Abstract
Background
Hematopoietic stem cell transplantation (HSCT) has been considered as an effective approach at inducing allogeneic hematopoietic reconstitution and immune tolerance. However, it remains critical to find the optimal HSCT delivery method and robust sources of hematopoietic stem cells (HSCs).
Material/Methods
We introduced a new method by infusing allogeneic endosteal bone marrow cells (BMCs) harvested from long bones endosteum through intra-bone marrow transplantation (IBBMT) into irradiated mice. Recipient mice that were transplanted with central BMCs or through intravenous bone marrow transplantation (IVBMT) were used as controls (n=6 per group). We compared the new method with each control group for allogeneic HSCs homing pattern, peripheral blood chimerism level, skin allograft survival time, and donor stromal cell percentage in recipient BM. AMD3100 was injected to determine whether chemokine stromal cell-derived factor-1 (CXCL-12) was critical for the new method.
Results
More allogeneic HSCs homed into spleen and bone marrow for the new method as compared to each control group. IBBMT of endosteal BMCs led to a higher peripheral blood chimerism and skin allograft survival. At 18 weeks, donor stromal cell percentage in recipient BMCs was higher for the new method than in each control group. By AMD3100 blockade at day 1, peripheral blood chimerism level and donor stromal cell percentage were significantly reduced as compared to the control group without AMD3100 blockade.
Conclusions
Our study suggests that IBBMT of endosteal BMCs is an effective approach for HSCT in inducing allogeneic hematopoietic reconstitution. The advantage is dependent upon the early expression of CXCL-12 after bone marrow transplantation.
MeSH Keywords: Bone Marrow Cells, Hematopoietic Stem Cells, Immune Tolerance, Skin Transplantation, Stem Cell Niche
Background
Allogeneic HSCT (hematopoietic stem cells transplantation) has been established as an effective approach at inducing allogeneic hematopoietic reconstitution and immune tolerance [1,2]. However, it remains very challenging to find a robust source of hematopoietic stem cells (HSCs) and effective delivering approach for allogeneic HSCs [3,4].
After HSCT, donor HSCs are closely related with the function of stromal microenvironment [5–8]. Donor-derived stromal cells can expedite the engraftment of donor HSCs in recipients [9]. All these studies suggest that it may be better to simultaneously establish donor-derived hematopoietic and stromal cells in recipient BM for successful allogeneic HSCT.
Endosteal region is a special region where HSCs reside [10,11]. In vivo tracking analyzes [12] found that allogeneic HSCs specifically home near BM endosteum, in which chemokine stromal cell derived factor-1 (also known as CXCL-12) plays a critical role. By enzyme digestion [13,14], bone marrow cells (BMCs) from the endosteal region can be harvested after central BMCs are flushed. As compared to the central BMCs, BMCs harvested from the endosteal region (endosteal BMCs) have a higher capacity of reconstituting irradiated recipient mice in vivo and a higher proliferative ability in vitro [13]. Furthermore, several independent experimental centers found that stromal stem cells were enriched in endosteal BMCs [15–19]. Consequently, endosteal BMCs may be a robust cell source to reconstitute both hematopoietic and stromal cell populations in recipient mice.
As compared to conventional IVBMT (intravenous bone marrow transplantation), IBBMT (intra-bone marrow transplantation) is considered as a more effective method of BMCs delivery for allogeneic hematopoietic reconstitution [20–22] and immune tolerance induction [23,24]. IBBMT induced a more efficient hematopoietic cell engraftment and higher percentage of immune regulatory cells without cells being trapped in the lungs or liver [21]. On the other hand, most stromal cells in donor BMCs transplant are lost after IVBMT [25–27], while donor-derived stromal cells successfully engrafted in recipient BM for IBBMT [28]. Consequently, IBBMT is potentially an effective approach to deliver allogeneic hematopoietic and stromal cells.
The aim of our study was to explore the possible usefulness of IBBMT of endosteal BMCs for inducing hematopoietic reconstitution and skin allograft survival in a mouse model. The mechanism was in part explained by assessing peripheral blood chimerism level and donor stromal percentage in recipient BMCs after AMD3100 blockade.
Material and Methods
Animals
All animals were purchased from the Fourth Military Medical University Animal Center. Mice used in the experiments were 8–12-week-old female mice. C57bl/6 mice were used as donors (MHC class I H-2kb) and Balb/c mice were used as recipients (MHC class I H-2kd). All recipient animals consumed acidified water for 2 weeks after total body irradiation.
