Abstract
This study investigated the anticancer effects of N-farnesyloxy-norcantharimide (NOC15), a newly synthesized norcantharidin (NCTD) analogue, on human leukemic Jurkat T cells and the signaling pathway underlying its effects. We found that the half maximal inhibitory concentration (IC50) of NOC15 on Jurkat T cells is 1.4 μmol/l, which is 11.14-fold (=15.6÷1.4) smaller than the 15.6 μmol/l of NCTD on Jurkat T cells, whereas the IC50 of NOC15 on human normal lymphoblast (HNL) is 207.9 μmol/l, which is 8.17-fold (=1698.0÷207.8) smaller than the 1698.0 μmol/l of NCTD on HNL cells. These results indicated that NOC15 exerts a higher anticancer effect on Jurkat T cells and has higher toxicity toward HNL cells than NCTD. Thus, NOC15 is 1.36-fold (=11.14÷8.17) beneficial as an anticancer agent toward Jurkat T cells compared with NCTD. Moreover, NOC15 can increase the percentage of cells in the sub-G1 phase and reduce the cell viability of Jurkat T cells, stimulate p38 and extracellular signal-regulated protein kinase 1/2 (ERK1/2) of mitogen-activated protein kinases (MAPKs) signaling pathway, and inhibit calcineurin expression and interleukin-2 (IL-2) production. However, NOC15 exerted no effects on the Jun-N-terminal kinase 1/2 (JNK1/2) signaling pathway, the production of IL-8, and tumor necrosis factor-α. We conclude that the anticancer activity of the newly synthesized NOC15 is 1.36-fold beneficial than NCTD as an anticancer agent and that NOC15 can increase the percentage of cells in the sub-G1 phase through the stimulation of p38 and ERK1/2 of the MAPK signaling pathway and the inhibition of calcineurin expression and IL-2 production. The NOC15 may have the potential of being developed into an anticancer agent in the future.
Keywords: calcineurin, interleukin-2, Jurkat T cell, mitogen-activated protein kinases, mononuclear cell, norcantharidin, norcantharimide
Introduction
Mylabris, a species of blister beetle (Mylabris phalerata Pall.), has been used in traditional Chinese medicine for over 2000 years for the treatment of malignant tumors such as hepatoma, breast cancer, colorectal cancer, and abdominal malignancy 1–4. Cantharidin (exo-2,3-dimethyl-7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid anhydride), which is one of the active compounds obtained from Mylabris, is a potent serine/threonine protein phosphatase 1, protein phosphates 2A, and protein phosphatase 2B [or calcineurin (CaN)] inhibitor 5–7. Cantharidin has anticancer properties both in vitro and in vivo 8,9, but the clinical applications of cantharidin are restricted by its side-effects in the urinary system and nephrotoxicity 10,11.
Norcantharidin (NCTD, exo-7-oxabicylo[2.2.1]heptane-2,3-dicarboxylic anhydride), a demethylated analog of cantharidin, is currently being used as an anticancer drug in China 12. This water-soluble synthetic small molecule has been shown to be an effective anticancer agent against certain cancers, including hepatoma 13, gallbladder carcinoma 14, leukemia 15, and colorectal carcinoma 16. It can also cause decreased tumor growth and prolonged survival in animal models 17. NCTD was designed to reduce the intrinsic toxicity and to exert anticancer activity with potency similar to that of cantharidin 18,19. It was found that NCTD has a lower toxicity toward normal cells compared with the cancer cells 12,19,20 and has fewer side effects and nephrotoxicity than cantharidin in the clinical settings 1.
The Jurkat T cell line is an eternalized T cell line that was established from the peripheral blood of a 14-year-old boy with acute T-cell leukemia in the late 1970s 21. As phorbol 12-myristate 13-acetate (PMA) plus ionomycin (ION) can activate Jurkat T cells to produce large amounts of interleukin-2 (IL-2), the PMA plus ION is useful in the screening of the anticancer capability and mechanism of drugs 22–25. The PMA plus ION is a T-cell activator, and the PMA, which is a protein kinase C (PKC) activator, is reported to protect T cells 22,23. ION can increase the calcium influx, which results in cytokine production. Treatment with PMA plus ION in Jurkat T cells can increase the PKC-Ras signaling pathway, resulting in cell activations 24,25.
N-Farnesyloxy-norcantharimide (NOC15, C23H33NO4), which is a newly synthesized NCTD derivative with the N-farnesyloxy group, has high anticancer activity toward hepatocellular carcinoma, bladder carcinoma, colorectal adenocarcinoma, and acute promyelocytic leukemia 26. NOC15 also has anticancer efficacy in a syngeneic mouse leukemia model by increasing the survival of mice and decreasing the tumor weight 27. However, the anticancer mechanism of NOC15 is not clear. Therefore, this study was designed to examine the effect of NOC15 on cell viability, cell cycle, mitogen-activated protein kinases (MAPKs), and CaN expression, and the production of IL-2, IL-8, and tumor necrosis factor-α (TNF-α) using Jurkat T cells.
Materials and methods
Cells and cell culture
Human normal lymphoblast (HNL, BCRC number: 08C0058) and human acute T-cell leukemia Jurkat T cells (Clone E6-1, BCRC number: 60424) were purchased from the Bioresource Collection and Research Center (BCRC), Taiwan. The HNL and Jurkat T cells were cultured in RPMI 1640 medium (Promocell, Heidelberg, Germany), supplemented with 10% certified fetal bovine serum (Biological Industries, Kibbutz Beit Haemek, Israel), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco, Grand Island, New York, USA), and 0.1% mycoplasma removal agent (AbD Serotec, Kidlington, Oxfordshire, UK) at 37°C in a humidified 5% CO2 incubator.
Chemical reagents
The PMA, ION, SB203580, PD98059, SP600125, anti-CaN antibody, anti-phospho-extracellular signal-regulated protein kinase 1/2 (anti-p-ERK1/2) antibody, and other chemicals used in this study were purchased from Sigma (St Louis, Missouri, USA). The anti-p38, anti-Jun-N-terminal kinase 1/2 (anti-JNK1/2), anti-ERK1/2, and anti-p-JNK1/2 antibodies were purchased from Upstate (Lake Placid, New York, USA). The anti-p-p38 antibody was purchased from Chemicon (Ramona, California, USA). The anti-β-actin and anti-rabbit IgG antibodies were purchased from Millipore (Billerica, Massachusetts, USA). The anti-mouse IgG antibody was purchased from Genetex (Irvine, California, USA).
