Abstract
Animals need to be able to rapidly and effectively respond to changes in their external and internal environment. To achieve this the nervous and immune systems need to coordinate their responses, integrating multiple cues including presence of potential pathogens, and availability of food. In our recent study 1 we demonstrate that signaling by sensory neurons in the head using the classical neurotransmitter serotonin can negatively regulate the rectal epithelial immune response upon infection of C. elegans with the naturally occurring bacterial pathogen Microbacterium nematophilum (M. nematophilum). The complicated nature of the mammalian brain and immune system has made it difficult to identify the molecular mechanisms mediating these interactions. With its simple, well described, nervous system and a rapidly growing understanding of its immune system, C. elegans has emerged as an excellent model to study the mechanisms by which animals recognize pathogens and coordinate behavioral and cellular immune responses to infection.
Keywords: G proteins, immune response, infection, sensory neurons, serotonin
Abbreviations
- C. elegans
Caenorhabditis elegans
- M. nematophilum
Microbacterium nematophilum
- GPCR
G-protein-coupled receptor
- CaMKII
Calcium/calmodulin-dependent protein kinase II
- DCV
Dense Core Vesicle
- NK cell
Natural Killer cell
- tph-1
tryptophan hydroxylase-1
Chemosensory Neuronal Signaling Acts Upstream of Epithelial Immune Responses
C. elegans commonly encounters many environmental hazards including pathogenic bacteria. In order to respond appropriately, the worm must integrate multiple environmental cues including food availability and pathogenicity to maximize its chances of survival. What are the molecular mechanisms that allow this finely tuned integration of information from both the nervous and immune systems? Previous work has shown that neuronal signaling can profoundly affect susceptibility to infection by mediating pathogen avoidance.2,3 Mutations in the neuronally expressed G protein coupled receptor (GPCR) npr-1 gene, which encodes a homolog of the neuropeptide Y receptor, can mediate the behavioral immune response of avoidance of a number of pathogens including Pseudomonas aeruginosa.3 In comparison, our recent work has shown that during M. nematophilum infection, signaling via the neurotransmitter serotonin can suppress the cellular immune response in the rectal epithelium (Fig. 1A).1
Figure 1.
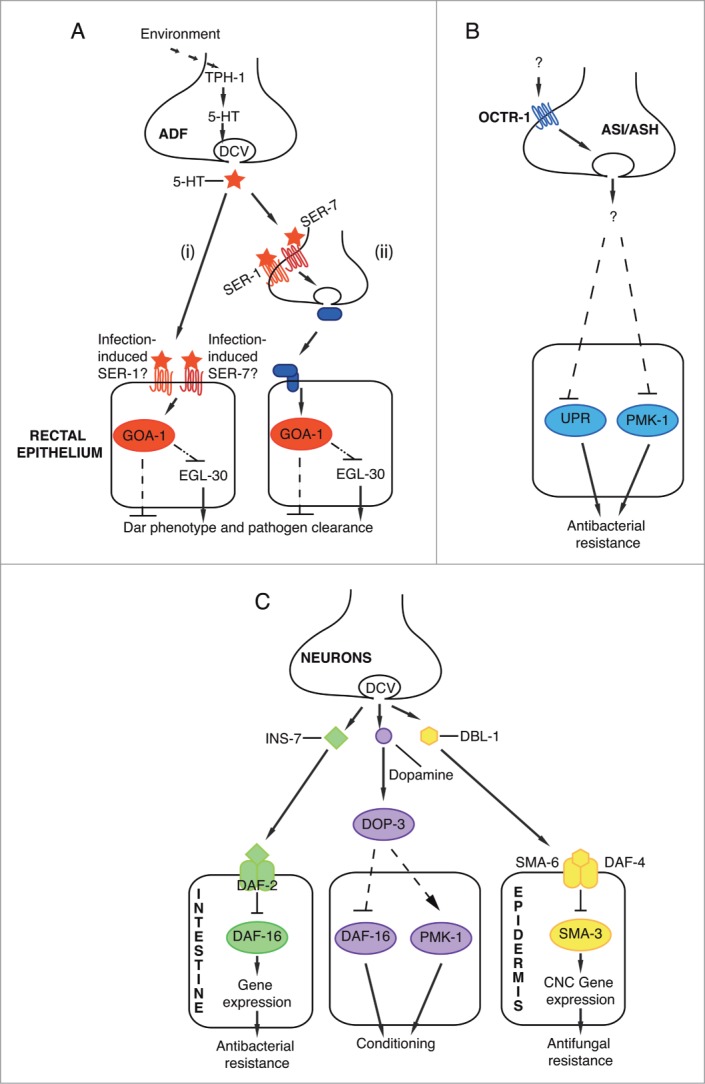
Neuronal signaling pathways that regulate C. elegans cellular immune responses. A number of molecules released from DCVs act non-autonomously to regulate the immune response. (A) Serotonin, synthesized by TPH-1, in ADF chemosensory neurons acts via SER-1 and SER-7 receptors to suppress the immune response to M. nematophilum infection. Serotonin acts, directly (i) or indirectly (ii), to regulate GOA-1 signaling in the rectal epithelium. Although