Abstract
Chemotherapy-induced neutropenia is a common complication in cancer treatment. In this study, we investigated chemotherapy-induced neutropenia that was recently detected in all patients with gynecologic malignancy. Between January 2009 and December 2011, we examined cases of chemotherapy-induced neutropenia reported in our hospital. We analyzed the incidence and clinical features of chemotherapy-induced neutropenia and febrile neutropenia in patients with gynecologic malignancy. During the study period, we administered over 1614 infusions (29 regimens) to 291 patients. The median age of the patients was 60 years (range 24–84 years). Chemotherapy-induced neutropenia occurred in 147 (50.5%) patients over 378 (23.4%) chemotherapy cycles. Febrile neutropenia occurred in 20 (6.9%) patients over 25 (1.5%) cycles. The mean duration of neutropenia and fever was 3.6 days (range 1–12 days) and 3.4 days (range 1–9 days), respectively. The source of fever was unexplained by examination or cultures in 14 (56.0%) cycles. There were two cases of neutropenia-related death. Chemotherapy-induced neutropenia was associated with older age (over 70 years) (P<0.0001), less than five previous chemotherapy cycles (P=0.02), disseminated disease (P=0.03), platinum-based regimens (P<0.0001), taxane-containing regimens (P<0.0001), and combined therapy (P<0.0001). Febrile neutropenia was associated with poor performance status (P<0.0001), no previous chemotherapy (P<0.05), disseminated disease (P<0.0001), and distant metastatic disease (P=0.03). Neither chemotherapy-induced neutropenia nor febrile neutropenia was associated with bone marrow metastases or previous radiotherapy. By identifying risk factors for febrile neutropenia, such as performance status, no previous chemotherapy, disseminated disease, and distant metastatic disease, the safe management of chemotherapy-induced neutropenia may be possible in patients with gynecologic malignancy.
Keywords: chemotherapy, febrile neutropenia, gynecologic malignancy, neutropenia
Introduction
Chemotherapy-induced neutropenia is one of the major dose-limiting toxicities in clinical trials and is a common complication in cancer treatment. Febrile neutropenia is associated with increased morbidity, mortality, and treatment costs 1–5. Therefore, febrile neutropenia is a clinically relevant problem that affects the patient’s quality of life.
Patients with gynecologic malignancy often receive several courses of systemic chemotherapy throughout primary therapy and recurrent therapy. Moreover, new drugs (pegylated liposomal doxorubicin, gemcitabine, etc.) have been used recently in patients with gynecologic malignancy 6,7. Chemotherapy-induced neutropenia may be more of a problem in the safe management of chemotherapy as outpatient chemotherapy is performed more frequently.
Currently, the standard treatment for chemotherapy-induced neutropenia is the use of a granulocyte colony-stimulating factor (G-CSF) to attenuate white blood cell counts and absolute neutrophil counts (ANCs). G-CSFs have been used frequently to reduce the incidence and duration of chemotherapy-induced neutropenia and febrile neutropenia. Kuderer et al. 8 reported that the use of prophylactic G-CSF led to a 46% decrease in a systematic review of randomized-controlled trials. Padilla and Ropka 9 reported that the use of prophylactic G-CSF also improved the patient’s quality of life. Vogel et al. 10 reported that the use of prophylactic G-CSF resulted in the reduced likelihood of prolonged hospitalization. However, G-CSF use is expensive, and adverse effects such as musculoskeletal pain and fever are frequently reported. G-CSF could also initiate or accelerate the development of myelodysplasia or acute myeloid leukemia 11. Identification of risk factors for febrile neutropenia may be important for the safe management of chemotherapy-induced neutropenia without unnecessary use of G-CSFs.
