Abstract
Para-toluenesulfonamide (PTS) has been implicated with anticancer effects against a variety of tumors. In the present study, we investigated the inhibitory effects of PTS on tongue squamous cell carcinoma (Tca-8113) and explored the lysosomal and mitochondrial changes after PTS treatment in vitro. High-performance liquid chromatography showed that PTS selectively accumulated in Tca-8113 cells with a relatively low concentration in normal fibroblasts. Next, the effects of PTS on cell viability, invasion, and cell death were determined. PTS significantly inhibited Tca-8113 cells’ viability and invasive ability with increased cancer cell death. Flow cytometric analysis and the lactate dehydrogenase release assay showed that PTS induced cancer cell death by activating apoptosis and necrosis simultaneously. Morphological changes, such as cellular shrinkage, nuclear condensation as well as formation of apoptotic body and secondary lysosomes, were observed, indicating that PTS might induce cell death through disturbing lysosomal stability. Lysosomal integrity assay and western blot showed that PTS increased lysosomal membrane permeabilization associated with activation of lysosomal cathepsin B. Finally, PTS was shown to inhibit ATP biosynthesis and induce the release of mitochondrial cytochrome c. Therefore, our findings provide a novel insight into the use of PTS in cancer therapy.
Keywords: apoptosis, lysosome, mitochondria, para-toluenesulfonamide, tongue squamous cell carcinoma
Introduction
Para-toluenesulfonamide (PTS) is a novel anticancer agent with good lipophilic ability. As an adjunct to chemotherapy and radiation therapy, PTS is usually delivered by an intravenous or an intratumoral injection. Recent studies suggested that PTS inhibits tumor progression by induction of tumor necrosis 1. A phase II clinical trial showed that chemotherapy with a concurrent PTS local injection was well tolerated and had efficient clinical outcomes in patients with peripherally advanced lung cancer 2. However, the mechanisms of anticancer effects of PTS remain elusive.
Lysosomes are highly dynamic cellular organelles that play critically important roles in endocytosis, autophagy, phagocytosis, and exocytosis 3. Disturbance of lysosomal stability induces cancer cell death 4. A limited release of lysosomal contents to the cytoplasm triggers apoptosis or apoptosis-like cell death, whereas generalized lysosomal rupture results in rapid cellular necrosis 5. Released lysosomal proteases including cathepsin B and D also cause mitochondrial damage and dysfunction, which further amplifies the cell death signals 6–8. It has been reported previously that PTS suppressed H460 lung cancer cells by necrotizing tumor in a nude mice model 1. However, the mechanisms of PTS-induced cell death remain unknown. To determine whether PTS treatment is associated with lysosome-mediated cell death, we examined the effects of PTS on the human tongue squamous cell carcinoma Tca-8113 cell line.
Materials and methods
Cell culture and reagents
The human tongue squamous cell carcinoma Tca-8113 cell line was purchased from the China Center for Type Culture Collection (Wuhan, China). Nontumor normal mucosa tissues were obtained from patients who underwent primary surgical resection of gingival squamous cell carcinoma with informed consent at West China Hospital of Stomatology (Chengdu, China). The age range of all individuals was 35–65 years. Mucosa tissues were washed with PBS, minced, and incubated for 4 h at 37°C in 5 ml of 10% collagenase type I (Sigma-Aldrich, St Louis, Missouri, USA). Cells were spun at 225g for 5 min, washed with PBS, and cultured. Human gingival fibroblast (HGF) cells were incubated in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum (FBS), 10 U/ml penicillin, 10 μg/ml streptomycin, and 200 mmol/l l-glutamine (Biochrom, Berlin, Germany). HGF and Tca-8113 cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Morphologic changes in HGF and Tca-8113 cells after PTS treatment were monitored using a phase-contrast microscope (Olympus, Tokyo, Japan). This study was approved by the Human Research Ethics Committee of West China Hospital of Stomatology of Sichuan University. PTS was purchased from Sigma-Aldrich and dissolved in dimethyl sulfoxide (DMSO).
High-performance liquid chromatography
Measurement of intracellular PST by high-performance liquid chromatography (HPLC) was performed as described previously 9. HPLC was performed using an Agilent 1100LC system (Agilent Technologies, Palo Alto, California, USA) equipped with a Zorbax C8 column (4.6×250 mm, 5 μm; Dupont, Wilmington, Delaware, USA). The mobile phase consisted of acetonitrile–water (15 : 85, v/v). The flow rate was 1.3 ml/min with UV detection at 268 nm. The protein concentration of samples was determined using the Bradford protein assay. Quantification of intracellular PST was expressed as PST content per microgram of protein (ng/μg).
