Abstract
Objective:
To assess differences in pitch-ranking ability across a range of speech understanding performance levels and as a function of electrode position.
Study Design:
An observational study of a cross-section of cochlear implantees.
Setting:
Tertiary referral center for cochlear implantation.
Patients:
A total of 22 patients were recruited. All three manufacturers’ devices were included (MED-EL, Innsbruck, Austria, n = 10; Advanced Bionics, California, USA, n = 8; and Cochlear, Sydney, Australia, n = 4) and all patients were long-term users (more than 18 months). Twelve of these were poor performers (scores on BKB sentence lists <60%) and 10 were excellent performers (BKB >90%).
Intervention:
After measurement of threshold and comfort levels, and loudness balancing across the array, all patients underwent thorough pitch-ranking assessments at 80% of comfort levels.
Main Outcome Measure:
Ability to discriminate pitch across the electrode array, measured by consistency in discrimination of adjacent pairs of electrodes, as well as an assessment of the pitch order across the array using the midpoint comparison task.
Results:
Within the poor performing group there was wide variability in ability to pitch rank, from no errors, to a complete inability to reliably and consistently differentiate pitch change across the electrode array. Good performers were overall significantly more accurate at pitch ranking (p = 0.026). Consistent pitch ranking was found to be a significant independent predictor of BKB score, even after adjusting for age. Users of the MED-EL implant experienced significantly more pitch confusions at the apex than at more basal parts of the electrode array.
Conclusions:
Many cochlear implant users struggle to discriminate pitch effectively. Accurate pitch ranking appears to be an independent predictor of overall outcome. Future work will concentrate on manipulating maps based upon pitch discrimination findings in an attempt to improve speech understanding.
Keywords: Cochlear implant—Cochlear implant performance—Pitch—Pitch discrimination
Some patients who receive cochlear implants (CIs) derive more benefit from them than others. Factors identified as influential to postoperative performance include duration of deafness (1), age of onset of deafness (2), age at implantation (3), etiology (4), surgical factors (5) (electrode placement and insertion depth), and psychological factors such as coexistence of depression (6), engagement with services (7), and intelligence level (IQ) (8). Speech recognition may be affected by an implantee's ability to perceive distinct pitch percepts across the array.
Pitch is a subjective psychophysical attribute of sound and corresponds closely to the frequency of sound waves. In normal, acoustic hearing, changes in frequency produce changes both in the temporal response of the auditory nerve (“temporal code”), and in which nerves fire (“place code”). In a cochlear hearing loss, frequency discrimination is impaired. Tonotopic organization of surviving nerve fibers in severely deafened individuals allows CI electrodes to stimulate distinct neural populations, allowing the place code to operate in a manner qualitatively similar to that in normal hearing.
It has been suggested that in patients with a poor ability to pitch rank, electrodes may be delivering information to areas of the cochlea that are “dead.” In patients with sensory hearing loss, dead regions correspond to local loss of inner hair cells and/or auditory nerve fibers (9), whereas for CI users the term refers to local neural loss.
Although the number of electrodes within CI arrays has expanded with technology, there is evidence that individuals can only usefully discriminate a limited number (10–13). Henry et al. (14) found a significant correlation between electrode discrimination ability and speech information perceived at four frequency regions between 170 and 2680 Hz, but not at higher frequencies.
METHODS OF ASSESSING PITCH PERCEPTION
Electrode discrimination is often assessed with a multiple-interval forced-choice task. This “oddman out” style of testing allows the participant to use any perceptual difference between stimuli (e.g., loudness) to detect differences between stimuli. The test and reference stimuli vary only in electrode position. It is therefore necessary to ensure careful loudness balancing.
In pitch-scaling tasks, an individual is asked to assign a number to a sequence of sounds depending on how high they perceive the pitch of each sound to be. For CI users, pitch should increase monotonically with stimulation of more basal electrodes; however, this is not always the case, and indistinguishable electrodes or electrode reversals may be identified. Collins et al. (15) found that pitch ranking and pitch scaling produced different ordering of electrode pitches. Perceived timbral differences may account for this. A problem with such methods is that they require the patient to assign numbers to sensations, and this process results in a number of nonsensory biases that can distort the pattern of results observed (16).
Multidimensional scaling is another means to assess pitch change across an array. A perceptual dissimilarity matrix is analyzed to produce a stimulus space in which the spatial distances between the stimuli best match the perceptual dissimilarities of the stimuli. Henshall and McKay (17) and Collins and Throckmorton (18) propose that this is a more accurate way to pinpoint indiscriminable electrodes than pitch ranking. However, mechanically detachable coil system (MDS) procedures require each stimulus to be compared to every other one, an equal number of times. This can be inefficient, because when the stimuli vary along a single perceptual dimension the participant spends a large amount of time comparing stimuli that are easily distinguishable.