Many clinical trials have reported the frequency of chemotherapy-induced neutropenia and febrile neutropenia in patients with gynecologic malignancy, but most reports did not include a comprehensive investigation of chemotherapy-induced neutropenia and febrile neutropenia 6,7. Moreover, there are insufficient data from patients who have received modern chemotherapy regimens to address these issues. Some reports comprehensively studied these issues in patients with solid tumors, but most patients in these reports had other solid tumors, including male patients, and only a small number of patients with gynecologic malignancy was included 4,5. Some reports comprehensively studied febrile neutropenia in patients with ovarian cancer. In these reports, only patients receiving first-line chemotherapy were included, and no patients with recurrent disease were included 12,13. To the best of our knowledge, the last comprehensive study of febrile neutropenia in patients with gynecologic malignancy was published in 1996 14. There are insufficient data from patients who have received modern chemotherapy regimens to address these issues.
In this study, we investigated chemotherapy-induced neutropenia recently performed in all patients with gynecologic malignancy.
Patients and methods
This retrospective study was approved by the Institutional Review Board of Graduate School of Medicine, Osaka City University. Using the available electronic medical record data between January 2009 and December 2011, we examined cases of chemotherapy-induced neutropenia reported in our hospital using the CTCAE v.4.0. Complete blood cell counts were performed on all patients at least once a week. We analyzed the incidence and clinical features of chemotherapy-induced neutropenia (grade 4: ANC<500/μl) in patients with gynecologic malignancy. Febrile neutropenia was defined as an oral temperature more than 38.3°C or two consecutive readings of more than 38.0°C for 2 h and ANC less than 0.5×109/l, or ANC that was expected to fall below 0.5×109/l. Performance status was measured on day 1 of each cycle using the Eastern Cooperative Oncology Group (ECOG) score. Performance status 3–4 was considered as poor performance status. Disease sites were categorized as no evidence of disease, local disease, distant metastatic disease, disseminated disease, and both distant metastatic and disseminated disease. Computed tomography examination of the abdomen and chest or MRI examination of the pelvis was performed on all patients at least once per three chemotherapy cycles. Disease sites and bone marrow metastases were evaluated by a variety of imaging modalities including PET. Transfusion was performed before chemotherapy in case of the patients with hemoglobin level less than 8.0. G-CSFs were used in our hospital basically according to the American Society of Clinical Oncology (ASCO) recommendations update 2006, which showed that primary prophylaxis was recommended for prevention of febrile neutropenia in patients who are at high risk on the basis of age, medical history, disease characteristics, and myelotoxicity of the chemotherapy regimen 15. The guideline of G-CSFs use in our hospital is as follows: (i) G-CSFs is used in all febrile neutropenia cases. (ii) Primary prophylaxis G-CSFs is used for prevention of febrile neutropenia in patients who are at high risk on the basis of age, medical history, disease characteristics. (iii) Secondary prophylaxis G-CSFs is not used for prevention of febrile neutropenia. (iv) If primary and secondary prophylaxis G-CSFs is not used, dose reduction or delay, or alternative regimen with less myelotoxicity is considered an alternative method for prevention of febrile neutropenia. This judgment is made by the clinician without restriction criteria. Treatment of febrile neutropenia is as follows: (i) all patients were admitted to our hospital and receive G-CSF and intravenous broad-spectrum antibiotics such as meropenem hydrate (MEP) within 1 h. (ii) In all cases, at least a laboratory investigation and radiography are performed. Cultures are performed whenever possible.
Statistical analysis
The relationship between each clinical group was analyzed using Fisher’s exact probability test. A P value of less than 0.05 was considered significant.
