Abstract
Background
The purpose of this study was to investigate the values of contrast-enhanced ultrasound (CEUS) in the diagnosis and differential diagnosis of hyperechoic liver lesions.
Material/Methods
The CEUS findings of 102 patients with hyperechoic liver lesions identified by 2-dimensional ultrasound in the Affiliated Tumor Hospital of Guangxi Medical University were reviewed and analyzed.
Results
A total of 135 lesions were analyzed, of which malignant lesions were found in 72 patients and benign lesions in 63, with a CEUS accuracy rate of 91.11%, which was significantly higher than that of conventional ultrasound (74.81%; P<0.05).
Conclusions
CEUS can improve the accuracy rate of ultrasonography in the diagnosis and differential diagnosis of hyperechoic liver lesions.
MeSH Keywords: Contrast Media; Liver; Ultrasonography, Doppler
Background
Focal liver lesions (FLLs) are common liver diseases in clinical practice. The 2-dimensional ultrasound (2DUS) examination is a preferred method used to assess FLLs. However, with the development and application of the second-generation ultrasound contrast agent (SonoVue®) and contrast-enhanced ultrasound (CEUS) imaging technology, the application of ultrasound contrast is increasing in the detection of liver diseases [1,2]. However, few studies have been conducted to investigate the hyperechoic FLLs by 2DUS. Thus, this study was undertaken to evaluate the value of CEUS in the diagnosis and differential diagnosis of hyperechoic liver lesions identified by 2DUS. The hyperechoic liver lesions were retrospectively reviewed and analyzed, and the accuracy rates of 2DUS and CEUS were compared in the diagnosis of hyperechoic liver lesions in this study.
Material and Methods
Clinical data
A total of 102 patients with 135 lesions were recruited from the Affiliated Tumor Hospital of Guangxi Medical University between March 2011 and October 2014. All the patients were diagnosed with slightly hyperechoic or hyperechoic lesions by 2DUS. There were 75 males and 27 females, with a mean age of 51.4±12.5 years (range: 21–87 years). In addition, single lesions were found in 76 patients and multiple lesions in 26. Of note, 2–3 large lesions were evaluated in patients with multiple liver lesions. The median diameter of lesions was 3.28 cm (range: 0.7–11 cm). Among them, pathological examination of liver lesions was done in 65 patients after surgery or biopsy; contrast-enhanced computed tomography (CECT) or magnetic resonance imaging (MRI) of the liver lesions, or 6–12-month follow-up was performed in 37 cases. The study protocol was approved by the institutional review board of the Cancer Hospital of Guangxi Medical University.
Instruments and methods
GE Logiq9 color Doppler ultrasonography was performed in this study with an abdominal convex array probe (frequency: 3.5–5 MHz). All facilities were equipped with low mechanical index CEUS technique (M=0.11). SonoVue® Contrast agent (Bracco company, Italy) was used, and 5 ml of 0.9% NaCl was used to dilute SonoVue into 5 mg/ml of sulfur hexafluoride microbubbles suspensions, followed by bolus injection through the cubital vein (1.5 ml per injection), and then 5 ml of 0.9% NaCl was used to flush the tube.
First, the liver was scanned with gray-scale 2DUS. After the detection of liver lesions, the lesions were contrasted by the surrounding liver tissues, and slightly hyperechoic or hyperechoic lesions were used to localize the lesions, and then following information of liver lesions was recorded: number, size, shape, boundary, etc. Subsequently, Color Doppler ultrasound was applied to observe the blood flow at the edge and center of these lesions and a preliminary diagnosis was made. The probe was kept still, and the contrast mode (M=0.11) was switched for continuous observation for 6 min. The whole videos ware preserved. There was a 15-min time window between the contrast observations of the lesions in different areas. Under a double-blind condition, 2 sonologists with more than 10 years experience made the etiologic diagnosis.
Statistical analysis
SPSS version 16.0 statistical software was employed for the statistical analysis. Chi-square test was applied for comparison of findings from 2DUS and CEUS. A value of P<0.05 was considered statistically significant.