The present study used two different procedures. One of these was a pitch-ranking task in which the listener was presented with a pair of sounds and was asked which has the higher pitch (two-alternative forced choice task). Because subjects have to make a judgment about the direction of the pitch difference between two sounds, this type of task is less susceptible to the use of loudness cues than when an odd-man-out task is used. However, the use of loudness cues cannot always be ruled out, particularly when the same pair of sounds is presented many times in a row and when correct answer feedback is provided. Here, we used a small number of comparisons and did not provide feedback. In order to obtain a more global picture of the variation of pitch across electrodes, we additionally adopted the optimally efficient pitch-ranking procedure described by Long et al. (19). This “midpoint comparison procedure,” which was originally validated with auditory brainstem implant users, and has subsequently been applied in studies of pitch perception by CI users (20,21), describes an efficient method of pitch ordering across the electrode array of auditory brainstem implants.
Many factors can influence the pitch-ranking ability of individual patients and on particular electrodes. These include the physical distance of the electrode from the surviving nerve cells, the phenomenon of “cross-turn stimulation” where electrodes in the apex of the cochlea stimulate neurons innervating more basal regions, and the presence of “dead regions.” Neural dead regions are more likely to coincide with regions of longstanding hair cell loss. Both cross-turn stimulation and neural dead regions could produce not only poor pitch-ranking ability but also “pitch reversals”; tuning CIs without consideration for these potential effects might result in poor speech perception. If this is true, then, conversely, patients that experience poor outcome would be more likely to have extended neural dead regions and poorer pitch perception. The key aim of this study was to examine differences in pitch perception abilities between the best and worst performing cochlear implantees, based upon their speech recognition scores.
METHODS
A total of 30 patients were contacted by post and 22 agreed to take part in the study. All device types (by manufacturer) were included.
Those with the highest and lowest Bamford-Kowal-Bench (BKB) (>90% included in good performing group, <60% grouped as poor performers) scores were targeted for participation. All were consistent implant users for over 1 year. Those with devices older than 6 years were excluded. Other exclusion criteria were age more than 16, known difficult surgical insertion, prelingual deafness/congenital deafness, and “non-users.”
Participants were allocated to two groups (excellent and poor performers) based upon their speech recognition scores. Before taking part in the study participants were asked to complete the Glasgow Benefit Inventory, Hospital Anxiety and Depression Scale and Hearing, and Tinnitus and Balance Handicap scores.
On the participant's first attendance, up-to-date BKB and VCV scores were obtained. Following this the participant's threshold and comfort levels were measured. Thresholds were obtained by finding the first sound the participant could detect and then moving down by 4 units (log spaced units for Cochlear but linearly spaced units for Advanced Bionics and MED-EL as governed by the clinical software) and up by increments of 2 units until a repeatable result was obtained. Sound was presented in increasing increments up to a loud but comfortable level and then this sound level was presented repeatedly to ensure comfort. Loudness balancing across the electrode array was then performed to avoid pitch-amplitude confusion. Following this all pitch-ranking tests were performed using 80% (in clinical units) of comfort levels (at a more normal listening level).
Pitch-Ranking Methods
Initially all adjacent pairs of activated electrodes were compared. Each electrode had its pitch compared five times with its neighbor before moving on to the next set of adjacent pair comparisons (forced choice) and the decision to present either the most apical or the most basal of each adjacent pairing first was random. Movement along the array for adjacent pair testing was randomized. When adjacent pairs scored 5/5 this was deemed to represent good discrimination, and 0/5 to represent a pitch reversal.
Once all adjacent pairs had been compared the midpoint comparison task was used to further analyze pitch order across the array.
Midpoint Comparison Task
This technique utilizes a method devised by the mathematician Hugo Steinhaus in 1950 to ascertain the correct order of any set of items efficiently. The algorithm involves comparing the pitch of pairs of electrodes, with the provisional pitch ordering of electrodes updated as more comparisons are made. This is a forced-choice procedure, in which each new electrode is initially compared with the middle-ranked one from the electrodes already tested. Following this additional comparisons are made based on the results of the previous comparison. For example, if at some point in the procedure the provisional ranking was [3 6 8 10 12] then a new electrode would be selected at random and initially compared to electrode 8; if judged higher it would then be compared to electrode 10, and, if then judged lower, would be ranked fourth (between 8 and 10); further details are provided by Long et al. (19). A picture is thus built up of the pitch order. This test was performed a minimum of three times to obtain a more accurate measure of pitch order (obtaining a mean pitch order and standard error for each electrode). Previous work by Long et al. suggested that at least three “runs” were required to accurately pitch order the electrodes.
RESULTS
One poor performer was only able to partially complete the pitch-ranking assessments due to cognitive problems in understanding the tasks. Only one poor performer performed perfectly on pitch ranking. Table 1 shows the mean values for BKB, VCV, age, duration of deafness, time since implantation, and pitch comparisons for participants in the good and poor performing groups (range in brackets). Interestingly, there were no significant differences between the good and poor performing groups with regard to the subjective questionnaire measures (see Table 2).
TABLE 1.
Comparisons between good and poor performers (averages shown, range in brackets)
| Measure | Good performers (n = 10) | Poor performers (n = 12) |
| BKB | 98 (91–100) | 43 (8–55) |
| VCV | 81 (75–90) | 36 (1–54) |
| Age | 61 (48–77) | 69 (49–78) |
| Duration of deafness | 31 (12–51) | 25 (4–61) |
| Time since implantation | 6 (1.5–14) | 4 (1.5–14) |
| 5/5 Pitch pairs | 71% (19–100) | 37% (0–90) |
| 0/5 Pitch pairs | 9% (0–27) | 5% (0–14) |
| Overall proportion correct pairs | 80% (57–100) | 62% (41–98) |
BKB indicates Bamford-Kowal-Bench; VCV, vowel consonant vowel.