Cell viability assay
Cell viability was measured in 96-well plates using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Life Technologies, Gaithersburg, Maryland, USA) as described previously 10. Briefly, the effects of the indicated concentration of PTS on the viability of Tca-8113 and HGF cells were assessed by mitochondrial activity indicator MTT. The assay was performed according to the manufacturer’s instructions and plates were read at 570 nm. Viability was calculated as percentage compared with untreated cells under basal conditions.
Flow cytometric analysis
The effects of PTS on cancer cell death were determined by flow cytometric analysis. In brief, cells were collected and washed twice with cold PBS. The cell collections were resuspended in Annexin V–FITC and propidium iodide (PI), and incubated for 15 min at room temperature in the dark. Fluorescence was measured at 488 nm in a flow cytometer (FACSCalibur; BD Biosciences, San Jose, California, USA).
Transwell migration assay
The effects of PTS on the invasive motility of Tca-8113 cells were determined using a transwell migration assay (BD Biosciences). In brief, after treatment with PTS for 1 h, cells (2.5×104) were seeded into the top chambers of a 24-well, 8-μm pore-size micropore polycarbonate membrane filter (BD Biosciences), and the bottom chambers were filled with 500 μl Dulbecco’s modified Eagle medium containing 10% FBS as a chemoattractant. Cells were allowed to migrate for 24 h at 37°C. Nonmigrated cells were removed with a cotton swab, and migrated cells were fixed with 4% paraformaldehyde and stained with 1% crystal violet. Data are represented as the average number of migrated cells per field (20 random ×20 magnification fields) per membrane filter.
Colony formation assay
Cells were seeded into 60 mm culture dishes at 200 cells/dish. After 24 h, cultures were replaced with fresh medium containing 10% FBS with or without PTS. After 1 h incubation, culture dishes were rinsed three times with PBS. Cells were further grown in fresh medium containing 10% FBS for 3 weeks. Colonies were stained with a solution containing 0.5% crystal violet and 25% methanol. Colonies were counted only if a single clone contained more than 50 cells.
Lactate dehydrogenase release assay
Lactate dehydrogenase (LDH) release from cells was measured by determining the activity of LDH released into the culture medium using an LDH assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocol. Briefly, Tca-8113 cells were treated with either control medium or PTS for 1 h. 100 μl culture medium was collected and mixed with an LDH reaction solution. Absorbance was measured at 450 nm using a microplate reader (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA). Necrotic cell death after PTS treatment was estimated by the activity of LDH released from necrotic cells with damaged plasma membrane integrity.
Transmission electron microscopy analysis
Tca-8113 cells were seeded on coverslips and treated with PTS for 1 h. The medium was replaced by fresh medium for a further 24-h incubation. At the end of the incubation period, cells were fixed in 3% glutaraldehyde in PBS for 2 h, washed, and fixed again in 1% osmium tetroxide. Samples were dehydrated in graded ethanol and embedded in epon 812. Serial ultrathin 60-nm sections were stained with uranyl acetate and lead citrate and then observed using a transmission electron microscope (Hitachi H-600; Hitachi, Tokyo, Japan).
Western blot
Preparation of total and cytosolic proteins as well as western blot analysis were carried out as described previously 11. For immunodetection, anticathepsin B polyclonal antibody (Abcam, Cambridge, Massachusetts, USA; 1 : 1000 dilution) and anticytochrome c monoclonal antibody (Cell Signaling Technology, Beverly, Massachusetts, USA; 1 : 1000 dilution) were used. Proteins were detected using HRP-conjugated secondary antibodies and an enhanced chemiluminescence reagent (Millipore, Bedford, Massachusetts, USA).
Assays for lysosomal integrity
Lysosomes of Tca-8113 cells were isolated using the Percoll gradient centrifugation method 12. The effects of PTS on lysosomal integrity were assessed by measuring the activity of lysosomal β-galactosidase using UMBG (4-methylumbelliferyl-β-D-galactoside) as described previously 13. The 4-methylumbelliferone released was determined by measuring its fluorescence (excitation: 365 nm, emission: 444 nm) on a fluorescence spectrophotometer (Hitachi F-4500; Hitachi). The activity of the enzyme measured in the presence and absence of 0.36% Triton X-100 was designated the free activity and the total activity, respectively.
ATP biosynthesis assay
Cellular mitochondria were isolated using the Percoll density gradient centrifugation method 14. Mitochondrial ATP biosynthesis was determined on the basis of the luciferin–luciferase reaction as described previously 15. Measurements of the chemiluminescence of the luciferin–luciferase reaction were performed in a Varioskan Flash microplate reader (Thermo Fisher Scientific Inc.).