Results
Findings from pathology, CECT, MRI, and 6–12-month follow-up
Of 135 hyperechoic lesions, hepatocellular carcinoma (HCC) was found in 57 lesions, intrahepatic cholangiocarcinoma (ICC) in 1, cirrhotic nodule in 38, hemangioma in 15, hepatic focal nodular hyperplasia (FNH) in 3, adenoma in 3, hepatic angiomyolipoma (HAML) in 1, inflammatory lesion in 1, degeneration nodules in 3, liver fat maldistribution in 2, and metastatic lesion in 2.
CEUS performance
HCC
A total of 57 lesions were identified as HCC in 50 patients. Among them, 50 lesions showed “rapid-filling in and rapid-washout”, 4 displayed “rapid-filling in, slow-washout”, 2 presented “equal enhancement of 3 phases”, and 1 had “high-enhancement of 3 phases” mode. In addition, 3 patients were misdiagnosed; among them, 2 patients were misdiagnosed as cirrhotic nodules with the size of 1.3×1.3 cm and 1.0×0.9 cm, respectively, and the “equal enhancement of 3 phases” in CEUS; 1 was misdiagnosed as hemangioma, with the size of 2.3×2.1 cm and “high-enhancement of 3 phases” in CEUS.
ICC
One patient had uniform echo in the lesion, posterior attenuation and dilation of intrahepatic bile duct in 2DUS; ring-like significant enhancement at the edge was found in arterial phase, rapid washout in portal phase, slight enhancement in delay phase, but the central region had no contrast during the whole CEUS.
Hemangioma
Hemangioma was found 13 patients with 15 lesions that showed even or slightly uneven hyperecho in 2DUS, and part of which showed features of “vascular perforating syndrome” in color Doppler imaging; a total of 8 lesions showed peripheral nodular enhancement and gradually filled the concentric in CEUS, 6 had overall significant enhancement in CEUS, and 1 displayed no contrast in arterial phase, ring-like significant enhancement in portal phase, and significant enhancement in delay phase in CEUS.
Adenoma
Three lesions in 1 patient showed even hyperecho, and the posterior echo remained changed in 2DUS. These 3 lesions showed significant enhancement in arterial phase, even enhancement in portal, and slight enhancement in delay phases. This patient was misdiagnosed as having a metastatic tumor.
FNH
Three patients were diagnosed with FNH, of whom 1 showed “spoke-like” enhancement in CEUS and 2 displayed even enhancement in 3 phases.
HAML
This lesion showed significant and even hyperecho in 2DUS, but no obvious blood flow signals were observed in the lesion in color Doppler imaging. This lesion displayed significant enhancement in 3 phases and was misdiagnosed as hemangioma.
Inflammatory lesion
This lesion showed uneven echoes and was mainly hyperechoic in 2DUS. No obvious blood flow signals were present in the lesion in color Doppler imaging. The lesion showed grid-like enhancement in arterial phase and slight enhancement in portal and delay phases in CEUS.
Degeneration nodule
This lesion showed blurred boundaries and even echoes in 2DUS, and no obvious blood flow signals were observed in the lesion in color Doppler imaging. No contrast was found in the lesion in 3 phases.
Abnormal liver fat distribution
It showed round hyperechoes in 2DUS, and there were no obvious blood flow signals in the lesion in color Doppler imaging. Even enhancement was observed around the liver parenchyma in CEUS.
Metastatic lesions
Metastatic lesions were found in 6 patients, of whom 4 were of gastrointestinal origin, 1 of esophageal origin, and 1 of breast origin. In addition, 5 out of 6 patients (9 lesions) showed typical “rapid-filling in, rapid-washout” in CEUS, and 2 lesions of transverse colon origin showed “slow- filling in, rapid-washout” in CEUS.
Cirrhotic nodules
Cirrhotic nodules were found in 19 patients (38 lesions). All patients had a background of cirrhosis; the diameter of these lesions ranged from 0.7 cm to 2.8 cm, they displayed even echoes in the lesions, and no obvious blood flow signals were observed in the lesions in color Doppler imaging. Even enhancement was present in 3 phases in CEUS (Figures 1, 2).