Chemical synthesis of NOC15
The NOC15 was synthesized by Dr Jin-Yi Wu in a previous report. Briefly, the key starting material in the study was the readily synthesizable 5,6-dehydronorcantharidin, which was prepared on a large scale through exo-selective cycloaddition by the Diels–Alder reaction, of the relatively cheap furan and maleic anhydride. Subsequent hydrogenation of 5,6-dehydronorcantharidin using 10% Pd/C as catalyst using a modified procedure of Hill et al. 28 provided the starting NCTD in an excellent yield. Then, the NCTD was made to react with hydroxylamine hydrochloride in the presence of sodium methoxide in dry methanol at room temperature to produce N-hydroxynorcantharimide, which was then reacted with farnesyl bromide in dry acetone in the presence of K2CO3, and the reaction mixture was refluxed for 8–10 h to obtain NOC15 in moderate yields. NOC15 was obtained as a colorless liquid; the details of the 1H and 13C NMR spectra have been described in a previous report. The molecular weight for C23H33NO4 calculated using LC-MS (ESI+, m/z) is 387.24, found for 410.21 [M+Na]+ 26,27.
Cell viability assay
The cell viability assays were performed in 96-well plates (SPL Life Sciences, Pocheon, Korea). A volume of 100 μl of cell suspensions (HNL or Jurkat T cells, 5×103 cells/well, serum-free medium) was inoculated in the wells and the plates were preincubated in the incubator for 24 h (preincubated for 22 h and stimulated with PMA 5 ng/ml plus ION 250 ng/ml for 2 h in PMA plus ION-treated groups). Then, various concentrations of NCTD (0, 2, 4, 15, 30, and 60 μmol/l) or NOC15 (0, 0.25, 0.5, 1, 2, and 4 μmol/l) were added to the culture media in the wells. After 24 h of incubation, the cell viability of HNL and Jurkat T cells was measured by cell counting kit-8 (CCK-8; Sigma). CCK-8 utilizes the highly water-soluble tetrazolium salt (WST-8) [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] colorimetric method. The optical density of each well was measured at 450 nm using a spectrophotometer. The detection sensitivity of CCK-8 is higher than that of other tetrazolium salts such as MTT.
The half maximal inhibitory concentration (IC50) indicates how much drug is needed to inhibit cell proliferation by half. The IC50 of NCTD and NOC15 on Jurkat T cells and HNL cells were calculated using the Probit regression analysis program of the SPSS 13.0 software (SPSS Inc., Chicago, Illinois, USA).
Net effect of anticancer agent
To facilitate the comparison of the net effects of anti-cancer activity on cancer cells and the toxic effects on normal cells between two anticancer agents, we define the anticancer activity ratio of agent X over agent Y toward cancer cells and the toxicity ratio of agent X over agent Y toward normal cells as the ratio of the reciprocals of the IC50 of agents X and Y toward cancer cells and normal cells, respectively.
The net effect of agent X over agent Y was then defined as follows:
Cell cycle analysis
The Jurkat T cells were collected and stained with propidium iodide to determine the DNA contents using a flow cytometer. The cell suspensions were preincubated in six-well plates for 24 h, stimulated with PMA 5 ng/ml plus ION 250 ng/ml for 2 h, and treated with NOC15 at IC50 for 24 or 48 h. After incubation, the cells were collected and washed with PBS. Then, the cells were fixed with ice-cold 70% ethanol for 1 h and incubated with 0.1% Triton X-100, 0.2 mg/ml RNaseA, and 20 μg/ml propidium iodide for 30 min at 4°C in the dark. Data acquisition and cell cycle analysis were carried out using a FACScan flow cytometer with CellQuest software (Becton Dickinson, Lincoln Park, New Jersey, USA). The samples were analyzed within 1 h of propidium iodide stain.
Protein extraction
To induce CaN and phosphorylated mitogen-activated protein kinase (p-MAPKs) production, the Jurkat T cells were preincubated for 22 h and stimulated with PMA plus ION for 2 h. After treatment with PMA plus ION, 0–4 μmol/l NOC15, and other chemical reagents, the Jurkat T cells were collected and washed with PBS. Then, the cells were resuspended in 100 μl lysis buffer, which was composed of RIPA lysis buffer (Millipore), protease inhibitor cocktail tablets (Roche Diagnostics, Indianapolis, Indiana, USA), phosphatase inhibitor cocktail tablets (Roche Diagnostics), and 1 mmol/l sodium orthovanadate, and homogenized at 4°C for 30 min. Finally, the cell suspensions were centrifuged and the supernatants were collected. The supernatants were stored at −80°C until use. The concentrations of proteins were determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, California, USA).
Western blot
Equal amounts of proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinylidene fluoride membrane (Millipore). The membrane was blocked with 5% non fat milk in TBST buffer (with 0.1% Tween-20; T-Pro Biotechnology, Taipei, Taiwan) and then incubated with primary antibodies against CaN, MAPKs (p38, ERK1/2, and JNK1/2), p-MAPKs (p-p38, p-ERK1/2, and p-JNK1/2), and β-actin (internal control) in TBST buffer. Subsequently, the membrane was washed with TBST buffer and incubated with secondary antibodies (anti-mouse or anti-rabbit IgG). After incubation with secondary antibodies, the polyvinylidene fluoride membrane was washed with TBST buffer. Determination of target proteins and their density was performed using enhanced chemiluminescence kits (Millipore) and the UVP biospectrum 600 imaging system (UVP, Upland, California, USA).
CaN cellular activity assay
The CaN cellular activity assay kit (Millipore) was used to evaluate the CaN activity. The Jurkat T cells were preincubated for 22 h and stimulated with PMA plus ION for 2 h. Then, various concentrations of NOC15 (0, 0.25, 0.5, 1, 2, and 4 μmol/l) were added to the culture media. After incubation for 24 h, the cells were collected to prepare a cell extract for the CaN activity assay.
Cytokine induction and measurement
To induce cytokine production, the Jurkat T cells were preincubated for 22 h and stimulated with PMA plus ION for 2 h. Subsequently, the activated cells were cultured with NOC15 at IC50 in the presence and absence of SB203580, PD98059, and SP600125 at 2.5, 10, and 10 μmol/l, respectively. After incubation for 24 h, the culture medium was centrifuged and collected for cytokine measurement. The concentrations of IL-2, IL-8, and TNF-α were measured using ELISA kits (eBioscience, San Diego, California, USA).
Statistical analysis
The experimental data were described as means±SD. Group comparison was performed using repeated-measures analysis of variance on ranks, followed by the Student–Newman–Keuls post-hoc test. P less than 0.05 was considered significantly different. All statistical analyses were carried out using SigmaStat software (SPSS Inc.).