Statistical analyses
Data are described by mean±SD and the statistical analysis was carried out using SPSS (version 13.0; SPSS Inc., Chicago, Illinois, USA). Statistical significance was evaluated using the Student’s t-test or two-way analysis of variance. P-values of less than 0.05 were defined as statistically significant.
Results
Para-toluenesulfonamide selectively accumulates in cancer cells without affecting normal fibroblasts
To examine the anticancer properties of PTS, we first measured the intracellular concentration of PTS in human tongue cancer Tca-8113 cells and gingival fibroblast HGF cells after PTS treatment. The HPLC method for the validation of PTS was performed with six calibration standards ranging from 10.0 to 320.0 μmol/l. The retention times of the internal standard and PTS were 2.0 and 12.6 min, respectively. As shown in Fig. 1a, PTS treatment increased the intracellular PTS concentration in Tca-8113 cells. We next assessed the accumulation of intracellular PTS after different doses and incubation periods of PTS treatment in Tca-8113 and HGF cells. Increased PTS treatment significantly induced a higher level of intracellular PTS accumulation in Tca-8113 and HGF cells. Prolonged PTS incubation also increased the intracellular PTS level. 80 μmol/l PTS treatment for 10 min induced maximal intracellular PTS accumulation in Tca-8113 and HGF cells (Fig. 1b and c). However, after 10 min of PTS treatment, intracellular PTS accumulation was significantly inhibited in HGF cells compared with that in Tca-8113 cells under the same PTS treatment condition (Fig. 1d). These results suggest that PTS selectively targets cancer cells, but exerts less effect on normal fibroblasts.
Fig. 1.
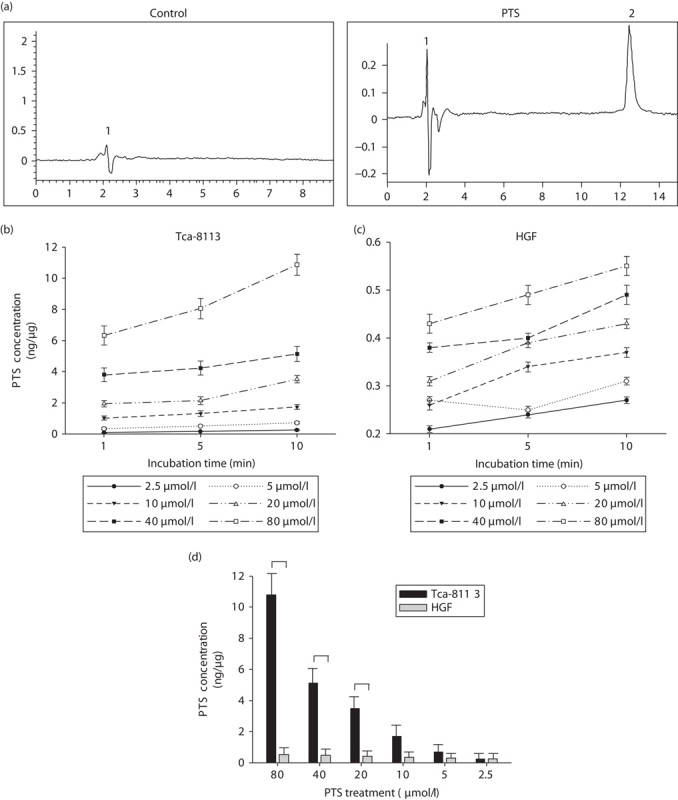
Para-toluenesulfonamide (PTS) selectively accumulates in Tca-8113 cells. (a) Tca-8113 were treated with PTS or dimethyl sulfoxide (control vehicle) and then subjected to high-performance liquid chromatography. Peak 1: antipyrine (internal standard), peak 2: PTS. (b) Plot of intracellular PTS concentration after 2.5–80 μmol/l PTS treatment for different periods of time in Tca-8113 cells. (c) Plot of intracellular PTS concentration after 2.5–80 μmol/l PTS treatment for different periods in human gingival fibroblast (HGF) cells. (d) Comparative evaluation of the intracellular PTS concentration under the same treatment condition in Tca-8113 and HGF cells. The 10 min time-point was chosen to observe the difference in the intracellular PTS concentration between Tca-8113 and HGF cells. Results are representative of three independent experiments. *P<0.05.