Results
Incidence of chemotherapy-induced neutropenia and febrile neutropenia
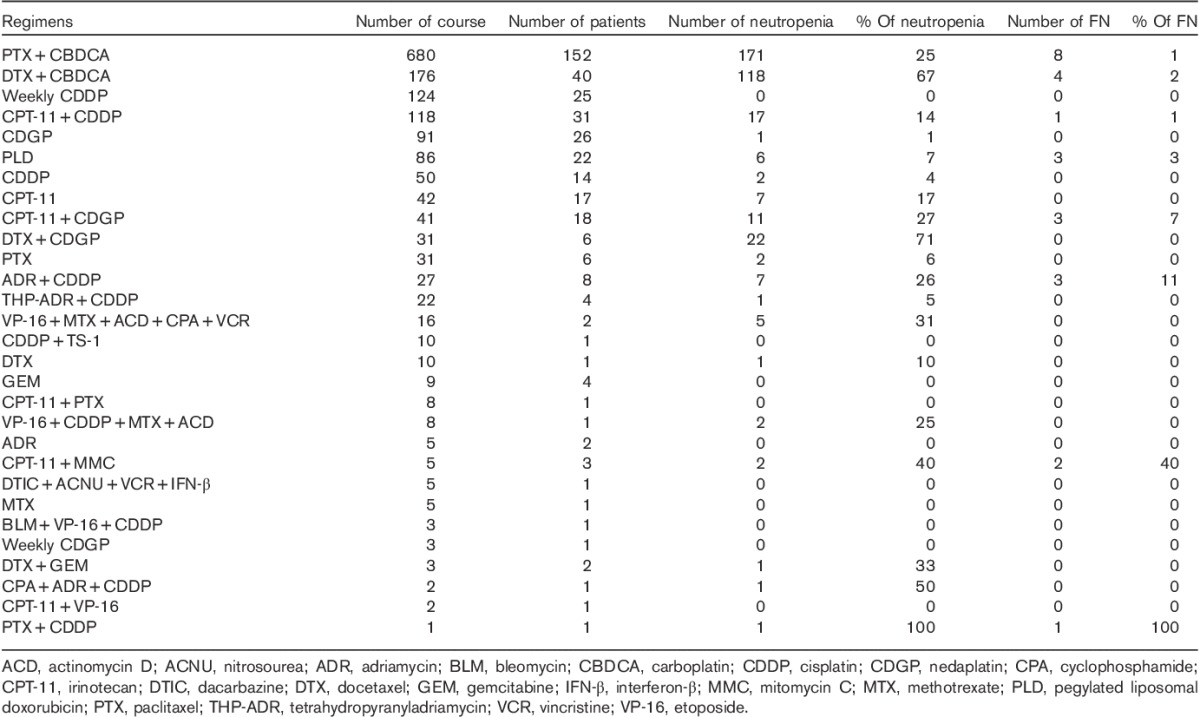
The number of patients with gynecologic malignancy and treatment regimens are shown in Tables 1 and 2, respectively. During the study period, we administered over 1614 infusions (29 regimens) to 291 patients with gynecologic malignancy. The median age of the patients was 60 years (range 24–84 years). The most common gynecologic malignancies were ovarian cancer [111 (38%) patients], endometrial cancer [75 (26%) patients], and cervical cancer [73 (25%) patients]. All patients received conventional cytotoxic chemotherapy. There was no use of targeted treatments, such as monoclonal antibodies or tyrosine kinase inhibitors. The most common chemotherapy regimen was paclitaxel and carboplatin (TC) therapy. A total of 152 (52%) patients received 680 (42%) courses of TC therapy. There were no primary and secondary prophylaxis G-CSF uses. Chemotherapy-induced neutropenia occurred in 147 (50.5%) patients over 378 (23.4%) chemotherapy cycles. Febrile neutropenia occurred in 20 (6.9%) patients over 25 (1.5%) cycles. Febrile neutropenia occurred after cycle 1 in five (20%) cycles and within cycles 1 and 2 in 14 (56.0%) cycles. There were two cases of neutropenia-related deaths.
Table 1.
