Abstract
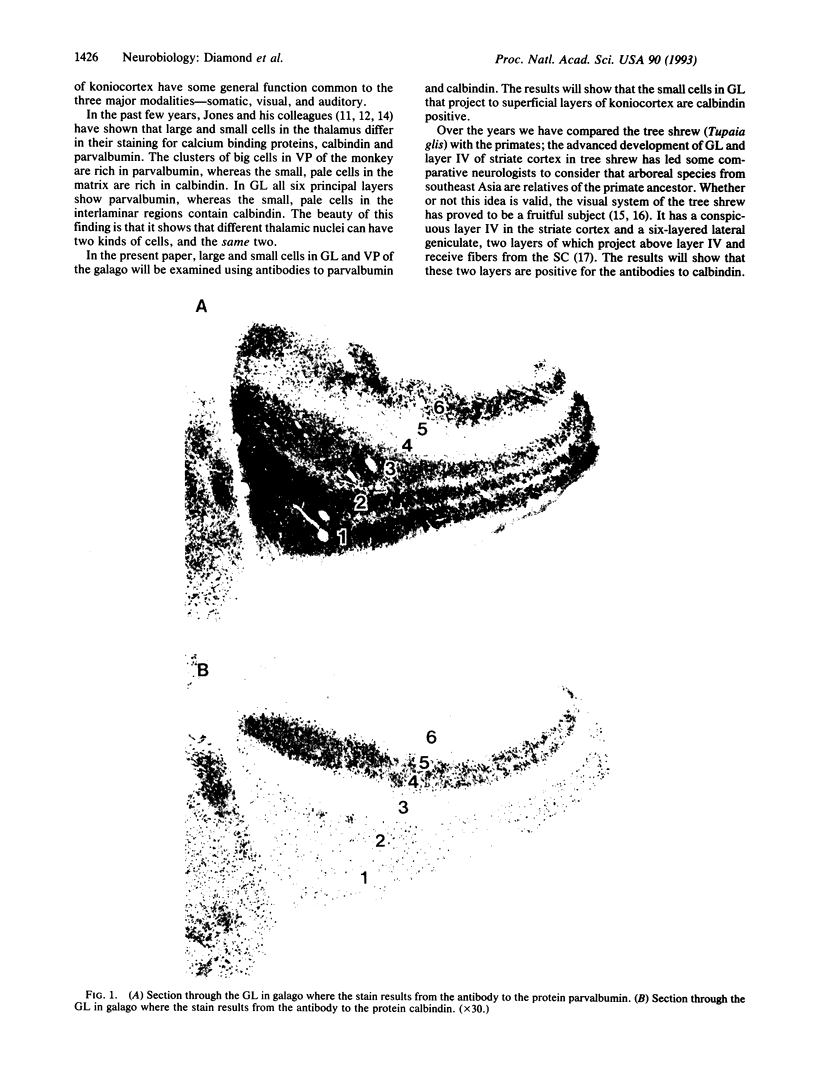
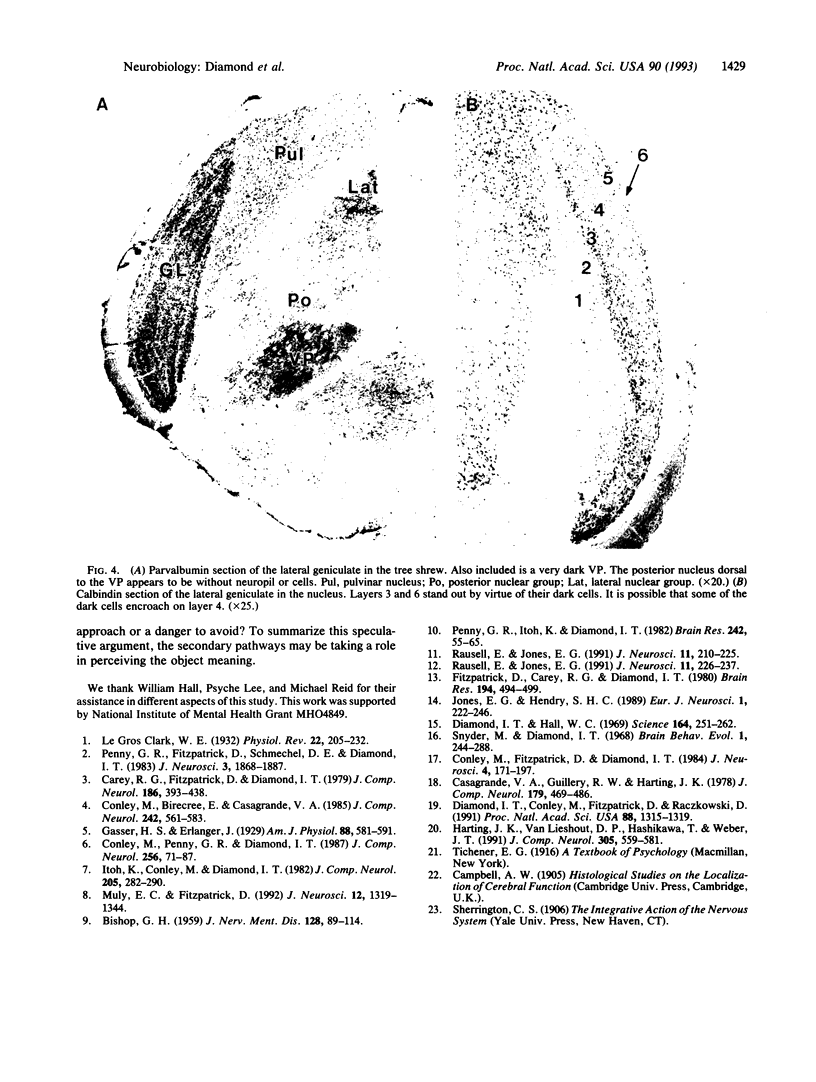
Two different cell types were identified in the thalamus of galago and Tupaia by using antibodies to two calcium binding proteins, calbindin and parvalbumin. In each species studied, the lateral geniculate nucleus consists of six layers, two of which have smaller relay cells. Previous studies have shown that the small cell layers receive fibers from the superior colliculus and project to the superficial layers of the striate cortex. These are the only geniculate layers that react to a calbindin antibody but not parvalbumin. The ventral posterior nucleus was included in the study and the results for both nuclei show that calbindin is a marker for thalamic cells that receive small fibers and project to superficial layers of koniocortex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP G. H. The relation between nerve fiber size and sensory modality: phylogenetic implications of the afferent innervation of cortex. J Nerv Ment Dis. 1959 Feb;128(2):89–114. [PubMed] [Google Scholar]
- Carey R. G., Fitzpatrick D., Diamond I. T. Layer I of striate cortex of Tupaia glis and Galago senegalensis: projections from thalamus and claustrum revealed by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979 Aug 1;186(3):393–437. doi: 10.1002/cne.901860306. [DOI] [PubMed] [Google Scholar]
- Casagrande V. A., Guillery R. W., Harting J. K. Differential effects of monocular deprivation seen in different layers of the lateral geniculate nucleus. J Comp Neurol. 1978 Jun 1;179(3):469–485. doi: 10.1002/cne.901790302. [DOI] [PubMed] [Google Scholar]
- Conley M., Birecree E., Casagrande V. A. Neuronal classes and their relation to functional and laminar organization of the lateral geniculate nucleus: a Golgi study of the prosimian primate, Galago crassicaudatus. J Comp Neurol. 1985 Dec 22;242(4):561–583. doi: 10.1002/cne.902420407. [DOI] [PubMed] [Google Scholar]
- Conley M., Fitzpatrick D., Diamond I. T. The laminar organization of the lateral geniculate body and the striate cortex in the tree shrew (Tupaia glis). J Neurosci. 1984 Jan;4(1):171–197. doi: 10.1523/JNEUROSCI.04-01-00171.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M., Penny G. R., Diamond I. T. Terminations of individual optic tract fibers in the lateral geniculate nuclei of Galago crassicaudatus and Tupaia belangeri. J Comp Neurol. 1987 Feb 1;256(1):71–87. doi: 10.1002/cne.902560107. [DOI] [PubMed] [Google Scholar]
