Abstract
The transcription factor Pancreatic and Duodenal Homeobox-1 (PDX-1) plays a major role in the development and function of pancreatic β−cells and its mutation results in diabetes. In adult β−cells, glucose stimulates transcription of the insulin gene in part by regulating PDX-1 expression, stability and activity. Glucose is also thought to modulate PDX-1 nuclear translocation but in vitro studies examining nucleo-cytoplasmic shuttling of endogenous or ectopically expressed PDX-1 in insulin-secreting cell lines have led to conflicting results. Here we show that endogenous PDX-1 undergoes translocation from the cytoplasm to the nucleus in response to glucose in dispersed rat islets but not in insulin-secreting MIN6, HIT-T15, or INS832/13 cells. Interestingly, however, we found that a PDX-1-GFP fusion protein can shuttle from the cytoplasm to the nucleus in response to glucose stimulation in HIT-T15 cells. Our results suggest that the regulation of endogenous PDX-1 sub-cellular localization by glucose is observed in primary islets and that care should be taken when interpreting data from insulin-secreting cell lines.
Keywords: glucose, glucose-stimulated insulin secretion, HIT-T15, INS832/13, MIN6, nucleo-cytoplasmic shuttling, pancreatic β cells, PDX-1
Abbreviations
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- DAPI
4′, 6-diamidino-2-phenylindole
- DMEM
dulbecco's modified eagle medium
- EDTA
ethylenediaminetetraacetic acid
- GFP
green fluorescent protein
- HDAC
histone deacetylase
- KRBH
krebs ringer bicarbonate hepes
- MODY
maturity-onset diabetes of the young
- PDX-1
pancreatic and duodenal homeobox-1
- SEM
standard error of the mean
- SUMO
small ubiquitin-like modifier
- T2D
type 2 diabetes
- ZDF
zucker diabetic fatty
Introduction
The worldwide prevalence of type 2 diabetes (T2D) has reached epidemic proportions as a result of abundant nutrient supply and sedentary lifestyle. T2D is characterized by fasting hyperglycaemia resulting from insufficient insulin secretion from pancreatic β−cells and peripheral insulin resistance. The transcription factor Pancreatic and Duodenal Homeobox-1 (PDX-1) is a homeodomain protein that activates insulin gene transcription as well as other pancreas-specific genes and plays a critical role in both pancreatic development and adult β−cell function (reviewed in1). Complete PDX-1 deficiency results in pancreatic agenesis in mice2 and humans,3 whereas β−cell specific disruption or heterozygous mutations result in T2D.4 In humans, mutations in pdx-1 are associated with the development of maturity-onset diabetes of the young type 4 (MODY 4), a monogenic form of T2D characterized by impaired β−cell function.5,6 Other mutations in human pdx-1 were shown to be associated with the development of adult-onset forms of T2D.7-9
Glucose induces post-translational modifications of PDX-1. These include O-GlcNAcylation,10,11 SUMOylation12 and phosphorylation13-25 that regulate PDX-1 protein stability,18,19,26 its transactivation potential,27 its interaction with co-factors such as the histone acetyl transferase p300,28 and its binding to the A boxes on the insulin gene promoter10,11,29 to trigger insulin gene transcription. The PDX-1 homeodomain contains a basic amino acid sequence, RRMKWKK, that also serves as a nuclear localization signal for PDX-1 nuclear import.30 The interest in the regulation of PDX-1 nucleo-cytoplasmic shuttling stems from the observation that β−cell dysfunction in islets from T2D patients correlates with the exclusion of PDX-1 from the nucleus.31,32 Similar results were obtained in rodent models of metabolic stress, such as in db/db mice,32 high fat diet in mice,31,33 ZDF rats34 nutrient infusion in rats,35 and in vitro oxidative and glucolipotoxic stress.35-38 Glucose16,35 and other factors promoting insulin secretion and β-cell survival, including GLP-1,31,39,40 TGF-β,41 nitric oxide42 and insulin15,43 increase nuclear PDX-1. The glucose-dependent shuttling of PDX-1 from the cytoplasm to the nucleus was established in vivo in glucose-infused rats35 and in vitro in cultured human islets.16 In contrast, studies using a number of insulin-secreting cell lines showed that PDX-1 is localized to the nuclear periphery,21,25,44 or restricted to the nucleus26,30 regardless of the glucose concentration. Since insulin-secreting cells are commonly used as in vitro models for studying the regulation of β-cell gene expression, this study aimed to determine whether PDX-1 nucleo-cytoplasmic shuttling is differently regulated by glucose in primary rat islets vs. the commonly used insulin-secreting MIN6, HIT-T15 and INS832/13 cell lines.
Results and Discussion
Glucose promotes the translocation of PDX-1 from the cytoplasm to the nucleus in dispersed rat islets
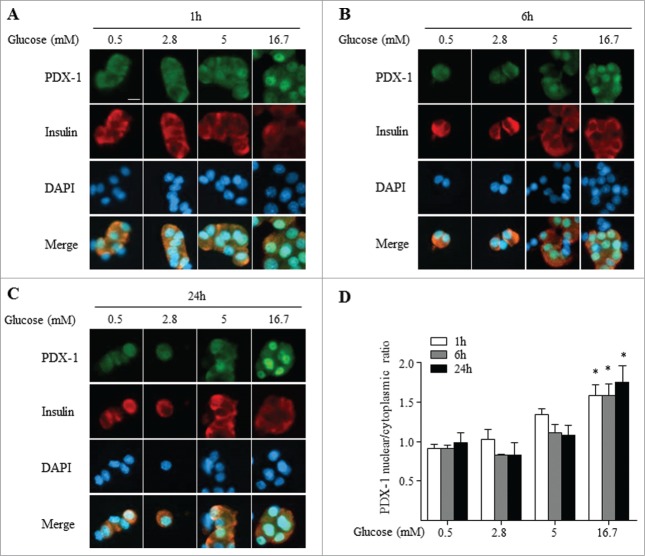
We first assessed PDX-1 nucleo-cytoplasmic shuttling by immunocytochemistry in dispersed rat islets exposed for 1, 6 and 24 h to increasing glucose concentrations (0.5 to 16.7 mM). After 1 h of treatment at 0.5 or 2.8 mM glucose, PDX-1 abundance was similar in the cytoplasmic and nuclear compartments (Fig. 1A and D). This distribution did not change after 6 and 24 h of low glucose exposure (Fig. 1B, C and D). Although not significant, there was a tendency for an increase in nuclear PDX-1 in the presence of 5 mM glucose in most cells as shown in Figure 1. After exposure to 16.7 mM glucose, however, PDX-1 was located predominantly in the nucleus already after 1 h and the distribution remained nuclear after 6 and 24 h (Fig. 1; p ≤ 0.05 at 16.7 vs. 0.5 mM glucose for all 3 time points). These results demonstrate that in dispersed rat islets, PDX-1 undergoes a cytoplasmic to nuclear shift in response to increasing glucose concentrations. These results are similar to previous reports in human islets ex vivo16 and in glucose-infused rats in vivo.35
Figure 1.