Para-toluenesulfonamide activates apoptosis and necrosis simultaneously to induce cancer cell death
To examine the effects of PTS on cell viability, Tca-8113 and HGF cells were treated with different doses of PTS for 1 h. As shown in Fig. 2a, PTS significantly reduced the viability of Tca-8113 cells in a dose-dependent manner. 80 μmol/l PTS treatment almost completely suppressed the number of viable cells. The inhibitory effects of PTS in HGF cells were significantly decreased compared with that in Tca-8113 cells, which is consistent with our HPLC results that HGF cells accumulated less intracellular PST. Observation of morphologic changes using a phase-contrast microscope showed that 40 μmol/l PTS treatment induced classical apoptotic features including cell shrinkage, nuclear condensation, cell density reduction, and apoptotic body formation (Fig. 2b). Colony formation assay further confirmed the cytotoxic effects of PTS on Tca-8113 cells (Fig. 2c). We next measured cancer cell death using flow cytometric analysis. PTS treatment for 1 h induced a significant increase in early apoptotic cells (Annexin V+/PI−) with 5.82, 20.2, and 64.1% for DMSO, and 40 and 80 μmol/l of PTS treatment. Late apoptotic/necrotic cells (Annexin V+/PI+) after DMSO, and 40 and 80 μmol/l of PTS treatment were 7.70, 11.5, and 15.9%, respectively. Viable cells (Annexin V−/PI−) decreased from 83.0% in the DMSO-treated group to 67.5 and 19.8% after 40 and 80 μmol/l of PTS treatment (Fig. 2d). The results of flow cytometric analysis showed that PTS induced cancer cell death by activating apoptosis and necrosis simultaneously. We further determined PTS-induced necrosis using the LDH release assay. LDH is a cytosolic enzyme that can be released into culture medium upon damage of the plasma membrane. Necrosis, which results in an early loss of plasma membrane integrity, can be determined using the LDH release assay 16,17. As shown in Fig. 2e, PTS induced a significant increase in the activity of LDH released from necrotic cells. 80 μmol/l PTS treatment for 1 h induced a two-fold increase in LDH release compared with the control group. In addition, we investigated the effects of PTS on the invasive ability of cancer cells. Using a transwell migration assay, we observed that 40 μmol/l PTS treatment for 1 h significantly reduced the invasive ability of Tca-8113 cells (Fig. 2f).
Fig. 2.
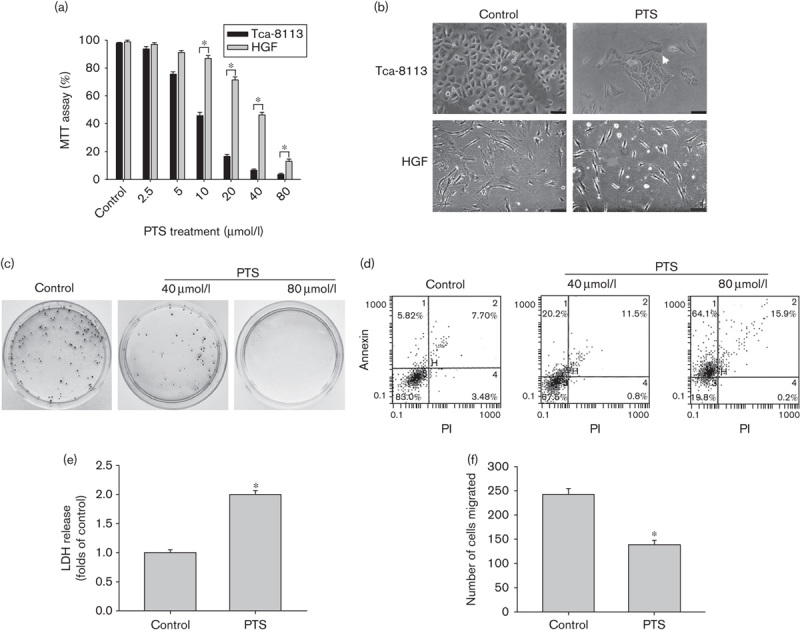
Para-toluenesulfonamide (PTS) suppresses cell viability and invasion, and induces apoptosis and necrosis simultaneously. (a) Tca-8113 and human gingival fibroblast (HGF) cells were treated with 2.5–80 μmol/l PTS for 1 h. The effects of PTS on cell viability were assessed using MTT 72 h after treatment. (b) Tca-8113 and HGF cells were treated with 40 μmol/l PTS or dimethyl sulfoxide (DMSO) (control vehicle) for 1 h; the morphologic changes were observed using