Abstract
After withdrawal from cocaine, chronic cocaine users often experience persistent reduction in total sleep time, which is accompanied by increased sleep fragmentation resembling chronic insomnia. This and other sleep abnormalities have long been speculated to foster relapse and further drug addiction, but direct evidence is lacking. Here, we report that after prolonged withdrawal from cocaine self-administration, rats exhibited persistent reduction in nonrapid-eye-movement (NREM) and rapid-eye-movement (REM) sleep, as well as increased sleep fragmentation. In an attempt to improve sleep after cocaine withdrawal, we applied chronic sleep restriction to the rats during their active (dark) phase of the day, which selectively decreased the fragmentation of REM sleep during their inactive (light) phase without changing NREM or the total amount of daily sleep. Animals with improved REM sleep exhibited decreased incubation of cocaine craving, a phenomenon depicting the progressive intensification of cocaine seeking after withdrawal. In contrast, experimentally increasing sleep fragmentation after cocaine self-administration expedited the development of incubation of cocaine craving. Incubation of cocaine craving is partially mediated by progressive accumulation of calcium-permeable AMPA receptors (CP-AMPARs) in the nucleus accumbens (NAc). After withdrawal from cocaine, animals with improved REM sleep exhibited reduced accumulation of CP-AMPARs in the NAc, whereas increasing sleep fragmentation accelerated NAc CP-AMPAR accumulation. These results reveal a potential molecular substrate that can be engaged by sleep to regulate cocaine craving and relapse, and demonstrate sleep-based therapeutic opportunities for cocaine addiction.
SIGNIFICANCE STATEMENT Sleep abnormalities are common symptoms in chronic drug users long after drug withdrawal. These withdrawal-associated sleep symptoms, particularly reduction in total sleep time and deteriorating sleep quality, have been speculated to foster relapse and further drug addiction, but direct evidence is lacking. Here we show in rats that the sleep pattern was persistently changed long after withdrawal from cocaine self-administration, and demonstrate that sleep interventions can bidirectionally regulate cocaine craving and seeking after withdrawal. We further demonstrate that glutamatergic synapses in the nucleus accumbens are potential neuronal targets for sleep intervention to influence cocaine craving after withdrawal. These results provide a strong rationale supporting sleep-based therapies for cocaine addiction.
Keywords: addiction, AMPA, cocaine, craving, EEG, REM sleep
Introduction
A general reduction in sleep is often observed in chronic cocaine users after withdrawal, accompanied by increased sleep fragmentation (SF; Kowatch et al., 1992; Matuskey et al., 2011; Angarita et al., 2014). These drug withdrawal-associated sleep abnormalities have been speculated to intensify drug craving and increase the likelihood of relapse (Roehrs et al., 2004; Teplin et al., 2006; Malcolm et al., 2007; Puhl et al., 2009, 2013). However, direct evidence supporting this speculation is lacking.
Rats are naturally nocturnal, having most (approximately two-thirds) of their total daily sleep during the light (inactive) phase, and the rest in the dark (active) phase (Borbély, 1977; Borbély and Neuhaus, 1978; Roky et al., 1999; Tang et al., 2007). As in humans, rats have nonrapid-eye-movement (NREM) and rapid-eye-movement (REM) sleep, both of which are under homeostatic regulation and exhibit rebound after deprivation (Mouret et al., 1969; Borbély and Neuhaus, 1979; Borbély and Achermann, 1999). In humans, increased wakefulness during the daytime improves night sleep, and restricting unnecessary bedtime is an effective treatment for chronic insomnia (Spielman et al., 1987). Similarly in rodents, sleep restriction (SR) during the dark phase limits scattered “naps” and improves the overall quality of light-phase sleep (Borbély and Neuhaus, 1979).
In an attempt to determine how sleep affects cocaine craving and the likelihood of relapse, we focused on incubation of cocaine craving — a rodent model depicting progressively intensified cocaine seeking after withdrawal (Neisewander et al., 2000; Grimm et al., 2001; Pickens et al., 2011). Our results show that after cocaine self-administration, rats exhibited persistent reduction in NREM and REM sleep. Experimentally improving light-phase REM sleep by dark-phase SR decreased incubation of cocaine craving, whereas increasing SF after cocaine self-administration expedited the development of incubation of cocaine craving. Incubation of cocaine craving is partially mediated by progressive accumulation of calcium-permeable AMPA receptors (CP-AMPARs) in the nucleus accumbens (NAc; Conrad et al., 2008; Loweth et al., 2014). After withdrawal from cocaine, dark-phase SR reduced NAc CP-AMPAR accumulation, and increasing SF accelerated NAc CP-AMPAR accumulation. These results reveal a potential molecular link through which sleep may influence cocaine craving and relapse, and demonstrate sleep-based therapeutic opportunities for cocaine addiction.
Materials and Methods
Subjects
Male Sprague Dawley rats (Harlan) at postnatal day 32–49 were used at the beginning of the experiments. Rats were singly housed under a 12 h reverse light/dark cycle (light off at 7:00 A.M.; light on at 7:00 P.M.). Temperature (22 ± 1°C) and humidity (60 ± 5%) were controlled. The rats were used in all experiments in accordance with protocols approved by the Institutional Animal Care and Use Committees at University of Pittsburgh.
Cocaine self-administration surgery and training
Rat jugular surgery was done as described previously (Lee et al., 2013; Ma et al., 2014). Briefly, a Silastic catheter was inserted into the right jugular vein, and the distal end was connected to a Quick Connect Harness (CamCaths) mounted at the back between the scapulae. Rats were allowed to recover for 7–14 d. During recovery, the catheter was flushed daily with heparin (1 ml/kg; 10 U/ml) and gentamicin antibiotics (5 mg/ml) in sterile saline to protect against infection and catheter occlusion.
Cocaine self-administration training was conducted in operant-conditioning chambers (Med Associates), each containing an active and an inactive nose-poke hole, a conditioned stimulus (CS) light in each nose-poke hole, a house light, and a cocaine-infusion line.
The self-administration training session was performed during the dark phase starting at postnatal day 42–56. On day 1, the rats were placed in the chamber for an overnight training session on a fixed ratio (FR) 1 reinforcement schedule. Nose poking in the active hole resulted in a cocaine infusion (0.75 mg/kg over 6 s) and illumination of a CS light inside the nose-poke hole. The CS light remained on for 6 s, whereas the house light was illuminated for 20 s, during which nose poking in the active hole was counted but resulted in no cocaine infusions. After this 20 s period, the house light was turned off, and the next nose poke in the active hole resulted in a cocaine infusion. Nose pokes in the inactive hole had no reinforcement consequences but were recorded. Animals with the training of saline self-administration were used as controls. Rats that received ≥40 cocaine infusions during the overnight session were subject to a subsequent 5 d self-administration procedure (2 h/session/d for 5 consecutive days on an FR1 reinforcement schedule). Same or similar cocaine self-administration procedures/standards were used in our previous studies (Lee et al., 2013; Ma et al., 2014). Rats that did not meet this standard (n = 5 of 116) were removed from data collection.
Cocaine HCl (provided by the National Institute on Drug Abuse Drug Supply Program) was dissolved in 0.9% NaCl saline. Ketamine (purchased from Drug Enforcement Administration-designated vendor at the University of Pittsburgh) was mixed with xylazine for anesthesia.
Measurement of cue-induced cocaine seeking after withdrawal
On withdrawal days 1 and 45 (or 21), extinction tests (0.5 h) were conducted in the same chambers where rats received cocaine self-administration training. However, active nose pokes resulted in contingent delivery of the CS light cue but not cocaine infusions. Within-subject assessment was used to measure incubation; the same rats were tested for cocaine seeking on withdrawal days 1 and 45 and, in some experiments, on withdrawal days 1 and 21. All training and testing were conducted during the dark phase, which is different from our previous studies (Lee et al., 2013; Ma et al., 2014), in which the extinction tests were performed during the light phase.
EEG surgery, recordings, and analysis
Surgery for installing EEG apparatus was similar as described previously (Krueger and Obál, 1993; Winters et al., 2011). Briefly, two stainless-steel wire electromyogram (EMG) electrodes were inserted into the nuchal neck muscle and two gold-plated wire EEG electrodes (Plastics One) were installed contralaterally through the skull over the parietal and frontal cortices. Electrode leads were gathered into a plastic socket (Plastics One) and fixed to the skull with dental cement. When the EEG surgeries were combined with the jugular surgeries, rats were allowed to recover for 14 d before experimentation. All rats were singly housed after surgery and during subsequent experimentation.