TABLE 2.
Comparisons between good and poor performers for subjective questionnaires (averages shown, range in brackets)
| Questionnaire | Good performers | Poor performers |
| Glasgow Benefit Inventory | 66 (54–72) | 65 (44–87) |
| Hearing Handicap | 35 (8–58) | 45 (0–84) |
| Tinnitus Handicap | 9 (0–48) | 15 (0–66) |
| Dizziness Handicap | 8 (0–36) | 13 (0–62) |
| HADS | 8 (0–14) | 6 (0–18) |
HADS indicates Hospital Anxiety and Depression Score.
Statistical analysis using univariate Mann-Whitney (exact) testing demonstrated that there were no significant differences between the two groups with regard to duration of deafness, time since implantation or preoperative BKB score. The good performers were however significantly younger than the poor performers (p = 0.014). Within the poor performing group there was wide variability in ability to pitch rank, from no errors, to a complete inability to reliably and consistently differentiate pitch change across the electrode array. When comparing average performance across the electrode array between subjects, we focused on the proportion of electrode pairs where the individual scored 5/5, rather than the overall number of correct pairs across the array. This is because summing scores would not distinguish between the case where all electrodes are at chance (3/3) and one where half gave perfect scores (5/5) and half showed possible reversals (0/5). In this study, a pitch confusion or unreliable discrimination is defined as a score out of 5 for adjacent pairs of electrodes of 1, 2, 3, or 4. Good performers were found to be significantly more accurate at pitch ranking, achieving a greater number of overall correct pairs as well as the proportion of times adjacent pairs scored 5/5 (p = 0.026).
There was no significant difference between the two groups with regard to the number of adjacent pairs where the subject scored 0/5 (assumed to represent electrode reversals). Tables 3 and 4 summarize the demographics and pitch perception findings of individuals in the poor and good performing groups, respectively.
TABLE 3.
Summary of poor performers (demographics and pitch ranking)
| Patient | BKB/VCV | Implant | Age | Duration deafness (years) | Cause | Time since implantation | Number 5/5 pairs | Number pairs scoring 1, 2, 3, or 4/5 | Number 0/5 pairs | Overall number correct pairs |
| 1 | 52%, 38% | MED-EL | 59 | 31 | Unknown | 3 years | 6/11 | 4/11 | 1/11 | 45/55 |
| 2 | 32%, 33% | AB | 72 | 12 | ?stroke | 1 year | 0/15 | 14/15 | 1/15 | 31/75 |
| 3 | 45%, 35% | AB | 66 | 26 | Progressive ?cause | 1 year | 0/15 | 14/15 | 1/15 | 39/75 |
| 4 | 36%, 35% | AB | 71 | 61 | Unknown | 3 years | 3/15 | 12/15 | 0/15 | 28/45 |
| 5 | 41%, 54% | MED-EL | 76 | 47 | CSOM, multiple mastoid ops | First implant 14 years; reimplant 2005 | 9/11 | 1/11 | 1/11 | 47/55 |
| 6 | 8%, 1% | MED-EL | 49 | 42 | Measles | 5 years | 1/10 | 9/10 | 0/10 | 28/50 |
| 7 | 54%, 44% | MED-EL | 81 | 34 | Unknown ?familial | 4 years | 1/11 | 9/11 | 1/11 | 30/55 |
| 8 | 17%, 20% | MED-EL | 70 | 16 | Streptomycin | 6 years | 6/9 | 3/9 | 0/9 | 39/45 |
| 9l | 55%, 39% | AB | 70 | 4 | Unknown | 3 years | 9/10 | 1/10 | 0/10 | 49/50 |
| 10 | 39%, 33% | Nucleus | 74 | Profound 15 years, some since age 18 | Progressive ?otosclerosis | 2 years | 6/21 | 13/21 | 2/21 | 60/105 |
| 11 | 55%, 38% | Nucleus | 78 | 38 | Progressive | 1 year | 10/21 | 11/21 | 0/21 | 84/105 |
| 12 | 41%, 43% | MED-EL | 71 | Profound 10 years, some loss 30 years | Progressive ?otosclerosis | 5 years | 2/7 | 4/7 | 1/7 | 24/35 |
BKB indicates Bamford-Kowal-Bench; VCV, vowel consonant vowel; AB, Advanced Bionics; ?, not certain; CSOM, chronic suppurative otitis media.
TABLE 4.
