Abstract
The aim of the present study was to measure gait velocity in cataract and simulated patients. The study was performed on 239 cataract patients, 115 age-matched subjects, and 11 simulated patients. We measured gait velocity and analyzed gait using a three-dimensional motion analysis system. Mean gait velocity before and 2 and 7 months after cataract surgery was 0.91 ± 0.19, 1.04 ± 0.21, and 1.06 ± 0.21 m/s, respectively, for males and 0.84 ± 0.22, 0.91 ± 0.24, and 0.92 ± 0.25 m/s, respectively, for females. The increase after surgery was significant in both groups at 7 months (P < 0.05). Gait velocity was significantly slower in cataract patients compared with controls before surgery, but no longer different after surgery. In simulated patients, mean velocity was 87.0 ± 11.4% of normal vision with a 3° visual field and 92.4 ± 12.3% of normal when counting fingers. Initial velocity was 89.1 ± 14.6% of normal vision with a 3° visual field and 92.7 ± 11.6% of normal when counting fingers. There was a significant difference between normal and impaired visual function (P < 0.05). The results demonstrate the close relationship between visual function and gait in cataract patients and simulated patients.
Age-related decline in motor function critically affects survival1,2,3,4,5,6. Healthy gait and posture are essential for the prevention of falls in older people, and they are controlled by the somatosensory, vestibular, and visual systems. Traditionally, neurology, otolaryngology, orthopedics, and rehabilitation medicine have been concerned with gait and posture, and ophthalmological evaluation of gait has been understudied even though motor function is directly associated with visual function7,8,9,10,11,12,13,14,15,16. A positive relationship between eye disease and motor function has been suggested in a number of cohort studies of patients with cataract17,18,19,20,21,22,23,24,25, macular degeneration15,26, or glaucoma27,28. The contribution of vision to mobility has also been examined in patients with simulated visual defects, such as closed eyes29 or wearing prism glasses30. Many of the previous studies into the link between cataract and mobility have been community-based epidemiological studies evaluating the risk of falls based on participants’ diaries and chart review. Two clinical studies have evaluated mobility in cataract patients. Schwartz et al.21 reported improved postural control for 23 cataract patients 1–4 months after surgery. In another study, Durmus et al.23 undertook comprehensive evaluation of motor function in 36 cataract patients before and 4 weeks after surgery and demonstrated improvements in gait analysis, as evaluated using the Biodex Stability System, time to “up and go”, the Tinetti assessment tool, and a functional reach test.
Cataract is a very common geriatric disease. As such, a clinical study into preoperative motor function decline and postoperative improvement in cataract patients could be of considerable benefit to public health. Ophthalmological interventions and experimental simulation of vision loss using eyewear could provide more useful information regarding the contribution of vision to motor function than conventional methods. In the present study we analyzed 239 cataract patients to explore correlations between gait velocity and ocular and systemic parameters. Gait velocity has been proposed as a relevant clinical indicator of well being in older adults and has been shown to predict survival1. Unlike fall frequency, gait velocity can be evaluated objectively in the eye clinic. All patients were examined and followed-up by board-certified ophthalmologists, certified orthoptists, and registered nurses throughout the study period. We also measured the gait velocity of subjects with normal vision in the same setting as cataract patients to compare gait velocity between the two groups. Finally, standardized technology for motion analysis (i.e. the point cluster method using a three-dimensional motion capture system) was used to evaluate gait velocity in subjects with simulated vision impairment (“simulated” patients) to determine whether the change in visual function, as a single factor, affected motor function.
Methods
Study institutions and Institutional Review Board approval
The Institutional Review Board and Ethics Committee of Keio University School of Medicine approved this study and the methods were carried out in accordance with the approved guidelines. All cataract and simulated patients provided written informed consent before taking part in the study. Subjects were recruited to the study from cataract patients and individuals with normal vision visiting eye clinics at the National Hospital Organization Saitama Hospital (Wako, Japan) and International Health and Welfare University Mita Hospital (Tokyo, Japan) between May 2011 and April 2013. Simulated patients were recruited from Keio University Hospital (Tokyo, Japan) between September 2013 and February 2014.
