14 years). The skeletal maturation of the distal attachment of the patellar tendon was examined using a LOGIQ e instrument (GE Health Care) with high-resolution linear-array probes at 12 MHz. The US evaluations were performed with both knees in 90° of flexion in the supine position. The tibial tuberosity was examined on longitudinal US images at the site at which the tuberosity was most clearly visualized. The tibial tuberosity development on US was divided into 3 stages: the cartilaginous stage (stage C) was characterized by a large amount of apophyseal cartilage (anechoic) (Figure 1A), the apophyseal stage (stage A) was characterized by apophyseal cartilage (Figure 1B), and the epiphyseal stage (stage E) was characterized by no detectable sign of apophyseal cartilage (Figure 1C).
Figure 1.
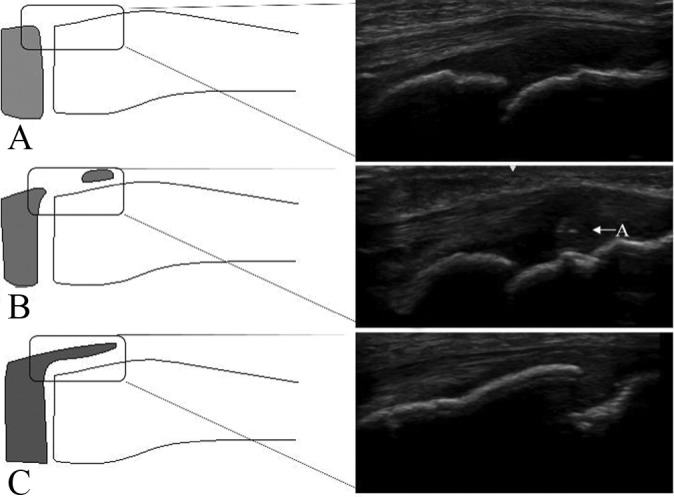
Ultrasound images of knees that are representative of the 3 stages of the maturation process of the patellar tendon attachment. (A) Stage C, (B) stage A, and (C) stage E. A, apophyseal cartilage.
We investigated whether the subjects had pain in the tibial tuberosity on application of pressure. The height was measured with a portable height scale using graduations of 0.1 cm. Weight was measured on an electronic scale. The heel-buttock distance (HBD, cm) was measured in the prone position, and the straight-leg raise angle (SLRA) was evaluated with the participant assuming the supine position and flexing the hip with the knee extended. The straight-leg raise angles were divided into 4 groups at 15° intervals: grade 1 = <60°, grade 2 = 60° to 74°, grade 3 = 75° to 89°, and grade 4 = >90°. To confirm the diagnosis of OSD, the participant had to fulfill the following clinical criteria: pain with direct pressure on the tibial apophysis; pain before, during, and after physical activities; enlargement or prominence of the tibial apophysis; pain with resisted knee extension; and pain caused by jumping.23 The following ultrasonographic findings were also required: fragmentation of the bone and irregularity of the ossification center in US (Figure 2).3,9,11,22,24,29 The physical findings were compared with the respective stages of tibial tuberosity development. Institutional review board approval for the study was provided by our institution.
Figure 2.
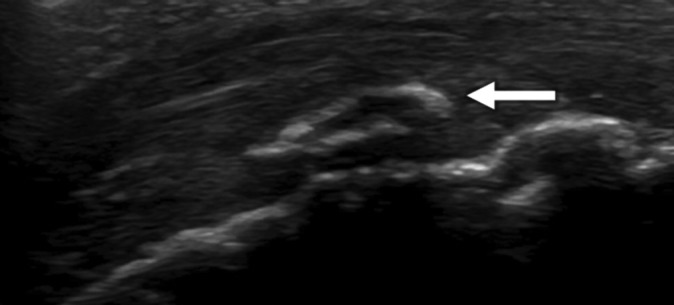
A longitudinal ultrasound image of the tibial tuberosity. The image shows fragmentation of the bone (arrow).
Statistical Analysis
A 1-way analysis of variance and the Tukey honestly significant difference test were used for comparisons between groups. The kappa statistic was used to assess intra- and interrater reliability of the ultrasound staging system. The degree of observer agreement was graded as follows: a kappa value of 0 to 0.20 indicated slight agreement, a value of 0.21 to 0.40 indicated fair agreement, a value of 0.41 to 0.60 indicated moderate agreement, a value of 0.61 to 0.80 indicated substantial agreement, and a value of 0.81 to 1.00 indicated almost perfect agreement. All statistical procedures were conducted using a computer software program (Statistical package for the Social Sciences v 19.0 J; SPSS Inc). Significance was inferred for values of P < .05.