Glucose induces PDX-1 nuclear translocation in dispersed rat islets. PDX-1 (green), insulin (red) and nuclei (DAPI, blue) were visualized by fluorescence microscopy (×20). Dispersed rat islets were exposed 1 h (A), 6 h (B) and 24 h (C) to increasing glucose concentrations as indicated. (D) Quantification of nuclear/cytoplasmic ratio of 4 replicate experiments. Results are expressed as mean ± SEM. *P ≤ 0.05: 16.7 vs 0.5 mM glucose at all time points. Scale bar, 10 μm.
PDX-1 subcellular localization is not regulated by glucose in MIN6 cells
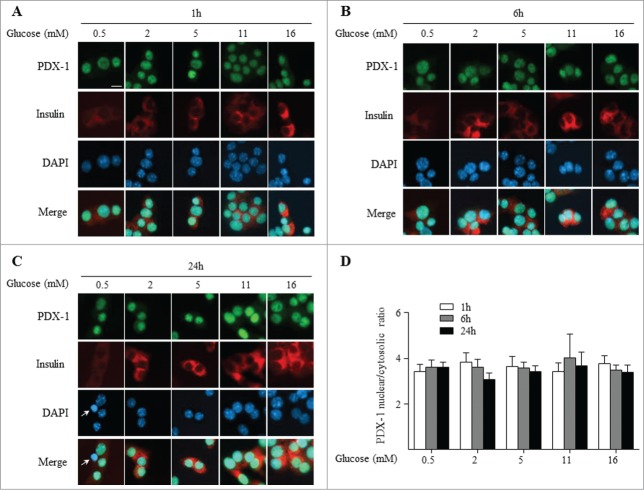
To determine whether insulin-secreting cells respond similarly to glucose as dispersed rat islets, the murine insulinoma MIN6 cell line was exposed for 1, 6 and 24 h to increasing glucose concentrations (0.5 to 16 mM) and endogenous PDX-1 sub-cellular localization was assessed as described above. PDX-1 immunostaining was already predominantly detected in the nucleus at 0.5 and 2 mM glucose and this pattern was maintained at 5, 11 and 16 mM glucose at all time points examined (Fig. 2). Even after 24 h of exposure to a low glucose concentration (0.5 mM) which started to trigger cell death (as shown by the presence of pyknotic nuclei (Fig. 2C)), PDX-1 was largely nuclear. We asked whether the constitutive nuclear localization of Pdx-1 in MIN6 was due to glucose hyper-responsiveness. To test this we analyzed glucose-stimulated insulin secretion over the same range of glucose concentrations (Suppl. Fig. 1A) and observed that glucose dose-dependently stimulates insulin secretion in MIN6 cells in a similar manner as in isolated islets, as previously published.45 Nuclear localization of Pdx-1 in MIN6 cells is consistent with our previous observation26 and in accordance with another reported study using an exogenous PDX-1-GFP fusion protein.30 However, other studies using MIN6 cells reported endogenous PDX-1 in the cytoplasm at low glucose levels and an increase in the nucleo-cytoplasmic ratio in the presence of high glucose.15,16,34 In some cases the discrepancy with our data may be due to differences in experimental approaches. For example, Macfarlane et al.16 measured the localization of specific PDX-1 isoforms by Western blotting. Rafiq et al.14 found that increasing glucose levels from 3 to 30 mM led to a small increase in the nuclear fraction of endogenous PDX-1. Although this differs from our results, we did not test glucose concentrations above the physiological range.
Figure 2.
PDX-1 is predominantly localized in the nucleus in MIN6 cells independent of the glucose concentration. PDX-1 (green), insulin (red) and nuclei (DAPI, blue) were visualized by fluorescence microscopy (×20). MIN6 cells were exposed 1 h (A), 6 h (B) and 24 h (C) to increasing glucose concentrations as indicated. Arrows indicate pyknotic nuclei. (D) Quantification of nuclear/cytoplasmic ratio of 3 replicate experiments. Results are expressed as mean ± SEM. Scale bar, 10 μm.