a phase-contrast microscope. The apoptotic body was shown (arrow) (bar=30 μm). (c) Tca-8113 cells were treated with 40–80 μmol/l PTS or DMSO for 1 h, the cytotoxic effects of PTS on Tca-8113 cells were assessed using the colony formation assay. (d) Tca-8113 cells were treated with 40–80 μmol/l PTS or DMSO for 1 h. Cells were stained with Annexin V–FITC and propidium iodide (PI), followed by flow cytometric analysis. (e) Tca-8113 cells were treated with 80 μmol/l PTS or DMSO for 1 h; PTS-induced necrosis was determined using the lactate dehydrogenase (LDH) release assay. (f) Tca-8113 cells were treated with 40 μmol/l PTS or DMSO for 1 h; the effects of PTS on cell migration were determined using a transwell migration assay. Data represent the mean±SD of three independent experiments. *P<0.05. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Para-toluenesulfonamide triggers cell death by inducing lysosomal instability
To investigate the mechanisms of PTS-induced cell death, we used transmission electron microscopy analysis to observe the changes in ultrastructures of Tca-8113 cells. As shown in Fig. 3a, cells showed shrunk nuclei and an irregular nuclear membrane after 40 μmol/l PTS treatment. More secondary lysosomes with high electron density were observed after PTS treatment. Lysosomal instability was proposed to control the fate of cells either through activation of apoptosis or necrosis 18,19. We therefore performed a lysosomal integrity assay to assess the effects of PTS on lysosomal membrane integrity. Using the UMBG assay for β-galactosidase activity, PTS was shown to significantly increase free enzyme activity in a dose-dependent manner, which suggests that PTS induces lysosomal membrane permeabilization (LMP) and lysosomal damage (Fig. 3b). LMP leads to release intralysosomal proteases such as cathepsin B and D and chymotrypsin B, which are suggested to be essential downstream effectors of caspases 20. Western blot showed PTS treatment significantly increased cleaved cathepsin B expression (Fig. 3c). These results suggest that the anticancer ability of PTS might be attributed to inducing lysosomal instability and activating lysosome-mediated cell death.
Fig. 3.
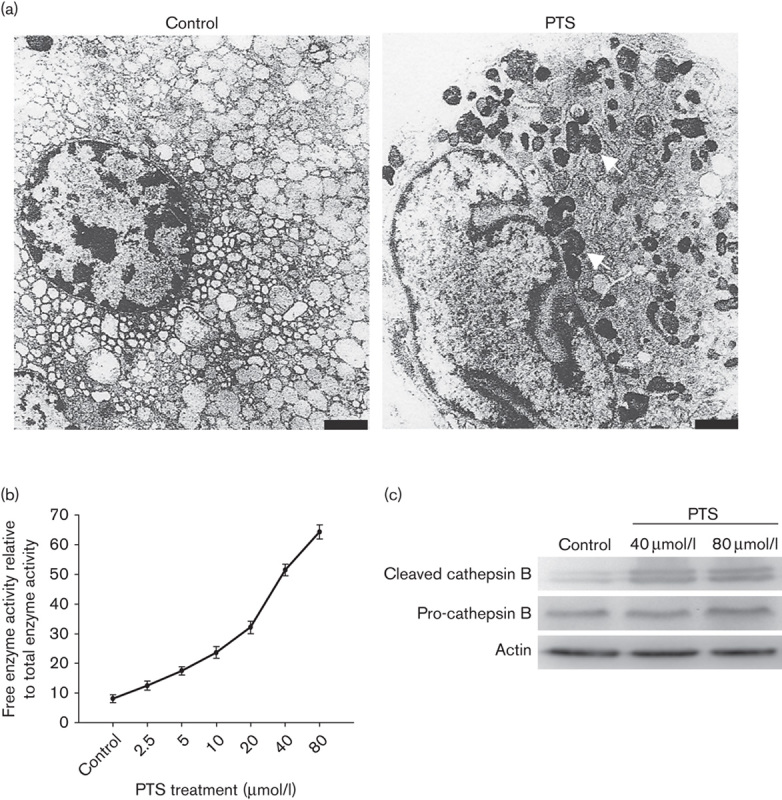
Para-toluenesulfonamide (PTS) induces lysosomal membrane permeabilization (LMP) and cathepsin B activation. (a) Tca-8113 cells were treated with 40 μmol/l PTS or dimethyl sulfoxide for 1 h; the ultrastructural changes were observed using a transmission electron microscope. The secondary lysosomes are shown (arrows) (bar=1 μm). (b) Plots of LMP after 40 μmol/l PTS for 1 h in Tca-8113 cells. (c) Western blot analysis of Tca-8113 cells after 40 μmol/l PTS treatment for 1 h. Cleaved, active cathepsin B and total cathepsin B are shown. β-Actin was used as a loading control. Data represent the mean±SD of three independent experiments. *P<0.05.