Summary of good performers (demographics and pitch ranking)
| Patient | BKB/VCV | Implant | Age | Duration deafness (years) | Cause | Time since implantation | Number 5/5 pairs | Number pairs scoring 1, 2, 3, or 4/5 | Number 0/5 pairs | Overall number correct pairs |
| 1 | 99%, 85% | MED-EL | 55 | 39 | Unknown | 6 years | 9/9 | 0/9 | 0/9 | 45/45 |
| 2 | 97%, 83% | MED-EL | 50 | 45 | Measles | 5 years | 9/11 | 0/11 | 2/11 | 45/55 |
| 3 | 100%,79% | MED-EL | 48 | 12 | Head injury | 6 years | 5/11 | 3/11 | 3/11 | 31/55 |
| 4 | 99%, 75% | AB | 67 | 38 | Otosclerosis | 3 years | 12/15 | 3/15 | 0/15 | 69/75 |
| 5 | 97%, 90% | AB | 64 | 30 | Progressive | 1.5 years | 15/15 | 0/15 | 0/15 | 75/75 |
| 6 | 99%, 80% | Nucleus | 61 | 51 | Unknown | 1 years | 4/21 | 14/21 | 3/21 | 60/105 |
| 7 | 98%, 71% | Nucleus | 68 | Profound 18 years, some loss 50 years | Unknown | First implant 1994; reimplanted 2007 | 13/21 | 8/21 | 0/21 | 92/105 |
| 8 | 91%, 85% | MED-EL | 77 | 15 | Meningitis | First implant 1994; reimplanted 2005 | 9/11 | 1/11 | 1/11 | 49/55 |
| 9 | 97%, 79% | AB | 49 | 20 | Progressive | 1.5 years | 13/18 | 4/18 | 1/18 | 75/90 |
| 10 | 96%, 85% | AB | 58 | 13 | Unknown | 1.5 years | 11/15 | 4/15 | 0/15 | 70/75 |
BKB indicates Bamford-Kowal-Bench; VCV, vowel consonant vowel; AB, Advanced Bionics.
Univariate linear regression models were constructed on the change in BKB scores variable (post–preoperatively) to investigate if the percentage of 5/5 pairs, the percentage of 0/5 pairs, age, duration, and preoperative BKB were each significant predictors of change in BKB score. We chose to focus on the change in BKB score, rather than the raw score, because we wanted to minimize the influence of effects such as overall education or IQ, that are unrelated to cochlear implantation. Although these factors may well influence the ability of a patient to process stimuli presented through their implant, we reasoned that this would also be true to some extent for their preoperative residual acoustic hearing, and so by taking the difference we would minimize their influence. Multiple linear regression models were then constructed to determine the effect of percentage of 5/5 pairs and percentage of 0/5 pairs on improvement in BKB scores after adjusting for (i) age, and (ii) age and duration. Age and duration were made available as covariates in the multiple regression models to make adjustment for potential confounders.
Only the percentage of 5/5 pairs was a significant predictor of change in BKB (p = 0.01); no other variables were significant at the 5% level. This positive relationship between the improvement in BKB score and the percentage of 5/5 pairs remained significant even after adjusting for the effects of age and of duration of deafness (p = 0.02). It remained significant even after adjusting the criterion to account for the fact that we studied the effects of two experimental variables (percentage of 5/5 and 0/5 pairs). The regression coefficient for percentage of 5/5 pairs was calculated to be 0.46 on average, with a 95% CI of 0.09 to 0.82, after adjusting for age. The R2 value for this analysis was 0.437, therefore the model accounted for 43.7% of variability across the group.
After adjusting for age and duration, the percentage of 5/5 pairs is still a significant predictor of improvement in BKB score (p = 0.02). A patient with a percentage of 5/5 pairs 10% higher than another patient is expected to have an improvement in BKB scores that is greater by 4.55 on average (95% CI 0.78–8.32), after adjusting for age and duration. The univariate analysis results show insufficient evidence for a relationship between percentage of 0/5 pairs and improvement in BKB scores. The same conclusion was reached even after (i) adjusting for age only (percentage of 0/5 pairs, coefficient 0.55, 95% CI −1.39 to 2.48, p = 0.56) and (ii) adjusting for age, duration, and preoperative BKB score (percentage of 0/5 pairs, coefficient 0.52, 95% CI −1.48 to 2.52, p = 0.59).
Results From the Midpoint Comparison Task
The midpoint comparison task added useful information over and above the initial adjacent electrode pair testing as it was able to provide an estimate of where along the array an anomalous electrode pitch percept might fit tonotopically. A, B, and C graphically represent the change in perceived pitch across the electrode array as found by three runs of the midpoint comparison task. An example from each device type is shown. Error bars represent the standard error for each mean rank.
FIG. 1.