EEG and EMG electrodes were connected to the amplifiers via lightweight cables and commutators (Plastics One). Rats were allowed to habituate for 3–6 d before data collection. Baseline EEG and EMG signals were typically recorded around postnatal day 50 and recorded for 3 d before the initial cocaine exposure. EEG and EMG signals were amplified using Grass model 15LT bipolar amplifiers (Grass Technologies) and an analog-to-digital converter (Kissei Comtec). The EEG was filtered <0.1 Hz and >100 Hz. The EMG was filtered <30 Hz and >3 kHz. All signals were digitized at 128 Hz and collected using Vital Recorder software (Kissei Comtec). All signals were manually scored for sleep states in 10 s epochs using Sleep Sign for Animal software (Kissei Comtec). Wakefulness was identified by desynchronized EEG and high EMG activity; NREM sleep exhibited high-amplitude slow waves and lower EMG; REM sleep exhibited regular EEG theta activity and extremely low EMG activity (Fig. 1A). Consolidated sleep durations were determined by the same sleep states in a stretch of time of ≥20 s (two epochs). For EEG power spectrum analysis, EEG signals underwent fast Fourier transformation using a 0.5 Hz frequency bin, and were normalized to the average delta power (0–4 Hz, for NREM) or theta power (5–10 Hz, for REM) of the baseline condition (before cocaine exposure). All rats were coded for sleep scoring, and then decoded for data compiling.
Figure 1.
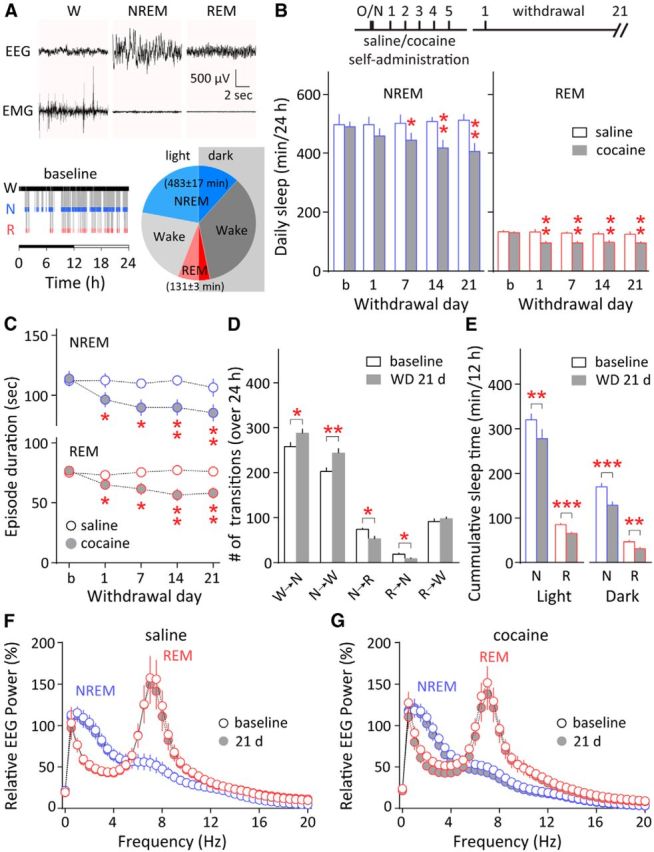
Persistent sleep abnormalities after withdrawal from cocaine self-administration. A, Top, Representative EEG and EMG recordings showing wakefulness (W), NREM, and REM sleep states. Bottom left, An example hypnogram from the same rat showing wakefulness (W), NREM sleep (N), and REM sleep (R) states across the 12 h dark and 12 h light phase (horizontal bar) on a baseline recording day. Bottom right, Summary pie chart showing the average proportion of time spent in wakefulness, NREM sleep, and REM sleep on baseline recording days (min/24 h), separated into the light and dark phases at midline. B, Top, Timeline of saline or cocaine self-administration training and withdrawal. Bottom, 24 h total NREM and REM sleep time persistently decreased during the initial 21 d after withdrawal from cocaine self-administration (24 h/d recordings on baseline day and on withdrawal days 1, 7, 14, and 21). *p < 0.05, **p < 0.01 (different from baseline). No significant changes were observed in the saline-exposed rats (compared with baseline). C, The average episode durations of both NREM and REM sleep were reduced during the same 21 d after withdrawal from cocaine self-administration. *p < 0.05, **p < 0.01 (different from baseline). No significant changes were observed in the saline-exposed rats (compared with baseline). D, After 21 d of withdrawal (WD 21 d) from cocaine self-administration, there were increased transitions between wakefulness and NREM sleep, and fewer transitions to REM sleep. *p < 0.05, **p < 0.01 (different from baseline); n = 12. E, Cumulative NREM and REM sleep times were decreased during both the light and dark phases by withdrawal day 21. **p < 0.01, ***p < 0.001 (different from baseline); n = 12. F, G, Relative EEG power spectra of NREM and REM episodes from saline-exposed (F) or cocaine-exposed (G) rats were compared between baseline and 21 d after the 5 d cocaine or saline self-administration. EEG signals were normalized to the average delta power (0–4 Hz, for NREM) or theta power (5–10 Hz, for REM) of the baseline condition (before cocaine exposure). No significant differences were detected in saline-exposed or cocaine-exposed rats between baseline and 21 d after self-administration. Error bars indicate SEM.
Dark-phase SR
Rats were singly housed in custom-made treadmill boxes with a flexible stainless-steel mesh floor covered with normal bedding. A cylinder-shaped object (∼2 cm in height), fixed to the treadmill belt underneath, moved quietly and bidirectionally with the belt across the entire mesh floor (Fig. 2A). Movement of the object underneath the mesh floor did not affect the gross movement of the rats but still disturbed their sleep (Fig. 2C,D). The treadmill was programmed to move continuously in alternating directions at the speed of 3 cm/s during the 12 h dark phase, and was kept off for the 12 h light phase. Rats housed in these boxes had access to food and water ad libitum. Control rats were housed in similar boxes without movement of the treadmill belts. This procedure lasted for 7–42 d at different time points after withdrawal as indicated.
Figure 2.
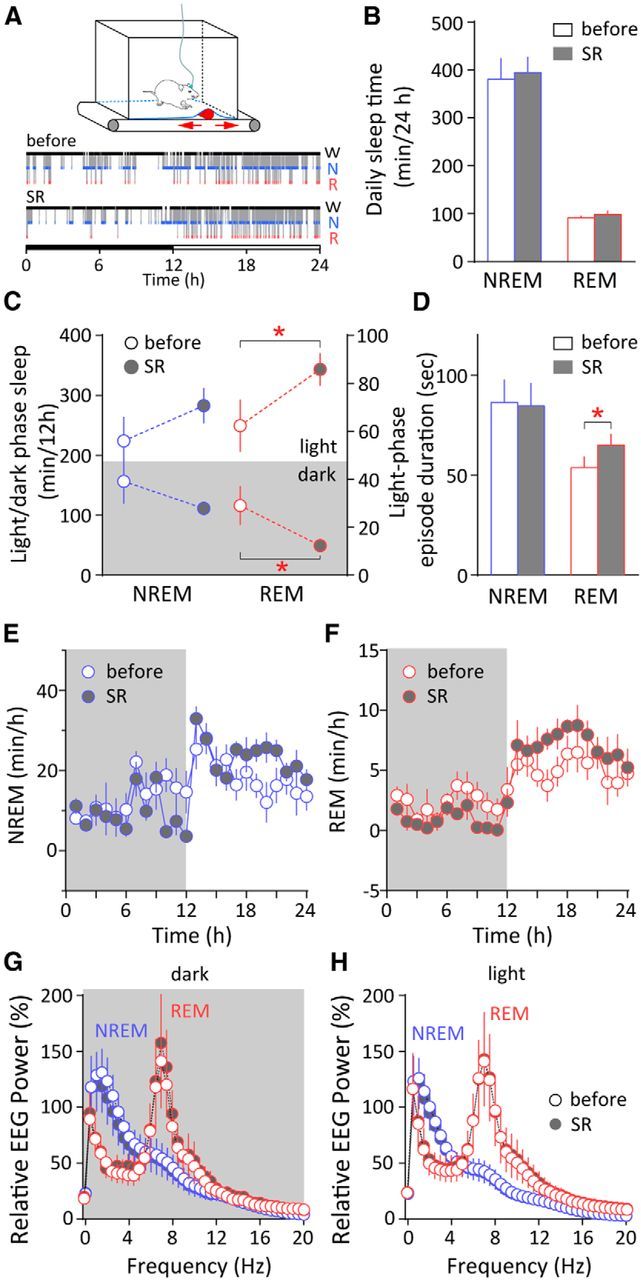
Consolidation of REM sleep by a single dark-phase SR after cocaine withdrawal. A, Top, Diagram of the custom-made treadmill system used for SR. It contains a programmable treadmill motor and belt; a bottomless housing cage sitting on top with a flexible stainless-steel mesh covered with normal bedding as the floor; and a cylinder-shaped object affixed to the treadmill belt moving bidirectionally (arrows) underneath the floor of the cage to sleep-restrict the rats (see Materials and Methods). Bottom, Example hypnograms showing the distribution of wakefulness (W), NREM sleep (N), and REM sleep (R) across the 12 h dark and 12 h light phase (horizontal bar) in a cocaine-exposed rat on withdrawal day 21 (before SR) and withdrawal day 22 (with dark-phase SR). B, In cocaine-exposed rats, a single dark-phase SR on withdrawal day 22 did not change the 24 h total NREM or REM sleep time compared with withdrawal day 21 (control). C, A single dark-phase SR on withdrawal day 22 did not significantly alter the cumulative NREM sleep in the dark or light phase, but selectively decreased REM sleep during the dark phase and enhanced REM sleep in the subsequent 12 h light phase. *p < 0.05 (different from withdrawal day 21); n = 6. D, The mean duration of REM episodes in the light phase was enhanced after a single dark-phase SR on withdrawal day 22. *p < 0.05 (different from withdrawal day 21); n = 6. No significant change was observed in NREM average episode duration. E, F, Hourly NREM sleep time (E) and REM sleep time (F) on withdrawal day 21 (before SR) and day 22 (with dark-phase SR) showed no overall alterations in the dark or light phase. G, H, Relative EEG power spectrum for NREM and REM sleep during the dark (G) or light phase (H) on withdrawal day 21 (before SR) and withdrawal day 22 (with dark-phase SR). No significant differences were detected. Error bars indicate SEM.