Gait velocity of cataract patients
The mean (±SD) age of the 239 cataract patients (145 women; 61%) was 74.5 ± 8.0 years. Patients were undergoing phacoemulsification and intra-ocular lens (IOL) implantation. Cataract patients were enrolled consecutively from the eye clinic and not biased. All surgeries were performed under topical anesthesia and a soft acrylic IOL (SN60WF [Alcon Laboratory, Fort Worth, TX, USA] n = 155; SA60AT [Alcon Laboratory] n = 84) was inserted by experienced surgeons. Best corrected visual acuity was converted to logarithm of minimal angle of resolution (LogMAR) units, and cataract opacity in the optical axis accounting for visual disturbance was classified as posterior subcapsular cataract, nuclear sclerosis (greater than Grade 2 on Emery–Little classification), or central cortical opacity. Some patients had mixed-type opacity. All patients completed the Japanese version31 of the National Eye Institute Visual Function Qestionnaire-25 (VFQ-25) before and 2 months after surgery. A trained technician measured 4-m gait velocity on a flat, straight track using a digital stopwatch. Patients were asked to walk at their preferred velocity from a standing position. This method has been used in many studies and meta-analyses1, and is considered appropriate for comparisons with the general population. Graders were not masked as to subject characteristics. Subjects who required a cane or walking aid and those with significant orthopedic and/or neurological complications were excluded from subsequent analysis.
Gait velocity of age-matched controls with normal vision
The control group consisted of 115 subjects with normal vision visiting the eye clinic for non-vision-related problems, including an eye health check, vitreous floaters, and ocular discomfort. Inclusion criteria for the control group were age between 64 and 84 years, not using a cane or walking aid, and normal vision (best corrected visual acuity >20/25 in both eyes). Exclusion criteria were vision-affecting eye disease, diabetes, brain infarction, and orthopedic and/or neurological complications, including paralysis, Parkinson’s disease, and a history of orthopedic surgery. Subjects were asked to walk at their preferred velocity in the examination room in the eye clinic, and 4-m gait velocity was measured once by a trained technician using a digital stopwatch.
Motion analysis of simulated patients
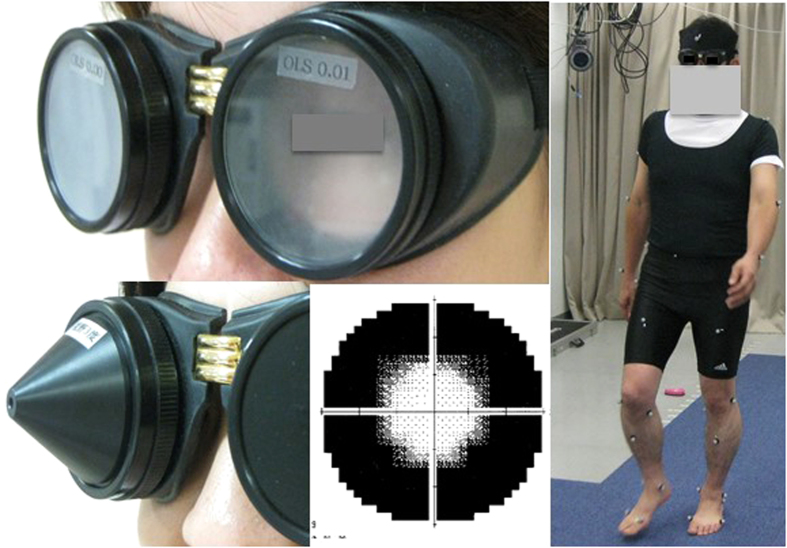
Eleven healthy male volunteers (aged 30–60 years; mean age 45 years; best corrected visual acuity 20/15) were recruited as simulated patients. Gait was evaluated in these subjects at the Gait Laboratory of the Department of Orthopedic Surgery using skin markers attached over the sacrum as part of a three-dimensional motion capture system (Qualysis®; Qualysis, Gothenburg, Sweden)32,33 with eight cameras synchronized to a force plate (frequency 600 Hz; Type 4060-10; Bertec, Columbus, OH, USA). This point cluster method enables measurement of velocity, vector, acceleration, step length, pitch, and many other parameters of motor function. Gait velocity was analyzed using the markers on the sacrum, which can best trace the motion of the center of gravity in a subject. The data sampling frequency was 120 Hz, and mean and initial (mean velocity during the first 0.1 s of the first step) velocities were calculated. Simulated patients wore goggles with a smoked surface or pin hole (M. Takata Optical, Tokyo, Japan) to reduce visual function to a visual acuity of counting fingers and 20/600, or to reduce the visual field to 3°, respectively (Fig. 1). The simulated effects were confirmed in 10 healthy volunteers with normal vision (>20/15). The goggles simulated visual acuity within an error range of 10% for counting fingers at 20 cm and 20/600. The visual field was evaluated in simulated patients wearing goggles with a Humphrey Field Analyzer (Carl Zeiss, Jena, Germany; 30-2 program) and the mean deviation was −29.9 ± 1.9 dB. The real visual field was ~3° (Fig. 1). This simulation is apparently equivalent to the visual field loss in advanced glaucoma; however, it is not very accurate and not a validated method for simulating advanced glaucomatous vision loss34.