SER-1 and SER-7 receptors are not expressed on rectal epithelial cells under standard conditions, it remains to be determined whether regulation of SER-1 and SER-7 expression by infection may allow serotonin to act directly on these cells (i). Alternatively serotonin may activate SER-1 and SER-7 expressed on neurons, which then release a signal to activate GOA-1 signaling in rectal epithelial cells (ii). GOA-1 signaling acts upstream of, or in parallel to, EGL-30 signaling to suppress the Dar phenotype and reduce pathogen clearance rates.1 (B) The octopamine receptor, OCTR-1, suppresses the immune response to infection with Pseudomonas aeruginosa and is required in ASH and ASI neurons to suppress PMK-1 signaling and the unfolded protein response in non-neuronal cells.15 (C) Release of INS-7 or DBL-1 from the DCVs of unidentified neurons regulate gene expression in the intestine and epithelial cells respectively to mediate antibacterial and antifungal resistance.13,14 In addition dopamine, released from DCVs protects animals from repeat infection by enteropathogenic E. Coli.16
How does neuronal signaling influence the immune response? The amphid chemosensory neuron pair ADF, which are exposed to the external environment, have the ability to modulate behavioral and cellular immune responses to Pseudomonas aeruginosa and M. nematophilum.1,2 These responses rely on the regulation of the biosynthetic enzyme tryptophan hydroxylase-1 (tph-1) in ADF neurons. TPH-1 is the rate-limiting enzyme in the synthesis of serotonin, and animals carrying a putative null allele for this gene are deficient for serotonin production and signaling.4 Transcription of tph-1 in ADF is regulated by a number of conditions including food quality, availability and heat stress.2,5,7 Our work shows that the cellular immune response to M. nematophilum is negatively regulated by tph-1 expression in ADF neurons and that this acts via the serotonin receptors SER-1 and SER-7 to regulate signaling by the G-protein goa-1(Gαo) in the rectal epithelium (Fig. 1A).1 M. nematophilum innately repels C. elegans and this behavioral immune response is not affected by mutations in tph-1.1,8 Using a transcriptional reporter we found that exposure to this pathogen does not increase tph-1 expression in ADF.1 In comparison, in response to contact with the pathogen Pseudomonas aeruginosa, C. elegans undergoes a behavioral immune response, learning within hours to avoid the smell of Pseudomonas. In this case, Pseudomonas ingestion increases intracellular calcium, which then increases tph-1 transcription and the levels of serotonin in ADF.9 This response is cell-autonomously regulated by the C. elegans homolog of calcium/calmodulin-dependent protein kinase II (CaMKII), UNC-43.9 This suggests that the same gene in the same chemosensory neurons can show differential regulation in response to different pathogens, illustrating the precise and subtle levels of control required.