Number of patients with gynecologic malignancy
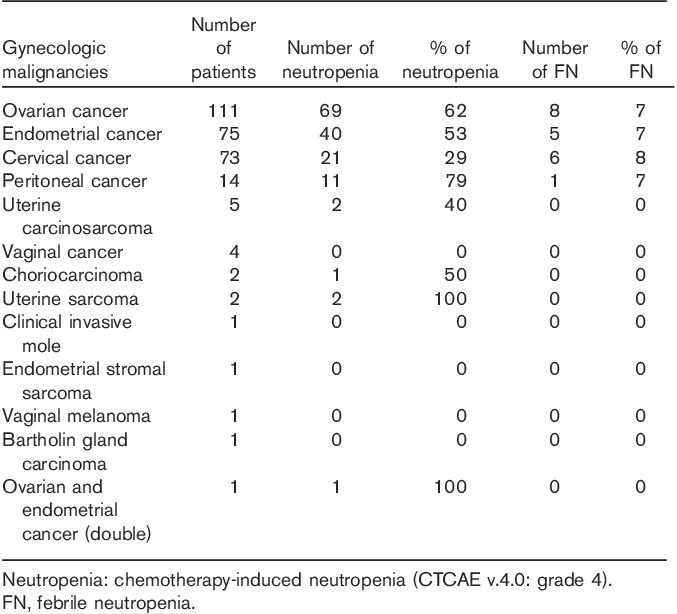
Table 2.
Regimen of chemotherapy performed between January 2009 and December 2011
Clinical features of chemotherapy-induced neutropenia and febrile neutropenia
Clinical characteristics related to the risk of chemotherapy-induced neutropenia and febrile neutropenia are shown in Table 3. Chemotherapy-induced neutropenia was associated with older age (over 70 years) (P<0.0001), less than five previous chemotherapy cycles (P=0.02), and disseminated disease (P=0.03). Febrile neutropenia was associated with poor performance status (P<0.0001), no previous chemotherapy (P<0.05), disseminated disease (P<0.0001), and distant metastatic disease (P=0.03). Neither chemotherapy-induced neutropenia nor febrile neutropenia was associated with bone marrow metastases or previous radiotherapy.
Table 3.
Risk of neutropenia and febrile neutropenia-related clinical characteristics
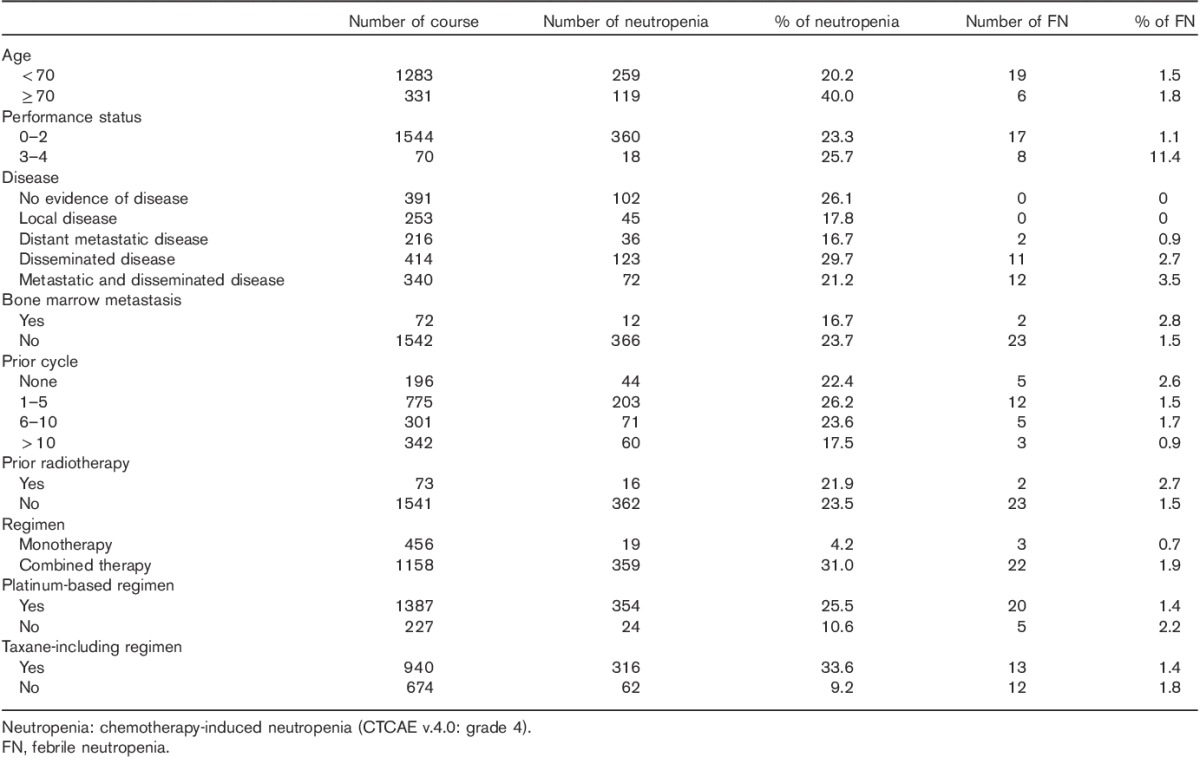
Chemotherapy-induced neutropenia was associated with platinum-based regimens (P<0.0001), taxane-containing regimens (P<0.0001), and the number of anticancer drugs in regimens (P<0.0001). Febrile neutropenia was not associated with platinum-based regimens, taxane-containing regimens, or the number of anticancer drugs in regimens.