Total SF
Rats were housed in the treadmill boxes described above. The treadmills were programmed to move for 26 s at one time at the speed of 3 cm/s before stopping for 30 s; the treadmill belt moved in alternating directions throughout the light and dark phases. This procedure lasted for 7 d. Control rats were housed in similar boxes but with treadmill belts that did not move.
Sucrose self-administration training
Forty-five days after cessation of cocaine self-administration, some rats underwent sucrose self-administration training using a different set of operant-conditioning chambers (Med Associates). These contained an active and an inactive lever, a CS light above each lever, a house light, and a food dispenser.
Pressing the active lever resulted in the delivery of a sucrose pellet (Bio-Serv) and illumination of a CS light above the active lever. The CS light remained on for 6 s, whereas the house light was illuminated for 20 s, during which time active lever-pressing actions were counted but resulted in no sucrose pellet delivery. After the 20 s, the house light was turned off, and the next press of the lever resulted in the delivery of a sucrose pellet. Pressing the inactive lever had no reinforcement consequences. Rats were trained for 1 h daily during the dark phase for 7 consecutive days on an FR1 reinforcement schedule.
In vitro electrophysiology
NAc acute slice preparation.
The rats were anesthetized with isoflurane and subsequently perfused transcardially with 4°C cutting solution (in mm: 135 N-methyl-d-glucamine, 1 KCl, 1.2 KH2PO4, 0.5 CaCl2, 1.5 MgCl2, 20 choline-HCO3, 11 glucose, pH adjusted to 7.4 with HCl, and saturated with 95% O2/5% CO2). The rat was decapitated and the brain was removed and sliced using a vibratome (Leica VT1200s) in 4°C cutting solution. Coronal slices of 300 μm thickness were cut containing the NAc subregions. Slices were allowed to recover in oxygenated aCSF (in mm: 119 NaCl, 2.5 KCl, 1 NaH2PO4, 1.3 MgCl2, 2.5 CaCl2, 26.2 NaHCO3, and 11 glucose, 290 mOsm, saturated with 95% O2/5% CO2) first in 37°C for 15–20 min and then at room temperature for a total of 1–2 h. For recordings, one slice was transferred from the holding chamber to a submerged recording chamber, where it was continuously perfused with oxygenated aCSF maintained at 31 ± 1°C.
Electrophysiological recordings.
Whole-cell voltage-clamp recordings were made under visual guidance (40×, differential interference contrast optics) from NAc neurons located in the dorsal-medial shell region. A patch electrode of 3–5 MΩ was filled with a Cs+-based internal solution (in mm: 108 Cs-methanesulfonate, 15 CsCl, 5 tetraethylammonium chloride, 20 HEPES, 0.4 EGTA, 2.5 MgATP, 0.25 Na3GTP, 1 QX-314, pH 7.25–7.30; 290 mOsm). Excitatory afferents were stimulated at 0.1 Hz by a constant-current isolated stimulator (DS3, Digitimer), using a monopolar electrode (glass pipet filled with aCSF) with pulse duration of 0.1 ms. At least 5 min of a stable baseline was recorded before data collection. In all recordings, series resistance was 8–14 MΩ and was left uncompensated. Series resistance was monitored continuously during all recordings, and a change beyond ±15% resulted in exclusion of the cell from data analysis. Synaptic currents were recorded with a MultiClamp 700B amplifier (Molecular Devices), filtered at 3 kHz, amplified 5 times, and then digitized at 20 kHz with a Digidata 1440A analog-to-digital converter (Molecular Devices). Picrotoxin (100 μm) was included in the recording bath to inhibit GABAAR-mediated responses. One-naplhthylacetyl spermine trihydrochloride (Naspm; 100 μm) was bath applied for 15–20 min to selectively inhibit CP-AMPARs. Naspm was purchased from R&D Systems. All other reagents were purchased from Sigma-Aldrich.
Data acquisition and analysis.
More than two-thirds of the electrophysiology data were analyzed without prior awareness of the treatment (cocaine or saline). The rest of the data (see Fig. 6I) were analyzed when no biased outcomes were expected. All results are shown as mean ± SEM. Each experiment was replicated in ≥4 rats (∼1–5 cells were recorded from each rat) for electrophysiological analysis, 5–12 rats for EEG analysis, and 8–14 rats in behavioral analysis. No data points were excluded unless specified in the experimental procedure. A total of 116 rats were used. Of these, four were excluded before data collection because of catheter leakage or clogging, two were excluded before data collection because of surgery-associated infection or significant (>15%) loss of body weight, five were excluded because they did not initiate cocaine self-administration, nine were excluded due to loss of socket connectors during EEG recordings, and one was excluded for EEG power analysis because of significant reduction in signal amplitude during the long-term recording.
Figure 6.
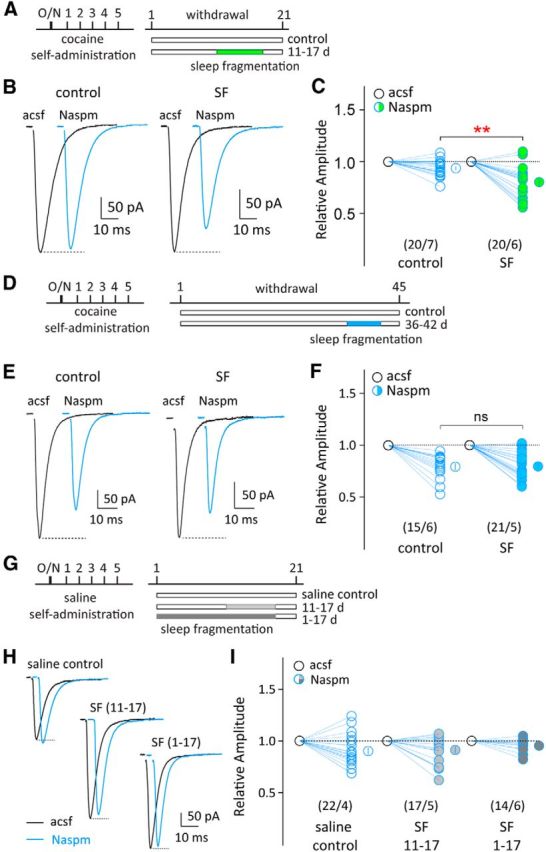
Total SF facilitates synaptic recruitment of CP-AMPARs in the NAc after cocaine withdrawal. A, Timeline of cocaine self-administration training, withdrawal, and total SF (on withdrawal days 11–17). Cocaine-exposed rats without SF were used as controls. B, Evoked EPSCs in example NAc MSNs before and during perfusion of Naspm (100 μm) from control group (left) and SF group (right) on withdrawal day 21. C, Summarized results showing that total SF on withdrawal days 11–17 significantly enhanced Naspm sensitivity in NAc MSNs on withdrawal day 21 compared with the control group. **p < 0.01 (compared with cocaine-exposed rats without SF). D, Timeline of cocaine self-administration training, withdrawal, and total SF (on withdrawal days 36–42). Cocaine-exposed rats without SF were used as controls. E, Evoked EPSCs in example NAc MSNs before and during perfusion of Naspm (100 μm) from cocaine control group (left) and SF group (right) on withdrawal day 45. F, Summarized results showing that total SF on withdrawal days 36–42 did not further increase Naspm-sensitive EPSC components in NAc MSNs (compared with cocaine-exposed rats without SF). G, Timeline of saline self-administration and total SF (on subsequent days 11–17 or 1–17). Saline-exposed rats without SF were used as controls. H, Evoked EPSCs in example NAc MSNs before and during perfusion of Naspm from saline control group (left), SF11–17 group (middle), and SF1–17 group (right) 21 d after cessation of saline self-administration. I, Summarized results showing that in saline-exposed rats, total SF over days 11–17 or 1–17 did not alter Naspm sensitivity in NAc MSNs on day 21 (compared with saline-exposed rats without SF). Numbers in parenthesis represent number of cells/number of rats. ns, Not significant. Error bars indicate SEM.