Experimental protocol
All recipients except those for homing assay analysis (detailed in Homing Assay section of this report) were irradiated at day 0 with 4 Gy total body irradiation at 1.06 Gy/min provided by the Fourth Military Medical University Irradiation Center (RS 2000 X-ray, Rad Source Technologies, Inc., 480 Brogdon Road, Suite 500 Suwanee, GA 30024). At day 1, recipient mice were transplanted with 3×106 total BMCs through IBBMT or IVBMT. The experimental group mice were transplanted with endosteal BMCs through IBBMT (abbreviated as IB-eBMCs group). There were 3 control groups: the first control group was transplanted with central BMCs through IBBMT (abbreviated as IB-cBMCs group); the second control group was transplanted with endosteal BMCs through IVBMT (abbreviated as IV-eBMCs group); the third control group was transplanted with central BMCs through IVBMT (abbreviated as IV-cBMCs group), which is also referred to as conventional IVBMT group. There were 6–8 mice in each group.
Antibody and staining reagents
Anti-mouse H-2kb (AF6-88.5.5.3), Sca-1 (D7), c-kit (ACK2) and corresponding isotypes antibodies for flow cytometry were purchased from eBioscience (San Diego, CA, USA). The anti-mouse hematopoietic lineage cocktail biotin (Ter-119, M1/70, RB6-8C5, 145-2C11, RA3-6B2), avidin-Percp cy5.5, CD31 (390), CD45 (30-F11), TER-119 (TER-119) antibodies were purchased from Biolegend (San Diego, CA, USA). 5-(and 6)-Carboxyfluorescein diacetate succinimidyl ester (CFSE) were purchased from eBioscience (San Diego, CA, USA).
Bone marrow cells harvest and transplantation
The bone marrow harvest was performed according to the protocol described by Nakamura [14]. Briefly, the central BMCs were flushed from donor femurs and tibias. The left bones were crushed with a pestle in a mortar. To obtain the endosteal BMCs, the crushing should be gently but thoroughly done because large amounts of fragments may affect the cell suspension later. The enzyme solution (Collagenase I (3 mg/ml) and Dispase (2 mg/ml)) was added into the bone fragments, and we put the solution on the orbital shaker with 300 rpm/min at 37°C for 30 min. PBS was added into the BM fragments to end the digestion, and the supernatant was filtered and centrifuged. In this paper, the BMCs flushed from long bones are referred to as central BMCs (abbreviated as cBMCs), while the BMCs harvested by digestion from bone fragments were referred to as endosteal BMCs (abbreviated as eBMCs). IBBMT was performed as Kushida [22] described. Briefly, 20 μl of cell suspension was injected into the left tibia of recipient mice. The same amount of BMCs in 200 μl of PBS was injected into the retro-orbital plexus of recipient mice.
Homing assay
Donor hematopoietic stem cells (HSCs) were isolated as described by Fujisaki [29]. Briefly, cells were incubated in a solution of a lineage cocktail conjugated with biotin. After washing, cells were incubated with MACS-SA beads (Miltenyi) following the manufacturer’s protocol, and then separated on an LD depletion column in a MidiMACS separation unit to remove lineage-specific cell populations. Lineage-negative cells from donor mice were then stained with c-kit and Sca-1 antibodies. Subsequently, c-kit+Sca-1+Lineage- cells were isolated by a FACS sorting machine (BD FACSAria). All isolated HSCs were stained with CFSE according to the manufacture’s protocol at a final concentration of 5 μM/ml at 37°C for 1 h [30]. CFSE labeled cells (30 000 cells per mouse) were injected with 300 000 unlabeled donor total BMCs through IBBMT or IVBMT, respectively, into unirradiated mice as described by Haylock [13]. At 24 h after transplantation, the recipient mice were sacrificed and single-cell suspension from spleen, thymus, and BM were prepared for flow cytometry analysis.