Results
Effects of NCTD and NOC15 on cell viability
To determine the effects of NCTD and NOC15 on the cell viability of Jurkat T cells with/without PMA plus ION, the Jurkat T cells were treated with NCTD (0, 2, 4, 15, 30, and 60 μmol/l) or NOC15 (0, 0.25, 0.5, 1, 2, and 4 μmol/l) for 24 h, respectively. The cell viability was assessed using the CCK-8 test. Figure 1 shows that both NCTD and NOC15 significantly inhibited the growth of Jurkat T cells in a dose-dependent manner. Moreover, the pretreatment with PMA plus ION can increase the viability of Jurkat T cells. The IC50 values of NCTD and NOC15 on Jurkat T cells without PMA plus ION pretreatment were estimated to be 15.6 and 1.4 μmol/l, respectively. Thus, the anticancer effect of NOC15 on Jurkat T cells is 11.14-fold (=15.6÷1.4) more potent than NCTD in terms of cell viability.
Fig. 1.
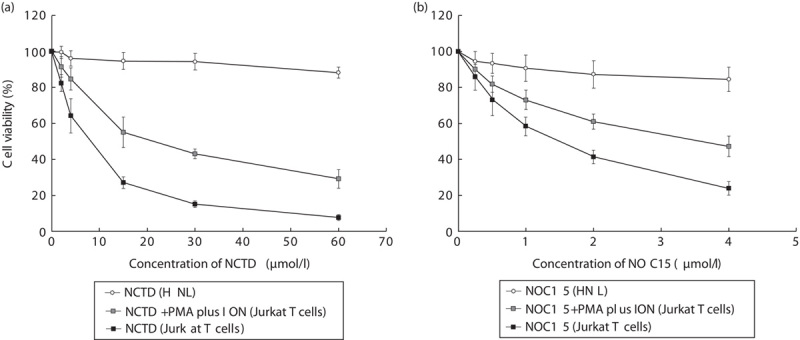
Effects of (a) NCTD and (b) NOC15 with/without PMA plus ION on the cell viability of HNL and Jurkat T cells as assessed using the CCK-8 test. The cells were preincubated for 22 h and stimulated with PMA plus ION for 2 h, and then NCTD (0, 2, 4, 15, 30, and 60 μmol/l) or NOC15 (0. 0.25, 0.5, 1, 2, and 4 μmol/l) were added to the culture media and incubated for 24 h. Cell viability was calculated using the CCK-8 test. The results are expressed as means±SD for six independent experiments. *P<0.05 versus NCTD+PMA plus ION (Jurkat T cell). NCTD and NOC15 significantly inhibited the growth of Jurkat T cells in a dose-dependent manner, and the pretreatment with PMA plus ION can increase the cell viability. The IC50 value of NCTD and NOC15 on Jurkat T cells without PMA plus ION pretreatment was estimated to be 15.6 and 1.4 μmol/l, respectively, and the IC50 of NCTD and NOC15 on HNL was estimated to be 1698.0 and 207.9 μmol/l, respectively. CCK-8, cell counting kit-8; HNL, human normal lymphoblast; IC50, half maximal inhibitory concentration; ION, ionomycin; NCTD, norcantharidin; NOC15, N-farnesyloxy-norcantharimide; PMA, phorbol 12-myristate 13-acetate.
The viability of HNL exposed to NCTD and NOC15 was also assessed using the CCK-8 test (Fig. 1). Both NCTD and NOC15 inhibited the growth of HNL slightly. The IC50 values of NCTD and NOC15 on HNL cells were estimated to be 1698.0 and 207.9 μmol/l, respectively. The toxic effect of NOC15 on HNL cells is 8.17-fold (=1698.0÷207.9) more potent than NCTD in terms of cell viability.
Taking together the anticancer effect on Jurkat T cells and the toxic effect on HNL cells, the NOC15 still exerts 1.36-fold (=11.14÷8.17) more beneficial effects than NCTD as an anticancer agent toward Jurkat T cells.
Effect of NOC15 on cell cycle
To examine the cell cycle variation of NOC15, the DNA histogram was determined with propidium iodide staining using flow cytometry. As shown in Fig. 2, NOC15 increased the percentage of cells in the sub-G1 phase and the G2/M phase, but decreased the percentage of cells in the S phase. This result indicates that NOC15 can inhibit cell growth by affecting the cell cycle.
Fig. 2.
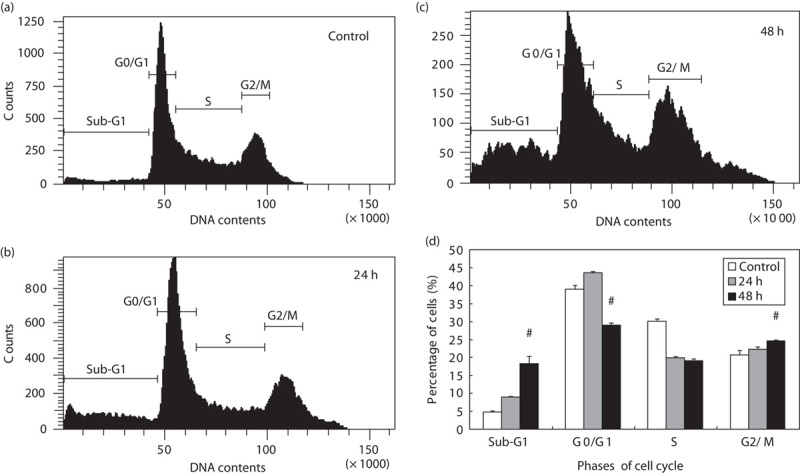
Cell cycle variation of NOC15 on human Jurkat T cell. (a) Control; (b) NOC15 (24 h); (c) NOC15 (48 h); (d) percent of cells in each cell cycle phase. The cells were preincubated for 22 h and stimulated with PMA plus ION for 2 h, and then treated with NOC15 (IC50) for 24 or 48 h. The cells were collected, fixed, and stained with propidium iodide to determine the DNA contents using a flow cytometer. The results are expressed as means±SD for three independent experiments. *P<0.05 versus untreated control. #P<0.05 versus NOC15 (24 h). NOC15 can increase the percentage of cells in the sub-G1 phase and the G2/M phase, but decrease the percentage of cells in the S phase. IC50, half maximal inhibitory concentration; ION, ionomycin; NOC15, N-farnesyloxy-norcantharimide; PMA, phorbol 12-myristate 13-acetate.