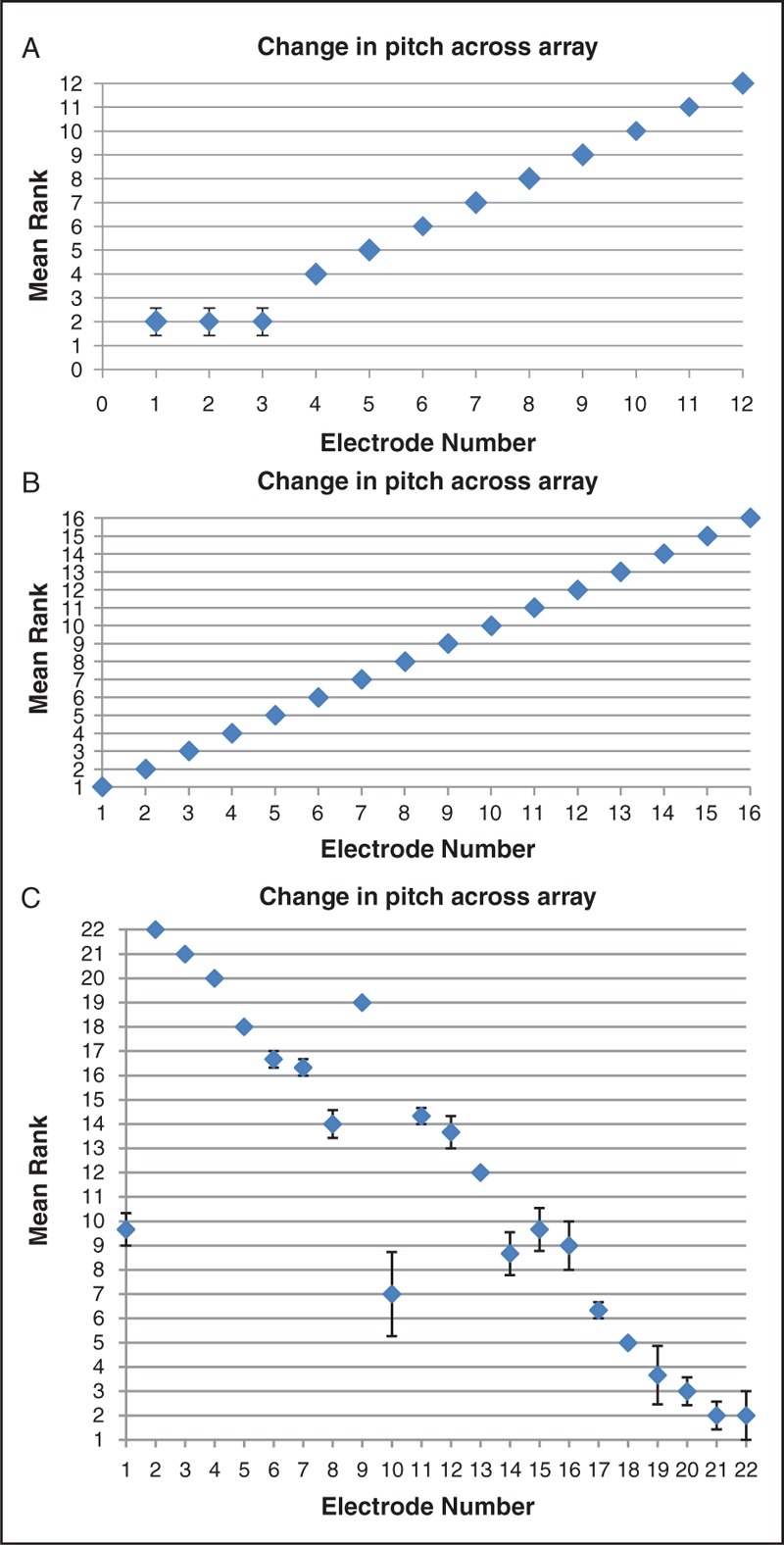
(A) MED-EL user, Subject 8, poor performer. Mean rank order of each electrode (y axis) plotted against each electrode (x axis). Error bars show standard error for each electrode rank order across 3 “runs” of the midpoint comparison task. (B) Advanced Bionics user, Subject 10, good performer. Mean rank order of each electrode (y axis) plotted against each electrode (x axis). Standard error is zero in this case. (C) Cochlear user, Subject 10, poor performer. Mean rank order of each electrode (y axis) plotted against each electrode (x axis). Error bars show standard error for each electrode rank order across 3 “runs” of the midpoint comparison task.
The results of the midpoint comparison pitch-ordering task revealed a high prevalence of anomalies. For the purpose of this study “apical” refers to the apical third of the array and “basal” to the basal third of electrodes. Of the MED-EL users 5 of 10 had apical pitch abnormalities (an abnormality being defined in this study as a score of less than 5 out 5 on adjacent pair testing), 3 of 10 had abnormalities across the array and one had normal pitch ranking. There were 8 Advanced Bionics users, 3 of which showed no abnormalities. Two of 8 had basal pitch abnormalities and 3 of 8 had problems throughout the array. Of the 4 cochlear devices, 2 had basal abnormalities only and 2 had pitch abnormalities evenly across the array.
Further statistical analysis was undertaken to try to identify whether any of the pitch discrimination abnormalities were more likely to occur at any particular part of the electrode array for any given device type. Firstly Chi-squared testing revealed that the distribution of 0/5 scores along the electrode arrays of MED-EL users differed significantly from chance (χ2 = 23, df = 10, p < 0.02; Fig. 2B). This finding was further supported by the analysis of variance testing described below. The distribution of 5/5 scores across the array did not differ significantly from chance for MED-EL devices. The distribution of 0/5 and 5/5 scores did not differ from chance in either the Cochlear or the Advanced Bionics devices.
FIG. 2.