Results
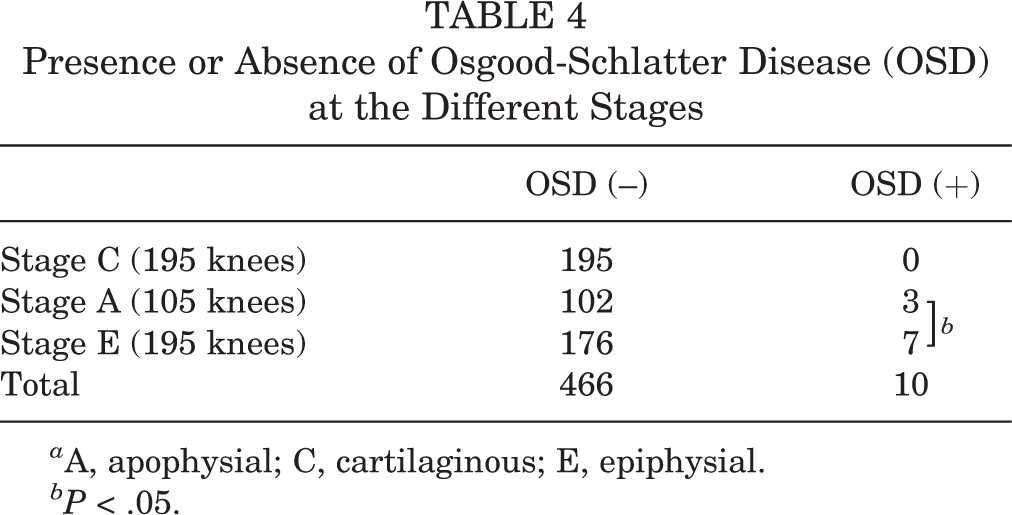
The tibial tuberosity was stage C in 195 knees, stage A in 105 knees, and stage E in 176 knees. The height, weight, and body mass index significantly increased with advancing development of the tibial tuberosity (Table 1). The HBD was significantly increased in stage E compared with other stages (P < .01) (Table 2), and was 0.41 ± 1.1 cm in stage C, 0.59 ± 1.4 cm in stage A, and 4.5 ± 4.4 cm in stage E. The SLRA was not significantly different among groups. There was fragmentation of the bone and irregularity of the ossification center in 32 knees (6.8%): 21 knees in stage A and 11 knees in stage E. Fragmentation of the bone and irregularity was observed significantly more often in stage A (P < .01) (Table 3). On the other hand, there were 10 knees with OSD (2.1%): 3 knees in stage A and 7 knees in stage E. Clinically symptomatic OSD was observed significantly more often in stage E than in the other stages (P < .05) (Table 4). The kappa values for the intrarater reliability of the evaluation of the ultrasound stage (0.92; P < .01) indicated almost perfect agreement. In contrast, the kappa values for the interrater reliability of the evaluation of the ultrasound stage (0.70; P < .01) indicated moderate agreement.
TABLE 1.
Age, Height, and Body Mass Index in Each Stagea
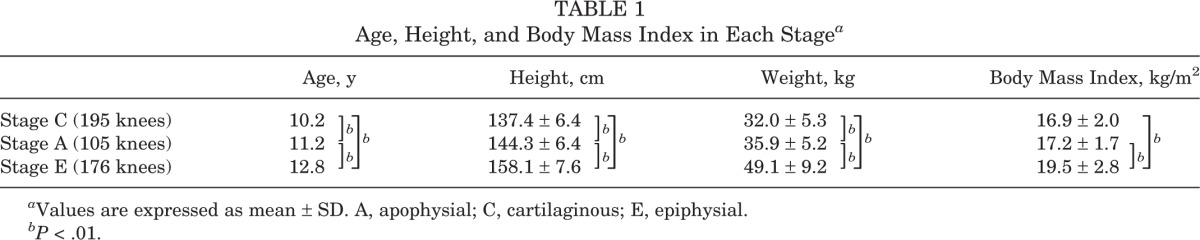
TABLE 2.