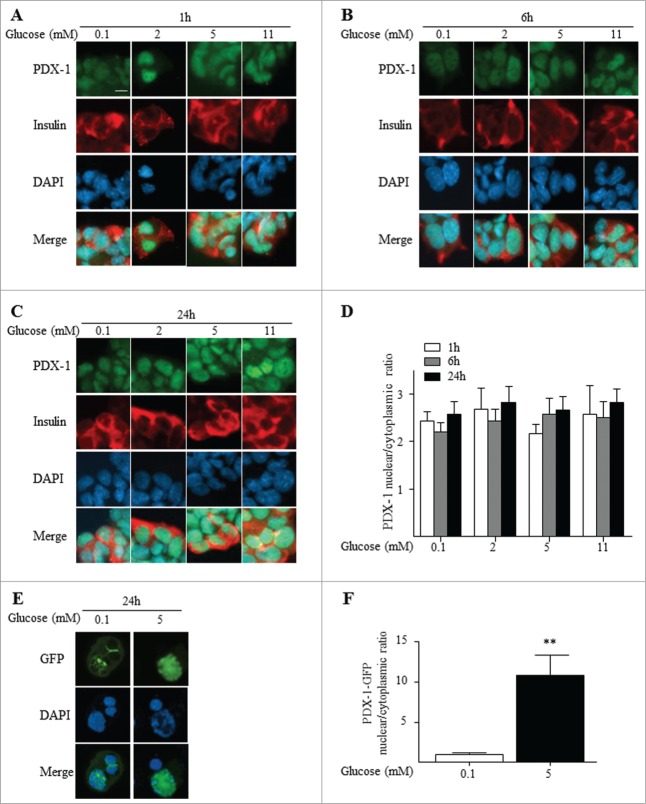
Glucose regulates exogenous but not endogenous PDX-1 nuclear translocation in HIT-T15 cells
To investigate the nucleo-cytoplasmic shuttling of PDX-1 in another rodent insulin-secreting cell line, hamster HIT-T15 cells were exposed for 1, 6 and 24 h to increasing glucose concentrations (0.1 to 11 mM). The glucose concentration range was chosen based on results from insulin secretion assays which revealed a dose-dependent but left-shifted (0.1–16 mM) increase in insulin secretion (Suppl. Fig. 1B), as previously observed.46 Similar to our observation in MIN6 cells (Fig. 2), PDX-1 was predominantly localized in the nucleus of HIT-T15 cells following exposure to low glucose (0.1 mM) at all time points, and increasing the glucose concentration had no detectable effect on PDX-1 localization (Fig. 3A-D). As numerous studies have described the shuttling of ectopically expressed PDX-1 fusion proteins from the cytoplasm or nuclear periphery to the nucleoplasm in response to increasing glucose concentrations,14,21,34,44 we tested whether this could be detected in HIT-T15 cells under the same conditions used to study endogenous PDX-1. Similar to our previous results,38 and unlike the endogenous protein (Fig. 3A-D), exogenous PDX-1 was predominantly expressed at the nuclear periphery and in the cytoplasm of HIT-T15 at low glucose concentrations and translocated to the nucleus in response to high glucose (Fig. 3E-F). This indicates that the mechanisms controlling glucose-dependent PDX-1 nuclear translocation are functional in HIT-T15 cells but are not operative with the endogenous protein. We speculate that nuclear import/export or retention signals, such as HDAC1 and HDAC225 and Importin β 1,34 may become saturated by overexpression of exogenous PDX-1 resulting in accumulation of the protein in the cytoplasm/nuclear periphery.
Figure 3.
In HIT-T15 cells, endogenous PDX-1 is localized to the nucleus independent of the glucose concentration, whereas ectopically expressed PDX-1 shuttles from the cytoplasm to the nucleus in a glucose-dependent manner. PDX-1 (green), insulin (red) and nuclei (DAPI, blue) were visualized by fluorescence microscopy (×20). HIT-T15 cells were exposed 1 h (A), 6 h (B) and 24 h (C) to increasing glucose concentrations as indicated. (D) Quantification of nuclear/cytoplasmic ratio of 3 replicate experiments. Results are expressed as mean ± SEM. (E) HIT-T15 cells were transfected with pcDNA3.1-PDX-1-GFP plasmid and exposed for 24 h to 0.1 or 5 mM glucose. PDX-1 (GFP, green) and nuclei (DAPI, blue) were visualized by confocal microscopy. (F) Quantification of nuclear/cytoplasmic ratio of 3 replicate experiments. Results are expressed as mean ± SEM. **P ≤ 0.01. Scale bar, 10 μm.
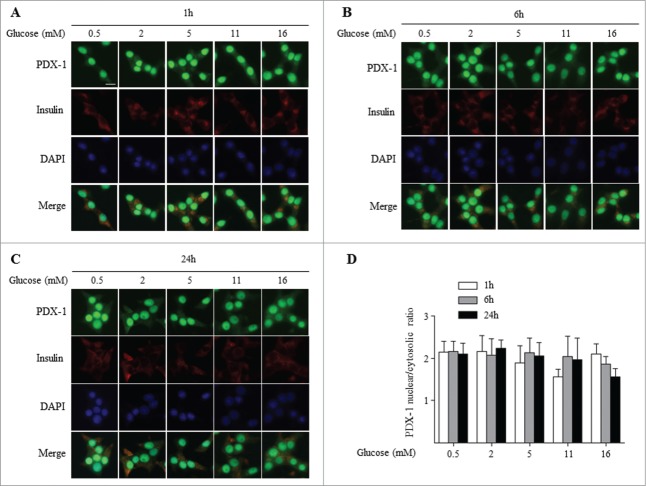
PDX-1 subcellular localization is not regulated by glucose in INS832/13 cells
As both MIN647 and HIT-T1548 cell lines were established by expression of the SV40 T antigen in islets, we wanted to exclude the possibility that T antigen transformation interferes with glucose-dependent nucleo-cytoplasmic shuttling of PDX-1. We analyzed PDX-1 localization in response to glucose in a clonal derivative of the X-ray-induced rat insulinoma INS-1 cells,49 INS832/13, that was selected for its glucose-stimulated insulin secretory responsiveness.50 INS832/13 cells were exposed for 1, 6 and 24 h to increasing glucose concentrations (0.5 to 16 mM). The glucose concentration range was chosen based on results from insulin secretion assays which revealed a dose-dependent (0.1–16 mM) increase in insulin secretion (Suppl. Fig. 1C), as previously described.50 Similar to our observation in MIN6 (Fig. 2) and HIT-T15 (Fig. 3) cells, PDX-1 was predominantly localized in the nucleus of INS832/13 cells following exposure to low glucose (0.5 mM) at all time points, and increasing the glucose concentration had no significant effect on PDX-1 localization (Fig. 4). Although a report by Sayo et al.41 found that glucose increased nuclear PDX-1 in the parental INS-1 cells, our data are not inconsistent as glucose also increased total cellular PDX-1 and the shuttling of PDX-1 was not addressed in that study.
Figure 4.
PDX-1 is predominantly localized in the nucleus in INS832/13 cells independent of the glucose concentration. PDX-1 (green), insulin (red) and nuclei (DAPI, blue) were visualized by fluorescence microscopy (×20). INS832/13 cells were exposed 1 h (A), 6 h (B) and 24 h (C) to increasing glucose concentrations as indicated. (D) Quantification of nuclear/cytoplasmic ratio of 3 replicate experiments. Results are expressed as mean ± SEM. Scale bar, 10 μm.