Figure 1. Simulation goggles and simulated visual field.
“Simulated patients” wore goggles (top left) to simulate reduced visual function to the vision of 20/600. Representative results of visual field analysis (Humphrey Field Analyzer 30-2 program; Carl Zeiss, Jena, Germany) with goggles restricting the visual field to 3° show a successful simulation effect (bottom left and center). Motion analyses were performed using a motion capture system comprising eight cameras and a force plate (right).
Simulated patients were asked to walk 6 m on a flat, straight track at their preferred velocity from a standing position. The floor was solid and without obstacles in order to evaluate gait under various simulated visual impairments in a simple environment. To exclude carry-over effects, participants were divided into two groups; the first group of six was examined under normal vision conditions first and then when wearing the goggles; the second group of five was examined in the reverse order. Based on preliminary experiments, we used three goggles simulating the lowest visual function to achieve significant differences and to avoid learning effects by using too many goggles.
Statistical analysis
Where appropriate, data are given as the mean ± SD. Data were analyzed using ANOVA and t-tests. Correlations were evaluated using Pearson’s product–moment correlation. All analyses were performed using StatFlex (Atech, Osaka, Japan) and SPSS version 21 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered significant.
Results
Demographics and gait velocity for cataract patients are given in Table 1. Generally, gait velocity was significantly greater for male than female patients before and after surgery. Mean gait velocity increased significantly up to 7 months after surgery in both groups (Fig. 2; P < 0.05, paired t-test). Mean gait velocity in male (n = 40; mean age 74.8 ± 5.6 years) and female (n = 75; mean age 75.0 ± 6.7 years) controls with normal vision was 0.92 ± 0.21 and 0.95 ± 0.17 m/s, respectively. The difference in gait velocity between cataract patients and controls was significant before surgery (P < 0.001, unpaired t-test and Mann–Whitney U-test), but not 2 months after surgery, in both gender groups.
Table 1. Demographics and gait velocity in cataract patients.
| All patients | Male | Female | P-value | |
|---|---|---|---|---|
| Gait velocity (m/s) | ||||
| Before surgery | 0.87 ± 0.21 | 0.91 ± 0.19 | 0.84 ± 0.22 | 0.01** |
| 2 months after surgery | 0.96 ± 0.23 | 1.04 ± 0.21 | 0.91 ± 0.24 | 0.04** |
| 7 months after surgery | 0.98 ± 0.24* | 1.06 ± 0.21* | 0.92 ± 0.25* | 0.04** |
| Model 1: systemic parameters | ||||
| No. patients | 239 | 95 | 144 | |
| Age (years) | 74.4 ± 8.0 | 72.1 ± 8.8 | 76.0 ± 7.1 | <0.001** |
| Height (cm) | 155 ± 9 | 163 ± 6 | 149 ± 6 | <0.001** |
| Weight (kg) | 56.2 ± 11.4 | 63.4 ± 11.0 | 51.3 ± 8.8 | <0.001** |
| BMI (kg/m2) | 23.3 ± 3.5 | 23.7 ± 3.6 | 22.9 ± 3.4 | 0.107 |
| Diabetes (%) | 23.8 | 23.7 | 22.9 | 0.310 |
| SBP (mmHg) | 131.3 ± 18.1 | 129.8 ± 18.7 | 132.4 ± 17.6 | 0.326 |
| Model 2: vision-related parameters | ||||
| VFQ-25 score | ||||
| Before surgery | 64.3 ± 16.4 | 68.4 ± 13.8 | 61.4 ± 17.6 | <0.001** |
| After surgery | 79.8 ± 12.5* | 80.6 ± 10.8* | 79.4 ± 13.6* | 0.477 |
| Visual acuity before surgery | ||||
| In the better eye | 0.16 ± 0.29 | 0.11 ± 0.19 | 0.20 ± 0.34 | 0.01** |
| In the worse eye | 0.63 ± 0.80 | 0.58 ± 0.68 | 0.67 ± 0.87 | 0.390 |
| Visual acuity 2 months after surgery | ||||
| In the better eye | −0.03 ± 0.09* | −0.05 ± 0.06* | −0.02 ± 0.10* | 0.003** |
| In the worse eye | 0.11 ± 0.46* | 0.09 ± 0.31* | 0.12 ± 0.53* | 0.579 |
| % Hyperopic (either eye ≥ +3·00D) | 7.1 | 7.4 | 6.9 | 0.900 |
| % Myopic (either eye ≤ −3·00D) | 25.5 | 31.6 | 21.5 | 0.081 |
| Model 3: type of cataract opacity | ||||
| Posterior subcapsular | 38.5% | 49.5% | 31.3% | 0.012** |
| Nuclear (>Grade 2) | 23.4% | 24.7% | 22.4% | 0.721 |
| Central cortical | 47.5% | 42.3% | 51.0% | 0.193 |
Data are given as the mean ± SD or as the percentage of patients in each group, as appropriate. *P < 0.05 compared with before surgery (paired t-test); **P < 0.05 for male versus female (unpaired t-test).