Muscle Tightness Based on the Skeletal Maturation Stagea
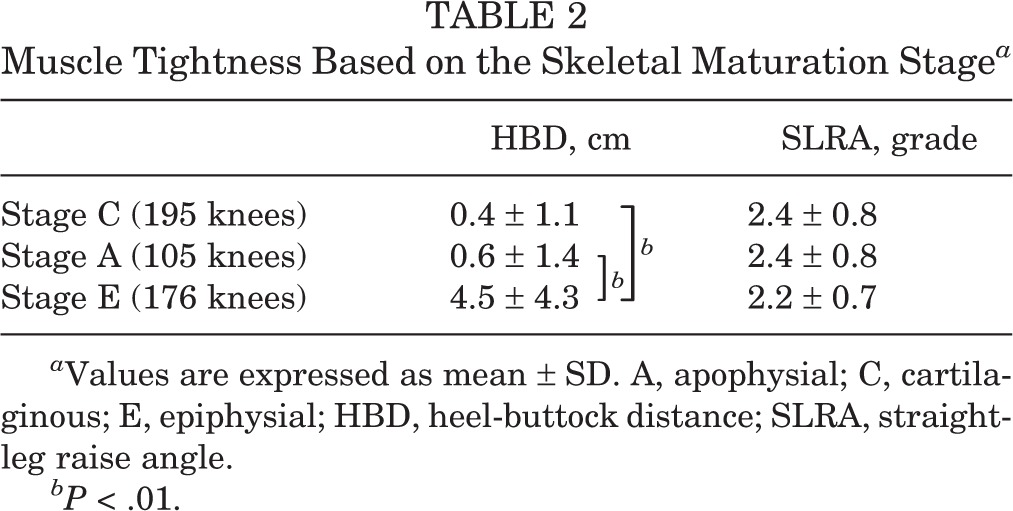
TABLE 3.
The Presence or Absence of Fragmentation of the Bone and Irregularity of the Ossification Center in the Tibial Tuberosity in the Different Stages
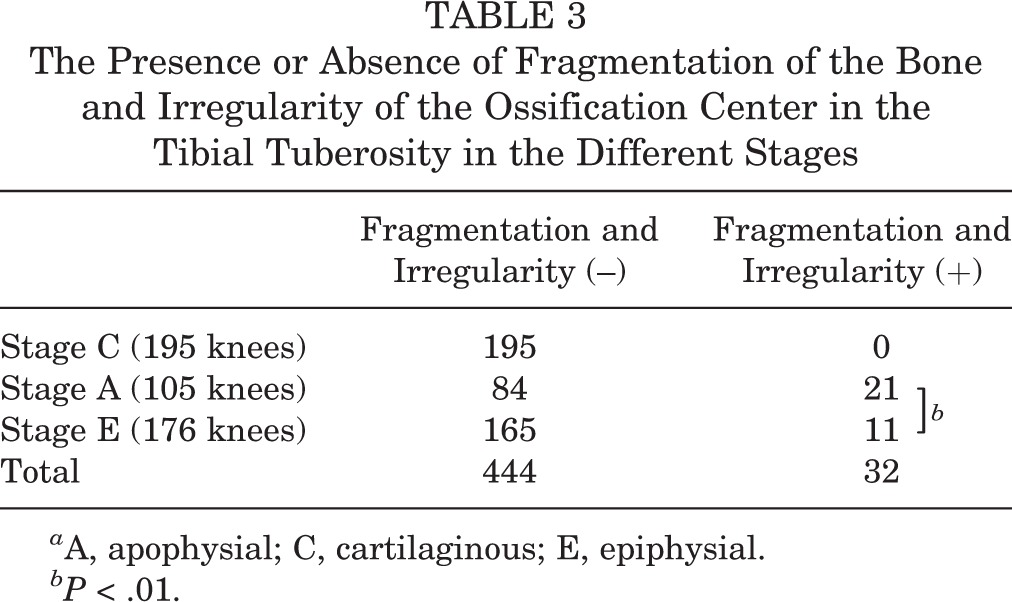
TABLE 4.
Presence or Absence of Osgood-Schlatter Disease (OSD) at the Different Stages
Discussion
The present study focused on the ultrasonographic stages of tibial tuberosity development, the prevalence of OSD, and in which stage it occurred in preadolescent males. The diagnosis of OSD is mostly clinical, but imaging methods are often used to confirm the diagnosis. Vreju et al29 reported that there were changes at the distal part of the tibial tuberosity during development and were able to diagnose OSD using US. They thought that US was a useful method to evaluate the immature tibial tuberosity.