MAPKs’ expression and its phosphorylation in NOC15-treated Jurkat T cells
Western blot was used to detect the expression of MAPKs and p-MAPKs in Jurkat T cells. As shown in Fig. 3a, the expressions of p-p38 and p-ERK1/2 were markedly increased in a dose-dependent manner by treatment with 0.5–4 μmol/l NOC15. Figure 3b shows that the expressions of p38, ERK1/2, and JNK1/2 were not significantly changed by NOC15 treatment, and that the expressions of p-p38 and p-ERK1/2 were significantly increased comparing with the untreated control. However, the p-JNK1/2 expression was not altered by NOC15 treatment (Fig. 3b).
Fig. 3.
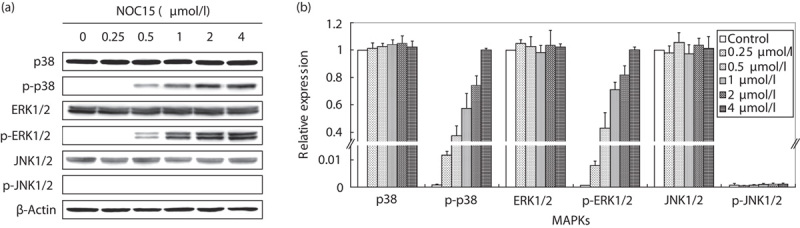
Expression of MAPKs and p-MAPKs in NOC15-treated Jurkat T cells. (a) Western blot. (b) Relative expression. The cells were preincubated for 22 h and then stimulated with PMA plus ION for 2 h. After the cells were treated by NOC15 (0. 0.25, 0.5, 1, 2, and 4 μmol/l) for 24 h, the cells were collected, lysed, and the proteins were extracted for western blot analysis. The β-actin was used as the internal control. The results are expressed as means±SD for three independent experiments. *P<0.05 versus untreated control. The expressions of p-p38 and p-ERK1/2 were significantly increased in a dose-dependent manner. ERK1/2, phospho-extracellular signal-regulated protein kinase 1/2; ION, ionomycin; MAPK, mitogen-activated protein kinase; NOC15, N-farnesyloxy-norcantharimide; PMA, phorbol 12-myristate 13-acetate.
Effects of MAPKs inhibitors on the viability of NOC15-treated Jurkat T cells
Figure 1 indicates that NOC15 effectively decreased the cell viability in Jurkat T cells. The expressions of p-p38 and p-ERK1/2 were significantly increased by NOC15 treatment (Fig. 3). Figure 4 further shows that the reduction in cell viability because of NOC15 could be inhibited by p38 inhibitor (SB203580) and ERK1/2 inhibitor (PD98059), but not by JNK1/2 inhibitor (SP600125).
Fig. 4.
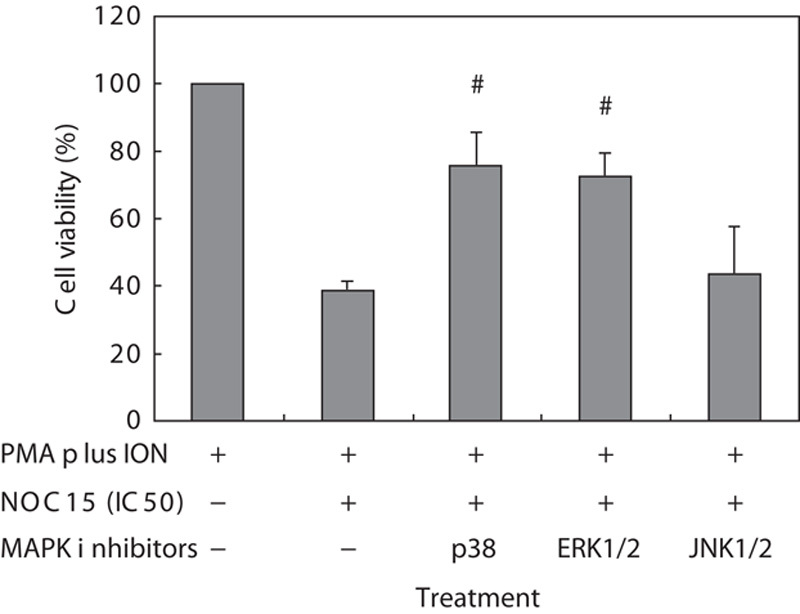
Effects of MAPK inhibitors on cell viability in NOC15-treated Jurkat T cells. The cells were preincubated for 22 h and then stimulated with PMA plus ION for 2 h. After the cells were incubated with NOC15 (IC50) or without NOC15 at the presence or absence of SB203580 (p38 inhibitor), PD98059 (ERK1/2 inhibitor), and SP600125 (JNK1/2 inhibitor) for 24 h, the cells were collected and the viability was calculated using the CCK-8 test. The results are expressed as means±SD for six independent experiments. *P<0.05 was considered significantly different from PMA plus ION control. #P<0.05 versus PMA plus ION+NOC15 (IC50). The p38 and ERK1/2 inhibitor, but not the JNK1/2 inhibitor, can increase the cell viability of NOC15-treated cells. CCK-8, cell counting kit-8; IC50, half maximal inhibitory concentration; ION, ionomycin; MAPK, mitogen-activated protein kinase; NOC15, N-farnesyloxy-norcantharimide; PMA, phorbol 12-myristate 13-acetate.
Effect of NOC15 on CaN expression
Western blot and CaN cellular activity assay kit were used to detect CaN expression in NOC15-treated Jurkat T cells. Figure 5a shows that NOC15 markedly decreased CaN expression in a dose-dependent manner, especially when the concentration of NOC15 was 4 μmol/l. In Fig. 5b, the CaN expression (by western blot) was slightly decreased when the concentration of NOC15 was in the range of 0–1 μmol/l, but was significantly decreased when the concentration of NOC15 was 2–4 μmol/l. The expression of CaN in Jurkat T cells was reduced by about 75% after treatment with 4 μmol/l NOC15 (Fig. 5b). Figure 5c shows the same result on the CaN activity assay. CaN activity was significantly decreased in a dose-dependent manner.
Fig. 5.