Dense Core Vesicle Release and the Immune Response
Unlike small synaptic vesicles, which are localized to synaptic zones, dense core vesicles (DCVs) are diffusely scattered throughout the nerve terminal.10 DCVs release a number of bioactive molecules including serotonin, and neuropeptides,11,12 many of which influence the immune response. Reducing DCV release by mutations in unc-31 (the calcium activator protein required for DCV secretion) results in increased resistance to Pseudomonas aeruginosa infections, suggesting that molecules released from DCV's suppress this immune response.13 Different DCV cargoes are required to modulate the response to different pathogen infections. Our work has shown that exogenous serotonin suppresses the rectal epithelial immune response to infection with M. nematophilum, while reductions in serotonin synthesis results in an increased immune response.1 In comparison, during Pseudomonas aeruginosa infections, loss of serotonin has no effect on cellular immunity, while mutations in the neuropeptide processing enzymes egl-3 and egl-21 result in enhanced pathogen resistance.2,13 This is due to the action of the insulin-like neuropeptide INS-7, acting as a DAF-2 agonist to negatively regulate resistance to Pseudomonas infection (Fig. 1C).13
Further DCV cargoes have also been implicated in modulating the immune response. Zugasti et al. have demonstrated that TGF-β signaling from the nervous system promotes expression of Caenacin peptides in the epidermis following infection with the fungal pathogen Drechmaria coniospora (Fig. 1C).14 Recent work by the Aballay lab has shown that during Pseudomonas infection OCTR-1, a putative catecholamine receptor whose ligand octopamine, is the invertebrate equivalent of noradrenaline, is required in ASH and ASI neurons to negatively regulate the p38 MAPK and unfolded protein response in pharyngeal and intestinal cells (Fig. 1B).15 However, whether octopamine acts as the OCTR-1 ligand to mediate this effect on the immune response is currently unclear.15 Another monoamine neurotransmitter found in DCVs is dopamine. Although direct evidence that this neurotransmitter can modulate the immune response is lacking, dopaminergic neurons have been shown to play a role in enabling the conditioning of C. elegans to enteropathogenic E. coli so that they are more resistant to infection upon a second exposure (Fig. 1C).16
Although there is strong evidence that molecules released from DCVs modulate the cellular immune response, the question of whether neurotransmitter release from small synaptic vesicles can also affect innate immune function remains to be addressed. The availability of C. elegans mutants in enzymes required to synthesize these neurotransmitters provides an excellent starting point to address this question.
Neuronal Signaling to Immune Cells; A Direct or Indirect Action?
One key question is how signals originating in neurons in the head are transmitted to distant targets such as the rectal epithelium or intestine. During M. nematophilum infection serotonin synthesized and released from ADF chemosensory neurons in the head acts via GOA-1 to modulate the response of rectal epithelial cells in the tail. Although ADF forms synapses with 17 interneurons and sensory neurons, as well as forming gap junctions with 2 additional sensory neurons (www.wormweb.org), the rectal epithelium is not reported to be a direct postsynaptic target. Changes in neuronal connectivity in response to M. nematophilum infection are possible, but our observations using a tph-1 transcriptional reporter suggest this not the case (unpublished data). Serotonin can act at sites microns away from its site of release to activate receptors,17 however whether it is possible that it could traverse the length of the worm at a high enough concentration to activate receptors in the rectal epithelium is unclear. An alternative explanation is that serotonin could mediate its actions on the rectal epithelium indirectly. This is not a new concept, indeed GOA-1 expressed on cholinergic motor neurons in the ventral nerve cord is known to act downstream of serotonin signaling in the regulation of locomotion.18
However, until recently it was not known whether serotonin signaled directly by binding to serotonin receptors expressed on these cholinergic neurons or indirectly by modulating the activity of interneurons, which subsequently activate GOA-1. Recent work by Gürel et al. revealed that the serotonin receptors controlling locomotion, MOD-1 and SER-4 are not expressed in cholinergic motor neurons, but are expressed in non-overlapping sets of interneurons in the head and tail. MOD-1 is also expressed in GABAergic motor neurons in the ventral nerve cord, suggesting that serotonin does indeed act indirectly on GOA-1 in cholinergic motor neurons.19 Similarly, serotonin may act indirectly on GOA-1 to modulate the immune response in the rectal epithelium. Our worked defined at least SER-1 and SER-7 receptors as playing a role in modulating the epithelial immune response.1 However, in the absence of infection, the reported expression for SER-1 and SER-7 places neither receptor in the rectal epithelium, suggesting that their action on the epithelium is likely to be indirect.
A Conserved Network of G Proteins Mediate Neurotransmission and Epithelial Immunity
Locomotion in C. elegans is dependent upon a network of G-proteins, including EGL-30(Gαq) and GOA-1(Gαo) which act antagonistically in cholinergic motor neurons to regulate acetylcholine release.20 Loss of egl-30 results in reduced acetylcholine release and locomotion. Animals lacking goa-1 show increased release and movement, as well as being resistant to the enhanced slowing on food response mediated by serotonin, suggesting this response is mediated by goa-1.18 Genetic data suggests that GOA-1 acts parallel to20 or upstream of 21 EGL-30 in locomotion. In 2012, we showed that the immune response to M. nematophilum in the rectal epithelium requires the EGL-30(Gαq)-UNC-73(TRIO)-RHO-1(RhoA) signaling pathway.22 Our most recent work shows that GOA-1 signaling acts antagonistically to this signaling pathway in the rectal epithelium, acting upstream of, or in parallel to, EGL-30.1 This demonstrates that the same conserved G-proteins can mediate different responses in different tissues.