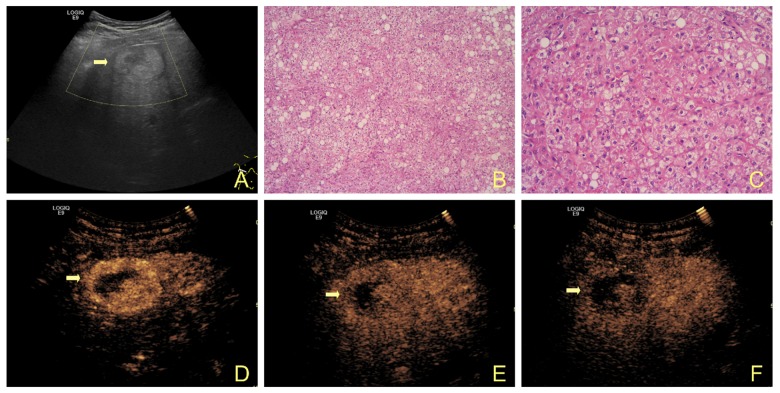
Figure 1.
A 52-year-old man with HCC. (A) Gray-scale ultrasound shows a hyperechoic mass (arrow) in the right lobe of the liver. (B, C) Post-operative pathological examination revealed the well-differentiated hepatocellular carcinoma (solid type) with obvious hepatic fatty degeneration, hemorrhage, and necrosis. (D–F) CEUS image shows hypervascularity in the arterial phase and washout in the portal and late phase.
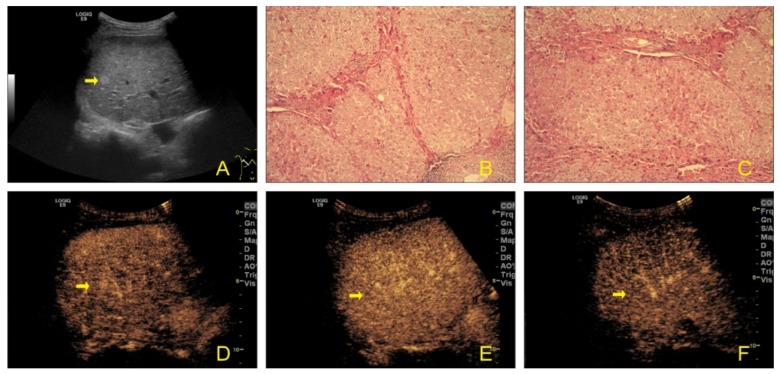
Figure 2.
A 50-year-old man with cirrhotic nodules. (A) Gray-scale ultrasound shows a hyperechoic mass (arrow) in the right lobe of the liver. (B, C) Post-operative pathological examination revealed cirrhotic nodules. (D–F) CEUS image shows even enhancement in 3 phases.
Pathological findings and clinical outcomes between conventional ultrasonography and CEUS
Hyperechoic lesions of 101 patients identified by conventional ultrasonography were confirmed by the pathological and clinical examinations. Thirty-four patients were misdiagnosed and the accuracy rate of diagnosis was 74.81% (34/135). Hyperechoic lesions of 123 patients identified by CEUS were confirmed by the pathological and clinical examinations and 12 patients were misdiagnosed, with a 91.11% (123/135) accuracy rate of diagnosis, which was significantly higher than that of conventional ultrasonography (χ2=15, P<0.005, (Tables 1, 2).
Table 1.
Pathological findings and clinical outcomes in 135 patients examined by 2DUS and CEUS.
| Pathology | Lesion (n) | 2DUS (n) | CEUS (n) | ||
|---|---|---|---|---|---|
| C | M | C | M | ||
| HCC | 57 | 35 | 22 | 53 | 4 |
| Cirrhotic nodules | 38 | 33 | 5 | 36 | 2 |
| Hemangioma | 15 | 14 | 1 | 13 | 2 |
| FNH | 3 | 2 | 1 | 3 | 0 |
| Metastases | 11 | 11 | 0 | 11 | 0 |
| Liver fat maldistribution | 2 | 2 | 0 | 2 | 0 |
| HAML | 1 | 0 | 1 | 0 | 1 |
| Inflammatory lesion | 1 | 1 | 0 | 1 | 0 |
| Degeneration nodular | 3 | 2 | 1 | 3 | 0 |
| Adenoma | 3 | 0 | 3 | 0 | 3 |
| ICC | 1 | 1 | 0 | 1 | 0 |
C – correct diagnosis; M – misdiagnosis.