BMI, body mass index; SBP, systolic blood pressure; LogMAR, logarithm of the minimum angle of resolution; VFQ-25, visual function questionnaire 25.
Figure 2.
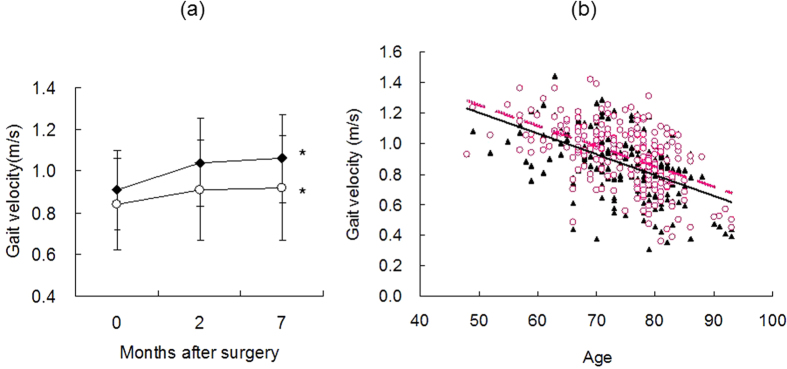
(a) Four-meter gait velocity in male (closed symbols) and female (open symbols) cataract patients before and 2 and 7 months after surgery. Gait velocity increased continuously up to 7 months after surgery with statistical significance in both groups (*P < 0.05 vs preoperative velocity, paired t-test). (b) Scatter plot of gait velocity versus age of cataract patients before (closed symbols) and 2 months after (open symbols) surgery. Note that age was strongly correlated with gait velocity. The regression lines were y = −0.0136x + 1.8845; R2 = 0.2753 (solid line) for before surgery and y = −0.0134x + 1.9175; R2 = 0.2743 (dashed line) for after surgery.
Stepwise multiple regression analysis of gait velocity and related parameters revealed that preoperative velocity was significantly correlated with sex (P < 0.01, Pearson’s product moment correlation), age (P < 0.001), height (P < 0.001), visual acuity in the worse eye (P < 0.05), and VFQ-25 score (P < 0.001) adjusted for age and sex (Table 2). The change in velocity 2 months after surgery was significantly correlated with the change in visual acuity in the worse eye (P < 0.05) and the presence of posterior subcapsular cataract adjusted for age and sex (P < 0.01). Graphic representation revealed a strong correlation between age and gait velocity (Fig. 2). Regression lines for before (y = −0.0136x + 1.8845; R2 = 0.2753) and 2 months after (y = −0.0134x + 1.9175; R2 = 0.2743) surgery demonstrate an increase in gait speed after surgery.
Table 2. Stepwise multiple regression analysis of gait velocity and related parameters.
| Preoperative gait velocity |
∆Gait velocityA |
|||
|---|---|---|---|---|
| β | P-value | Β | P-value | |
| Model 1: vision-related parameters | ||||
| LogMAR in the better eye | −0.11 | 0.06 | 0.02 | 0.79 |
| LogMAR in the worse eye | −0.12 | 0.04* | 0.07 | 0.29 |
| ∆LogMAR in the worse eye | — | — | 0.55 | 0.04* |
| Myopia > −3.0D in either eye | 0.02 | 0.70 | 0.08 | 0.27 |
| Preoperative VFQ-25 score | 0.28 | 0.00* | −0.05 | 0.46 |
| ∆VFQ-25 score | — | — | 0.04 | 0.55 |
| Model 2: cataract opacity | ||||
| Nuclear sclerosis | −0.09 | 0.12 | 0.02 | 0.74 |
| Posterior subcapsular | −0.07 | 0.30 | 0.20 | 0.01* |
| Central cortical | 0.09 | 0.19 | 0.02 | 0.81 |
| Model 3: systemic parameters | ||||
| Age | −0.52 | 0.00* | −0.06 | 0.50 |
| Sex | 0.19 | 0.01* | −0.02 | 0.72 |
| Height | 0.39 | 0.00* | −0.12 | 0.32 |
| BMI | −0.05 | 0.41 | −0.12 | 0.11 |
| Diabetes | −0.03 | 0.68 | 0.02 | 0.81 |
| SBP | −0.07 | 0.31 | 0.11 | 0.14 |
*P < 0.05, Pearson’s product–moment correlation.