Data from the repeated experiments for the same substudy were pooled together for statistical analyses. Sample size for each experiment was based on our previous experience with similar experiments or on sizes that have been routinely used in similar studies published in this journal. Sample size in electrophysiology experiments was presented as n/m, where “n” refers to the number of cells examined and “m” refers to the number of rats. Normal distribution was assumed for all statistics. Variance was estimated for most major results and no significant difference was found between control and manipulation groups. Statistical significance was assessed using t tests (for two-group comparisons, two-tailed tests unless otherwise specified), one-way ANOVA (single-factor multiple groups), or two-way repeated-measure (RM) ANOVAs (two-factor multiple groups with repeated measurements), followed by Fisher's least significant difference test. For two-factor ANOVA, Factor A was assigned for the treatments (e.g., cocaine vs saline or sleep restricted vs control) and Factor B was assigned for withdrawal time. The statistical results were primarily presented in the F and p values of the main effect of Factor A, which was the primary research interest. Degrees of freedom of between (b) and within (w) treatments were presented as F(b,w). p < 0.05 was considered statistically significant. For all experiments involving electrophysiology using treated animals, both cell-based and animal-based statistics were performed and reported, with results of cell-based analysis provided in graphic presentations. In animal-based analyses, electrophysiological parameters of all recorded cells from a single rat were averaged and the mean was used to represent this parameter of this rat for subsequent animal-based analysis.
Results
Sleep loss and fragmentation after cocaine withdrawal
In experimental rats, we used the signature EEG and EMG signals to determine sleep and wakefulness (Fig. 1A) before and after 5 d cocaine self-administration. Rats before cocaine exposure (baselines) spent 614 ± 16 min/d asleep, with 483 ± 17 min in NREM sleep and 131 ± 3 min in REM sleep, and with ∼68% of NREM sleep and ∼66% of REM sleep occurring during the daily light phase (Fig. 1A). These parameters are comparable to the published results in adult rats (Tang et al., 2007). After withdrawal from cocaine self-administration, rats exhibited a sustained reduction in total NREM and REM sleep (NREM, F(4,44) = 11.43, p < 0.001; REM, F(4,44) = 7.98, p < 0.001, one-way RM ANOVA), recapitulating the human situation; whereas the age-matched, saline-exposed rats showed no significant changes (NREM, F(4,16) = 0.22, p = 0.93; REM, F(4,16) = 0.35, p = 0.84, one-way RM ANOVA; Fig. 1B). In addition, we detected persistent shortening of individual NREM and REM episodes in cocaine-exposed rats after withdrawal (NREM, F(4,44) = 7.72, p < 0.001; REM, F(4,44) = 10.84, p < 0.001, one-way RM ANOVA), suggesting increased SF; whereas no significant changes were observed in the saline-exposed rats (NREM, F(4,16) = 0.28, p = 0.88; REM, F(4,16) = 0.57, p = 0.69, one-way RM ANOVA; Fig. 1C). Twenty-one days after cessation of cocaine self-administration, there were more frequent transitions between wakefulness and NREM sleep (wakefulness→NREM, p < 0.05; NREM→wakefulness, p < 0.01, n = 12, paired t test), and fewer transitions to REM sleep (NREM→REM, p < 0.05; REM→NREM, p < 0.05, n = 12, paired t test; Fig. 1D), suggesting that the decrease in total NREM sleep was mainly because of shortened NREM episodes, whereas the loss in REM was because of a decrease in both the number and duration of REM episodes. Cocaine withdrawal-associated sleep loss occurred in both light and dark phases by withdrawal day 21 (light phase: NREM p < 0.01, REM p < 0.001; dark phase: NREM p < 0.001, REM p < 0.01, n = 12, paired t test; Fig. 1E). Finally, the power spectrum of NREM and REM episodes was not altered in saline-exposed or cocaine-exposed rats 21 d after cessation of self-administration (saline: NREM(0–4 Hz) F(1,4) = 2.26, p = 0.21; REM(5–10 Hz) F(1,4) = 1.68, p = 0.26; cocaine: NREM(0–4 Hz) F(1,10) = 0.41, p = 0.54; REM(5–10 Hz) F(1,10) = 0.54, p = 0.48, two-way RM ANOVA; Fig. 1F,G). These results reveal that cocaine withdrawal-associated sleep disturbance is primarily expressed as persistent reduction in total sleep and increased SF.
Consolidation of REM sleep by dark-phase SR after cocaine withdrawal
Although cocaine withdrawal-associated sleep loss occurred in both light and dark phases in rats (Fig. 1E), sleep loss during the light phase can have a more adverse effect in these nocturnal animals. In an attempt to improve their sleep after withdrawal, we applied an SR procedure to the rats selectively during the dark phase so as to enhance sleep during the light phase while preserving their daily activity rhythm (Fig. 2A; see Materials and Methods). After cocaine self-administration, a single SR applied on withdrawal day 22 during the 12 h dark phase did not change the total (light plus dark phase) NREM or REM sleep on this withdrawal day compared with withdrawal day 21 (NREM p = 0.82; REM p = 0.42, n = 6, paired t test; Fig. 2B). However, the daily REM sleep exhibited prominent redistribution such that it was reduced during the dark-phase SR period, but compensatorily increased during the subsequent light phase (dark phase p < 0.05; light phase p < 0.05, n = 6, one-tailed paired t test; Fig. 2C). On the other hand, the cumulative NREM sleep was not altered during either the dark or light phase despite SR (dark phase p = 0.17; light phase p = 0.18, n = 6, one-tailed paired t test; Fig. 2C). Importantly, dark-phase SR also increased the duration of individual REM episodes in the subsequent light phase (p < 0.05, n = 6, paired t test), but not that of NREM episodes (p = 0.32, n = 6, paired t test; Fig. 2D), suggesting that dark-phase SR selectively improved REM sleep consolidation during the light phase in cocaine-exposed rats. Within-subject comparisons before and after dark-phase SR revealed no overall changes in the hourly amount of NREM or REM sleep across dark and light phases on withdrawal day 22 (dark phase: NREM F(1,5) = 1.11, p = 0.34; REM F(1,5) = 3.53, p = 0.12; light phase: NREM F(1,5) = 0.98, p = 0.37; REM F(1,5) = 3.94, p = 0.10, two-way RM ANOVA; Fig. 2E,F), and no change was detected in the EEG power spectrum of either NREM or REM episodes after SR (dark phase: NREM(0–4 Hz) F(1,4) = 6.25, p = 0.07; REM(5–10 Hz) F(1,4) = 1.10, p = 0.35; light phase: NREM(0–4 Hz) F(1,4) = 3.23, p = 0.15; REM(5–10 Hz) F(1,4) = 0.18, p = 0.69, two-way RM ANOVA; Fig. 2G,H). Together, these results indicate that our dark-phase SR procedure selectively affected REM sleep; it not only shifted the distribution of REM sleep over the light/dark cycle, but indeed improved REM sleep by increasing the episode duration in rats after withdrawal from cocaine self-administration.
We then repeatedly applied dark-phase SR over withdrawal days 22–42 in cocaine-exposed rats (Fig. 3A), attempting to improve their sleep in a long-term manner. As an age-matched control, saline-exposed rats did not show changes in the 24 h total NREM or REM sleep time from baseline till 42 d after cessation of saline self-administration (NREM F(7,28) = 0.22, p = 0.98; REM F(7,28) = 1.15, p = 0.36, one-way ANOVA), suggesting no significant age-dependent changes over this period. In cocaine-exposed rats, similar to single SR, repeated SR did not alter the total amount of daily (light plus dark phase) NREM or REM sleep (NREM F(1,10) = 0.02, p = 0.90; REM F(1,10) = 0.10, p = 0.76, two-way RM ANOVA; Fig. 3B), the dark-phase or light-phase NREM sleep (dark phase, F(1,10) = 1.58, p = 0.24; light phase, F(1,10) = 0.75, p = 0.41, two-way RM ANOVA; Fig. 3C), or the duration of light-phase NREM episodes (p = 0.12, n = 6, paired t test; Fig. 3E). In contrast, repeated dark-phase SR profoundly redistributed the daily REM sleep over the light and dark phases in cocaine-exposed rats by suppressing REM sleep during the dark phase (F(1,10) = 17.51, p < 0.01) and increasing REM sleep during the light phase (F(1,10) = 5.37, p < 0.05, two-way RM ANOVA; Fig. 3D). Furthermore, repeated SR also increased the duration of REM episodes during light phase (p < 0.05, n = 6, paired t test; Fig. 3E). Thus, repeated dark-phase SR after cocaine withdrawal preferentially increased total REM sleep and REM episode duration during the light phase.