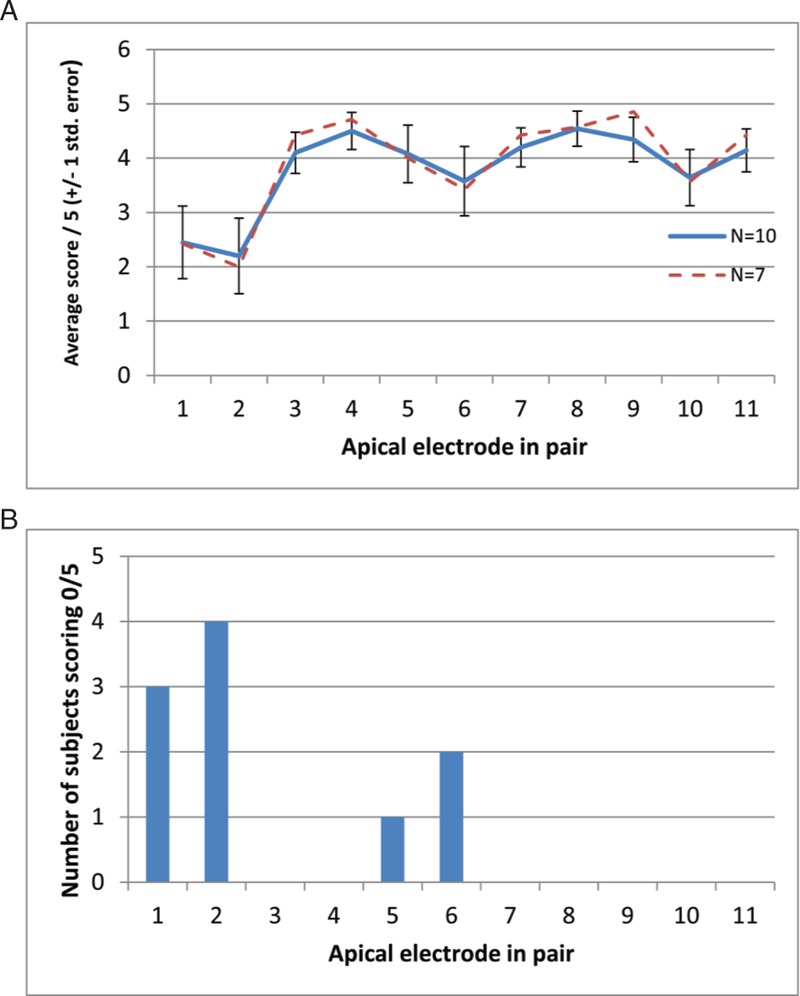
(A) The abscissa shows the more apical member of the adjacent pairs to be discriminated. The solid blue line shows the average score out of 5, as a function of electrode position, for the 10 users of the MED-EL device who took part. Error bars show +/− one standard error. Missing values have been replaced by the mean score for each subject. The dashed red line shows the average score only for the seven subjects for whom there were no missing data. (B) The total number of listeners scoring 0/5 for each electrode pair.
Separate one-way repeated-measures analysis of variances were performed on adjacent-pair comparison data for each device, with electrode position as the independent variable, and proportion of correct responses as the dependent variable. The Huynh-Feldt sphericity correction was used, and the corrected degrees of freedom are reported. In a few cases, individual subjects were not tested on all electrode pairs. When this occurred the score for the missing pair was replaced with the mean score across all pairs for that subject. This conservative treatment of missing values will reduce the chances of observing a significant effect of electrode position. It happened for a total of 11 out of 120 combinations of electrode and subject for the Advanced Bionics device, for 7 out of 110 such combinations for the MED-EL device, and not at all for the Cochlear device.
No significant effect of electrode position was observed for the Advanced Bionics (F(14,94) = 1.058, p > 0.05) or Nucleus (F(8.11,23.3) = 1.7, p > 0.05) devices. However, for the MED-EL device there was a significant effect of electrode position (F(5.0,45.0) = 3.255, p < 0.02), and also significant linear (F = 5.623, p < 0.05) and quadratic (F = 6.414, p < 0.05) trends. As shown in A, this reflects the fact that performance was worse for the apical than for the basal electrodes. A significant effect of electrode position was also obtained when we included only the seven MED-EL patients for whom there was no missing data (F(4.8,28.8) = 2.943, p < 0.05).
DISCUSSION
These findings indicate that accurate pitch ranking is an important, independent factor in overall speech perception performance, accounting for 43.7% of variance across subjects. The study also demonstrates a high prevalence of pitch anomalies particularly in poor performers. Such a prevalence of pitch confusions is in keeping with the work of Zwolan et al. (22) who demonstrated great variability in electrode discrimination ability between subjects. They did not however find a correlation between electrode discrimination and speech recognition performance as demonstrated in this study and other similar work by Nelson et al. (23) and Dorman et al. (24). In these studies, speech-understanding performance seemed to be correlated with place-pitch sensitivity (Nelson) and the range of available pitch through the Ineraid implant (Dorman).
A number of limitations to this study should be recognized. Many factors can contribute to functional speech recognition and could act as confounding variables. However, statistical analysis of the differences between the two groups (specifically preoperative BKB, duration of deafness, time since implantation) would suggest that these key variables did not impact on this study. Loudness balancing was carefully performed, but as this is a subjective measure any “inaccuracy” by the participant may have led to electrode pitch differences being erroneously perceived as detectable or not. This could either lead to an over or underestimate of pitch perception ability. During prolonged testing individuals can also become easily fatigued, which could produce spurious inaccuracies later in testing. We attempted to minimize the influence of this effect by comparing electrode pairs randomly across the array rather than from basal to apical or vice versa.