Para-toluenesulfonamide induces mitochondrial damage and inhibits ATP biosynthesis
Increased lysosomal permeability is reported to induce mitochondrial damage and the release of proapoptotic factors 6. We therefore investigated the cytosolic cytochrome c released from the mitochondria after PTS treatment. Western blot showed that 40 μmol/l PTS treatment significantly induced cytosolic cytochrome c expression in Tca-8113 cells (Fig. 4a). As the mitochondria are at the core of cellular energy metabolism and also the major organelles for ATP generation, we then asked whether PTS regulates mitochondrial ATP biosynthesis. As shown in Fig. 4b, PTS treatment significantly inhibited mitochondrial ATP biosynthesis in a dose-dependent manner. 40 μmol/l PTS treatment for 1 h attenuated ATP biosynthesis to 65.8%. These results suggest that PTS induces mitochondrial damage and exerts a metabolic arrest effect on cancer cells.
Fig. 4.
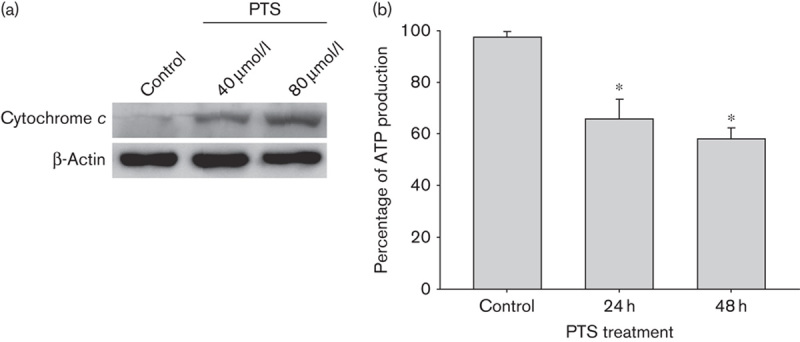
Para-toluenesulfonamide (PTS) induces the release of mitochondrial cytochrome c and inhibits ATP biosynthesis. (a) Western blot analysis of Tca-8113 cells after 40 μmol/l PTS treatment for 1 h. Cytosolic cytochrome c released from mitochondria is shown. β-Actin was used as a loading control. (b) Tca-8113 cells were treated with 40 μmol/l PTS or dimethyl sulfoxide for 24–48 h. The effects of PTS on ATP biosynthesis were assessed by measuring chemiluminescence. Data represent the mean±SD of three independent experiments. *P<0.05.
Discussion
In the present study, we tested the anticancer effects of a novel agent PTS on tongue cancer cells in vitro. PTS selectively accumulated in Tca-8113 cells, with fewer poisonous effects on normal fibroblasts. PTS inhibits tumor progression by simultaneously inducing apoptosis, and necrosis and suppressing invasive ability in Tca-8113 cells. Moreover, our results suggest that PTS triggers cell death through disturbing lysosomal stability and inducing mitochondrial dysfunction.
PTS is reported to exert anticancer effects in many types of cancer including hepatocarcinoma and non-small-cell lung cancer 1,2. Puncture injection of PTS combined with transcatheter arterial chemoembolization resulted in significant tumor control without severe complications in a patient with advanced hepatocellular carcinoma 21. Previous studies showed that PTS induced necrosis in lung cancer cells or in hepatocellular carcinoma cells in vivo 1,22. However, an in-vitro study of the mechanisms of PTS-induced cell death is still lacking. In this study, we showed that PTS treatment significantly suppressed the cell viability and invasive ability of Tca-8113 cells in vitro. Results from flow cytometric analysis and the LDH release assay showed that PTS induced apoptosis and necrosis simultaneously, which suggests that the anticancer effects of PTS can be attributed to the combined action of apoptosis and necrosis. In addition, PTS is reported to exert a selective killing effect on lung cancer cells compared with normal bronchial epithelium cells, or result in a smaller injury area to normal tissue than does ethanol in a xenograft mouse model 23. We also observed that PTS induced more significant cell death in Tca-8113 cells than that in normal gingival fibroblasts, and found that this might be partially attributed to different intracellular PTS accumulation between cancer cells and normal cells. However, the mechanisms of the selectively cytotoxic effects of PTS need further investigation.
Lysosomes and their enzymes promote cancer progression by breaking down the extracellular matrix, and stimulating angiogenesis and migration through releasing the lysosomal proteases into the extracellular space 20,24. However, disturbed lysosomal stability and increased LMP sensitize cells to the lysosome-mediated cell death pathway 25,26. The lysosomal proteases released regulate cell death either in a caspase-dependent or caspase-independent manner 20. Cytosolic cathepsin B released from lysosomes cleaves and activates proapoptotic Bcl-2 family member Bid and PARP-1 27–29. In this study, the formation of secondary lysosomes in cancer cells was observed after PTS treatment.