Male = 1, female = 0, diabetic = 1, non-diabetic = 0.
AChanges in values (Δ) were calculated by subtracting preoperative values from values obtained 2 months after surgery. All values were adjusted for age and sex.
LogMAR, logarithm of the minimum angle of resolution; VFQ-25, visual function questionnaire 25; BMI, body mass index; SBP, systolic blood pressure.
Simulated patients exhibited significantly reduced gait velocity when visual function was impaired compared with normal-vision conditions (Fig. 3). Mean velocity under simulated low-vision conditions was 86.0 ± 15.1% of normal (P <0.05 vs normal vision, paired t-test) with a visual acuity of 20/600, 81.8 ± 23.6% (P < 0.05) when counting fingers, and 79.8 ± 15.4% (P < 0.01) with a visual field of 3° (P < 0.01). Initial velocity in simulated patients was 90.5 ± 8.8% of normal (P < 0.05) with a visual acuity of 20/600, 82.5 ± 13.8% (P < 0.05) when counting fingers, and 84.3 ± 9.7% (P < 0.05) with a visual field of 3° (P < 0.05).
Figure 3.
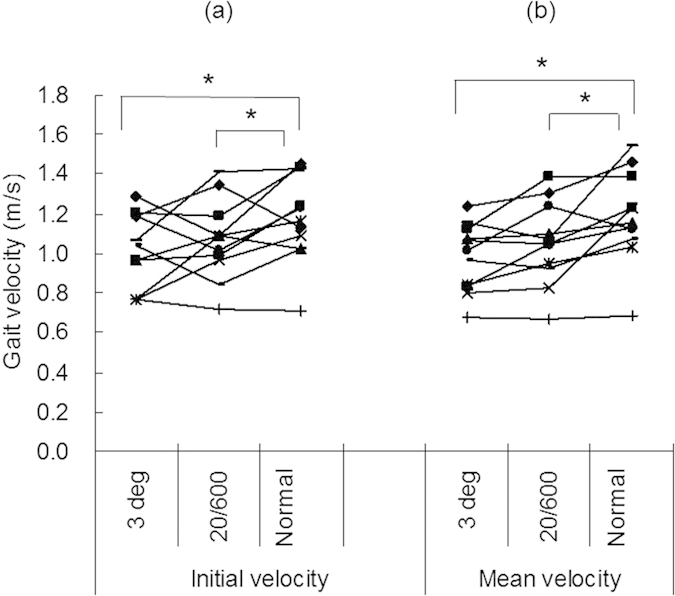
(a) Initial velocity (mean velocity during the first 0.1 s of the first step) and (b) mean velocity in subjects in whom low visual function was simulated by reducing visual acuity to 20/600 and counting fingers, and reducing the visual field to 3°. Note that gait velocity was correlated with visual function. There was a significant difference between values obtained under normal vision conditions (visual acuity > 20/15) and each simulated low-vision condition (*P < 0.05, paired t-test).
Discussion
The results of the present study demonstrate that gait velocity increases 2 months after cataract surgery and further increases at 7 months, despite the fact that these elderly patients are also 7 months older. To the best of our knowledge, the present study is the largest clinical case series of gait velocity in patients undergoing cataract surgery. The improvement in gait velocity was not dependent on age, sex, or height, indicating that sensory, neural, or psychological robustness may be more important than musculoskeletal components for achieving and maintaining a healthy gait in older people. Therefore, age-related declines in gait may be due not only to physical frailty as a consequence of aging, but also to a fear of falling as a result of low visual recognition exacerbated by eye disease. It was interesting that, prior to surgery, the cataract patients in the present study had significantly slower gait velocity than age-matched subjects with normal vision without cataract but, after surgery, cataract patients walked faster than the control subjects even through some of them had diabetes and other comorbidities that could possibly suppress gait. Our clinical impression was that the cataract patients decided to undergo surgery only when they were mentally and physically robust enough to tolerate the procedure. According to recent reports, postoperative improvements in sleep quality or mental status are no longer evident in some cases 1 year after surgery35,36,37, and we speculate that the sustainability of non-physical improvements after cataract surgery may depend on individual patients. Nevertheless, improvements in gait are supported, at the very least, by sustained restoration of the visual sensory system, and so longer effects may be expected compared with improvements in mental status.