A significant proportion of MED-EL implants in this study showed poor apical discrimination. Five of 6 poor-performing MED-EL implantees showed apical pitch confusions as well as 3 of 4 good performers. The prevalence of apical pitch confusions identified in this study correspond with the work of Gani et al. (25), who additionally noted improvements in overall performance when apical electrodes were deactivated. Pitch reversals did not appear to be predictive of performance. This may suggest that when a pitch reversal occurs the implantee is still obtaining discrete pitch percepts, and that a reversal is therefore less detrimental to performance than when different pitches are not distinguishable.
The severe electrode discrimination deficits highlighted in some individuals would suggest some implantees receive extremely limited information by way of pitch change across the array. Cochlear hearing loss itself is thought to result in changes to the representation of sound in the auditory system by way of reduced frequency selectivity and precision of phase locking, as well as the presence of dead regions and changes in the propagation time of the traveling wave across the basilar membrane (26). Additionally CIs are limited in their ability to encode sound. Incomplete insertion of the device can lead to a frequency-to-place mismatch of greater than three octaves at the apical electrode (27). CI processing strategies generally apply pulse trains with the same rate to each electrode, unlike acoustic hearing where the auditory nerve phase-locks to resolved frequency components. A correspondence between temporal and place-of-excitation cues to frequency may be important for pitch perception (28–30). Phase transitions around peaks of the traveling wave are also not encoded by implant processors (26).
CONCLUSIONS AND CLINICAL APPLICABILITY
Key Findings:
The methods of assessing pitch perception used in this study provide an efficient detailed picture of an individual's abilities, using equipment commonly available to clinicians.
Ability to pitch rank accurately is an independent predictor of overall performance, even after adjusting for age, duration of deafness, and time since implantation.
MED-EL users, at all performance levels, showed a significantly greater tendency for pitch confusions at the apex of the array, compared to other locations.
One implication of these findings may be that removal of nontonotopic electrodes, or frequency reallocations to match pitch ranking, might improve overall performance.
Henshall and McKay (17) found pitch-ranking deficiencies in a group of patients and studied the effects of switching off nontonotopic electrodes and extending the high-frequency range beyond that normally allocated to electrodes in clinical speech processor maps. They did not find improvements in performance after such changes, perhaps due to the deleterious effects of automatic frequency reallocation when this is attempted. Di Nardo et al. (31) similarly found no correspondence between acoustic pitch and assigned frequency ranges and propose that mapping procedures should include a comparison with homolateral residual hearing if possible. The same author also describes a case study using Digimap technology where an individual's performance was increased by reallocating frequencies based upon electric to acoustic matching (32). Zwolan et al. (22) used an experimental map based upon electrode discrimination and found that seven of nine subjects showed significant improvement in at least one speech recognition measure.
Future work by the authors of this study will concentrate on the effect of manipulating maps based upon pitch-ranking findings to try to improve overall performance.
ACKNOWLEDGMENTS
The authors are grateful to the Cambridge Hearing Trust for provision of funding for the purposes of participant travel expenses.
Footnotes
The authors disclose no conflicts of interest.
REFERENCES
- 1. Green KM, Bhatt YM, Mawman DJ, et al. Predictors of audiological outcome following cochlear implantation in adults. Cochlear Implants Int 2007; 8:1–11. [DOI] [PubMed] [Google Scholar]
- 2. Gantz BJ, Woodworth GG, Knutson JF, et al. Multivariate predictors of success with cochlear implants. Adv Otorhinolaryngol 1993; 48:153–167. [DOI] [PubMed] [Google Scholar]
- 3. Blamey P, Arndt P, Bergeron F, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol Neurootol 1996; 1:293–306. [DOI] [PubMed] [Google Scholar]
- 4. Blamey PJ, Pyman BC, Gordon M, et al. Factors predicting postoperative sentence scores in postlinguistically deaf adult cochlear implant patients. Ann Otol Rhinol Laryngol 1992; 101:342–348. [DOI] [PubMed] [Google Scholar]
- 5. Finley CC, Holden TA, Holden LK, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol 2008; 29:920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Summerfield AQ, Marshall DH. Preoperative predictors of outcomes from cochlear implantation in adults: performance and quality of life. Ann Otol Rhinol Laryngol Suppl 1995; 166:105–108. [PubMed] [Google Scholar]
- 7. Knutson JF, Hinrichs JV, Tyler RS, et al. Psychological predictors of audiological outcomes of multichannel cochlear implants: preliminary findings. Ann Otol Rhinol Laryngol 1991; 100:817–822. [DOI] [PubMed] [Google Scholar]