Neuro-Immune Signaling in Mammalian Systems
The concept of neuro-immune communication is not restricted to C. elegans. Humans undergoing psychological stress show changes in immune measures and are known to become more susceptible to new infections or reactivation of a latent infection.23,24 Clinical depression is associated with changes in cellular immunity, including changes in lymphocyte proliferation and natural killer (NK) cell activity.25 In mammals both the innate and adaptive immune systems show interactions with the nervous system. Mammalian immune cells express receptors for neurotransmitters, including serotonin, and can be influenced by neuronal signaling. Serotonin is released in response to injury and pro-inflammatory signals26 and many immune cells express receptors for serotonin including dendritic cells,27 mast cells28 and macrophages.29 Treating T-cells with exogenous serotonin can inhibit proliferation and promote T-cell activation.26 Serotonin can also act induce mast cell migration28 and proliferation of NK cells.30 Dopamine is also implicated in mediating immune responses in mammals. Induction of dopamine release has been shown to suppress systemic inflammation and improve survival in a mouse model of sepsis.31
Evidence is also accumulating that this communication is bidirectional and that the immune system can also modulate neuronal signaling. In response to infection, mammalian immune cells can produce neuropeptides, cytokines and neurotransmitters that influence the nervous system and can lead to the development of sickness syndrome and depression.32-34 This bidirectional communication means that dysfunction in the nervous system can have a significant impact on the immune system and vice versa. Indeed analysis of people with the autoimmune condition rheumatoid arthritis reveals an increased incidence of depression.35
The role of the immune response in regulating neuronal function is yet to be explored in C. elegans, however detailed characterization of the neuronal circuits underlying behavior, coupled with our growing understanding of C. elegans immunity, provides an excellent starting point for this work.
Future Perspectives
Our recent publication together with previous data highlights the profound interrelationship between the nervous and immune systems required for optimal survival. Balancing the inputs of the 2 systems is complex. For example, when food is scarce expending resources mounting an immune response may not always be the most appropriate response. Likewise, mutants defective in DVC secretion are better at combating an opportunistic Pseudomonas infection, but this is often accompanied by defects in locomotion that would likely prove detrimental to survival in their natural environment. As classical neurotransmitters become recognized as immunomodulators, it will be interesting to determine whether synaptic vesicle release can also influence the immune response or if this is restricted to DCVs.
Broadening our understanding of the dialog between the nervous and immune systems has the opportunity to provide new treatments options for those developing mood disorders post-infection, or reducing susceptibility to infection for those experiencing psychological stress. The complexity of the mammalian nervous and immune systems mean that dissecting out the signaling pathways important in integrating these systems is extremely difficult. This is why, since a near complete connectome for the nervous system of C. elegans exists, the simple worm is likely to continue to play a significant role in understanding the complex interplay between the nervous and immune systems in the future.
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
Funding
AA and RM are funded by a Wellcome Trust Career Development Fellowship to RM.