Flow cytometry
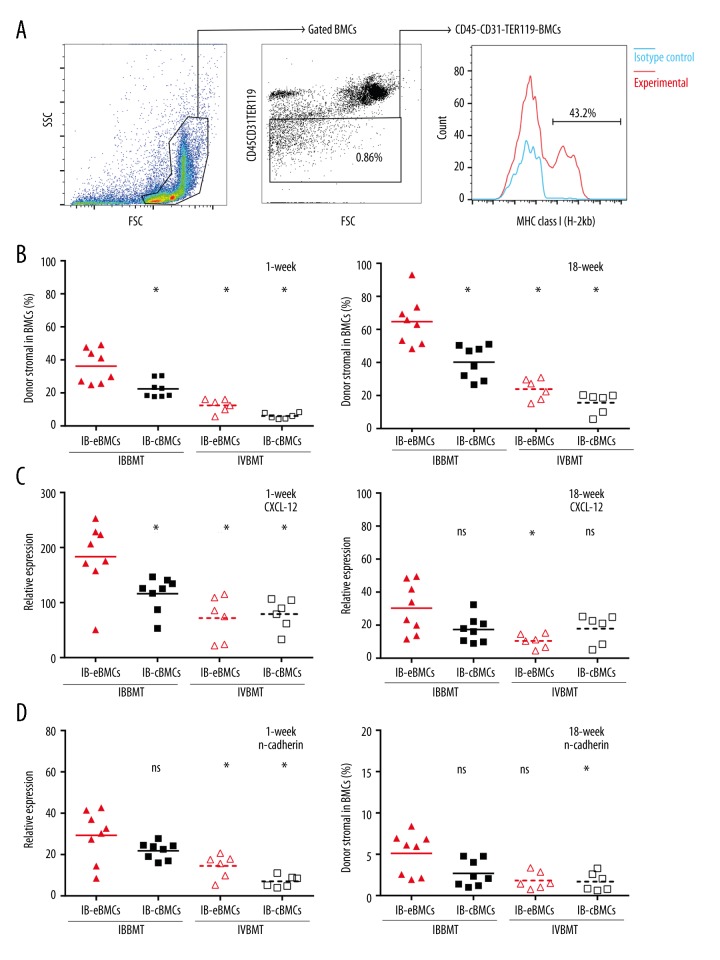
All staining was performed at a cell concentration of 1×106/ml. All peripheral blood marrow cells (PBMCs) were gated according to the protocol described by Krause [31]. The peripheral blood chimerism level was calculated as H-2kb + cells percentage (%) in all PBMCs. The donor HSCs phenotype was H-2kb+ c-kit+ Sca-1+ Lineage- BMCs. Donor CFSE-positive cells were calculated as CFSE-positive cells in CD45+ cell population. The gate was set according to isotype control. The flow cytometry gating protocol for donor stromal cells in recipient BMCs is shown in Figure 1A. Donor stromal cell population are represented as H-2kb+CD45-CD31-TER119- cells in all BMCs (%).
Figure 1.
The analysis of recipient stromal cell replacement and gene expressions of recipient BMCs. (A) The gating protocol for donor-derived stromal cells in recipient BMCs. FSC, Forward Scatter. SSC, Side Scatter. Blue line, isotype control. (B, C) Donor stromal percentage in recipient BMCs were assessed at 1 (B) and 18 (C) weeks after BMT. Donor stromal cell population were H-2kb+ CD45-CD31-TER119- cells in BMCs harvested from the left tibia BMs. *, p value for IB-eBMCs versus each control group, p<0.05. Each dot represents an individual mouse result and bars are the mean for each group (%), n=6 per group. (D–G) Quantitative PCR analyzes of CXCL-12 (D, E) and n-cadherin (F, G) expression of recipient BMCs were performed at 1 week (D, F) and 18 weeks (E, G) after BMT. The corresponding gene expression level of BMCs from normal untreated mice was taken as control after normalization to GAPDH. All the bars represent the mean folds increase relative to that of normal mice BMCs for each group, n=6–8 per group. *, p value of IB-eBMCs group versus each control group (IB-cBMCs, IV-eBMCs, IV-cBMCs group respectively), p<0.05. ns is for not significant.
Skin Transplantation
Skin transplantation was performed according to IACUC-approved procedures. Briefly, the skin graft recipient site was prepped with iodine. A 1×1 cm area was marked in the left lateral thoracic area. The marked skin was incised with a surgical scissor just above the panniculus carnosus. Then the skin pad was gently pulled off the underlying panniculus carnosus where the blood supply should be kept intact. The donor tail skin was put on the bed and fixed with sutures after the hemostasis was assured. A firm bandage was added for protection and immobilization of the skin graft. The bandage was removed at 10 days after skin transplantation. Time of rejection was defined as the first day on which the entire epidermal surface of the graft was necrotic [32].