Figure 3.
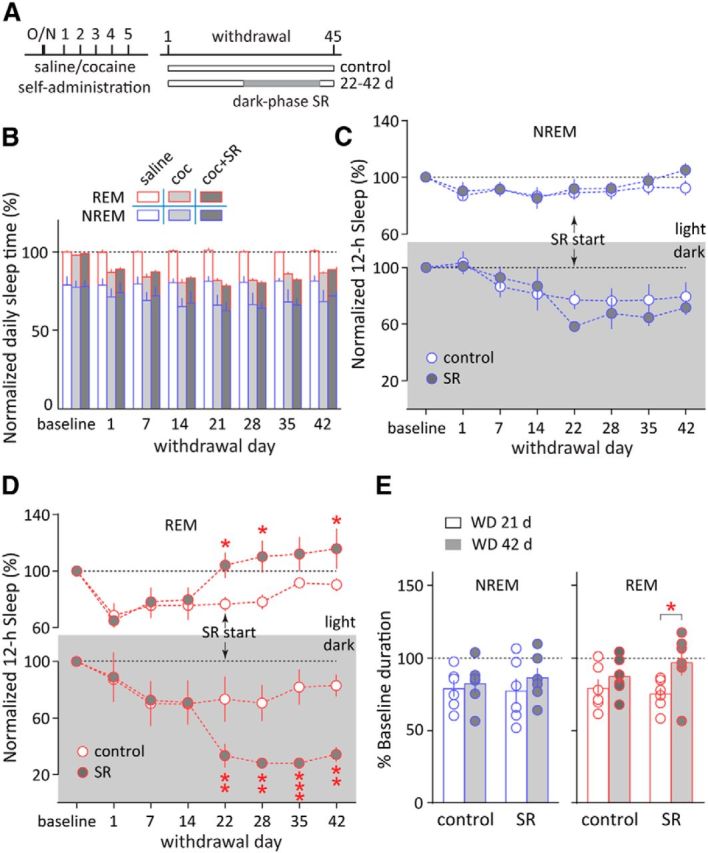
Consolidation of REM sleep by repeated dark-phase SR after cocaine withdrawal. A, Timeline of saline or cocaine self-administration training, withdrawal, and repeated dark-phase SR (on withdrawal days 22–42). Cocaine-exposed rats without SR were used as controls. B, 24 h total NREM and REM sleep times were not altered by dark-phase SR in cocaine-withdrawal rats between withdrawal days 22 and 42 (compared with cocaine-exposed rats without SR). Saline-exposed rats did not show changes in the 24 h total NREM or REM sleep time from baseline till 42 d after saline self-administration (compared with baseline). C, Repeated dark-phase SR on withdrawal days 22–42 did not alter the cumulative NREM sleep time in the dark or light phase (compared with cocaine-exposed rats without SR). D, Repeated dark-phase SR on withdrawal days 22–42 persistently reduced the cumulative REM sleep time in the dark phase and increased REM sleep time in the light phase. *p < 0.05, **p < 0.01, ***p < 0.001 (different from cocaine-exposed rats without SR). E, Repeated dark-phase SR on withdrawal days 22–42 increased the mean duration of REM episodes on withdrawal day 42 (WD 42 d) compared with withdrawal day 21 (WD 21 d; before dark-phase SR), but did not alter that of NREM sleep. *p < 0.05; n = 6. Error bars indicate SEM.
Dark-phase SR decreases incubation of cocaine craving
To test whether dark-phase SR, which improved REM sleep, affects withdrawal-associated cocaine seeking and relapse, we used the rat model of incubation of cocaine craving, in which rats exhibited progressive increase in cue-induced cocaine seeking over the drug-withdrawal period (Grimm et al., 2001). In cocaine-exposed rats without SR (controls), cue-induced cocaine seeking (measured by nose pokes in active holes; see Materials and Methods) was elevated by ∼60% on withdrawal day 45 compared with withdrawal day 1 in a 30 min extinction test (p < 0.001, n = 10, paired t test; Fig. 4A,B). Interestingly, dark-phase SR (applied on withdrawal days 36–42, 29–42, 22–42, or 1–42; Fig. 4A) reduced incubation of cocaine craving in a “dose”-dependent manner, with longer durations of SR exhibiting larger anti-incubation effects (F(4,49) = 2.79, p < 0.05, one-way ANOVA; Fig. 4B). Within-group comparisons revealed that cue-induced cocaine seeking on withdrawal day 45 was not significantly different from that on withdrawal day 1 in two of the longer SR groups (SR 22–42: p = 0.094, n = 14; SR 1–42: p = 0.730, n = 8, paired t test), suggesting suppressed incubation of cocaine craving by prolonged dark-phase SR. In addition, the levels of nose pokes in inactive holes were not affected by SR (F(4,49) = 1.20, p = 0.32, one-way ANOVA; Fig. 4B), suggesting no memory deficit or decreased basal exploratory behaviors after SR. Importantly, natural reward-associated learning, assessed by sucrose self-administration 45 d after cocaine withdrawal, was not altered in these cocaine-exposed, dark-phase sleep-restricted rats (active lever press: F(1,9) = 0.55, p = 0.48; percentage active/total lever presses: F(1,9) = 1.35, p = 0.28, two-way RM ANOVA; Fig. 4C–E), suggesting no effects of SR on natural reward seeking. These results reveal that repeated dark-phase SR, which may improve certain aspects of REM sleep, prevents the progressive intensification (incubation) of cocaine craving after drug withdrawal.
Figure 4.
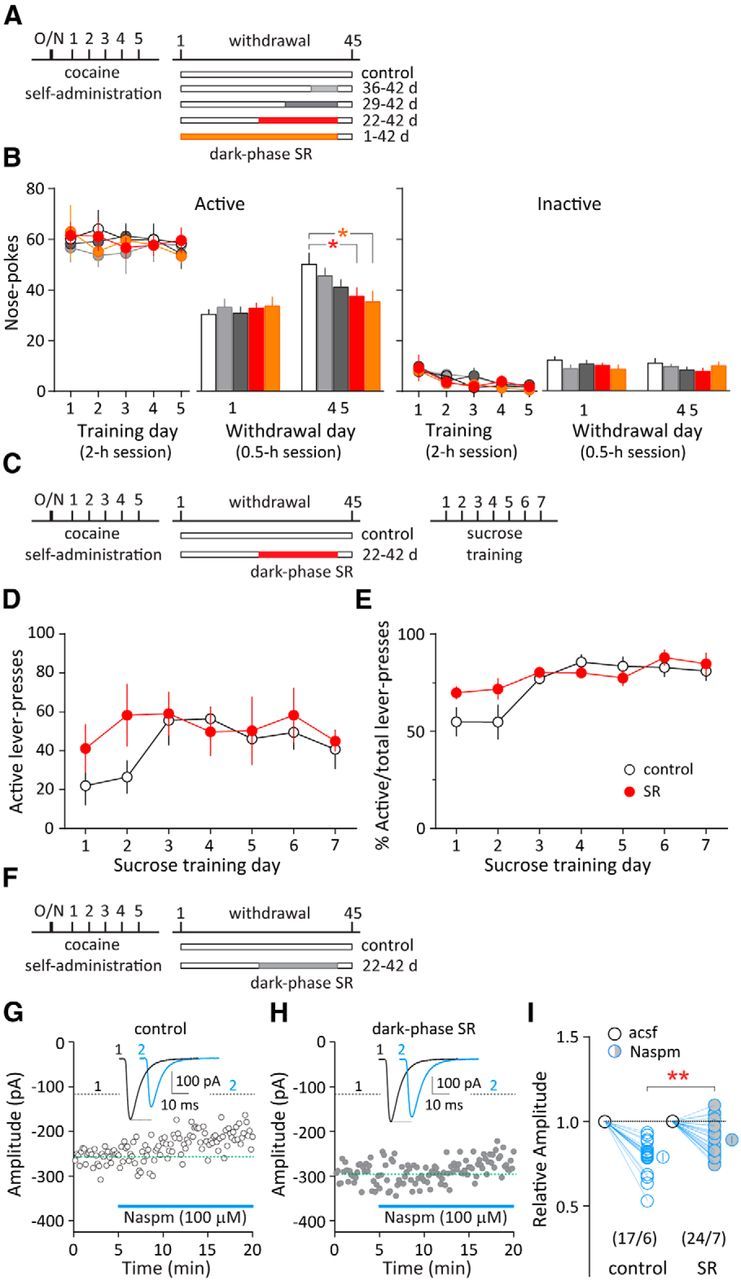
Dark-phase SR reduces incubation of cocaine craving and prevents synaptic accumulation of CP-AMPARs in the NAc. A, Timeline of cocaine self-administration training, withdrawal, and repeated dark-phase SR during different periods after withdrawal (on withdrawal days 36–42, 29–42, 22–42, 1–42). Cocaine-exposed rats without SR were used as controls. The same rats were tested on withdrawal days 1 and 45. B, Left, Cue-induced cocaine seeking (measured by the number of nose pokes in the active hole during the daily 2 h training session or the 0.5 h test session) in the control group was enhanced on withdrawal day 45 compared with day 1, suggesting incubation of cocaine craving. Following different periods of dark-phase SR, cue-induced cocaine seeking was reduced in a “dose”-dependent manner on withdrawal day 45, with longer durations of SR exhibiting larger anti-incubation effects. *p < 0.05 (different from control group on withdrawal day 45). Color coding is the same as in A. Right, Cue-induced nose pokes in the inactive hole (not associated with cocaine delivery) remained low as in controls. C, Timeline of cocaine self-administration training, withdrawal, repeated dark-phase SR (on withdrawal days 22–42), and sucrose self-administration training. Cocaine-exposed rats without SR were used as controls. D, E, Sucrose self-administration learning following long-term cocaine withdrawal was not altered in the SR group compared with the control, as measured by the number of lever presses on the active lever (D) or percentage active/total lever presses (E), suggesting no effect of SR on natural reward seeking. F, Timeline of cocaine self-administration training, withdrawal, and repeated dark-phase SR (on withdrawal days 22–42). G, Evoked EPSCs from an example NAc MSN before and during perfusion of CP-AMPAR-selective antagonist Naspm (100 μm) in an NAc slice from a rat on withdrawal day 45. H, Evoked EPSCs from an example NAc MSN before and during perfusion of Naspm from a rat that received dark-phase SR between withdrawal days 22 and 42. I, Summarized results showing that the evoked EPSCs in NAc MSNs were less sensitive to Naspm inhibition in the dark-phase SR group. **p < 0.01 (different from cocaine-exposed rats without SR). Numbers in parenthesis represent number of cells/number of rats. Error bars indicate SEM.