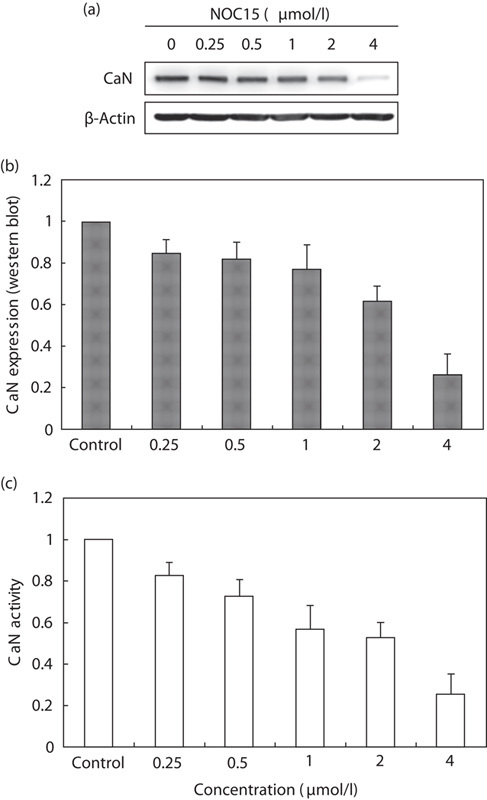
Expression of CaN in NOC15-treated Jurkat T cells. (a) Western blot; (b) CaN expression (western blot); and (c) CaN activity. The cells were preincubated for 22 h and then stimulated with PMA plus ION for 2 h. After the cells were treated by NOC15 (0. 0.25, 0.5, 1, 2, and 4 μmol/l) for 24 h, the cells were collected, lysed, and the proteins were extracted for western blot analysis and the CaN cellular activity assay. The β-actin was used as the internal control. The results are expressed as means±SD for three independent experiments. *P<0.05 versus untreated control. The expression of CaN was significantly decreased by NOC15 treatment. CaN, calcineurin; ION, ionomycin; NOC15, N-farnesyloxy-norcantharimide; PMA, phorbol 12-myristate 13-acetate.
Effect of NOC15 on cytokine production
As shown in Fig. 6, IL-2 and IL-8 production was markedly increased by PMA plus ION stimulation in Jurkat T cells. Conversely, the NOC15 could inhibit the IL-2 production, but exerted no effect on IL-8 and TNF-α production in PMA plus ION-treated Jurkat T cells. To further show the effect of MAPKs on IL-2 production, three inhibitors (SB203580, PD98059, and SP600125) were added to the cell culture media. Figure 7 shows that p38 inhibitor (SB203580) and ERK1/2 inhibitor (PD98059) could effectively increase the IL-2 production in NOC15 and PMA plus ION-treated cells. This experiment indicates that NOC15 can inhibit IL-2 production by the regulation of p38 and ERK1/2.
Fig. 6.
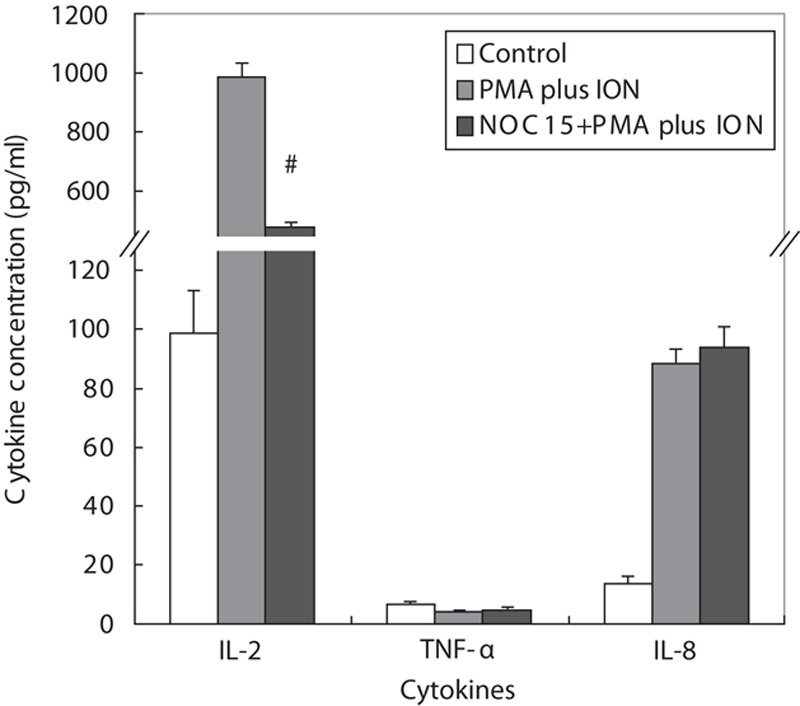
Production of cytokines in NOC15-treated Jurkat T cells. The cells were preincubated for 22 h and then stimulated with PMA plus ION for 2 h. After the cells were treated by NOC15 (IC50) for 24 h, the supernatants were transferred to new tubes for cytokine measurement by ELISA. The results are expressed as means±SD for six independent experiments. *P<0.05 was considered significantly different from the control. #P<0.05 versus PMA plus ION control. NOC15 could inhibit the IL-2 production, but exerted no effect on IL-8 and TNF-α production in PMA plus ION-treated Jurkat T cells. IC50, half maximal inhibitory concentration; IL-2, interleukin-2; ION, ionomycin; NOC15, N-farnesyloxy-norcantharimide; PMA, phorbol 12-myristate 13-acetate; TNF-α, tumor necrosis factor-α.
Fig. 7.
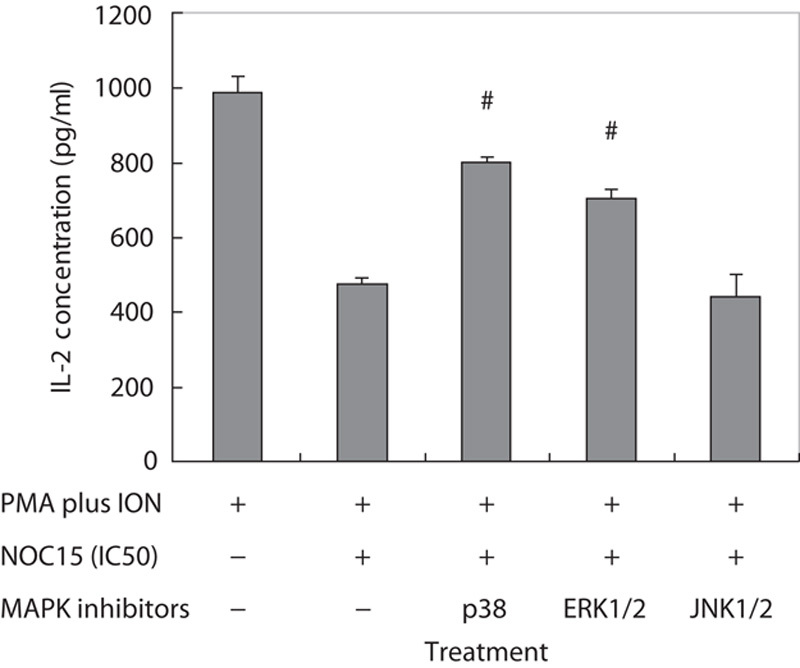
Effects of MAPK inhibitors on the IL-2 concentration in NOC15-treated Jurkat T cells. The cells were preincubated for 22 h and then stimulated with PMA plus ION for 2 h. After the cells were incubated with NOC15 (IC50) or without NOC15 and at the presence or absence of SB203580 (p38 inhibitor), PD98059 (ERK1/2 inhibitor), and SP600125 (JNK1/2 inhibitor) for 24 h, the supernatants were transferred to new tubes for cytokine measurement by ELISA. The results are expressed as means±SD for six independent experiments. *P<0.05 versus PMA plus ION control. #P<0.05 versus PMA plus ION+NOC15 (IC50). The SB and PD, but not SP, can increase the IL-2 concentration in NOC15-treated cells. ERK1/2, extracellular signal-regulated protein kinase 1/2; IC50, half maximal inhibitory concentration; IL-2, interleukin-2; ION, ionomycin; JNK1/2, c-Jun-N-terminal kinase; MAPK, mitogen-activated protein kinase; NOC15, N-farnesyloxy-norcantharimide; PMA, phorbol 12-myristate 13-acetate.