Table 2.
Diagnosis of HFLLs by 2DUS and CEUS in 102 patients with 135 lesions.
| Diagnostic method | Correct diagnosis | Misdiagnosis | Accuracy rate (%) |
|---|---|---|---|
| 2DUS | 101 | 34 | 74.81% |
| CEUS | 123 | 12 | 91.11% |
CEUS vs. 2DUS: χ2=15, P<0.005.
Discussion
Many types of liver diseases may show hyperechoic lesions in ultrasonography. The hyperechoic lesions can be divided into 2 categories: benign and malignant lesions. Most benign and malignant lesions can be identified by CEUS with a low mechanical index mode, and the accuracy rate is 85–95% [3,4]. Therefore, to understand the pathology of hyperechoic lesions in the liver, the characteristics of liver lesions of different pathological types can provide evidence for the diagnosis and differential diagnosis of hyperechoic lesions in the liver by CEUS.
Hemangioma and FNH are the common benign lesions in the liver. Both can be easily identified by the typical characteristics in CEUS. Liver hemangioma has “peripheral nodular concentric enhancement” or “significant enhancement in 3 phases”, and FNH usually displays “spoke-like enhancement”. However, in our study, 1 patient with hemangioma was misdiagnosed with small HCC, because this patient had a background of cirrhosis and CEUS showed significant enhancement in arterial phase, and even enhancement in portal and delay phases. These features were similar to those of well-differentiated HCC. Adenoma is a rare benign lesion in the liver, and is usually found in young women. It has a close relationship with use of oral contraceptives in women and with diabetes, glycogen storage disease, and use of male hormones in men. The patient with adenoma in the present study was a young man, and had no other diseases or use of androgen. Multiple lesions were found in 2DUS, and CEUS revealed significant enhancement in arterial phase, even enhancement in portal phase, and slight enhancement in delay phase. These characteristics were inconsistent with those of typical adenoma in CEUS (significant enhancement in arterial phase, even enhancement or slight enhancement in portal and delay phases [5–7]. According to the European Imaging Guideline [8], most malignant lesions in delay phase showed slight enhancement, and most benign lesions displayed enhancement or significant enhancement. Thus, adenoma is likely to be misdiagnosed as a malignant lesion when slight enhancement is present in delay phase of CEUS.
HAML is rare and is easily misdiagnosed as benign liver lesions in ultrasonography. According to previous reports [9–11], due to fat content and rich vascular component, HAML mainly shows hyperecho-based mixed-echo in conventional 2DUS. It is difficult to differentiate HAML from other hyperechoic liver lesions [12]; thus, the pre-operative rate of accurate diagnosis is very low for conventional ultrasound [13]. The typical enhancement pattern (“significant heterogeneous enhancement in 3 phases”) [14] is overlapped with hemangioma to a certain extent (“significant enhancement in 3 phases”), thereby increasing the difficulty in differential diagnosis of hemangioma. In the present study, 1 patient with HAML showing “significant enhancement in 3 phases” in CEUS was misdiagnosed as hemangioma, and the pathological examination showed HAML mainly composed of adipocytes. According to previous reports [9], of 8 lesions predominantly composed of mature adipocytes, 7 showed homogeneous hyperechoes in conventional 2DUS and 6 displayed “heterogeneous significant enhancement in 3 phases” in CEUS, which was similar to HAML in our study.