References
- 1. Anderson A, Laurenson-Schafer H, Partridge FA, Hodgkin J, McMullan R. Serotonergic chemosensory neurons modify the C. elegans immune response by regulating G-protein signaling in epithelial cells. PLoS Pathogens 2013; 9:e1003787; PMID:24348250; http://dx.doi.org/ 10.1371/journal.ppat.1003787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 2005; 438:179-84; PMID:16281027; http://dx.doi.org/ 10.1038/nature04216 [DOI] [PubMed] [Google Scholar]
- 3. Reddy KC, Andersen EC, Kruglyak L, Kim DH. A polymorphism in npr-1 is a behavioral determinant of pathogen susceptibility in C. elegans. Science 2009; 323:382-4; PMID:19150845; http://dx.doi.org/ 10.1126/science.1166527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 2000; 403:560-4; PMID:10676966; http://dx.doi.org/ 10.1038/35000609 [DOI] [PubMed] [Google Scholar]
- 5. Moussaif M, Sze JY. Intraflagellar transport/Hedgehog-related signaling components couple sensory cilium morphology and serotonin biosynthesis in Caenorhabditis elegans. J Neurosci:Off J Soc Neurosci 2009; 29:4065-75; PMID:19339602; http://dx.doi.org/ 10.1523/JNEUROSCI.0044-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Estevez AO, Cowie RH, Gardner KL, Estevez M. Both insulin and calcium channel signaling are required for developmental regulation of serotonin synthesis in the chemosensory ADF neurons of Caenorhabditis elegans. Dev Biol 2006; 298:32-44; PMID:16860310; http://dx.doi.org/ 10.1016/j.ydbio.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 7. Estevez M, Estevez AO, Cowie RH, Gardner KL. The voltage-gated calcium channel UNC-2 is involved in stress-mediated regulation of tryptophan hydroxylase. J Neurochem 2004; 88:102-13; PMID:14675154; http://dx.doi.org/ 10.1046/j.1471-4159.2003.02140.x [DOI] [PubMed] [Google Scholar]
- 8. Yook K, Hodgkin J. Mos1 mutagenesis reveals a diversity of mechanisms affecting response of Caenorhabditis elegans to the bacterial pathogen Microbacterium nematophilum. Genet 2007; 175:681-97; PMID:17151260; http://dx.doi.org/ 10.1534/genetics.106.060087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qin Y, Zhang X, Zhang Y. A neuronal signaling pathway of CaMKII and Gqalpha regulates experience-dependent transcription of tph-1. J Neurosci:Off J Soc Neurosci 2013; 33:925-35; PMID:23325232; http://dx.doi.org/ 10.1523/JNEUROSCI.2355-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salio C, Lossi L, Ferrini F, Merighi A. Neuropeptides as synaptic transmitters. Cell Tissue Res 2006; 326:583-98; PMID:16847638; http://dx.doi.org/ 10.1007/s00441-006-0268-3 [DOI] [PubMed] [Google Scholar]
- 11. Desai C, Garriga G, McIntire SL, Horvitz HR. A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature 1988; 336:638-46; PMID:3200316; http://dx.doi.org/ 10.1038/336638a0 [DOI] [PubMed] [Google Scholar]
- 12. Sulston J, Dew M, Brenner S. Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol 1975; 163:215-26; PMID:240872; http://dx.doi.org/ 10.1002/cne.901630207 [DOI] [PubMed] [Google Scholar]
- 13. Kawli T, Tan MW. Neuroendocrine signals modulate the innate immunity of Caenorhabditis elegans through insulin signaling. Nat Immunol 2008; 9:1415-24; PMID:18854822; http://dx.doi.org/ 10.1038/ni.1672 [DOI] [PubMed] [Google Scholar]
- 14. Zugasti O, Ewbank JJ. Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-beta signaling pathway in Caenorhabditis elegans epidermis. Nat Immunol 2009; 10:249-56; PMID:19198592; http://dx.doi.org/ 10.1038/ni.1700 [DOI] [PubMed] [Google Scholar]
- 15. Sun J, Singh V, Kajino-Sakamoto R, Aballay A. Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science 2011; 332:729-32; PMID:21474712; http://dx.doi.org/ 10.1126/science.1203411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anyanful A, Easley KA, Benian GM, Kalman D. Conditioning protects C. elegans from lethal effects of enteropathogenic E. coli by activating genes that regulate lifespan and innate immunity. Cell Host Microbe 2009; 5:450-62; PMID:19454349; http://dx.doi.org/ 10.1016/j.chom.2009.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bunin MA, Wightman RM. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J Neurosci: Off J Soc Neurosci 1998; 18:4854-60; PMID:9634551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nurrish S, Segalat L, Kaplan JM. Serotonin inhibition of synaptic transmission: Galpha(0) decreases the abundance of UNC-13 at release sites. Neuron 1999; 24:231-42; PMID:10677040; http://dx.doi.org/ 10.1016/S0896-6273(00)80835-1 [DOI] [PubMed] [Google Scholar]