Discussion
NOC15, a newly synthesized derivative of NCTD, has stronger anticancer activity than NCTD on cell proliferation in Jurkat T cells. Moreover, the NOC15 is similar to NCTD in the molecular mechanism of anti-cancer effects. NOC15 can reduce the viability of the Jurkat T cell line, increase the percentage of cells in the sub-G1 phase, and inhibit CaN expression and IL-2 production. NOC15 can also activate p38 and ERK1/2 to become p-p38 and p-ERK1/2. As previous investigations have proved the anticancer effects of NCTD and its derivatives for several carcinomas 2–4,14,15,26,29–33, it is possible that NOC15 may be developed as a new drug for the treatment of cancer in the future.
In this study, the IC50 of NCTD on Jurkat T cells was estimated to be 15.6 μmol/l and the IC50 of NOC15 on the same cells was estimated to be 1.4 μmol/l (Fig. 1). Therefore, the NOC15 is 11.14-fold (=15.6÷1.4) more potent than NCTD in terms of anticancer activity toward Jurkat T cells. However, both NCTD and NOC15 can slightly inhibit the growth of HNL (Fig. 1). The IC50 of NCTD and NOC15 on HNL were estimated to be 1698.0 and 207.9 μmol/l, respectively. Thus, the NOC15 is 8.17-fold (=1698.0÷207.9) more toxic than NCTD toward HNL cells. These results suggest that NOC15 may have higher anticancer capability toward Jurkat T cells and higher toxicity toward HNL cells than NCTD, with an overall 1.36-fold (=11.14÷8.17) beneficial effect than NCTD as an anticancer agent toward Jurkat T cells. Hence, NOC15 might be developed into a new compound with a better therapeutic potential than NCTD for leukemia and other cancers.
In this study, we showed that NOC15 could inhibit the viability of Jurkat T cells by increasing the percentage of cells in the sub-G1 phase (Fig. 2). This result is similar to the findings of Liao et al. 34. We speculate that NOC15 may have a pharmacological mechanism similar to that of NCTD. To confirm the speculation, we further investigated the effect of NOC15 on MAPKs and CaN expression and the production of IL-2, TNF-α, and IL-8. MAPKs are proline-directed serine/threonine kinases, which respond to chemical and physical stress by connecting cell-surface receptor responses to the activity of regulatory proteins 35. MAPKs are composed of three major members including ERK, JNK, and p38. ERK is mostly activated by growth factor signals 36, and promotes cell growth, differentiation, and proliferation 37. JNK and p38 are activated by considerable stress-related stimulation, and mediate apoptotic signals 37,38. By regulating innate immunity and activating adaptive immunity 39,40, the MAPK signaling pathway plays an important role in the regulation of the growth and survival of cancer cells 41. Our study showed that the expressions of p-p38 and p-ERK1/2 were increased by NOC15 treatment (Fig. 3a and b). We also found that NOC15 could inhibit IL-2 production, but had no effect on IL-8 and TNF-α production in PMA plus ION-treated Jurkat T cells. Further studies using the p38 inhibitor (SB203580) and the ERK1/2 inhibitor (PD98059) showed that both p38 and ERK1/2 inhibitors could increase the cell viability of NOC15-treated Jurkat T cells (Fig. 4). As the p-MAPKs and the subsequent activation of transcription factors can lead to the genes’ expression for cell viability reduction 42, it is possible that p-p38 and p-ERK1/2 are the major proteins of the MAPK signaling pathway for cell viability reduction after NOC15 treatment, and the inhibition of IL-2 production through activation of p38 and ERK1/2, similar to that for NCTD.
PMA plus ION is a T-cell activator. PMA, a PKC activator, is reported to protect T cells from cell death 22,23. ION can increase the calcium influx, and result in CaN activation and cytokine production. Treatment with PMA plus ION in leukemia Jurkat T cells can induce calcium influx and increase the PKC-Ras signaling pathway, resulting in cell activations 24,25. CaN, a calcium-dependent serine/threonine phosphatase, can be activated by a change in the intracellular calcium concentration 43. CaN causes dephosphorylation of the nuclear factor of activated T cell-cytoplasm (NFAT-c) when the T-cell receptor-mediated pathway is activated. NFAT-c, which is a transcription factor, can be translocated into nucleus to upregulate gene expression and induce cytokine production (e.g. IL-2, IL-6, IL-8, MCP-1, and TGF-b1) 44. Our study showed that the expression of CaN could be decreased by NOC15 treatment (Fig. 5). Because the activation of CaN can cause IL-2 and IL-8 production by activating NFAT-c, the production of IL-2 and IL-8 can also be increased by PMA plus ION pretreatment. However, only IL-2 was found to be decreased by NOC15 treatment (Fig. 6). These results suggest that the inhibitory effect of NOC15 may be through the regulation of CaN and IL-2, but not IL-8. A similar result was found in the studies of Iacobelli et al. 45 and Liao et al. 34, which showed that IL-2 is required for cell cycle progression and apoptosis inhibition in primary T cells. To further investigate the interrelation of IL-2 and the MAPK signaling pathway, we used MAPK inhibitors to prove the effect of p-MAPKs expression in IL-2 production. Figure 7 shows that p38 and the ERK1/2 inhibitor could increase the IL-2 production in NOC15-treated cells. These results imply that the p-p38 and p-ERK signaling pathways may inhibit CaN or IL-2 expression, resulting in cell growth inhibition by NOC15 treatment. In addition, Liu et al. 46 reported that NCTD can stimulate TNF-α production. However, the TNF-α exerted no effect after NOC15 treatment in this study. This result indicates that TNF-α may not be the main cytokine that mediates the inhibitory effect of NOC15.