PDX-1 localization is affected by oxidative stress in dispersed rat islets, MIN6 and INS832/13 cells but not in HIT-T15 cells
PDX-1 is excluded from the nucleus of β cells in islets isolated from rats infused with glucose + Intralipid,35 from db/db mice or mice fed a high fat – high sucrose diet31 and in ZDF rats51 and in vitro in islets exposed to glucose and palmitate.37 Reactive oxygen species are thought to be the main trigger as oxidative stress leads to PDX-1 nuclear exclusion in HIT-T15 cells37 and anti-oxidant treatment of ZDF rats restores PDX-1 nuclear localization.46 To investigate whether PDX-1 nucleo-cytoplasmic shuttling remains sensitive to oxidative stress, we exposed dispersed rat islets (Fig. 5A) or MIN6, INS832/13 and HIT-T15 cells (Fig. 5B) to 50 μM H2O2 in the presence of 16.7 mM glucose for 1 h and examined PDX-1 localization. As a positive control for PDX-1 nuclear exclusion, palmitate prevented glucose-induced PDX-1 nuclear import in isolated rat islets (compare Fig. 5A to Fig. 1C) as shown previously.37 H2O2 treatment led to PDX-1 nuclear exclusion in dispersed rat islets (compare Fig. 5A to Fig. 1A) and MIN6 cells (compare Fig. 5B to Fig. 2A). H2O2 treatment of INS832/13 led to an approximately 3-fold decrease in the nuclear/cytoplasmic ratio of PDX-1 (0.66 ± 0.08 vs 2.1 ± 0.24, p < 0 .01; compare Fig. 5B to Fig. 4A). Surprisingly, H2O2 exposure did not affect PDX-1 localization in HIT-T15 cells (compare Fig. 5B to Fig. 3A), in contrast to a previous study.37 This discrepancy may be due to the difference in the passage number of HIT-T15 which may affect their susceptibility to oxidative stress. While ours were used at 78–86 passages, cells used by Kawamori et al.37 were of higher passages.
Figure 5.
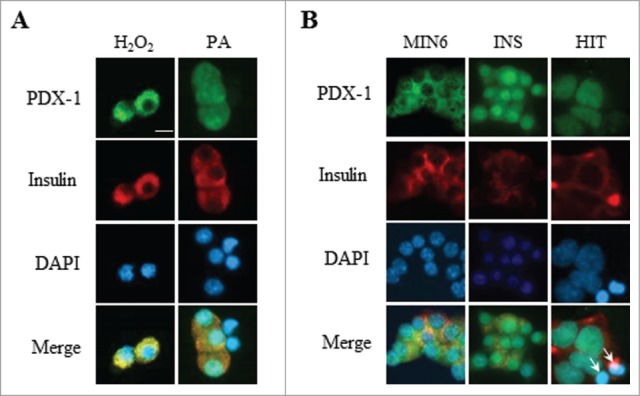
PDX-1 localization is affected by oxidative stress in dispersed rat islets and MIN6 and INS832/13 cells but not in HIT-T15 cells. PDX-1 (green), insulin (red) and nuclei (DAPI, blue) were visualized by fluorescence microscopy (×20). (A) Dispersed rat islets were exposed to 16.7 mM glucose in the presence of 50 μM H2O2 for 1h or 0.5 mM palmitate (PA) for 24 h. (B) MIN6, INS832/13 (INS) and HIT-T15 (HIT) cells were exposed to 16.7 mM glucose in the presence of 50 μM H2O2 for 1 h. Arrows indicate pyknotic nuclei. Scale bar, 10 μm.
In summary, these data show that PDX-1 nuclear localization is regulated by glucose in dispersed rat islets, consistent with previous ex vivo16 and in vivo studies.35 In contrast, using the same experimental conditions we found endogenous PDX-1 sub-cellular localization to be independent of glucose in the insulin-secreting MIN6, HIT-T15 and INS832/13 cells. These data highlight a defect in the mechanism regulating the localization of PDX-1 and its glucose responsiveness in a number of commonly used β-cell lines which we speculate may have arisen during the transformation process. In support of this, PDX-1 is highly expressed in human pancreatic tumors52-54 and controls their oncogenic properties.55-57 It would be interesting to examine the localization of PDX-1 and its glucose responsiveness in insulin-secreting cell lines under restricted growth conditions. In conclusion, these results raise a note of caution regarding the use of transformed insulin-secreting cell lines to examine the regulation of PDX-1 nucleo-cytoplasmic shuttling.
Materials and Methods
Generation of PDX-1-GFP expression plasmid
A pcDNA3.1-PDX-1-GFP plasmid was generated as previously described.38,44 Briefly, after stop codon removal, the mouse PDX-1-cMyc sequence was excised from the pcDNA3.1-PDX-1-cMyc plasmid (provided by Dr. Guy Rutter, Imperial College London, London, United Kingdom) using Hind III and Kpn I and re-inserted into the mammalian expression vector pcDNA3.1-GFP provided by Dr. Marc Prentki (Montreal Diabetes Research Center, CRCHUM, Montreal, QC, Canada).
Rat islet isolation, dispersion and glucose stimulation
All procedures using animals were approved by the Institutional Committee for the Protection of Animals at the Center Hospitalier de l’Université de Montréal. 250–275 g male Wistar rats (Charles River) were housed on a 12 h light/dark cycle with free access to water and standard laboratory chow. Islets were isolated as previously described.35 After overnight recovery, islets were dispersed by treatment with Trypsin – 0.05 EDTA (Invitrogen) for 5 min and plated on poly-L-lysine-coated (Sigma) coverslips. Dispersed islets were cultured in RPMI-1640 media (Invitrogen) supplemented with 10 % Fetal Bovine Serum (FBS; Invitrogen) and 5.5 mM glucose for 24 h. Media was then changed to 0.5 % fatty acid-free BSA (Equitech-Bio) and 2.8 mM glucose for 2 h after which cells were treated with glucose in the presence or absence of palmitate (Sigma) or H2O2 (Invitrogen) as described in results.