- 19. Gurel G, Gustafson MA, Pepper JS, Horvitz HR, Koelle MR. Receptors and other signaling proteins required for serotonin control of locomotion in Caenorhabditis elegans. Genet 2012; 192:1359-71; PMID:23023001; http://dx.doi.org/ 10.1534/genetics.112.142125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lackner MR, Nurrish SJ, Kaplan JM. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 1999; 24:335-46; PMID:10571228; http://dx.doi.org/ 10.1016/S0896-6273(00)80848-X [DOI] [PubMed] [Google Scholar]
- 21. Hajdu-Cronin YM, Chen WJ, Patikoglou G, Koelle MR, Sternberg PW. Antagonism between G(o)alpha and G(q)alpha in Caenorhabditis elegans: the RGS protein EAT-16 is necessary for G(o)alpha signaling and regulates G(q)alpha activity. Genes Dev 1999; 13:1780-93; PMID:10421631; http://dx.doi.org/ 10.1101/gad.13.14.1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McMullan R, Anderson A, Nurrish S. Behavioral and immune responses to infection require Galphaq- RhoA signaling in C. elegans. PLoS Pathogens 2012; 8:e1002530; PMID:22359503; http://dx.doi.org/ 10.1371/journal.ppat.1002530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nature Rev Immunol 2005; 5:243-51; PMID:15738954; http://dx.doi.org/ 10.1038/nri1571 [DOI] [PubMed] [Google Scholar]
- 24. Herbert TB, Cohen S. Stress and immunity in humans: a meta-analytic review. Psychosom Med 1993; 55:364-79; PMID:8416086; http://dx.doi.org/ 10.1097/00006842-199307000-00004 [DOI] [PubMed] [Google Scholar]
- 25. Herbert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychol Bull 1993; 113:472-86; PMID:8316610; http://dx.doi.org/ 10.1037/0033-2909.113.3.472 [DOI] [PubMed] [Google Scholar]
- 26. O'Connell PJ, Wang X, Leon-Ponte M, Griffiths C, Pingle SC, Ahern GP. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood 2006; 107:1010-7; PMID:16223770; http://dx.doi.org/ 10.1182/blood-2005-07-2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Idzko M, Panther E, Stratz C, Muller T, Bayer H, Zissel G, Durk T, Sorichter S, Di Virgilio F, Geissler M, et al. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol 2004; 172:6011-9; PMID:15128784; http://dx.doi.org/ 10.4049/jimmunol.172.10.6011 [DOI] [PubMed] [Google Scholar]
- 28. Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, Brown JM, Bruening S, Toth M, Metcalfe DD. 5-hydroxytryptamine induces mast cell adhesion and migration. J Immunol 2006; 177:6422-32; PMID:17056574; http://dx.doi.org/ 10.4049/jimmunol.177.9.6422 [DOI] [PubMed] [Google Scholar]
- 29. Mikulski Z, Zaslona Z, Cakarova L, Hartmann P, Wilhelm J, Tecott LH, Lohmeyer J, Kummer W. Serotonin activates murine alveolar macrophages through 5-HT2C receptors. Am J Physiol Lung Cell Mol Physiol 2010; 299:L272-80; PMID:20495077; http://dx.doi.org/ 10.1152/ajplung.00032.2010 [DOI] [PubMed] [Google Scholar]
- 30. Hernandez ME, Martinez-Fong D, Perez-Tapia M, Estrada-Garcia I, Estrada-Parra S, Pavon L. Evaluation of the effect of selective serotonin-reuptake inhibitors on lymphocyte subsets in patients with a major depressive disorder. Eur Neuropsychopharm: J Eur Coll Neuropsychopharm 2010; 20:88-95; PMID:20005081; http://dx.doi.org/ 10.1016/j.euroneuro.2009.11.005 [DOI] [PubMed] [Google Scholar]
- 31. Torres-Rosas R, Yehia G, Pena G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 2014; 20:291-5; PMID:24562381; http://dx.doi.org/ 10.1038/nm.3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9:46-56; PMID:18073775; http://dx.doi.org/ 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moreau M, Andre C, O'Connor JC, Dumich SA, Woods JA, Kelley KW, Dantzer R, Lestage J, Castanon N. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav Immunity 2008; 22:1087-95; PMID:18479887; http://dx.doi.org/ 10.1016/j.bbi.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiat 2009; 14:511-22; PMID:18195714; http://dx.doi.org/ 10.1038/sj.mp.4002148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Godha D, Shi L, Mavronicolas H. Association between tendency towards depression and severity of rheumatoid arthritis from a national representative sample: the Medical Expenditure Panel Survey. Curr Med Res Opin 2010; 26:1685-90; PMID:20455827; http://dx.doi.org/ 10.1185/03007991003795808 [DOI] [PubMed] [Google Scholar]