Quantitative PCR
The harvested BMCs were resuspended into the RNA isolation lysis buffer of TaKaRa MiniBEST Universal RNA Extraction Kit (Code No. 9767, TaKaRa, Dalian, China). The reverse transcription was performed using the Takara PrimeScriptTM RT Master Mix (Perfect Real Time) according to the manufacturer’s instructions. Quantitative real-time PCR was performed as previously described [33]. The relative mRNA quantity was calculated using the ΔΔCt method [34]. Gene expression was normalized to GAPDH and normal mouse BMCs gene expression was taken as baseline. The sequences of the primers used in this paper are listed in Table 1.
Table 1.
Primer sequence used in this paper.
| Gene name | Forward/reverse | Sequence |
|---|---|---|
| n-Cadherin | Forward | 5′-CAATGATGGGCTAGTCACAGTGG-3′ |
| n-Cadherin | Reverse | 5′-CGACTGAGGTGGGTGCTGAA-3′ |
| CXCL-12 | Forward | 5′-CCCGAAATTAAAGTGGATCCAAGAG-3′ |
| CXCL-12 | Reverse | 5′-GCGAGTTACAAAGCGCCAGAG-3′ |
| GAPDH | Forward | 5′-TGTGTCCGTCGTGGATCTGA-3′ |
| GAPDH | Reverse | 5′-TTGCTGTTGAAGTCGCAGGAG-3′ |
AMD3100 blockade
AMD3100 blockade was performed according to the method described by Winkler [35]. AMD3100 (Selleckchem) was injected intraperitoneally as a single 16 mg/kg dose at day 1 or 7 for IB-eBMCs and IB-cBMCs groups.
Statistical analyzes
Data were analyzed using Prism GraphPad 6.0 software (GraphPad Software, Inc., San Diego, CA). The paired t test was used for comparison between the injected BM and non-injected BM data. The Student’s t test was used for comparison between other non-paired data. The log-rank (Mantel-Cox) test was performed to compare the survival curves of 2 groups. P<0.05 was considered statistically significant.
Results
Allogeneic HSCs efficiently homed to spleen and BMs through intra-bone injection of endosteal BMCs
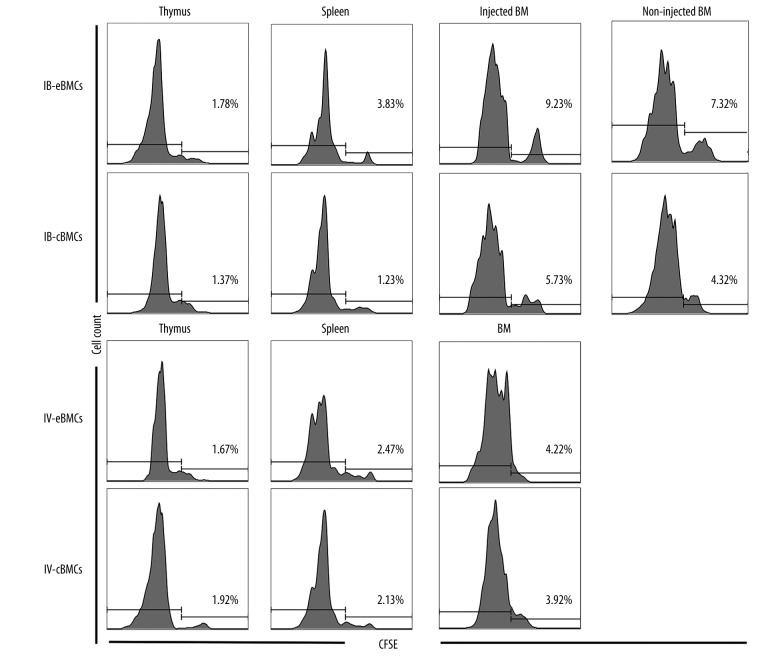
The previous work by Haylock [13] showed that HSCs from endosteal region had a higher capacity of homing into recipient BMs. To determine whether IBBMT delivery of endosteal BMCs improved donor HSCs homing, we performed a homing assay by tracking CFSE-labeled allogeneic HSCs. We found that more donor-derived cells in the IB-eBMCs group than in either control group homed in spleen and BMs (both the injected and non-injected BM) but not in thymus (Table 2, Figure 2). Of note, the frequency of donor-derived cells was highest in the injected BM for both IB-eBMCs and IB-cBMCs groups. IBBMT of endosteal BMCs had a higher efficiency of homing into recipient lymphoid organ and BMs.
Table 2.