MIN6, INS832/13 and HIT-T15 cell culture, transfection and glucose stimulation
MIN6 cells (passage 25–30; provided by Dr. Jun-ichi Miyazaki, Osaka University Graduate School of Medicine, Suita, Osaka, Japan) were maintained in Hyclone DMEM (ThermoFisher Scientific) containing 25 mM glucose and supplemented with 10 % FBS and 0.005 % β-mercaptoethanol. INS832/13 cells (passages 50–56; provided by Dr. Christopher Newgard, Duke University School of Medicine, Durham, NC) were maintained in RPMI-1640 containing 11 mM glucose and supplemented with 10 % FBS. For PDX-1 localization studies MIN6 and INS832/13 culture medium was changed to 5.5 mM glucose overnight then to 2 mM glucose and 0.5 % BSA for an additional 2 h after which cells were exposed to glucose in the presence or absence of H2O2 as described in results. For MIN6 static insulin secretion assays cells were cultured overnight in culture medium containing 5.5 mM glucose and then pre-incubated twice for 30 min in Krebs Ringer bicarbonate HEPES buffer (KRBH) + 0.1 % BSA supplemented with 1 mM glucose prior to stimulation with 1, 5, 9, 12, 16 or 20 mM glucose. For INS832/13 static insulin secretion assays cells were pre-incubated in culture medium containing 1 mM glucose for 2 hours and then in KRBH + 0.1 % BSA supplemented with 1 mM glucose for 1 hour prior to stimulation with 1, 2, 5, 11 and 16 mM glucose. Each condition was run in triplicate. Intracellular insulin content was measured after acid-alcohol extraction. Insulin was quantified using an AlphaLISA immunoassay kit (PerkinElmer). HIT-T15 cells (passages 74–86; provided by Dr. R. Paul Robertson, Pacific Northwest Diabetes Research Institute, Seattle, WA, USA) were maintained in RPMI-1640 containing 11.1 mM glucose and supplemented with 10 % FBS. Medium was changed to 1 mM glucose for 24 h then to 0.1 mM glucose + 0.5 % BSA for an additional 2 h prior to treatment with glucose in the presence or absence of H2O2 as described in results. For static insulin secretion assays cells were cultured overnight in 0.1 mM glucose and then pre-incubated twice for 30 min in KRBH + 0.1 % BSA supplemented with 0.1 mM glucose prior to stimulation with either 0.1, 0.5, 1, 1.5, 2, 5, 11 or 16 mM glucose and treated as described above. Transfection was performed using Lipofectamine 2000 (Invitrogen) as per the manufacturer's instructions. Following transfection in RPMI-1640 supplemented with 11.1mM glucose and 10 % FBS the medium was changed to 1 mM glucose and after 24 hours to 0.1 mM glucose + 0.5 % BSA for an additional 2 h prior to treatment with either 0.1 or 5 mM glucose.
Immunohistochemistry
PDX-1 immunostaining was performed as previously described.26 Cells were incubated with rabbit anti-PDX-1 (Abcam) and guinea pig anti-insulin (Dako) antibodies followed by Alexa Fluor® 488 (Invitrogen) and Rhodamine (Jackson ImmunoResearch) fluorophore-conjugated secondary antibodies. Coverslips were then mounted using VectaShield mounting media containing DAPI for nuclei staining (VectorLab). Images were acquired and quantified using a fluorescence microscope (×20) and Zen Imaging Software (Carl Zeiss). Quantification was performed on 3 acquired images of each of the 3–4 independent experiments, and counting 5 cells per image. For exogenous PDX-1 immunostaining, GFP signal was visualized using a Leica confocal microscope (×40).
Statistics
Results are expressed as mean ± SEM and significance was tested using Student's paired t test or a 2-way ANOVA with Bonferroni post hoc adjustment for multiple comparisons, as appropriate, using GraphPad Instat (GraphPad Software). A value of p < 0.05 was considered significant.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr Guy Rutter for the pcDNA3.1-PDX-1-cMyc plasmid, Dr. Marc Prentki for the pcDNA3.1-GFP plasmid, Dr. Christopher Newgard for the INS832/13 cells, Dr. Jun-ichi Miyazaki for the MIN6 cells and Dr. R. Paul Robertson for the HIT-T15 cells.
Funding
This study was supported by the US. National Institutes of Health (R01DK58096 to V.P.) and the Canadian Institutes of Health Research (MOP 77686 to V.P.). M.S. is the recipient of a doctoral studentship from the Fonds de Recherche Québec - Santé. B.Z. is supported by a postdoctoral fellowship from Eli Lilly. V.P. holds the Canada Research Chair in Diabetes and Pancreatic Beta cell Function.