Most previous studies of gait speed were performed as part of a health check for a large population, so very simple methods (in terms of efficacy and safety) were used to determine gait speed, such as measurements made over a short distance using a stopwatch. In the present study, we chose to measure walking velocity over a distance of 4 m because the reproducibility and standardization of this technique have been established in many studies1, even when measured by different graders. It is very important to evaluate another control group of cataract patients who did not undergo surgery, to determine if repeat testing results in improvements in gait velocity.
In the present study, visual acuity in the worse eye was correlated with preoperative gait velocity and postoperative improvement, which accords with the study of Matsuo et al.30, who found a close relationship between binocular function and postural stability. Thus, binocular vision may be more important for gait than good monocular vision. Vision and mobility studies also suggest contrast sensitivity21, visual field27, illumination16, and the use of sedatives10 are significantly correlated with postural stability. Geriatricians and eye care providers are advised to take care of visual function in older people to enable them to walk safely.
With regard to the type of cataract opacity, patients with posterior subcapsular cataract exhibited a greater increase in gait velocity compared with patients with other types of opacities. These findings support those of previous studies that posterior subcapsular cataract is a significant prognostic determinant of cataract surgery for quality of life and sleep31,37. This type of cataract has a greater adverse effect on vision under conditions of bright ambient lighting. Visual acuity is usually tested in an indoor examination room, using black letters on a white background. Patients with posterior subcapsular cataract usually have much worse acuity in a brightly lit environment than in a darker examination room. Based on the clinical experiences of eye surgeons, we speculate that there is response shift phenomenon38 whereby patients with posterior subcapsular cataract do not recognize their preoperative disability until their visual function recovers after surgery. Consequently, patients with posterior subcapsular cataract may enjoy the best outcomes after surgery, even though preoperative parameters do not differ among the different types of cataract opacities.
Three-dimensional motion capture is a modern technology for analyzing motion under normal and pathological conditions. It is commonly used in orthopedic evaluation by capturing the motion of each joint and bone32,33. We attempted to exclude the possible bias of age and other systemic conditions on gait velocity by simulating low-vision conditions. The results from simulated patients demonstrated that motor function decreased significantly with visual impairment alone and confirmed that visual function plays an important role in postural control. Visual restoration may potentially contribute to longer survival since recent epidemiological studies indicating that the mortality rate is lower in postoperative cataract patients than in those without surgery39,40. Cataract surgery is also suggested to be a potential anti-aging therapy in terms of vision, mobility17,18,19,20,21,22,23,24,25, cognitive function41,42, and sleep24,25,36,37,43,44. Further investigations should be performed to evaluate neural and musculoskeletal improvements after cataract surgery that could sustain improvements in gait velocity.
The limitations of the present study include the type of control subjects for old cataract patients, simulated patients, and the learning curve of participants. There are many systemic conditions that can potentially affect gait velocity and most older adults may have age-related disabilities and comorbidities. Physicians and nurses observed the gait and sitting and standing posture of subjects with normal vision to confirm that they were eligible to be enrolled in the study as normal controls. It was not ethical to ask older participants to walk while wearing simulation goggles because of safety concerns. However, we believe the healthy simulated patients in the present study successfully served as a relevant experimental model and contributed to reasonable results. With regard to the possible learning curve of participants, gait velocity measurements were performed only once using a very simple method that required no skill or practice. Measurements after surgery were performed 2 and 7 months after the initial measurement and, after this time, the patients may not remember how they walked in the initial assessment. Although graders were not masked as to subject characteristics, we do not believe this resulted in bias because the graders used the same simple method for all subjects.
In conclusion, we found that the gait velocity of cataract patients increased over 7 months after surgery and exceeded that of age-matched controls with normal vision. Three-dimensional motion analysis of simulated patients also demonstrated a significant correlation between visual function and gait velocity. The results of the present study confirm motor function benefits after visual restoration by cataract surgery.
Additional Information
How to cite this article: Ayaki, M. et al. Motor function benefits of visual restoration measured in age-related cataract and simulated patients: Case-control and clinical experimental studies. Sci. Rep. 5, 14595; doi: 10.1038/srep14595 (2015).
Acknowledgments
The authors thank Dr Takayuki Abe for help with the statistical analysis. The authors acknowledge the assistance of Inter-Biotech (http://www.inter-biotech.com) with the English language editing of this manuscript. This work was supported by a grant from JSPS KAKENHI (no. 24592643).