Dark-phase SR prevents synaptic accumulation of NAc CP-AMPARs after cocaine withdrawal
Changes in glutamatergic transmission within the NAc, particularly synaptic accumulation of CP-AMPARs in NAc medium spine neurons (MSNs) after prolonged withdrawal from cocaine self-administration, are core neuronal bases underlying the expression of incubation of cocaine craving (Conrad et al., 2008; Purgianto et al., 2013; Loweth et al., 2014). To examine whether these NAc CP-AMPARs are a neuronal target of dark-phase SR, we measured synaptic CP-AMPARs in the NAc MSNs on withdrawal day 45 with dark-phase SR between withdrawal days 22 and 42 (Fig. 4F). In cocaine-exposed rats without SR, NAc AMPAR EPSCs recorded on withdrawal day 45 were sensitive to the CP-AMPAR-selective antagonist Naspm (Fig. 4G,I), recapitulating cocaine withdrawal-induced accumulation of synaptic CP-AMPARs shown in previous studies (Conrad et al., 2008). Interestingly, dark-phase SR over withdrawal days 22–42 reduced the Naspm-sensitive AMPAR EPSCs in NAc MSNs in cocaine-exposed rats (p < 0.01, t test; Fig. 4H,I). Thus, repeated dark-phase SR reduced withdrawal-induced accumulation of CP-AMPARs, and this effect may, among other SR-induced cellular consequences, contribute to the reduced incubation of cocaine craving.
SF accelerates the development of incubation of cocaine craving
After finding that dark-phase SR led to sleep improvement and decreased incubation of cocaine craving, we asked whether worsened sleep would produce the opposite effect. To address this, we adopted a total SF procedure, which attempted to interrupt sleep episodes throughout the entire 24 h each day. We first applied this SF procedure over withdrawal days 36–42 in cocaine-exposed rats. Both control and SF groups exhibited enhanced cue-induced cocaine seeking on withdrawal day 45 than day 1 (control p < 0.01, n = 9; SF p < 0.01, n = 8, paired t test), indicating incubation of cocaine craving. However, SF between withdrawal days 36 and 42 did not further increase incubation of cocaine craving tested on withdrawal day 45 in cocaine-exposed rats (p = 0.97, control n = 9, SF n = 8, t test; Fig. 5A,B). By contrast, the same SF procedure, when applied between withdrawal days 11 and 17, caused a clear incubation of cocaine craving on withdrawal day 21, a time point at which incubation of cocaine craving would otherwise still be under development before reaching the detection threshold (p < 0.05, n = 8 each group, t test; Fig. 5C,D). Thus, SF expedites the development of incubation of cocaine craving after withdrawal. Note that the above rats were subject to a relatively weak cocaine incubation regimen and tested on both withdrawal days 1 and 21 for within-subject comparisons (see Materials and Methods).
Figure 5.
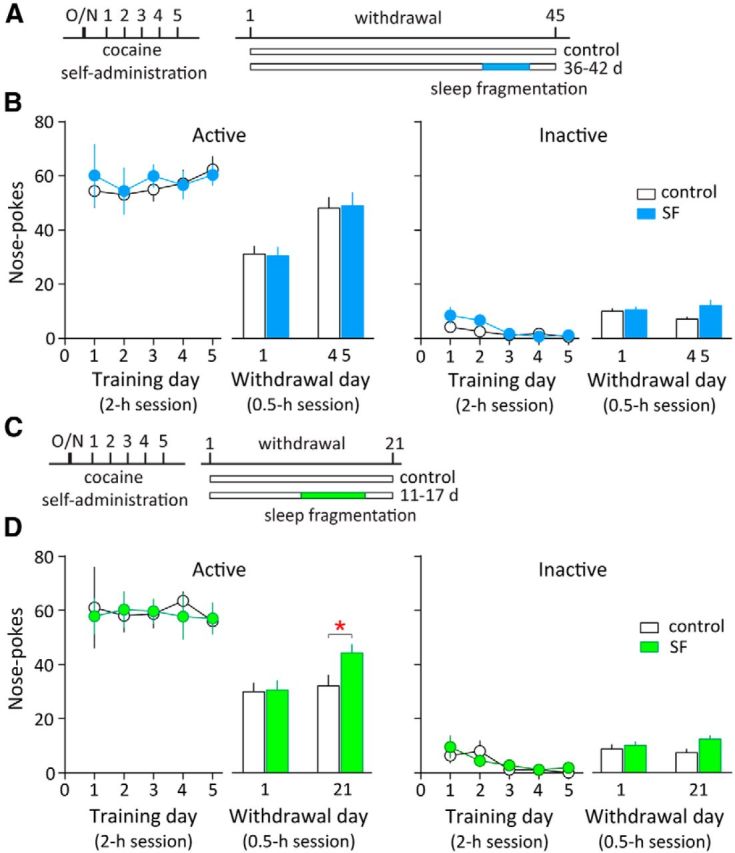
Total SF accelerates the development of incubation of cocaine craving. A, Timeline of cocaine self-administration training, withdrawal, and total SF (on withdrawal days 36–42). Cocaine-exposed rats without SF were used as controls. The same rats were tested on withdrawal days 1 and 45. B, Summarized results showing that both control and SF groups exhibited enhanced active nose pokes (cue-induced cocaine seeking) on withdrawal day 45 than day 1, suggesting incubation of cocaine craving. Total SF on withdrawal days 36–42 did not further increase the number of active nose pokes on withdrawal day 45 compared with the control group (cocaine-exposed rats without SF), suggesting similar levels of incubation of cocaine craving. Inactive nose pokes remained low. C, Timeline of cocaine self-administration training, withdrawal, and total SF (on withdrawal days 11–17). Cocaine-exposed rats without SF were used as controls. D, Total SF on withdrawal days 11–17 significantly increased the number of active nose pokes (cue-induced cocaine seeking) on withdrawal day 21, suggesting increased cocaine craving in the SF group. *p < 0.05 (different from cocaine-exposed rats without SF); n = 8 each group. Inactive nose pokes remained low. The same rats were tested on withdrawal days 1 and 21. Error bars indicate SEM.