The apophysis is relatively weak and a common area of injury in sports participants.1 Nakase et al24 evaluated the stages of tibial tuberosity development and the physical features of 200 knees in 100 male football players aged 10 to 15 years using ultrasound. They found that quadriceps muscle tightness increased with skeletal maturation, while hamstring tightness did not. Similar to this study, they found that quadriceps muscle tightness increased with skeletal maturation while hamstring tightness did not. It is not clear why such a mismatch would occur between the quadriceps muscle and hamstring muscle, and further studies are needed on this topic.
After performing magnetic resonance imaging studies, Hirano et al15 stated that some type of injury occurs in the secondary ossifiation center of the tibial tuberosity during the apophyseal stage in patients with OSD. Czyrny8 and Gholve et al14 reported that OSD is caused by overuse during the period from stage A to stage E, and that the tibial tuberosity changes dramatically in the short period between these stages. The present study shows that the fragmentation of the bone and irregularity of the ossification center was observed significantly more often in stage A; however, OSD was observed significantly more often in stage E. Lucena et al23 reported that the major factor associated with the presence of OSD was the shortening of the rectus femoris muscle. Shortening of the rectus femoris may substantially affect the biomechanical function of the knee with respect to the lever arm and peak torque.2,10,18,28 Evidence in the literature suggests that muscle stretching could help to protect against musculoskeletal lesions, including the predisposition or development of OSD.16,19
Kujala et al21 reported that the prevalence of OSD with adolescent athletes was 12.9% (mean age, 13.1 years). Lucena et al23 reported that the prevalence of OSD in the sample population was 9.8% (mean age, 13.7 years; range, 12-15 years). The reason that the prevalence of OSD in the present study is lower may be because this study included a younger cohort (mean age, 11.4 years; range, 7-14 years).
The results of this study should be interpreted while keeping the associated limitations in mind. First, this study was a cross-sectional study of preadolescent baseball players. Therefore, the results may not be generalizable to other sports activities. Second, although we used the HBD to evaluate quadriceps tightness, a more reproducible method for measuring quadriceps tightness involves the degree of prone knee flexion of the child. Third, because this study was based on preparticipation physical examination of preadolescent baseball players, radiographic imaging was not performed. Therefore, we were unable to compare US with radiographic findings in this study.
Conclusion
The present study showed that the HBD increased from stage A to stage E. The prevalence of OSD was highest in stage E.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
References
- 1. Auringer ST, Anthony EY. Common pediatric sports injuries. Semin Musculoskelet Radiol. 1999; 3:247–256. [DOI] [PubMed] [Google Scholar]
- 2. Bandy WD, Irion JM, Briggler M. The effect of time and frequency of static stretching on flexibility of the hamstring muscles. Phys Ther. 1997;77:1090–1096. [DOI] [PubMed] [Google Scholar]
- 3. Blankstein A, Cohen I, Heim M, et al. Ultrasonography as a diagnostic modality in Osgood-Schlatter disease. A clinical study and review of the literature. Arch Orthop Trauma Surg. 2001;121:536–539. [DOI] [PubMed] [Google Scholar]
- 4. Bloom OJ, Mackler L, Barbee J. Clinical inquiries. What is the best treatment for Osgood-Schlatter disease? J Fam Pract. 2004;53:153–156. [PubMed] [Google Scholar]
- 5. Cassas KJ, Cassettari-Wayhs A. Childhood and adolescent sports-related overuse injuries. Am Fam Physician. 2006;73:1014–1022. [PubMed] [Google Scholar]
- 6. Caton J, Mironneau A, Walch G, Levigne C, Michel CR. Idiopathic high patella in adolescents. Apropos of 61 surgical cases [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1990;76:253–260. [PubMed] [Google Scholar]