Treatment of chemotherapy-induced neutropenia and febrile neutropenia
All patients were admitted to our hospital and received G-CSF and intravenous antibiotics within 1 h. Broad-spectrum antibiotics such MEP were used as first-line antibiotics in all cases. In all cases, two (8%) cases required second-line antibiotics for persisting intermittent fever and one (4%) case received an additional antifungal regimen. The mean duration of neutropenia and fever was 3.6 days (range 1–12 days) and 3.4 days (range 1–9), respectively. In all cases, at least a laboratory investigation and radiography were performed. Cultures were performed in 22 (88%) cycles, and cultures were positive in 11 (50%) cycles. The source of fever was unexplained by examination or cultures in 14 (56.0%) cycles. In all patients, five (25%) patients had a history of bowel resection and 15 (75%) patients had undergone a radical procedure such as lymphadenectomy. There were two (8%) cases of neutropenia-related deaths during their admission.
Chemotherapy-induced neutropenia without fever occurred over 353 (21.9%) chemotherapy cycles. In all cases, G-CSFs were used in 25 (7.1%) cycles, and oral or intravenous antibiotics were used in 26 (7.4%) cycles. Both G-CSFs and antibiotics were used in 15 (4.2%) cycles.
Discussion
Patients with gynecologic malignancy often receive systemic chemotherapy as a component of primary therapy. Moreover, most patients with recurrent disease also receive chemotherapy. As a result, patients with gynecologic malignancy receive several types of chemotherapy and receive frequent chemotherapy per patient in clinical practice.
Chemotherapy-induced neutropenia may be more of a problem in the safe management of chemotherapy as outpatient chemotherapy is performed more frequently. Chemotherapy-induced neutropenia is a known source of major stress for physicians and patients. Febrile neutropenia is a serious clinical problem 1–5. In many cases, G-CSFs are administered to patients with malignancy to prevent such events. International guidelines for the use of G-CSF include the ASCO recommendations update 2006, the EORTC guideline 2010, and the NCCN guideline update 2011 15–17. Although these guidelines differ from each other slightly, including the definition or risk factors of febrile neutropenia, the clinical benefits of G-CSF use are evident in specific chemotherapy, with a threshold rate of febrile neutropenia of 20%. Identification of risk factors for febrile neutropenia may be important for the safe management of chemotherapy-induced neutropenia without the unnecessary use of G-CSFs. The primary purpose of our study was to identify risk factors for febrile neutropenia.
In our study, chemotherapy-induced neutropenia occurred in 50.5% of patients over 23.4% of chemotherapy cycles. The reported incidence of chemotherapy-induced neutropenia varies widely. Smith et al. 15 reported chemotherapy-induced neutropenia in 6–50% of patients depending on the cancer type, disease staging, patient functional status, and chemotherapy regimen. Laskey et al. 13 reported that chemotherapy-induced neutropenia was observed in 43% of patients with ovarian cancer during primary chemotherapy. Our findings were similar to those of previous reports.