SF precipitates synaptic accumulation of NAc CP-AMPARs after cocaine withdrawal
To examine whether NAc CP-AMPARs are a potential molecular target for SF to influence incubation of cocaine craving, we recorded AMPAR EPSCs in acute NAc slices from cocaine-exposed rats with SF after withdrawal. Accumulation of NAc CP-AMPARs occurs gradually after cocaine withdrawal, and a significant increase is normally detected only after prolonged, but not short-term (e.g., 21 d), withdrawal (Wolf and Tseng, 2012). Consistently, on withdrawal day 21, we did not detect an increase in Naspm-sensitive components in NAc AMPAR EPSCs (Fig. 6B,C). However, SF between withdrawal days 11 and 17 elevated the Naspm-sensitive component of NAc AMPAR EPSCs on withdrawal day 21 (p < 0.01, t test; Fig. 6A–C). On the other hand, on withdrawal day 45, a prominent Naspm-sensitive component was detected in NAc AMPAR EPSCs in cocaine-exposed rats without SF; SF applied on withdrawal days 36–42 did not further affect the magnitude of Naspm sensitivity of AMPAR EPSCs (percentage inhibition by Naspm: control, 20.42 ± 2.69%; SF, 20.77 ± 3.03%, p = 0.93, t test; Fig. 6D–F). Thus, although SF does not further increase the level of NAc CP-AMPARs when they have already accumulated after long-term withdrawal, it may accelerate this withdrawal-induced synaptic pathophysiology during early periods of withdrawal. It is important to note that SF alone did not elevate the level of CP-AMPARs in NAc MSNs. In rats with SF over days 1–17 or 11–17 after saline self-administration, no alterations in the Naspm sensitivity of AMPAR EPSCs in NAc MSNs were detected (percentage inhibition by Naspm: saline, 9.71 ± 3.10%, SF11–17, 8.64 ± 3.29%, SF1–17, 4.57 ± 1.56%; F(2,50) = 0.75, p = 0.48, one-way ANOVA; Fig. 6G–I).
Discussion
Although sleep disturbance has long been thought to promote the development of drug addiction, it remains elusive how drug experience influences sleep states over prolonged periods, and how drug-induced sleep disturbance influences the addictive state at mechanistic levels (Dackis and O'Brien, 2002; Morgan et al., 2010). Our present study reveals that (1) cocaine withdrawal persistently reduced both NREM and REM sleep and increased SF; (2) SF after cocaine withdrawal expedited the development of incubation of cocaine craving, whereas dark-phase SR reduced REM sleep fragmentation and suppressed the development of incubation of cocaine craving; and (3) NAc CP-AMPARs may be one of the molecular targets through which sleep and sleep disturbance regulate the development of incubation of cocaine craving. These results suggest that sleep disturbance is not only a symptom of cocaine use, but is also an important factor in promoting cocaine craving and increasing the likelihood of relapse.
Sleep abnormalities after cocaine exposure
Using a repeated intraperitoneal cocaine injection procedure, previous studies detected increased NREM and decreased REM sleep during the first 2 weeks of cocaine withdrawal, with no change in total sleep time (Yang et al., 2011). Using cocaine self-administration procedure, our present study shows a persistent reduction in both NREM and REM sleep as well as enhanced SF after cocaine withdrawal (Fig. 1). The patterns of sleep changes induced by our contingent cocaine exposure in rats largely recapitulate the sleep changes in human chronic cocaine users after drug withdrawal (Matuskey et al., 2011).
Sleep manipulations after cocaine withdrawal
Without altering the total amount of NREM or REM sleep every day, redistribution of sleep to enhance light-phase sleep reduced incubation of cocaine craving after long-term withdrawal (Fig. 4B). On the other hand, exacerbating withdrawal-associated sleep disturbance expedited the development of incubation of cocaine craving (Fig. 5D). These results suggest that sleep may not only be a symptomatic manifestation of cocaine dependence, but an integral part of the illness.
More importantly, the results suggest that manipulating sleep architecture, rather than the total amount of sleep, may serve as a behavioral therapy to reduce drug craving and relapse. Although intervention of sleep-episode durations has been rarely factored out singly in sleep-based therapies, the notion is reminiscent of a recent finding that a minimal unit of consolidated sleep, rather than the total amount of sleep or sleep intensity, is essential for memory consolidation (Rolls et al., 2011).
Like other sleep manipulations, the dark-phase SR procedures that we used in this study are inevitably associated with other consequences, such as stress, in addition to changes in sleep per se. The current treadmill system is designed to maximally reduce the stress associated with the physical disturbance of the animals—rats were not physically restrained or forced to move during SR. Indeed, within-subject comparisons indicate that the amount as well as intensity of NREM sleep was not altered before and after treadmill was turned on (Figs. 2, 3), suggesting minimal physical disturbance. Nonetheless, sleep loss per se, independent of the SR method, is a stressful event, which has a complex role in addiction-associated behaviors (Koob and Kreek, 2007; Sinha, 2008; Karoly and Hutchison, 2012; Al-Hasani et al., 2013). Thus, stress, or the relief of which following sleep rebound, could be a potential contributor to altered cocaine-seeking behaviors. Whereas the current study identifies the correlation between sleep and withdrawal-associated cocaine seeking, future studies are needed to determine whether sleep per se or the physiological consequences of sleep regulates addiction-associated behaviors.
In searching for effective treatment for cocaine dependence, there have been promising behavioral approaches identified from animal model studies, including voluntary chronic aerobic exercise and environmental enrichment (Smith et al., 2008; Chauvet et al., 2009; Thiel et al., 2009, 2011; Zlebnik et al., 2010; Ogbonmwan et al., 2015). It is intriguing to note that these treatments, when administered properly, can often directly or indirectly affect sleep. For example, exercise during the active phase of the day could enhance sleep during the inactive phase (Hobson, 1968; Matsumoto et al., 1968; Horne, 1981). Similarly, an enriched environment also enhances a rat's sleep during the inactive phase (van Gool and Mirmiran, 1986). Thus, questions arise as to whether these treatments become effective partly through their effects on sleep, and whether targeting insomnia after cocaine withdrawal could have direct therapeutic effects on relapse beyond being a treatment for just the sleep symptoms. Indeed, a candidate class of pharmacotherapies for cocaine dependence targets GABAergic transmission, which positively affects sleep (Kampman, 2008). In addition, modafinil, a morning-dosed psychostimulant for reducing early withdrawal symptoms, increases the total amount of sleep as well as enhances sleep efficiency in the evening in chronic cocaine users (Morgan et al., 2010). These results indicate that manipulating sleep may serve as a new angle of behavioral therapy for addiction treatment.
Targeting REM sleep in cocaine withdrawal
Although both NREM and REM sleep were compromised after cocaine withdrawal, mending REM sleep alone appeared sufficient to reduce incubation of cocaine craving (Figs. 3, 4B). These results suggest that REM sleep consolidation and improvement are of particular interest in developing treatment for cocaine relapse. In light of this, it is worth noting that many of the agents tested for treating cocaine relapse, including the above-mentioned agents, have a predominant effect on NREM sleep. For example, tiagabine is a GABA reuptake blocker that enhances slow-wave sleep in chronic cocaine users (Morgan and Malison, 2008); modafinil has been shown to restore NREM sleep with less restoring effect on REM sleep in chronic cocaine users (Morgan et al., 2010). These preclinical and clinical results invite further investigation of whether drugs that effectively target REM sleep could be advantageous in preventing relapse.
Sleep and NAc CP-AMPARs
CP-AMPARs progressively accumulate in the NAc after cocaine withdrawal along with the development of incubation of cocaine craving, while blocking or downregulating NAc CP-AMPARs effectively reduces incubation of cocaine craving (Conrad et al., 2008; Lee et al., 2013; Loweth et al., 2014; Ma et al., 2014). Here we show that sleep bidirectionally regulates NAc CP-AMPARs after cocaine withdrawal: dark-phase SR decreased NAc CP-AMPARs whereas SF accelerated NAc CP-AMPAR accumulation (Figs. 4F–I, 6A–C). These results suggest that NAc CP-AMPARs are important molecular correlates of sleep-mediated regulation of incubation of cocaine craving.
It appears that sleep-based regulation of AMPARs is not confined to the NAc. In the rodent cortex and hippocampus, a general downregulation of AMPARs is detected right after the sleep/light phase (Vyazovskiy et al., 2008; Winters et al., 2011). In the somatosensory cortex layer V pyramidal neurons, a selective removal of CP-AMPARs occurs through an LTD-like process during sleep (Lanté et al., 2011). Conversely, strengthening of AMPAR-mediated synaptic transmission by an LTP-like process during sleep has also been observed in the primary visual cortex (Aton et al., 2009). These results suggest that the effects of sleep on synaptic AMPARs are bidirectional, and are possibly dependent on prior experience of different neurocircuits.
Whereas a critical role of NREM sleep is suggested in the regulation of AMPARs in cortical regions (Vyazovskiy et al., 2008; Lanté et al., 2011; Aton et al., 2014), our present results show that REM sleep may be particularly important in regulating synaptic accumulation of CP-AMPARs in the NAc. Furthermore, regulation of NAc CP-AMPARs can serve as a heuristic mechanism bridging the neuronal effects of sleep and cocaine experience (Figs. 4F–I, 6A–C). Thus it reiterates the importance of specifying brain regions and the experience/activity history of local neural ensembles in defining the cellular consequences of sleep (Krueger et al., 2013; Frank, 2015). Future studies will focus on the specific components of sleep—the unique combinations of neural activity and neuromodulator levels—that orchestrate AMPAR trafficking at NAc synapses.