References
- 1. Melloul D. Transcription factors in islet development and physiology: role of PDX-1 in beta-cell function. Ann N Y Acad Sci 2004; 1014:28-37; PMID:15153417; http://dx.doi.org/ 10.1196/annals.1294.003 [DOI] [PubMed] [Google Scholar]
- 2. Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter factor 1 is required for pancreatic development in mice. Nature 1994; 371:606-9; PMID:7935793; http://dx.doi.org/ 10.1038/371606a0 [DOI] [PubMed] [Google Scholar]
- 3. Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet 1997; 15:106-10; PMID:8988180; http://dx.doi.org/ 10.1038/ng0197-106 [DOI] [PubMed] [Google Scholar]
- 4. Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev 1998; 12:1763-8; PMID:9637677; http://dx.doi.org/ 10.1101/gad.12.12.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet 1997; 17:138-9; PMID:9326926; http://dx.doi.org/ 10.1038/ng1097-138 [DOI] [PubMed] [Google Scholar]
- 6. Weng J, Macfarlane WM, Lehto M, Gu HF, Shepherd LM, Ivarsson SA, Wibell L, Smith T, Groop LC. Functional consequences of mutations in the MODY4 gene (IPF1) and coexistence with MODY3 mutations. Diabetologia 2001; 44:249-58; PMID:11270685; http://dx.doi.org/ 10.1007/s001250051608 [DOI] [PubMed] [Google Scholar]
- 7. Cockburn BN, Bermano G, Boodram LL, Teelucksingh S, Tsuchiya T, Mahabir D, Allan AB, Stein R, Docherty K, Bell GI. Insulin promoter factor-1 mutations and diabetes in Trinidad: identification of a novel diabetes-associated mutation (E224K) in an Indo-Trinidadian family. J Clin Endocrinol Metab 2004; 89:971-8; PMID:14764823; http://dx.doi.org/ 10.1210/jc.2003-031282 [DOI] [PubMed] [Google Scholar]
- 8. Hani EH, Stoffers DA, Chevre JC, Durand E, Stanojevic V, Dina C, Habener JF, Froguel P. Defective mutations in the insulin promoter factor-1 (IPF-1) gene in late-onset type 2 diabetes mellitus. J Clin Invest 1999; 104:R41-8; PMID:10545531; http://dx.doi.org/ 10.1172/JCI7469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen L, Urioste S, Petersen HV, Jensen JN, Eiberg H, Barbetti F, Serup P, Hansen T, Pedersen O. Missense mutations in the human insulin promoter factor-1 gene and their relation to maturity-onset diabetes of the young and late-onset type 2 diabetes mellitus in caucasians. J Clin Endocrinol Metab 2000; 85:1323-6; PMID:10720084 [DOI] [PubMed] [Google Scholar]
- 10. Gao Y, Miyazaki J, Hart GW. The transcription factor PDX-1 is post-translationally modified by O-linked N-acetylglucosamine and this modification is correlated with its DNA binding activity and insulin secretion in min6 beta-cells. Arch Biochem Biophys 2003; 415:155-63; PMID:12831837; http://dx.doi.org/ 10.1016/S0003-9861(03)00234-0 [DOI] [PubMed] [Google Scholar]
- 11. Kebede M, Ferdaoussi M, Mancini A, Alquier T, Kulkarni RN, Walker MD, Poitout V. Glucose activates free fatty acid receptor 1 gene transcription via phosphatidylinositol-3-kinase-dependent O-GlcNAcylation of pancreas-duodenum homeobox-1. Proc Natl Acad Sci U S A 2012; 109:2376-81; PMID:22308370; http://dx.doi.org/ 10.1073/pnas.1114350109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kishi A, Nakamura T, Nishio Y, Maegawa H, Kashiwagi A. Sumoylation of Pdx1 is associated with its nuclear localization and insulin gene activation. Am J Physiol Endocrinol Metab 2003; 284:E830-40; PMID:12488243 [DOI] [PubMed] [Google Scholar]
- 13. An R, da Silva Xavier G, Semplici F, Vakhshouri S, Hao HX, Rutter J, Pagano MA, Meggio F, Pinna LA, Rutter GA. Pancreatic and duodenal homeobox 1 (PDX1) phosphorylation at serine-269 is HIPK2-dependent and affects PDX1 subnuclear localization. Biochem Biophys Res Commun 2010; 399(2):155-61; PMID:20637728; http://dx.doi.org/ 10.1016/j.bbrc.2010.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rafiq I, da Silva Xavier G, Hooper S, Rutter GA. Glucose-stimulated preproinsulin gene expression and nuclear trans-location of pancreatic duodenum homeobox-1 require activation of phosphatidylinositol 3-kinase but not p38 MAPK/SAPK2. J Biol Chem 2000; 275:15977-84; PMID:10821851; http://dx.doi.org/ 10.1074/jbc.275.21.15977 [DOI] [PubMed] [Google Scholar]
- 15. Elrick LJ, Docherty K. Phosphorylation-dependent nucleocytoplasmic shuttling of pancreatic duodenal homeobox-1. Diabetes 2001; 50:2244-52; PMID:11574405; http://dx.doi.org/ 10.2337/diabetes.50.10.2244 [DOI] [PubMed] [Google Scholar]
- 16. Macfarlane WM, McKinnon CM, Felton-Edkins ZA, Cragg H, James RF, Docherty K. Glucose stimulates translocation of the homeodomain transcription factor PDX1 from the cytoplasm to the nucleus in pancreatic beta-cells. J Biol Chem 1999; 274:1011-6; PMID:9873045; http://dx.doi.org/ 10.1074/jbc.274.2.1011 [DOI] [PubMed] [Google Scholar]
- 17. Petersen HV, Peshavaria M, Pedersen AA, Philippe J, Stein R, Madsen OD, Serup P. Glucose stimulates the activation domain potential of the PDX-1 homeodomain transcription factor. FEBS Lett 1998; 431:362-6; PMID:9714543; http://dx.doi.org/ 10.1016/S0014-5793(98)00776-5 [DOI] [PubMed] [Google Scholar]
- 18. Boucher MJ, Selander L, Carlsson L, Edlund H. Phosphorylation marks IPF1/PDX1 protein for degradation by glycogen synthase kinase 3-dependent mechanisms. J Biol Chem 2006; 281:6395-403; PMID:16407209; http://dx.doi.org/ 10.1074/jbc.M511597200 [DOI] [PubMed] [Google Scholar]