In conclusion, NOC15 reduces cell viability, increases the percentage of cells in the sub-G1 phase, stimulates p38 and ERK1/2 signaling pathways, and inhibits CaN expression and IL-2 production in the human acute leukemia Jurkat T cell line. The new compound NOC15 has stronger anticancer activity than that of NCTD and low cytotoxicity against HNL. NOC15 might be developed as a new drug to treat human leukemia and other cancers.
Acknowledgements
This study was supports by grants V101E5-004, V102E5-001, and V103E5-001 of the Taipei Veterans General Hospital, Taipei, Taiwan.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Jin-Yi Wu and Ming-Che Chang contributed equally to the writing of this article.
References
- 1.Wang GS. Medical uses of mylabris in ancient China and recent studies. J Ethnopharmacol 1989; 26:147–162. [DOI] [PubMed] [Google Scholar]
- 2.Chen YN, Chen JC, Yin SC, Wang GS, Tsauer W, Hsu SF, Hsu SL. Effector mechanisms of norcantharidin-induced mitotic arrest and apoptosis in human hepatoma cells. Int J Cancer 2002; 100:158–165. [DOI] [PubMed] [Google Scholar]
- 3.Chen YJ, Shieh CJ, Tsai TH, Kuo CD, Ho LT, Liu TY, Liao HF. Inhibitory effect of norcantharidin, a derivative compound from blister beetles, on tumor invasion and metastasis in CT26 colorectal adenocarcinoma cells. Anticancer Drugs 2005; 16:293–299. [DOI] [PubMed] [Google Scholar]
- 4.Chen YJ, Chang WM, Liu YW, Lee CY, Jang YH, Kuo CD, Liao HF. A small-molecule metastasis inhibitor, norcantharidin, downregulates matrix metalloproteinase-9 expression by inhibiting Sp1 transcriptional activity in colorectal cancer cells. Chem Biol Interact 2009; 181:440–446. [DOI] [PubMed] [Google Scholar]
- 5.Honkanen RE. Cantharidin, another natural toxin that inhibits the activity of serine/threonine protein phosphatases types 1 and 2A. FEBS Lett 1993; 330:283–286. [DOI] [PubMed] [Google Scholar]
- 6.McCluskey A, Walkom C, Bowyer MC, Ackland SP, Gardiner E, Sakoff JA. Cantharimides: a new class of modified cantharidin analogues inhibiting protein phosphatases 1 and 2A. Bioorg Med Chem Lett 2001; 11:2941–2946. [DOI] [PubMed] [Google Scholar]
- 7.Baba Y, Hirukawa N, Sodeoka M. Optically active cantharidin analogues possessing selective inhibitory activity on Ser/Thr protein phosphatase 2B (calcineurin): implications for the binding mode. Bioorg Med Chem 2005; 13:5164–5170. [DOI] [PubMed] [Google Scholar]
- 8.Chen RT, Hua Z, Yang JL, Han JX, Zhang SY, Lü FL, Xü B. Studies on antitumor actions of cantharidin. Chin Med J (Engl) 1980; 93:183–187. [PubMed] [Google Scholar]
- 9.McCluskey A, Ackland SP, Bowyer MC, Baldwin ML, Garner J, Walkom CC, Sakoff JA. Cantharidin analogues: synthesis and evaluation of growth inhibition in a panel of selected tumour cell lines. Bioorg Chem 2003; 31:68–79. [DOI] [PubMed] [Google Scholar]
- 10.Tagwireyi D, Ball DE, Loga PJ, Moyo S. Cantharidin poisoning due to "Blister beetle" ingestion. Toxicon 2000; 38:1865–1869. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Sun X, Zhang ZR. An investigation on liver-targeting microemulsions of norcantharidin. Drug Deliv 2005; 12:289–295. [DOI] [PubMed] [Google Scholar]
- 12.Chen YJ, Kuo CD, Tsai YM, Yu CC, Wang GS, Liao HF. Norcantharidin induces anoikis through Jun-N-terminal kinase activation in CT26 colorectal cancer cells. Anticancer Drugs 2008; 19:55–64. [DOI] [PubMed] [Google Scholar]
- 13.Wu LT, Chung JG, Chen JC, Tsauer W. Effect of norcantharidin on N-acetyltransferase activity in HepG2 cells. Am J Chin Med 2001; 29:161–172. [DOI] [PubMed] [Google Scholar]
- 14.Fan YZ, Fu JY, Zhao ZM, Chen CQ. Effect of norcantharidin on proliferation and invasion of human gallbladder carcinoma GBC-SD cells. World J Gastroenterol 2005; 11:2431–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li JL, Cai YC, Liu XH, Xian LJ. Norcantharidin inhibits DNA replication and induces apoptosis with the cleavage of initiation protein Cdc6 in HL-60 cells. Anticancer Drugs 2006; 17:307–314. [DOI] [PubMed] [Google Scholar]
- 16.Hill TA, Stewart SG, Sauer B, Gilbert J, Ackland SP, Sakoff JA, McCluskey A. Heterocyclic substituted cantharidin and norcantharidin analogues – synthesis, protein phosphatase (1 and 2A) inhibition, and anti-cancer activity. Bioorg Med Chem Lett 2007; 17:3392–3397. [DOI] [PubMed] [Google Scholar]
- 17.Yang EB, Tang WY, Zhang K, Cheng LY, Mack PO. Norcantharidin inhibits growth of human HepG2 cell-transplanted tumor in nude mice and prolongs host survival. Cancer Lett 1997; 117:93–98. [DOI] [PubMed] [Google Scholar]
- 18.Tsauer W, Lin JG, Lin PY, Hsu FL, Chiang HC. The effects of cantharidin analogues on xanthine oxidase. Anticancer Res 1997; 17 (3C):2095–2098. [PubMed] [Google Scholar]
- 19.Massicot F, Dutertre-Catella H, Pham-Huy C, Liu XH, Duc HT, Warnet JM. In vitro assessment of renal toxicity and inflammatory events of two protein phosphatase inhibitors cantharidin and nor-cantharidin. Basic Clin Pharmacol Toxicol 2005; 96:26–32. [DOI] [PubMed] [Google Scholar]
- 20.Kok SH, Hong CY, Kuo MY, Lee CH, Lee JJ, Lou IU, et al. Comparisons of norcantharidin cytotoxic effects on oral cancer cells and normal buccal keratinocytes. Oral Oncol 2003; 39:19–26. [DOI] [PubMed] [Google Scholar]
- 21.Schneider U, Schwenk HU, Bornkamm G. Characterization of EBV-genome negative "null" and "T" cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer 1977; 19:621–626. [DOI] [PubMed] [Google Scholar]