We also showed that PTS treatment induced LMP and the release of cathepsin B. Collectively, our findings suggest that the anticancer effects of PTS might be attributed to its ability to activate lysosome-mediated cell death. Considering that LMP can be partially induced in apoptosis, but massively in necrosis, blocking experiments using an apoptosis inhibitor (e.g. z-VAD-fmk) or a necrosis inhibitor (e.g. IM-54) are needed in the future.
A growing number of studies suggest that LMP could induce mitochondrial dysfunction, associated with the release of apoptogenic factors, such as cytochrome c, from the mitochondria, followed by caspase activation 7,20,30. Reactive oxygen species generated after mitochondrial damage feeds back to the lysosomes, leading to further lysosomal breakdown and exacerbation of apoptosis 31,32. Induction of mitochondrial membrane permeabilization and subsequent mitochondrial dysfunction are critical steps in lysosome-mediated cell death. The anticancer effects of hydroxychloroquine, a lysosomotropic amine with cytotoxic properties, were abolished after inhibition of the mitochondrial translocation of Bax 33. In this study, we identified that PTS treatment induced the release of mitochondrial cytochrome c and inhibited ATP biosynthesis. These findings suggest that PTS might induce mitochondrial damage and cell death by disturbing lysosomal stability and activating LMP.
Conclusion
Our present results suggest that PTS is capable of inducing Tca-8113 cells cell death by induction of lysosomal instability and further mitochondrial damage. These results may provide a new insight into the use of PTS in the treatment of tongue squamous cell carcinoma. However, the molecular mechanisms downstream of lysosomal instability mediating the anticancer effects of PTS need to be further identified.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81172578 and no. 81472532).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gao Y, Gao Y, Guan W, Huang L, Xu X, Zhang C, et al. Antitumor effect of para-toluenesulfonamide against lung cancer xenograft in a mouse model. J Thorac Dis 2013; 5:472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He J, Ying W, Yang H, Xu X, Shao W, Guan Y, et al. Gemcitabine plus cisplatin chemotherapy with concurrent para-toluenesulfonamide local injection therapy for peripherally advanced nonsmall cell lung cancer larger than 3 cm in the greatest dimension. Anticancer Drugs 2009; 20:838–844. [DOI] [PubMed] [Google Scholar]
- 3.Cesen MH, Pegan K, Spes A, Turk B. Lysosomal pathways to cell death and their therapeutic applications. Exp Cell Res 2012; 318:1245–1251. [DOI] [PubMed] [Google Scholar]
- 4.Neuzil J, Zhao M, Ostermann G, Sticha M, Gellert N, Weber C, et al. Alpha-tocopheryl succinate, an agent with in vivo anti-tumour activity, induces apoptosis by causing lysosomal instability. Biochem J 2002; 362 (Pt 3):709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W, Yuan X, Nordgren G, Dalen H, Dubowchik GM, Firestone RA, Brunk UT. Induction of cell death by the lysosomotropic detergent MSDH. FEBS Lett 2000; 470:35–39. [DOI] [PubMed] [Google Scholar]
- 6.Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene 2004; 23:2881–2890. [DOI] [PubMed] [Google Scholar]
- 7.Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest 2000; 106:1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberg K, Johansson U, Ollinger K. Lysosomal release of cathepsin D precedes relocation of cytochrome c and loss of mitochondrial transmembrane potential during apoptosis induced by oxidative stress. Free Radic Biol Med 1999; 27:1228–1237. [DOI] [PubMed] [Google Scholar]
- 9.Zhou JQ, Tang ZQ, Zhang JN, Tang JC. Metabolism and effect of para-toluene-sulfonamide on rat liver microsomal cytochrome P450 from in vivo and in vitro studies. Acta Pharmacol Sin 2006; 27:635–640. [DOI] [PubMed] [Google Scholar]
- 10.Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett 2006; 160:171–177. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Xia Y, Li B, Xu H, Wang C, Liu Y, et al. Induction of ER stress-mediated apoptosis by ceramide via disruption of ER Ca2+ homeostasis in human adenoid cystic carcinoma cells. Cell Biosci 2014; 4:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pertoft H. Fractionation of cells and subcellular particles with Percoll. J Biochem Biophys Methods 2000; 44:1–30. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Zhao L, Wei T, Zhao Y, Chen C. The inhibition of death receptor mediated apoptosis through lysosome stabilization following internalization of carboxyfullerene nanoparticles. Biomaterials 2011; 32:4030–4041. [DOI] [PubMed] [Google Scholar]