- 8. Waltzman SB, Fisher SG, Niparko JK, et al. Predictors of postoperative performance with cochlear implants. Ann Otol Rhinol Laryngol Suppl 1995; 165:15–18. [PubMed] [Google Scholar]
- 9. Moore BC, Vickers DA, Glasberg BR, et al. Comparison of real and simulated hearing impairment in subjects with unilateral and bilateral cochlear hearing loss. Br J Audiol 1997; 31:227–245. [DOI] [PubMed] [Google Scholar]
- 10. Dorman MF, Loizou PC, Rainey D. Speech intelligibility as a function of the number of channels of stimulation for signal processors using sine-wave and noise-band outputs. J Acoust Soc Am 1997; 102:2403–2411. [DOI] [PubMed] [Google Scholar]
- 11. Hill FJ, McRae LP, McClellan RP. Speech recognition as a function of channel capacity in a discrete set of channels. J Acoust Soc Am 1968; 44:13–18. [DOI] [PubMed] [Google Scholar]
- 12. Lawson DT, Wilson BS, Finley CC. New processing strategies for multichannel cochlear prostheses. Prog Brain Res 1993; 97:313–321. [DOI] [PubMed] [Google Scholar]
- 13. Fishman KE, Shannon RV, Slattery WH. Speech recognition as a function of the number of electrodes used in the SPEAK cochlear implant speech processor. J Speech Lang Hear Res 1997; 40:1201–1215. [DOI] [PubMed] [Google Scholar]
- 14. Henry BA, McKay CM, McDermott HJ, et al. The relationship between speech perception and electrode discrimination in cochlear implantees. J Acoust Soc Am 2000; 108 (3 Pt 1):1269–1280. [DOI] [PubMed] [Google Scholar]
- 15. Collins LM, Zwolan TA, Wakefield GH. Comparison of electrode discrimination, pitch ranking, and pitch scaling data in postlingually deafened adult cochlear implant subjects. J Acoust Soc Am 1997; 101:440–455. [DOI] [PubMed] [Google Scholar]
- 16. Poulton EC. Models for the biases in judging sensory magnitude. Psychological Bull 1979; 86:777–803. [PubMed] [Google Scholar]
- 17. Henshall KR, McKay CM. Optimizing electrode and filter selection in cochlear implant speech processor maps. J Am Acad Audiol 2001; 12:478–489. [PubMed] [Google Scholar]
- 18. Collins LM, Throckmorton CS. Investigating perceptual features of electrode stimulation via a multidimensional scaling paradigm. J Acoust Soc Am 2000; 108 (5 Pt 1):2353–2365. [DOI] [PubMed] [Google Scholar]
- 19. Long CJ, Nimmo-Smith I, Baguley DM, et al. Optimizing the clinical fit of auditory brain stem implants. Ear Hear 2005; 26:251–262. [DOI] [PubMed] [Google Scholar]
- 20. Kong YY, Carlyon RP. Temporal pitch perception at high rates in cochlear implants. J Acoust Soc Am 2009; 127:3114–3123. [DOI] [PubMed] [Google Scholar]
- 21. Carlyon RP, Deeks JM, McKay CM. The upper limit of temporal pitch: Stimulus duration, conditioner pulses, and the number of electrodes stimulated. J Acoust Soc Am 2010; 127:1469–1478. [DOI] [PubMed] [Google Scholar]
- 22. Zwolan TA, Collins LM, Wakefield GH. Electrode discrimination and speech recognition in postlingually deafened adult cochlear implant subjects. J Acoust Soc Am 1997; 102:3673–3685. [DOI] [PubMed] [Google Scholar]
- 23. Nelson DA, Van Tasell DJ, Schroder AC, et al. Electrode ranking of “place pitch” and speech recognition in electrical hearing. J Acoust Soc Am 1995; 98:1987–1999. [DOI] [PubMed] [Google Scholar]
- 24. Dorman MF, Smith L, McCandless G, et al. Pitch scaling and speech understanding by patients who use the Ineraid cochlear implant. Ear Hear 1990; 11:310–315. [DOI] [PubMed] [Google Scholar]
- 25. Gani M, Valentini G, Sigrist A, et al. Implications of deep electrode insertion on cochlear implant fitting. J Assoc Res Otolaryngol 2007; 8:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carlyon R, Moore B. Perception of pitch by people with cochlear hearing loss and by cochlear implant users. In: PLack C, Oxenham A, Fay R. eds. Pitch: Neural Coding and Perception. New York: Springer Handbook of Auditory Research, 2005; 234–77. [Google Scholar]
- 27. Ketten DR, Skinner MW, Wang G, et al. In vivo measures of cochlear length and insertion depth of nucleus cochlear implant electrode arrays. Ann Otol Rhinol Laryngol Suppl 1998; 175:1–16. [PubMed] [Google Scholar]
- 28. Loeb GE, White MW, Merzenich MM. Spatial cross-correlation. A proposed mechanism for acoustic pitch perception. Biol Cybern 1983; 47:149–163. [DOI] [PubMed] [Google Scholar]
- 29. Carlyon RP, van Wieringen A, Long CJ, et al. Temporal pitch mechanisms in acoustic and electric hearing. J Acoust Soc Am 2002; 112:621–633. [DOI] [PubMed] [Google Scholar]
- 30. Oxenham AJ, Bernstein JGW, Penagos H. Correct tonotopic representation is necessary for complex pitch perception. Proc Natl Acad Sci U S A 2004; 101:1421–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Di Nardo W, Cantore I, Cianfrone F, et al. Differences between electrode-assigned frequencies and cochlear implant recipient pitch perception. Acta Otolaryngol 2007; 127:370–377. [DOI] [PubMed] [Google Scholar]
- 32. Di Nardo W, Cantore I, Marchese MR, et al. Electric to acoustic pitch matching: a possible way to improve individual cochlear implant fitting. Eur Arch Otorhinolaryngol 2008; 265:1321–1328. [DOI] [PubMed] [Google Scholar]