Febrile neutropenia occurred in 6.9% of patients over 1.5% of chemotherapy cycles in our study. Shama et al. 12 reported febrile neutropenia in 12% patients with epithelial ovarian cancer during first-line adjuvant chemotherapy and concluded that the rate of febrile neutropenia was higher than reported previously. Laskey et al. 13 reported febrile neutropenia in 7% of patients with ovarian cancer during primary chemotherapy. A lower rate of febrile neutropenia was observed in our study compared with these reports. In these reports, febrile neutropenia occurred frequently in early cycles, particularly after cycle 1. Similarly, 56.0% of febrile neutropenia cases occurred after cycles 1 and 2 in our study. Our study included a number of patients with recurrent disease who received chemotherapy. This may have led to the lower rate of febrile neutropenia in our study.
We investigated the clinical characteristics related to the risk of chemotherapy-induced neutropenia and febrile neutropenia. Chemotherapy-induced neutropenia was associated with older age and less than five previous chemotherapy cycles. Febrile neutropenia was associated with poor performance status and no previous chemotherapy. Several reports concluded that increasing age was an independent predictor of the development of febrile neutropenia 2,15,16. Although older age was a risk factor for chemotherapy-induced neutropenia, it was not a risk factor for febrile neutropenia in our study. G-CSFs were used in our hospital according to the ASCO recommendations update 2006 15 and we administered G-CSFs to elderly patients with chemotherapy-induced neutropenia. The use of prophylactic G-CSF may reduce the incidence of febrile neutropenia in elderly patients. In our study, febrile neutropenia was associated with no previous chemotherapy and 56.0% of febrile neutropenia cases occurred after cycles 1 and 2. Okera et al. 5 reported that 50% of all episodes of febrile neutropenia occurred at or near the initiation of chemotherapy courses (cycles 1 and 2) in patients with solid tumors. Shama et al. 12 reported that 60% of febrile neutropenia cases occurred after cycle 1 in patients receiving first-line adjuvant chemotherapy for epithelial ovarian cancer. Our data were similar to data of these reports and led to the recommendation for prophylactic G-CSF use in high-risk groups 15–17. Febrile neutropenia was associated with poor performance status in our study. In G-CSF guidelines such as the ASCO recommendations update 2006, the EORTC guideline 2010, and the NCCN guideline update 2011, poor performance status was not clearly identified as a risk factor for febrile neutropenia 15–17. There are no reports identifying poor performance status as a risk factor for febrile neutropenia in patients with gynecologic malignancy; therefore, our data are the first to report poor performance status as a risk factor. Neither chemotherapy-induced neutropenia nor febrile neutropenia was associated with bone marrow metastases or previous radiotherapy in our study. Although bone marrow involvement in tumors and previous radiotherapy were identified as risk factors for febrile neutropenia in the NCCN guideline update 2011, bone marrow metastases and previous radiotherapy were not considered risk factors for febrile neutropenia in the ASCO recommendations update 2006 and the EORTC guideline 2010 15–17. Our data are in accordance with these recommendations. To the best of our knowledge, there has been no comprehensive study of the association between the disease site and chemotherapy-induced neutropenia and febrile neutropenia in patients with gynecologic malignancy. Hence, we investigated the association between the disease site and chemotherapy-induced neutropenia and febrile neutropenia. In our study, disease sites were categorized into no evidence of disease, local disease, distant metastatic disease, disseminated disease, and both distant metastatic and disseminated disease. Chemotherapy-induced neutropenia was associated with disseminated disease. Febrile neutropenia was associated with disseminated disease and distant metastatic disease. In G-CSF guidelines, advanced disease or metastasis was not identified as risk factors for febrile neutropenia in patients with solid tumors 15–17. Advanced disease, such as disseminated and distant metastatic diseases, may be an important risk factor for febrile neutropenia in patients with gynecologic malignancy. Although chemotherapy-induced neutropenia was associated with platinum-based regimens, taxane-containing regimens, and the number of anticancer drugs in regimens, febrile neutropenia was not related to the regimens in our study. In G-CSF guidelines, platinum-based regimens and taxane-containing regimens were not identified as chemotherapy regimens with a high risk of febrile neutropenia in patients with malignancy 15–17. In patients with gynecologic malignancy, chemotherapy regimens, such as platinum-based regimens and taxane-containing regimens, may not be risk factors for febrile neutropenia, but may be risk factors for chemotherapy-induced neutropenia.