- 19. Humphrey RK, Yu SM, Flores LE, Jhala US. Glucose regulates steady-state levels of PDX1 via the reciprocal actions of GSK3 and AKT kinases. J Biol Chem 2010; 285:3406-16; PMID:19833727; http://dx.doi.org/ 10.1074/jbc.M109.006734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khoo S, Griffen SC, Xia Y, Baer RJ, German MS, Cobb MH. Regulation of insulin gene transcription by ERK1 and ERK2 in pancreatic beta cells. J Biol Chem 2003; 278:32969-77; PMID:12810726; http://dx.doi.org/ 10.1074/jbc.M301198200 [DOI] [PubMed] [Google Scholar]
- 21. An R, da Silva Xavier G, Hao HX, Semplici F, Rutter J, Rutter GA. Regulation by Per-Arnt-Sim (PAS) kinase of pancreatic duodenal homeobox-1 nuclear import in pancreatic beta-cells. Biochem Soc Trans 2006; 34:791-3; PMID:17052199; http://dx.doi.org/ 10.1042/BST0340791 [DOI] [PubMed] [Google Scholar]
- 22. Lebrun P, Montminy MR, Van Obberghen E. Regulation of the pancreatic duodenal homeobox-1 protein by DNA-dependent protein kinase. J Biol Chem 2005; 280:38203-10; PMID:16166097; http://dx.doi.org/ 10.1074/jbc.M504842200 [DOI] [PubMed] [Google Scholar]
- 23. Meng R, Al-Quobaili F, Muller I, Gotz C, Thiel G, Montenarh M. CK2 phosphorylation of Pdx-1 regulates its transcription factor activity. Cell Mol Life Sci 2010; 67:2481-9; PMID:20339896; http://dx.doi.org/ 10.1007/s00018-010-0348-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu H, MacFarlane WM, Tadayyon M, Arch JR, James RF, Docherty K. Insulin stimulates pancreatic-duodenal homoeobox factor-1 (PDX1) DNA-binding activity and insulin promoter activity in pancreatic beta cells. Biochem J 1999; 344(Pt 3):813-8; PMID:10585868; http://dx.doi.org/ 10.1042/0264-6021:3440813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mosley AL, Ozcan S. The pancreatic duodenal homeobox-1 protein (Pdx-1) interacts with histone deacetylases Hdac-1 and Hdac-2 on low levels of glucose. J Biol Chem 2004; 279:54241-7; PMID:15496408; http://dx.doi.org/ 10.1074/jbc.M410379200 [DOI] [PubMed] [Google Scholar]
- 26. Semache M, Zarrouki B, Fontes G, Fogarty S, Kikani C, Chawki MB, Rutter J, Poitout V. Per-Arnt-Sim kinase regulates pancreatic duodenal homeobox-1 protein stability via phosphorylation of glycogen synthase kinase 3beta in pancreatic beta-cells. J Biol Chem 2013; 288:24825-33; PMID:23853095; http://dx.doi.org/ 10.1074/jbc.M113.495945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shushan EB, Cerasi E, Melloul D. Regulation of the insulin gene by glucose: stimulation of trans-activation potency of human PDX-1 N-terminal domain. DNA Cell Biol 1999; 18:471-9; PMID:10390156; http://dx.doi.org/ 10.1089/104454999315196 [DOI] [PubMed] [Google Scholar]
- 28. Ohneda K, Mirmira RG, Wang J, Johnson JD, German MS. The homeodomain of PDX-1 mediates multiple protein-protein interactions in the formation of a transcriptional activation complex on the insulin promoter. Mol Cell Biol 2000; 20:900-11; PMID:10629047; http://dx.doi.org/ 10.1128/MCB.20.3.900-911.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Melloul D, Ben-Neriah Y, Cerasi E. Glucose modulates the binding of an islet-specific factor to a conserved sequence within the rat I and the human insulin promoters. Proc Natl Acad Sci U S A 1993; 90:3865-9; PMID:8483904; http://dx.doi.org/ 10.1073/pnas.90.9.3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moede T, Leibiger B, Pour HG, Berggren P, Leibiger IB. Identification of a nuclear localization signal, RRMKWKK, in the homeodomain transcription factor PDX-1. FEBS Lett 1999; 461:229-34; PMID:10567702; http://dx.doi.org/ 10.1016/S0014-5793(99)01446-5 [DOI] [PubMed] [Google Scholar]
- 31. Ardestani A, Sauter NS, Paroni F, Dharmadhikari G, Cho JH, Lupi R, Marchetti P, Oberholzer J, Conte JK, Maedler K. Neutralizing interleukin-1beta (IL-1beta) induces beta-cell survival by maintaining PDX1 protein nuclear localization. J Biol Chem 2011; 286:17144-55; PMID:21393239; http://dx.doi.org/ 10.1074/jbc.M110.210526 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest 2013; 123:3305-16; PMID:23863625; http://dx.doi.org/ 10.1172/JCI65390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reimer MK, Ahren B. Altered beta-cell distribution of pdx-1 and GLUT-2 after a short-term challenge with a high-fat diet in C57BL/6J mice. Diabetes 2002; 51(Suppl 1):S138-43; PMID:11815473; http://dx.doi.org/ 10.2337/diabetes.51.2007.S138 [DOI] [PubMed] [Google Scholar]
- 34. Guillemain G, Da Silva Xavier G, Rafiq I, Leturque A, Rutter GA. Importin beta1 mediates the glucose-stimulated nuclear import of pancreatic and duodenal homeobox-1 in pancreatic islet beta-cells (MIN6). Biochem J 2004; 378:219-27; PMID:14632628; http://dx.doi.org/ 10.1042/BJ20031549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hagman DK, Latour MG, Chakrabarti SK, Fontes G, Amyot J, Tremblay C, Semache M, Lausier JA, Roskens V, Mirmira RG, et al. . Cyclical and alternating infusions of glucose and intralipid in rats inhibit insulin gene expression and Pdx-1 binding in islets. Diabetes 2008; 57:424-31; PMID:17991758; http://dx.doi.org/ 10.2337/db07-1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hagman DK, Hays LB, Parazzoli SD, Poitout V. Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J Biol Chem 2005; 280:32413-8; PMID:15944145; http://dx.doi.org/ 10.1074/jbc.M506000200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kawamori D, Kajimoto Y, Kaneto H, Umayahara Y, Fujitani Y, Miyatsuka T, Watada H, Leibiger IB, Yamasaki Y, Hori M. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes 2003; 52:2896-904; PMID:14633849; http://dx.doi.org/ 10.2337/diabetes.52.12.2896 [DOI] [PubMed] [Google Scholar]