HCC is one of the most common malignant lesions in the liver. The development of HCC is often accompanied by hepatitis or a background of cirrhosis, and about 1/5 of small HCC lesions are hyperechoic in ultrasonography [15]. In the present study, 46 patients with small HCC had similar findings in 2DUS to the cirrhotic nodules in 38 patients. Therefore, it is difficult to distinguish small HCC from cirrhotic nodules by conventional 2DUS. However, CEUS is helpful for the early diagnosis and improves the accuracy rate of diagnosis of sclerosis nodules and small HCC in patients with cirrhosis and multiple nodular backgrounds because it is able to detect the microvascular perfusion. In our study, the accuracy rate of CEUS in the diagnosis of HCC (94.04%) was significantly higher than that of 2DUS (73.81%). Of note, 5 patients were still misdiagnosed in this study, and 4 were misdiagnosed for sclerosis nodules due to the “even enhancement in 3 phases” in CEUS. The misdiagnosis may be associated with the inadequate angiogenesis of the tumor, which is supplied mainly by the portal vein. Thus, the tumor exhibited similar enhancement to sclerosis nodules. In addition, 1 patient was pathologically diagnosed with well-differentiated HCC, which showed “significant-enhancement in 3 phases” in CEUS and was misdiagnosed as hemangioma. According to the findings of Takahashi et al. [16], well-differentiated tumors usually lack a pseudo-fibrous capsule and newly generated blood vessels, and are often supplied by the hepatic artery and portal vein. These pathological features lead to prolonged arterial phase. However, the blood supply by portal vein in small HCC has a close relationship with the state and the differentiation degree. According to previous reports [17,18] on the degree of differentiation of different HCC, there was significant difference in the time of contrast washout.
The metastatic lesions with hyperecho in 2DUS were mainly of gastrointestinal origin (66.7%). CEUS showed “rapid-filling in, rapid-washout” and “slow-filling in, rapid-washout” patterns. The diagnosis can be easily made according to the primary disease. Another rare malignant tumor, ICC, is difficult to diagnose due to the varied performance and absence of obvious characteristics in 2DUS. According to the findings of Xu et al. [19–21], after administration of contrast, 4 enhancement patterns were observed in arterial phase as follows: peripheral irregular ring-like hyperenhancement, heterogeneous hyperenhancement, homogeneous hyperenhancement, and heterogeneous hypo-enhancement. Among them, the most common pattern is irregular ring-like hyperenhancement (58.8–59.4%). In the present study, the CEUS performance of ICC was similar to previous reports. In addition, 1 inflammatory lesion was misdiagnosed as malignant tumor due to the “grid-like enhancement in arterial phase and low-enhancement in portal and delay phases” in CEUS. On the basis of previous findings [22,23] (disease history, hemogram, tumor markers, irregular morphology, and no significant mass effect in 2DUS), we made the correct diagnosis.
Conclusions
Although liver lesions with hyperecho are diverse in pathology, CEUS can make a definite diagnosis in a majority of these lesions, and the diagnostic accuracy rate is significantly higher than that of traditional 2DUS. Therefore, when the quality of hyperechoic liver lesions cannot be determined by 2DUS, CEUS is an alternative that can improve the diagnostic accuracy, especially in the identification of hyperechoic cirrhotic nodules and small HCC.
Footnotes
Source of support: Departmental sources
References
- 1.Kim TK, Jang HJ. Contrast-enhanced ultrasound in the diagnosis of nodules in liver cirrhosis. World J Gastroenterol. 2014;20(13):3590–96. doi: 10.3748/wjg.v20.i13.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y, Huang GJ. Application progress of contrast-enhanced ultrasound in diagnosis of focal liver lesions. Clin Ultras Med. 2013 [Epub ahead of print] [Google Scholar]