We also investigated the treatment of chemotherapy-induced neutropenia and febrile neutropenia. In our study, febrile neutropenia occurred in 6.9% of patients over 1.5% of cycles. All patients were admitted to our hospital and received G-CSF and intravenous antibiotics within 1 h. The specific features of our hospital were as follows: 24-h telephone support, 24-h investigation and treatment by senior medical staff and consultation to an oncologist, urgent assessment and treatment facility, and 24-h admission to our hospital and ICU. The National Chemotherapy Advisory Group (NCAG) recommendation ensures that patients receive antibiotics within 1 h of presentation (door-to needle) 18. Our hospital complies with these recommendations. Broad-spectrum antibiotics such MEP were used as first-line antibiotics in all cases. In all cases, two (8%) cases required second-line antibiotics for persisting intermittent fever and one (4%) case received additional antifungal treatment. The mean duration of neutropenia and fever was 3.6 days (1–12 days) and 3.4 days (1–9) days, respectively. In all cases, at least a laboratory investigation and radiography were performed. Cultures were performed in 22 (88%) cycles. In three cycles, cultures were not performed because of transfer to another palliative care hospital. Cultures were positive in 11 (50%) cycles. The source of fever was unexplained by examination or cultures in 14 (56.0%) cycles. McMeekin et al. 14 reported that the source of fever was unexplained by examination or cultures in 56% of episodes in patients with gynecologic oncology. Shama et al. 12 reported positive cultures in 47% of patients receiving first-line adjuvant chemotherapy for epithelial ovarian cancer. Our data were similar to data of these reports. In all patients, five (25%) patients had a history of bowel resection and 15 (75%) patients had undergone a radical procedure such as lymphadenectomy. Shama et al. 12 reported that in patients with ovarian cancer, 53% had undergone bowel resection and 100% had undergone radical or supraradical procedures. They concluded that patients with advanced ovarian cancer who underwent supraradical procedures should be identified as having a high risk of developing febrile neutropenia 12. Our data were in accordance with the data of their report. There were two (8%) neutropenia-related deaths during admission. In both cases, sepsis-related death occurred following palliative chemotherapy. Chemotherapy-induced neutropenia without fever occurred over 21.9% of chemotherapy cycles. In all cases, G-CSFs were used in 7.1% of cycles, and oral or intravenous antibiotics were used in 7.4% of cycles. Both G-CSFs and antibiotics were used in 4.2% of cycles. These treatments may have contributed toward the lower incidence of febrile neutropenia in this study. International guidelines for the use of G-CSF include the ASCO recommendations update 2006, the EORTC guideline 2010, and the NCCN guideline update 2011 15–17. Although these guidelines differ from each other slightly, including the definition or risk factors of febrile neutropenia, the clinical benefits of G-CSF use are evident in specific chemotherapy, with a threshold rate of febrile neutropenia of 20%. G-CSFs were used in our hospital according to the ASCO recommendations update 2006 15. The ASCO recommendations update is a guideline for various diseases with malignancy. The threshold rate of febrile neutropenia of 20% seems to differ in diseases with gynecologic malignancy. For this reason, there were no primary and secondary prophylaxis G-CSF use, and dose reduction or delay, or alternative regimen with less myelotoxicity was considered an alternative method for the prevention of febrile neutropenia in our hospital. G-CSFs have only been used therapeutically. In our study, febrile neutropenia occurred only in 6.9% of patients over 1.5% of cycles. This means that the threshold rate of febrile neutropenia of 20% differs in disease with gynecologic malignancy including the definition or risk factors of febrile neutropenia. Therefore, our study seems to make some contribution to a new guideline for the use of G-CSF, which is necessary for patients with gynecologic malignancy.