- 38. Amyot J, Semache M, Ferdaoussi M, Fontes G, Poitout V. Lipopolysaccharides impair insulin gene expression in isolated islets of Langerhans via Toll-Like Receptor-4 and NF-kappaB signalling. PLoS One 2012; 7:e36200; PMID:22558381; http://dx.doi.org/ 10.1371/journal.pone.0036200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang X, Cahill CM, Pineyro MA, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 regulates the beta cell transcription factor, PDX-1, in insulinoma cells. Endocrinology 1999; 140:4904-7; PMID:10499550; http://dx.doi.org/ 10.1210/endo.140.10.7158 [DOI] [PubMed] [Google Scholar]
- 40. Wang X, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 causes pancreatic duodenal homeobox-1 protein translocation from the cytoplasm to the nucleus of pancreatic beta-cells by a cyclic adenosine monophosphate/protein kinase A-dependent mechanism. Endocrinology 2001; 142:1820-7; PMID:11316746 [DOI] [PubMed] [Google Scholar]
- 41. Sayo Y, Hosokawa H, Imachi H, Murao K, Sato M, Wong NC, Ishida T, Takahara J. Transforming growth factor beta induction of insulin gene expression is mediated by pancreatic and duodenal homeobox gene-1 in rat insulinoma cells. Eur J Biochem 2000; 267:971-8; PMID:10672004; http://dx.doi.org/ 10.1046/j.1432-1327.2000.01080.x [DOI] [PubMed] [Google Scholar]
- 42. Campbell SC, Richardson H, Ferris WF, Butler CS, Macfarlane WM. Nitric oxide stimulates insulin gene transcription in pancreatic beta-cells. Biochem Biophys Res Commun 2007; 353:1011-6; PMID:17210120; http://dx.doi.org/ 10.1016/j.bbrc.2006.12.127 [DOI] [PubMed] [Google Scholar]
- 43. Johnson JD, Bernal-Mizrachi E, Alejandro EU, Han Z, Kalynyak TB, Li H, Beith JL, Gross J, Warnock GL, Townsend RR, et al. . Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc Natl Acad Sci U S A 2006; 103:19575-80; PMID:17158802; http://dx.doi.org/ 10.1073/pnas.0604208103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rafiq I, Kennedy HJ, Rutter GA. Glucose-dependent translocation of insulin promoter factor-1 (IPF-1) between the nuclear periphery and the nucleoplasm of single MIN6 beta-cells. J Biol Chem 1998; 273:23241-7; PMID:9722555; http://dx.doi.org/ 10.1074/jbc.273.36.23241 [DOI] [PubMed] [Google Scholar]
- 45. Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M, Kikuchi M, Yazaki Y, Miyazaki JI, Oka Y. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 1993; 36:1139-45; PMID:8270128; http://dx.doi.org/ 10.1007/BF00401058 [DOI] [PubMed] [Google Scholar]
- 46. Zhang HJ, Walseth TF, Robertson RP. Insulin secretion and cAMP metabolism in HIT cells. Reciprocal and serial passage-dependent relationships. Diabetes 1989; 38:44-8; PMID:2535825; http://dx.doi.org/ 10.2337/diab.38.1.44 [DOI] [PubMed] [Google Scholar]
- 47. Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. Establishment of a pancreatic ß cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 1990; 127:126-32; PMID:2163307; http://dx.doi.org/ 10.1210/endo-127-1-126 [DOI] [PubMed] [Google Scholar]
- 48. Santerre RF, Cook RA, Crisel RMD, Sharp JD, Scmhidt RJ, Williams DC, Wilson CP. Insulin synthesis in a clonal cell line of simian virus 40-transformed hamster pancreatic beta cell. Proc Natl Acad Sci U S A 1981; 78:4339-43; PMID:6270673; http://dx.doi.org/ 10.1073/pnas.78.7.4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 1992; 130:167-78; PMID:1370150 [DOI] [PubMed] [Google Scholar]
- 50. Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 2000; 49:424-30; PMID:10868964; http://dx.doi.org/ 10.2337/diabetes.49.3.424 [DOI] [PubMed] [Google Scholar]
- 51. Mahadevan J, Parazzoli S, Oseid E, Hertzel AV, Bernlohr DA, Vallerie SN, Liu CQ, Lopez M, Harmon JS, Robertson RP. Ebselen treatment prevents islet apoptosis, maintains intranuclear Pdx-1 and MafA levels, and preserves beta-cell mass and function in ZDF rats. Diabetes 2013; 62:3582-8; PMID:23801580; http://dx.doi.org/ 10.2337/db13-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koizumi M, Doi R, Toyoda E, Masui T, Tulachan SS, Kawaguchi Y, Fujimoto K, Gittes GK, Imamura M. Increased PDX-1 expression is associated with outcome in patients with pancreatic cancer. Surgery 2003; 134:260-6; PMID:12947327; http://dx.doi.org/ 10.1067/msy.2003.231 [DOI] [PubMed] [Google Scholar]
- 53. Wang XP, Li ZJ, Magnusson J, Brunicardi FC. Tissue MicroArray analyses of pancreatic duodenal homeobox-1 in human cancers. World J Surg 2005; 29:334-8; PMID:15706433; http://dx.doi.org/ 10.1007/s00268-004-7823-4 [DOI] [PubMed] [Google Scholar]
- 54. Liu T, Gou SM, Wang CY, Wu HS, Xiong JX, Zhou F. Pancreas duodenal homeobox-1 expression and significance in pancreatic cancer. World J Gastroenterol 2007; 13:2615-8; PMID:17552012; http://dx.doi.org/ 10.3748/wjg.v13.i18.2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu X, Yu X, Zack DJ, Zhu H, Qian J. TiGER: a database for tissue-specific gene expression and regulation. BMC Bioinformatics 2008; 9:271; PMID:18541026; http://dx.doi.org/ 10.1186/1471-2105-9-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu SH, Patel S, Gingras MC, Nemunaitis J, Zhou G, Chen C, Li M, Fisher W, Gibbs R, Brunicardi FC. PDX-1: demonstration of oncogenic properties in pancreatic cancer. Cancer 2011; 117:723-33; PMID:20886630; http://dx.doi.org/ 10.1002/cncr.25629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu SH, Rao DD, Nemunaitis J, Senzer N, Zhou G, Dawson D, Gingras MC, Wang Z, Gibbs R, Norman M, et al. . PDX-1 is a therapeutic target for pancreatic cancer, insulinoma and islet neoplasia using a novel RNA interference platform. PLoS One 2012; 7:e40452; PMID:22905092; http://dx.doi.org/ 10.1371/journal.pone.0040452 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.