- 22.Meng XW, Heldebrant MP, Flatten KS, Loegering DA, Dai H, Schneider PA, et al. Protein kinase Cbeta modulates ligand-induced cell surface death receptor accumulation: a mechanistic basis for enzastaurin-death ligand synergy. J Biol Chem 2010; 285:888–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smirnova IS, Chang S, Forsthuber TG. Prosurvival and proapoptotic functions of ERK1/2 activation in murine thymocytes in vitro. Cell Immunol 2010; 261:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin M, Park S, Pyo MY. Suppressive effects of T-412, a flavone on interleukin-4 production in T cells. Biol Pharm Bull 2009; 32:1875–1879. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Zhou CL, Lei H, Wei Q. Inhibition of calcineurin by quercetin in vitro and in Jurkat cells. J Biochem 2010; 147:185–190. [DOI] [PubMed] [Google Scholar]
- 26.Wu JY, Kuo CD, Chu CY, Chen MS, Lin JH, Chen YJ, Liao HF. Synthesis of novel lipophilic N-substituted norcantharimide derivatives and evaluation of their anticancer activities. Molecules 2014; 19:6911–6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang MC, Tsai ET, Wu JY, Liao HF, Chen YJ, Kuo CD. N-Farnesyloxy-norcantharimide and N-farnesyl-norcantharimide inhibit the progression of leukemia and increase survival days in a syngeneic mouse leukemia model. Anticancer Drugs 2015; 26:508–517. [DOI] [PubMed] [Google Scholar]
- 28.Hill TA, Stewart SG, Ackland SP, Gilbert J, Sauer B, Sakoff JA, McCluskey A. Norcantharimides, synthesis and anticancer activity: Synthesis of new norcantharidin analogues and their anticancer evaluation. Bioorg Med Chem 2007; 15:6126–6134. [DOI] [PubMed] [Google Scholar]
- 29.Peng F, Wei YQ, Tian L, Yang L, Zhao X, Lu Y, et al. Induction of apoptosis by norcantharidin in human colorectal carcinoma cell lines: involvement of the CD95 receptor/ligand. J Cancer Res Clin Oncol 2002; 128:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang PY, Chen MF, Kao YH, Hu DN, Chang FR, Wu YC. Norcantharidin induces apoptosis of breast cancer cells: involvement of activities of mitogen activated protein kinases and signal transducers and activators of transcription. Toxicol In Vitro 2011; 25:699–707. [DOI] [PubMed] [Google Scholar]
- 31.Cimmino F, Scoppettuolo MN, Carotenuto M, Antonellis PD, Dato VD, et al. Norcantharidin impairs medulloblastoma growth by inhibition of Wnt/β-catenin signaling. J Neurooncol 2012; 106:59–70. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu T, Iizuka M, Matsukura H, Hashizume D, Sodeoka M. Synthesis of optically pure norcantharidin analogue NCA-01, a highly selective protein phosphatase 2B inhibitor, and its derivatives. Chem Asian J 2012; 7:1221–1230. [DOI] [PubMed] [Google Scholar]
- 33.Tarleton M, Gilbert J, Sakoff JA, McCluskey A. Synthesis and anticancer activity of a series of norcantharidin analogues. Eur J Med Chem 2012; 54:573–581. [DOI] [PubMed] [Google Scholar]
- 34.Liao HF, Chen YJ, Chou CH, Wang FW, Kuo CD. Norcantharidin induces cell cycle arrest and inhibits progression of human leukemic Jurkat T cells through mitogen-activated protein kinase-mediated regulation of interleukin-2 production. Toxicol In Vitro 2011; 25:206–212. [DOI] [PubMed] [Google Scholar]
- 35.Ramírez CJ, Haberbusch JM, Soprano DR, Soprano KJ. Retinoic acid induced repression of AP-1 activity is mediated by protein phosphatase 2A in ovarian carcinoma cells. J Cell Biochem 2005; 96:170–182. [DOI] [PubMed] [Google Scholar]
- 36.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol 1999; 11:211–218. [DOI] [PubMed] [Google Scholar]
- 37.Nelson JM, Fry DW. Akt, MAPK (Erk1/2), and p38 act in concert to promote apoptosis in response to ErbB receptor family inhibition. J Biol Chem 2001; 276:14842–14847. [DOI] [PubMed] [Google Scholar]
- 38.Davis RJ. MAPKs: new JNK expands the group. Trends Biochem Sci 1994; 19:470–473. [DOI] [PubMed] [Google Scholar]
- 39.Hidenori I. From receptors to stress-activated MAP kinases. Oncogene 1999; 18:6087–6093. [DOI] [PubMed] [Google Scholar]
- 40.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol 2002; 20:55–72. [DOI] [PubMed] [Google Scholar]
- 41.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer 2004; 4:937–947. [DOI] [PubMed] [Google Scholar]
- 42.English J, Pearson G, Wilsbacher J, Swantek J, Karandikar M, Xu S, Cobb MH. New insights into the control of MAP kinase pathways. Exp Cell Res 1999; 253:255–270. [DOI] [PubMed] [Google Scholar]
- 43.Crabtree GR. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell 1999; 96:611–614. [DOI] [PubMed] [Google Scholar]
- 44.Yu H, Sliedregt-Bol K, Overkleeft H, van der Marel GA, van Berkel TJ, Biessen EA. Therapeutic potential of a synthetic peptide inhibitor of nuclear factor of activated T cells as antirestenotic agent. Arterioscler Thromb Vasc Biol 2006; 26:1531–1537. [DOI] [PubMed] [Google Scholar]
- 45.Iacobelli M, Rohwer F, Shanahan P, Quiroz JA, McGuire KL. IL-2-mediated cell cycle progression and inhibition of apoptosis does not require NF-?B or activating protein-1 activation in primary human T cells. Map kinases in the immune response. J Immunol 1999; 162:3308–3315. [PubMed] [Google Scholar]
- 46.Liu XH, Blazsek I, Comisso M, Legras S, Marion S, Quittet P, et al. Effects of norcantharidin, a protein phosphatase type-2A inhibitor, on the growth of normal and malignant haemopoietic cells. Eur J Cancer 1995; 31A:953–963. [DOI] [PubMed] [Google Scholar]