In conclusion, although chemotherapy-induced neutropenia is fairly common, febrile neutropenia is relatively uncommon in patients with gynecologic malignancy. By identifying risk factors for febrile neutropenia, such as performance status, no previous chemotherapy, disseminated disease, and distant metastatic disease, the safe management of chemotherapy-induced neutropenia may be possible in patients with gynecologic malignancy.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lyman GH, Kuderer N, Greene J, Balducci L. The economics of febrile neutropenia: implications for the use of colony-stimulating factors. Eur J Cancer 1998; 34:1857–1864. [DOI] [PubMed] [Google Scholar]
- 2.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 2004; 100:228–237. [DOI] [PubMed] [Google Scholar]
- 3.Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006; 106:2258–2266. [DOI] [PubMed] [Google Scholar]
- 4.López-Pousa A, Rifà J, Casas de Tejerina A, González-Larriba JL, Iglesias C, Gasquet JA, Carrato A. DELFOS Study Group. Risk assessment model for first-cycle chemotherapy-induced neutropenia in patients with solid tumours. Eur J Cancer Care (Engl) 2010; 19:648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okera M, Chan S, Dernede U, Larkin J, Popat S, Gilbert D, et al. A prospective study of chemotherapy-induced febrile neutropenia in the South West London Cancer Network. Interpretation of study results in light of NCAG/NCEPOD findings. Br J Cancer 2011; 104:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mutch DG, Orlando M, Goss T, Teneriello MG, Gordon AN, McMeekin SD, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol 2007; 25:2811–2818. [DOI] [PubMed] [Google Scholar]
- 7.Ferrandina G, Ludovisi M, Lorusso D, Pignata S, Breda E, Savarese A, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol 2008; 26:890–896. [DOI] [PubMed] [Google Scholar]
- 8.Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 2007; 25:3158–3167. [DOI] [PubMed] [Google Scholar]
- 9.Padilla G, Ropka ME. Quality of life and chemotherapy-induced neutropenia. Cancer Nurs 2005; 28:167–171. [DOI] [PubMed] [Google Scholar]
- 10.Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas-Figueroa LJ, Wiens BL, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 2005; 23:1178–1184. [DOI] [PubMed] [Google Scholar]
- 11.Lyman GH, Dale DC, Wolff DA, Culakova E, Poniewierski MS, Kuderer NM, Crawford J. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony-stimulating factor: a systematic review. J Clin Oncol 2010; 28:2914–2924. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Rezai K, Driscoll D, Odunsi K, Lele S. Characterization of neutropenic fever in patients receiving first-line adjuvant chemotherapy for epithelial ovarian cancer. Gynecol Oncol 2006; 103:181–185. [DOI] [PubMed] [Google Scholar]
- 13.Laskey RA, Poniewierski MS, Lopez MA, Hanna RK, Secord AA, Gehrig PA, et al. Predictors of severe and febrile neutropenia during primary chemotherapy for ovarian cancer. Gynecol Oncol 2012; 125:625–630. [DOI] [PubMed] [Google Scholar]
- 14.McMeekin DS, Gazzaniga C, Berman M, DiSaia P, Manetta A. Retrospective review of gynecologic oncology patients with therapy-induced neutropenic fever. Gynecol Oncol 1996; 62:247–253. [DOI] [PubMed] [Google Scholar]
- 15.Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 2006; 24:3187–3205. [DOI] [PubMed] [Google Scholar]
- 16.Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. European Organisation for Research and Treatment of Cancer. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 2011; 47:8–32. [DOI] [PubMed] [Google Scholar]
- 17.Crawford J, Allen J, Armitage J, Blayney DW, Cataland SR, Heaney ML, et al. Myeloid growth factors. J Natl Compr Canc Netw 2011; 9:914–932. [DOI] [PubMed] [Google Scholar]
- 18.NCAG. Chemotherapy services in England: ensuring quality, safety: a report from the National Chemotherapy Advisory Group. UK: National Chemotherapy Advisory Group; 2009. [Google Scholar]