- 7. Commandre FA, Gagnerie G, Zakarian M, Alaoui M, Fourre JM, Bouzayen A. The child, the spine and sport. J Sports Med Phys Fitness. 1988;28:11–19. [PubMed] [Google Scholar]
- 8. Czyrny Z. Osgood-Schlatter disease in ultrasound diagnostic—a pictorial essay. Med Ultrason. 2010;12:323–335. [PubMed] [Google Scholar]
- 9. De Flaviis L, Nessi R, Scaglione P, Balconi G, Albisetti W, Derchi LE. Ultrasonic diagnosis of Osgood-Schlatter and Sinding-Larsen-Johansson diseases of the knee. Skeletal Radiol. 1989;18:193–197. [DOI] [PubMed] [Google Scholar]
- 10. Demirag B, Ozturk C, Yazici Z, Sarisozen B. The pathophysiology of Osgood-Schlatter disease: a magnetic resonance investigation. J Pediatr Orthop B. 2004;13:379–382. [DOI] [PubMed] [Google Scholar]
- 11. Ducher G, Cook J, Spurrier D, Coombs P, Black J, Bass S. Ultrasound imaging of the patellar tendon attachment to the tibia during puberty: a 12-month follow up in tennis players. Scand J Med Sci Sports. 2010;20:e35–e40. [DOI] [PubMed] [Google Scholar]
- 12. Dupuis CS, Westra SJ, Makris J, Wallace EC. Injuries and conditions of the extensor mechanism of the pediatric knee. Radiographics. 2009;29:877–886. [DOI] [PubMed] [Google Scholar]
- 13. Ehrenborg G. The Osgood–Schlatter lesion: a clinical study of 170 cases. Acta Chir Scand. 1962;124:89–105. [PubMed] [Google Scholar]
- 14. Gholve PA, Scher DM, Khakharia S, Widmann RF, Green DW. Osgood Schlatter syndrome. Curr Opin Pediatr. 2007;19:44–50. [DOI] [PubMed] [Google Scholar]
- 15. Hirano A, Fukubayashi T, Ishii T, Ochiai N. Magnetic resonance imaging of Osgood–Schlatter disease: the course of the disease. Skeletal Radiol. 2002;31:334–342. [DOI] [PubMed] [Google Scholar]
- 16. Jakob RP, von Gumppenberg S, Engelhardt P. Does Osgood-Schlatter disease influence the patella? J Bone Joint Surg Br. 1981;63B:579–582. [DOI] [PubMed] [Google Scholar]
- 17. Kaya DO, Toprak U, Baltaci G, Yosmaoglu B, Ozer H. Long-term functional and sonographic outcomes in Osgood-Schlatter disease. Knee Surg Sports Traumatol Arthrosc. 2013;21:1131–1139. [DOI] [PubMed] [Google Scholar]
- 18. Kerssemakers SP, Fotiadou AN, de Jonge MC, Karantanas AH, Maas M. Sport injuries in the paediatric and adolescent patient: a growing problem. Pediatr Radiol. 2009;39:471–484. [DOI] [PubMed] [Google Scholar]
- 19. Koh TJ, Herzog W. Excursion is important in regulating sarcomere number in the growing rabbit tibialis anterior. J Physiol. 1998;508:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krause BL, Williams JP, Catterall A. Natural history of Osgood-Schlatter disease. J Pediatr Orthop. 1990;10:65–68. [PubMed] [Google Scholar]
- 21. Kujala UM, Kvist M, Heinonen O. Osgood-Schlatter’s disease in adolescent athletes. Retrospective study of incidence and duration. Am J Sports Med. 1985;13:236–241. [DOI] [PubMed] [Google Scholar]
- 22. Lanning P, Heikkinen E. Ultrasonic features of the Osgood-Schlatter lesion. J Pediatr Orthop. 1991;11:538–540. [DOI] [PubMed] [Google Scholar]
- 23. Lucena GL, dos Santos Gomes C, Guerra RO. Prevalence and associated factors of Osgood-Schlatter syndrome in a population-based sample of Brazilian adolescents. Am J Sports Med. 2011;39:415–420. [DOI] [PubMed] [Google Scholar]
- 24. Nakase J, Aiba T, Goshima K, et al. Relationship between the skeletal maturation of the distal attachment of the patellar tendon and physical features in preadolescent male football players. Knee Surg Sports Traumatol Arthrosc. 2014;22:195–199. [DOI] [PubMed] [Google Scholar]
- 25. Ogden JA. Radiology of postnatal skeletal development. X. Patella and tibial tuberosity. Skeletal Radiol. 1984;11:246–257. [DOI] [PubMed] [Google Scholar]
- 26. Osgood RB. Lesions of the tibial tubercle occurring during adolescence. Boston Med Surg J. 1903;148:114–117. [PubMed] [Google Scholar]
- 27. Schlatter C. Verletzungen der schnabelformigen fortsatzes der obseren tibia epiphyse. Beitr Klin Chir. 1903;38:874–887. [Google Scholar]
- 28. Visuri T, Pihlajamaki HK, Mattila VM, Kiuru M. Elongated patellae at the final stage of Osgood-Schlatter disease: a radiographic study. Knee. 2007;14:198–203. [DOI] [PubMed] [Google Scholar]
- 29. Vreju F, Ciurea P, Rosu A. Osgood-Schlatter disease—ultrasonographic diagnostic. Med Ultrason. 2010;12:336–339. [PubMed] [Google Scholar]