Concluding remarks
Sleep is not only a daily ritual, but likely also the most important means for the brain to readjust all aspects of its function every day. Unveiling the cellular and behavioral interaction between sleep and cocaine experience may allow clinical strategy to unleash the power of sleep for correcting important aspects of the addictive state.
Footnotes
This work was supported by National Institutes of Health Grants MH101147 (Y.H.H.) DA035805 (Y.H.H.), DA029565 (Y.H.H.), DA023206 (Y.D.), DA034856 (Y.D.), and DA030379 (Y.D.); and the Pennsylvania Department of Health (Y.H.H.). Cocaine was supplied by the Drug Supply Program of National Institutes of Health National Institute on Drug Abuse. We thank Rong Guo, Kimberly G. Gagnon, and Bradley D. Winters for technical support.
The authors declare no competing financial interests.
References
- Al-Hasani R, McCall JG, Bruchas MR. Exposure to chronic mild stress prevents kappa opioid-mediated reinstatement of cocaine and nicotine place preference. Front Pharmacol. 2013;4:96. doi: 10.3389/fphar.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angarita GA, Canavan SV, Forselius E, Bessette A, Pittman B, Morgan PT. Abstinence-related changes in sleep during treatment for cocaine dependence. Drug Alcohol Depend. 2014;134:343–347. doi: 10.1016/j.drugalcdep.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Suresh A, Broussard C, Frank MG. Sleep promotes cortical response potentiation following visual experience. Sleep. 2014;37:1163–1170. doi: 10.5665/sleep.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbély AA. Sleep in the rat during food deprivation and subsequent restitution of food. Brain Res. 1977;124:457–471. doi: 10.1016/0006-8993(77)90947-7. [DOI] [PubMed] [Google Scholar]
- Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- Borbély AA, Neuhaus HU. Daily pattern of sleep, motor activity and feeding in the rat: effects of regular and gradually extended photoperiods. J Comp Physiol. 1978;124:1–14. [Google Scholar]
- Borbély AA, Neuhaus HU. Sleep-deprivation: effects on sleep and EEG in the rat. J Comp Physiol. 1979;133:71–87. doi: 10.1007/BF00663111. [DOI] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, O'Brien CP. Cocaine dependence: the challenge for pharmacotherapy. Curr Opin Psychiatry. 2002;15:261–267. doi: 10.1097/00001504-200205000-00006. [DOI] [Google Scholar]
- Frank MG. Sleep and synaptic plasticity in the developing and adult brain. Curr Top Behav Neurosci. 2015;25:123–149. doi: 10.1007/7854_2014_305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson JA. Sleep after exercise. Science. 1968;162:1503–1505. doi: 10.1126/science.162.3861.1503. [DOI] [PubMed] [Google Scholar]
- Horne JA. The effects of exercise upon sleep: a critical review. Biol Psychol. 1981;12:241–290. doi: 10.1016/0301-0511(81)90001-6. [DOI] [PubMed] [Google Scholar]
- Kampman KM. The search for medications to treat stimulant dependence. Addict Sci Clin Pract. 2008;4:28–35. doi: 10.1151/ascp084228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Hutchison KE. Does stress contribute to the incubation of craving? Biol Psychiatry. 2012;71:e39. doi: 10.1016/j.biopsych.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowatch RA, Schnoll SS, Knisely JS, Green D, Elswick RK. Electroencephalographic sleep and mood during cocaine withdrawal. J Addict Dis. 1992;11:21–45. doi: 10.1300/J069v11n04_03. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obál F. A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Huang YH, Rector DM, Buysse DJ. Sleep: a synchrony of cell activity-driven small network states. Eur J Neurosci. 2013;38:2199–2209. doi: 10.1111/ejn.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanté F, Toledo-Salas JC, Ondrejcak T, Rowan MJ, Ulrich D. Removal of synaptic Ca(2)+-permeable AMPA receptors during sleep. J Neurosci. 2011;31:3953–3961. doi: 10.1523/JNEUROSCI.3210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, Neumann PA, Graziane NM, Brown TE, Suska A, Guo C, Lobo MK, Sesack SR, Wolf ME, Nestler EJ, Shaham Y, Schlüter OM, Dong Y. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, Li X, Ford KA, Le T, Olive MF, Szumlinski KK, Tseng KY, Wolf ME. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci. 2014;17:73–80. doi: 10.1038/nn.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schlüter OM, Huang YH, Dong Y. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm R, Myrick LH, Veatch LM, Boyle E, Randall PK. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3:24–32. [PubMed] [Google Scholar]
- Matsumoto J, Nihisho T, Suto T, Sadahiro T, Miyoshi M. Influence of fatigue on sleep. Nature. 1968;218:177–178. doi: 10.1038/218177a0. [DOI] [PubMed] [Google Scholar]
- Matuskey D, Pittman B, Forselius E, Malison RT, Morgan PT. A multistudy analysis of the effects of early cocaine abstinence on sleep. Drug Alcohol Depend. 2011;115:62–66. doi: 10.1016/j.drugalcdep.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Malison RT. Pilot study of lorazepam and tiagabine effects on sleep, motor learning, and impulsivity in cocaine abstinence. Am J Drug Alcohol Abuse. 2008;34:692–702. doi: 10.1080/00952990802308221. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott E, Pittman B, Stickgold R, Malison RT. Normalizing effects of modafinil on sleep in chronic cocaine users. Am J Psychiatry. 2010;167:331–340. doi: 10.1176/appi.ajp.2009.09050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouret J, Pujol JF, Kiyono S. Paradoxical sleep rebound in the rat. Effects of physical procedures involved in intracisternal injection. Brain Res. 1969;15:501–506. doi: 10.1016/0006-8993(69)90170-X. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonmwan YE, Schroeder JP, Holmes PV, Weinshenker D. The effects of post-extinction exercise on cocaine-primed and stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2015;232:1395–1403. doi: 10.1007/s00213-014-3778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Fang J, Grigson PS. Acute sleep deprivation increases the rate and efficiency of cocaine self-administration, but not the perceived value of cocaine reward in rats. Pharmacol Biochem Behav. 2009;94:262–270. doi: 10.1016/j.pbb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Boisvert M, Guan Z, Fang J, Grigson PS. A novel model of chronic sleep restriction reveals an increase in the perceived incentive reward value of cocaine in high drug-taking rats. Pharmacol Biochem Behav. 2013;109:8–15. doi: 10.1016/j.pbb.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgianto A, Scheyer AF, Loweth JA, Ford KA, Tseng KY, Wolf ME. Different adaptations in AMPA receptor transmission in the nucleus accumbens after short vs long access cocaine self-administration regimens. Neuropsychopharmacology. 2013;38:1789–1797. doi: 10.1038/npp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrs T, Johanson CE, Meixner R, Turner L, Roth T. Reinforcing and subjective effects of methylphenidate: dose and time in bed. Exp Clin Psychopharmacol. 2004;12:180–189. doi: 10.1037/1064-1297.12.3.180. [DOI] [PubMed] [Google Scholar]
- Roky R, Kapás L, Taishi TP, Fang J, Krueger JM. Food restriction alters the diurnal distribution of sleep in rats. Physiol Behav. 1999;67:697–703. doi: 10.1016/S0031-9384(99)00137-7. [DOI] [PubMed] [Google Scholar]
- Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC, de Lecea L. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci U S A. 2011;108:13305–13310. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–135. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–56. [PubMed] [Google Scholar]
- Tang X, Yang L, Sanford LD. Sleep and EEG spectra in rats recorded via telemetry during surgical recovery. Sleep. 2007;30:1057–1061. doi: 10.1093/sleep/30.8.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplin D, Raz B, Daiter J, Varenbut M, Tyrrell M. Screening for substance use patterns among patients referred for a variety of sleep complaints. Am J Drug Alcohol Abuse. 2006;32:111–120. doi: 10.1080/00952990500328695. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol. 2009;12:1151–1156. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Engelhardt B, Hood LE, Peartree NA, Neisewander JL. The interactive effects of environmental enrichment and extinction interventions in attenuating cue-elicited cocaine-seeking behavior in rats. Pharmacol Biochem Behav. 2011;97:595–602. doi: 10.1016/j.pbb.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool WA, Mirmiran M. Effects of aging and housing in an enriched environment on sleep–wake patterns in rats. Sleep. 1986;9:335–347. doi: 10.1093/sleep/9.2.335. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Winters BD, Huang YH, Dong Y, Krueger JM. Sleep loss alters synaptic and intrinsic neuronal properties in mouse prefrontal cortex. Brain Res. 2011;1420:1–7. doi: 10.1016/j.brainres.2011.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SL, Han JY, Kim YB, Nam SY, Song S, Hong JT, Oh KW. Increased nonrapid eye movement sleep by cocaine withdrawal: possible involvement of A2A receptors. Arch Pharm Res. 2011;34:281–287. doi: 10.1007/s12272-011-0214-0. [DOI] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology. 2010;209:113–125. doi: 10.1007/s00213-010-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