Footnotes
Author Contributions M.A. and T.N. collected and analyzed the data and wrote the manuscript. M.A., T.N. and K.T. designed the study. K.N., Y.T. and T.K. reviewed and approved the final version of the manuscript.
References
- Studenski S. et al. Gait speed and survival in older adults. JAMA 305, 50–58 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abellan van Kan G. et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people; an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging. 13, 881–889 (2009) (Review). [DOI] [PubMed] [Google Scholar]
- Ostir G. V. et al. Measures of lower body function and risk of mortality over 7 years of follow-up. Am. J. Epidemiol. 166, 599–605 (2007). [DOI] [PubMed] [Google Scholar]
- Cooper R. et al. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ 341, c4467 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai S. et al. Walking speed as a good predictor for the onset of functional dependence in a Japanese rural community population. Age Aging. 29, 441–446 (2000). [DOI] [PubMed] [Google Scholar]
- Auyeung T. W. et al. Age-associated decline of muscle mass, grip strength and gait velocity: a 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatr. Gerontol. Int. 14, Suppl 1: 76–84 (2014). [DOI] [PubMed] [Google Scholar]
- Lord S. R. & Menz H. B. Visual contributions to postural stability in older adults. Gerontology 46, 306–310 (2000). [DOI] [PubMed] [Google Scholar]
- Klein B. E. et al. Associations of visual function with physical outcomes and limitations 5 years later in an older population: the Beaver Dam eye study. Ophthalmology 110, 644–650 (2003). [DOI] [PubMed] [Google Scholar]
- Patel I. et al. Measures of visual function and percentage of preferred walking speed in older adults: the Salisbury Eye Evaluation Project. Invest. Ophthalmol. Vis. Sci. 47, 65–71 (2006). [DOI] [PubMed] [Google Scholar]
- Tinetti M. E., Speechley M. & Ginter S. F. Risk factors for falls among elderly persons living in the community. N. Eng. J. Med. 319, 1701–1707 (1988). [DOI] [PubMed] [Google Scholar]
- Swenor B. K., Muñoz B. & West S. K. Does visual impairment affect mobility over time? The Salisbury Eye Evaluation Study. Invest. Ophthalmol. Vis. Sci. 54, 7683–7690 (2013). . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenor B. K., Bandeen-Roche K., Muñoz B. & West S. K. Does walking speed mediate the association between visual impairment and self-report of mobility disability? The Salisbury eye evaluation study. J. Am. Geriatr. Soc. 62, 1540–1545 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. J. et al. Randomized controlled trial of prevention of falls in people aged ≥75 with severe visual impairment: the VIP trial. BMJ 10.1136/bmj.38601.447731.55 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenor B. K. et al. Visual Impairment and Incident Mobility Limitations: The Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 63, 46–54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding S. J. et al. Waterloo Vision and Mobility Study: gait adaptations to altered surfaces in individuals with age-related maculopathy. Optom. Vis. Sci. 71, 770–777 (1994). [DOI] [PubMed] [Google Scholar]
- Choi J. S., Kang D. W., Shin Y. H. & Tack G. R. Differences gait pattern between the elderly and the young during level walking under low illumination. Acta Bioeng. Biomech. 16, 3–9 (2014). [PubMed] [Google Scholar]
- Desapriya E. et al. Vision improvement and reduction in falls after expedited cataract surgery Systematic review and metaanalysis. J. Cataract Refract. Surg. 36, 13–19 (2010). [DOI] [PubMed] [Google Scholar]
- Harwood R. H. et al. Falls and health status in elderly woman following first eye cataract surgery: a randomized controlled trial. Br. J. Ophthalmol. 89, 53–59 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuleners L. B., Fraser M. L., Ng J. & Morlet N. The impact of first- and second-eye cataract surgery on injurious falls that require hospitalisation: a whole-population study. Age Ageing 43, 341–346 (2014). [DOI] [PubMed] [Google Scholar]
- Ishikawa T. et al. Evaluating the benefits of second-eye cataract surgery among the elderly. J. Cataract Refract. Surg. 39, 1593–1603 (2013). [DOI] [PubMed] [Google Scholar]
- Schwartz S. et al. The effect of cataract surgery on postural control. Invest. Ophthalmol. Vis. Sci. 46, 920–924 (2005). [DOI] [PubMed] [Google Scholar]
- Lee B. S., Munoz B. E., West S. K. & Gower E. W. Functional Improvement after One- and Two-Eye Cataract Surgery in the Salisbury Eye Evaluation. Ophthalmology 120, 949–955 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmus B. et al. Gain in visual acuity after cataract surgery improves postural stability and mobility. Bratisl. Lek. Listy. 112, 701–705 (2011). [PubMed] [Google Scholar]