- Diamond I. T., Conley M., Fitzpatrick D., Raczkowski D. Evidence for separate pathways within the tecto-geniculate projection in the tree shrew. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1315–1319. doi: 10.1073/pnas.88.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I. T., Hall W. C. Evolution of neocortex. Science. 1969 Apr 18;164(3877):251–262. doi: 10.1126/science.164.3877.251. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D., Carey R. G., Diamond I. T. The projection of the superior colliculus upon the lateral geniculate body in Tupaia glis and Galago senegalensis. Brain Res. 1980 Aug 4;194(2):494–499. doi: 10.1016/0006-8993(80)91230-5. [DOI] [PubMed] [Google Scholar]
- Harting J. K., Van Lieshout D. P., Hashikawa T., Weber J. T. The parabigeminogeniculate projection: connectional studies in eight mammals. J Comp Neurol. 1991 Mar 22;305(4):559–581. doi: 10.1002/cne.903050404. [DOI] [PubMed] [Google Scholar]
- Itoh K., Conley M., Diamond I. T. Retinal ganglion cell projections to individual layers of the lateral geniculate body in Galago crassicaudatus. J Comp Neurol. 1982 Mar 1;205(3):282–290. doi: 10.1002/cne.902050308. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Hendry S. H. C. Differential Calcium Binding Protein Immunoreactivity Distinguishes Classes of Relay Neurons in Monkey Thalamic Nuclei. Eur J Neurosci. 1989 May;1(3):222–246. doi: 10.1111/j.1460-9568.1989.tb00791.x. [DOI] [PubMed] [Google Scholar]
- Muly E. C., Fitzpatrick D. The morphological basis for binocular and ON/OFF convergence in tree shrew striate cortex. J Neurosci. 1992 Apr;12(4):1319–1334. doi: 10.1523/JNEUROSCI.12-04-01319.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny G. R., Fitzpatrick D., Schmechel D. E., Diamond I. T. Glutamic acid decarboxylase-immunoreactive neurons and horseradish peroxidase-labeled projection neurons in the ventral posterior nucleus of the cat and Galago senegalensis. J Neurosci. 1983 Sep;3(9):1868–1887. doi: 10.1523/JNEUROSCI.03-09-01868.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny G. R., Itoh K., Diamond I. T. Cells of different sizes in the ventral nuclei project to different layers of the somatic cortex in the cat. Brain Res. 1982 Jun 17;242(1):55–65. doi: 10.1016/0006-8993(82)90495-4. [DOI] [PubMed] [Google Scholar]
- Rausell E., Jones E. G. Chemically distinct compartments of the thalamic VPM nucleus in monkeys relay principal and spinal trigeminal pathways to different layers of the somatosensory cortex. J Neurosci. 1991 Jan;11(1):226–237. doi: 10.1523/JNEUROSCI.11-01-00226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausell E., Jones E. G. Histochemical and immunocytochemical compartments of the thalamic VPM nucleus in monkeys and their relationship to the representational map. J Neurosci. 1991 Jan;11(1):210–225. doi: 10.1523/JNEUROSCI.11-01-00210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]