- 14.Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem 1990; 55:698–707. [DOI] [PubMed] [Google Scholar]
- 15.Loh KP, Qi J, Tan BK, Liu XH, Wei BG, Zhu YZ. Leonurine protects middle cerebral artery occluded rats through antioxidant effect and regulation of mitochondrial function. Stroke 2010; 41:2661–2668. [DOI] [PubMed] [Google Scholar]
- 16.Lu CC, Yang JS, Huang AC, Hsia TC, Chou ST, Kuo CL, et al. Chrysophanol induces necrosis through the production of ROS and alteration of ATP levels in J5 human liver cancer cells. Mol Nutr Food Res 2010; 54:967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kern JC, Kehrer JP. Acrolein-induced cell death: a caspase-influenced decision between apoptosis and oncosis/necrosis. Chem Biol Interact 2002; 139:79–95. [DOI] [PubMed] [Google Scholar]
- 18.Olejnicka BT, Andersson A, Tyrberg B, Dalen H, Brunk UT. Beta-cells, oxidative stress, lysosomal stability, and apoptotic/necrotic cell death. Antioxid Redox Signal 1999; 1:305–315. [DOI] [PubMed] [Google Scholar]
- 19.Kågedal K, Zhao M, Svensson I, Brunk UT. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem J 2001; 359 (Pt 2):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkegaard T, Jäättelä M. Lysosomal involvement in cell death and cancer. Biochim Biophys Acta 2009; 1793:746–754. [DOI] [PubMed] [Google Scholar]
- 21.He Q, Kuang AR, Guan YS, Liu YQ. Puncture injection of para-toluenesulfonamide combined with chemoembolization for advanced hepatocellular carcinoma. World J Gastroenterol 2012; 18:6861–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li MY, Meng H, Zhu WL, Zhou SZ, Li HY, Zhang JR. Dose-effect relationship of para-toluenesulfonamide for treatment of hepatocellular carcinoma in rats. Nan Fang Yi Ke Da Xue Xue Bao 2008; 28:249–251. [PubMed] [Google Scholar]
- 23.Wang T, Li Y, Liu M, Xu J, Zhong N. Anti-cancer effect of PTS in vitro. Practic J Cancer 2004; 19:1–4. [Google Scholar]
- 24.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer 2006; 6:764–775. [DOI] [PubMed] [Google Scholar]
- 25.Fehrenbacher N, Bastholm L, Kirkegaard-Sørensen T, Rafn B, Bøttzauw T, Nielsen C, et al. Sensitization to the lysosomal cell death pathway by oncogene-induced down-regulation of lysosome-associated membrane proteins 1 and 2. Cancer Res 2008; 68:6623–6633. [DOI] [PubMed] [Google Scholar]
- 26.Boya P, Andreau K, Poncet D, Zamzami N, Perfettini JL, Metivier D, et al. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med 2003; 197:1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Zhong C, Shi L, Guo Y, Fan Z. Granulysin induces cathepsin B release from lysosomes of target tumor cells to attack mitochondria through processing of bid leading to necroptosis. J Immunol 2009; 182:6993–7000. [DOI] [PubMed] [Google Scholar]
- 28.Conus S, Simon HU. Cathepsins: key modulators of cell death and inflammatory responses. Biochem Pharmacol 2008; 76:1374–1382. [DOI] [PubMed] [Google Scholar]
- 29.Gobeil S, Boucher CC, Nadeau D, Poirier GG. Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Cell Death Differ 2001; 8:588–594. [DOI] [PubMed] [Google Scholar]
- 30.Repnik U, Stoka V, Turk V, Turk B. Lysosomes and lysosomal cathepsins in cell death. Biochim Biophys Acta 2012; 1824:22–33. [DOI] [PubMed] [Google Scholar]
- 31.Zhao M, Antunes F, Eaton JW, Brunk UT. Lysosomal enzymes promote mitochondrial oxidant production, cytochrome c release and apoptosis. Eur J Biochem 2003; 270:3778–3786. [DOI] [PubMed] [Google Scholar]
- 32.Wei J, Liu M, Liu H, Wang H, Wang F, Zhang Y, et al. Oleanolic acid arrests cell cycle and induces apoptosis via ROS-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells. J Appl Toxicol 2013; 33:756–765. [DOI] [PubMed] [Google Scholar]
- 33.Boya P, Gonzalez-Polo RA, Poncet D, Andreau K, Vieira HL, Roumier T, et al. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 2003; 22:3927–3936. [DOI] [PubMed] [Google Scholar]