- 3.Jiang YF, Zhou QC, Zhu CY. [Contrast-enhanced ultrasound in the diagnosis of benign and malignant hepatic tumors]. Centr Sou Univ (Med Sci) 2012;37(1):53–56. doi: 10.3969/j.issn.1672-7347.2012.01.010. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 4.Luo W, Numata K, Kondo M, et al. Sonazoid-enhanced ultrasonography for evaluation of the enhancement patterns of focal liver tumors in the late phase by intermittent imaging with a high mechanical index. J Ultrasound Med. 2009;28(4):439–48. doi: 10.7863/jum.2009.28.4.439. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht T, Blomley M, Bolondi L, et al. Guidelines for the use of contrast agents in ultrasound. January 2004. Ultraschall Med. 2004;25(4):249–56. doi: 10.1055/s-2004-813245. [DOI] [PubMed] [Google Scholar]
- 6.Quaia E, Calliada F, Bertolotto M, et al. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232(2):420–30. doi: 10.1148/radiol.2322031401. [DOI] [PubMed] [Google Scholar]
- 7.Zhang JW, Wang XY, Wang Q, Li TT, Yang XH. Contrast enhanced ultrasonographic features of benign focal liver lesions. Chin J Med Ultrasoun (Elec Edn) 2013 [Google Scholar]
- 8.Zheng YY, Ran HT, Wang ZG. The European clinical contrast enhanced ultrasound guide. J Clin Ultras Med. 2008 [Google Scholar]
- 9.Zhang Y, Li R, ZHang XH, et al. Hepatic angiomyolipoma: correlation of contrast-enhanced ultrasound, contrast-enhanced CT and pathologic findings. Chin J Med Ultrasoun (Elec Edn) 2013 [Google Scholar]
- 10.Wang YD, Jing X, Ding JM, et al. Contrast enhanced ultrasound features of hepatic angiomyolipoma. Chin J Med Ultrasoun (Elec Edn) 2013 [Google Scholar]
- 11.Wang Z, Xu HX, Xie XY, et al. Contrast enhanced ultrasound features of hepatic angiomyolipoma. Chin J Ultrasonogra. 2009 [Epub ahead of print] [Google Scholar]
- 12.Prasad SR, Wang H, Rosas H, et al. Fat-containing lesions of the liver: radiologic-pathologic correlation. Radiographics. 2005;25(2):321–31. doi: 10.1148/rg.252045083. [DOI] [PubMed] [Google Scholar]
- 13.Chang Z, Zhang JM, Ying JQ, Ge YP. Characteristics and treatment strategy of hepatic angiomyolipoma: a series of 94 patients collected from four institutions. J Gastrointestin Liver Dis. 2011;20(1):65–69. doi: 10.1007/s11749-010-0230-2. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Zhang X, Hua X, et al. Real-time contrast-enhanced ultrasonography of resected and immunohistochemically proven hepatic angiomyolipomas. Abdom Imaging. 2010;35(6):676–82. doi: 10.1007/s00261-009-9592-x. [DOI] [PubMed] [Google Scholar]
- 15.Chen MH, Dai Y, Yan K, et al. [Early diagnosis of small hepatocellular carcinoma in patients with cirrhosis using contrast-enhanced ultrasound]. Chin J Ultrasonogra. 2005;37(5):458–62. [in Chinese] [PubMed] [Google Scholar]
- 16.Takahashi M, Maruyama H, Ishibashi H, et al. Contrast-enhanced ultrasound with perflubutane microbubble agent: evaluation of differentiation of hepatocellular carcinoma. Am J Roentgenol. 2011;196(2):W123–31. doi: 10.2214/AJR.10.4242. [DOI] [PubMed] [Google Scholar]
- 17.Pei XQ, Liu LZ, Liu M, et al. Contrast-enhanced ultrasonography of hepatocellular carcinoma: correlation between quantitative parameters and histological grading. Br J Radiol. 2012;85(1017):e740–47. doi: 10.1259/bjr/20402927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao JA, Wang AY, Zhang HC, Jin XX. Contrast-enhanced ultrasound analysis of different differentiation degree and pathological types of small hepatocellular carcinoma. Chin J Med Ultrasoun (Elec Edn) 2012 [Google Scholar]
- 19.Xu HX, Chen LD, Liu LN, et al. Contrast-enhanced ultrasound of intrahepatic cholangiocarcinoma: correlation with pathological examination. Br J Radiol. 2012;85(1016):1029–37. doi: 10.1259/bjr/21653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010;51(6):2020–29. doi: 10.1002/hep.23600. [DOI] [PubMed] [Google Scholar]
- 21.Chen LD, Xu HX, Xie XY, et al. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol. 2010;20(3):743–53. doi: 10.1007/s00330-009-1599-8. [DOI] [PubMed] [Google Scholar]
- 22.Zhao HQ, Yang H, He Y, et al. Characteristics of liver focal inflammatory lesions on gray-scal ultrasound and contrast-enhanced imaging. J Guangxi Med Univ. 2013 [Google Scholar]
- 23.Wang S, Yan K, Yang W, Chen MH. Quantitative analysis of liver inflammatory lesions with contrast enhanced ultrasound. Chin J Med Ultrasoun (Elec Edn) 2013 [Google Scholar]