- Ayaki M., Muramatsu M., Negishi K. & Tsubota K. Improvements in sleep quality and gait speed after cataract surgery. Rejuvenation Res. 16, 35–42 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaki M., Negishi K. & Tsubota K. Rejuvenation effects of cataract surgery with UV blocking intra-ocular lens on circadian rhythm and gait speed. Rejuvenation Res. 17, 359–365 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M. et al. Risk of falls, injurious falls, and other injuries resulting from visual impairment among older adults with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 52, 5088–5092 (2011). [DOI] [PubMed] [Google Scholar]
- Friedman D. S. et al. Glaucoma and mobility performance: the Salisbury Eye Evaluation Project. Ophthalmology 114, 2232–2237 (2007). [DOI] [PubMed] [Google Scholar]
- Yuki K. et al. The association between visual field defect severity and fear of falling in primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci. 54, 7739–7745 (2013). [DOI] [PubMed] [Google Scholar]
- Reynard F. & Terrier P. Role of visual input in the control of dynamic balance: variability and instability of gait in treadmill walking while blindfolded. Exp. Brain Res. 2014. Dec 23. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Matsuo T. et al. Postural stability changes during the prism adaptation test in patients with intermittent and constant exotropia. Invest. Ophthalmol. Vis. Sci. 51, 6341–6347 (2010). [DOI] [PubMed] [Google Scholar]
- Suzukamo Y. et al. Psychometric properties of the 25-item National Eye Institute Visual Function Questionnaire (NEI VFQ-25), Japanese version. Health Qual. Life Outcomes 3, 65 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K. et al. Kinematic motion of the anterior cruciate ligament deficient knee during functionally high and low demanding tasks. J. Biomech. 47, 2526–2530 (2014). [DOI] [PubMed] [Google Scholar]
- Harato K. et al. A gait analysis of simulated knee flexion contracture to elucidate knee-spine syndrome. Gait Posture 28, 687–692 (2008). [DOI] [PubMed] [Google Scholar]
- Crabb D. P., Smith N. D., Glen F. C., Burton R. & Garway-Heath D. F. How does glaucoma look?: patient perception of visual field loss. Ophthalmology. 120, 1120–1126 (2013). [DOI] [PubMed] [Google Scholar]
- Espindle D. et al. Quality-of-life improvements in cataract patients with bilateral blue light-filtering intraocular lenses: clinical trial. J. Cataract Refract. Surg. 31, 1952–1959 (2005). [DOI] [PubMed] [Google Scholar]
- Alexander I. et al. Impact of cataract surgery on sleep in patients receiving either ultra-violet blocking or blue-filtering intraocular lens implants. Invest. Ophthalmol. Vis. Sci. 55, 4999–5004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaki M., Negishi K., Suzukamo Y. & Tsubota K. Color of intra-ocular lens and cataract opacity type are prognostic determinants for health indices after photoreceptive restoration by surgery. Rejuvenation Res. 18, 145–152 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ridder D., Geenen R., Kuijer R. & van Middendorp H. Psychological adjustment to chronic disease. Lancet 372, 246–255 (2008). [DOI] [PubMed] [Google Scholar]
- Blundell M. S. et al. Reduced mortality compared with national averages following phacoemulsification cataract surgery: a retrospective observational study. Br. J. Ophthalmol. 93, 290–295 (2009). [DOI] [PubMed] [Google Scholar]
- Fong C. S. et al. Correction of visual impairment by cataract surgery and improved survival in older persons: the Blue Mountains Eye Study cohort. Ophthalmology 120, 1720–1727 (2013). [DOI] [PubMed] [Google Scholar]
- McGwin G. Jr. et al. Effect of Cataract Surgery on Cognitive Function in Older Adults. J. Am. Geriatr. Soc. 54, 1089–1094 (2006). [DOI] [PubMed] [Google Scholar]
- Ishii K., Kabata T. & Oshika T. The impact of cataract surgery on cognitive impairment and depressive mental status in elderly patients. Am. J. Ophthalmol. 146, 404–409 (2008). [DOI] [PubMed] [Google Scholar]
- Wei. X. et al. Blue-light-blocking intraocular lens implantation improves the sleep quality of cataract patients. J. Clin. Sleep Med. 9, 741–745 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmack I. et al. Visual quality assessment in patients with orange-tinted blue light-filtering and clear ultraviolet light-filtering intraocular lenses. J. Cataract Refract. Surg. (United States). 38, 823–832 (2012). [DOI] [PubMed] [Google Scholar]