HSCs homing analysis after CFSE-labeled HSCs injection at Day 1 after BMT.
| Groups | CFSE positive cells (%) | |||
|---|---|---|---|---|
| Thymus | Spleen | Injected BM | Non-injected BM | |
| IB-eBMCs (n=6) | 1.98±0.44 | 3.34±0.63 | 9.93±1.36 | 7.2±1.48 |
| IB-cBMCs (n=6) | 2.05±0.33 | 1.06±0.28* | 5.30±1.05* | 4.9±0.47# |
| IV-eBMCs (n=6) | 1.81±0.42 | 2.35±0.39* | 4.94±1.08*,# | |
| IV-cBMCs (n=6) | 1.66±0.18 | 2.17±0.52* | 4.13±0.85*,# | |
Donor HSCs were isolated and labeled with CFSE. Suspended CFSE-labeled HSCs and unlabeled total BMCs were injected into the unirradiated recipient mice of different groups as described in Materials and Methods. 24 hours after HSCT, all animals were sacrificed to harvest cells from thymus, spleen and BMs for flow cytometry analysis. For IBBMT groups, BMs were distinguished as injected BM or non-injected BM. The results were expressed as the Mean ± SD (%) of 6 mice.
Represented p value for CFSE positive cells (%) in locations other than non-injected BM (Thymus, Spleen, Injected BM) of IB – eBMCs group versus each control group (IB – cBMCs, IV – eBMCs, IV – cBMCs group).
Represented p value for CFSE positive cells (%) in non-injected BM of IB-eBMCs versus each control group;
, p<0.05.
Figure 2.
Representative data of homing assay analyzes. CFSE-labeled donor HSCs were injected into unirradiated recipient mice at 0-day. Recipient mice were sacrificed 24 hours later for CFSE-positive cell percentage in thymus, spleen, bone marrows (injected BM and non-injected BM, respectively, for IB-eBMCs and IB-cBMCs group). CFSE-positive cell percentage was calculated as CFSE positive cells in CD45+ cells. Each diagram represents the CFSE-positive cell percentage data of 6 mice for 1 specific location.
Intra-bone delivery of endosteal BMCs increased donor PBMCs chimerism and prolonged skin transplantation survival
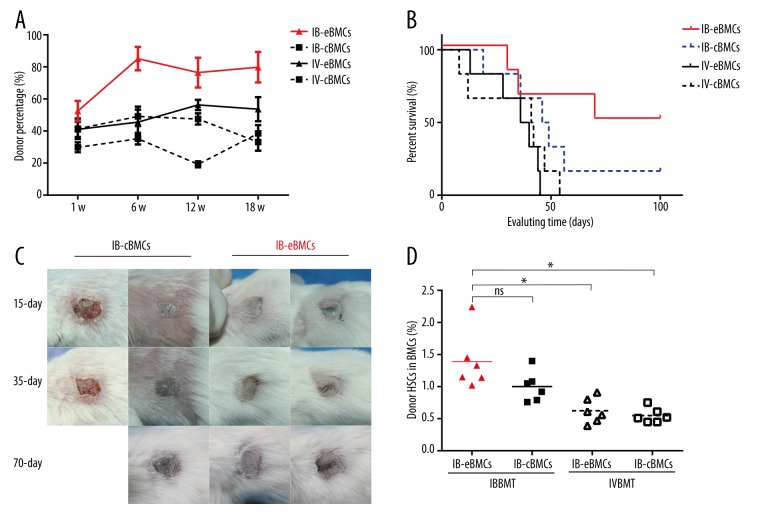
To test whether endosteal BMCs through IBBMT can improve donor hematopoietic cell engraftment after BMT, we assessed PBMCs chimerism level at weeks 1, 6, 12, and 18 and after BMT. We found that only through IBBMT of endosteal BMCs, the PBMCs chimerism can be kept at a relative higher level than that of each control group (Figure 3A). In addition, we found that the donor HSCs percentage for the IB-eBMCs group was higher than that for IVBMT control groups (Figure 3D).
Figure 3.
Peripheral blood mixed chimerism and skin allograft survival curve analyzes. (A) Donor-derived cell percentage in recipient PBMCs was assessed at 1, 6, 12, and 18 weeks after BMT. All the results were represented as Mean ±SD (%), n=6 for each group. (B) At 12 weeks after BMT, donor tail skin allotransplant was transplanted. Every plotted point in the diagram represented an individual skin graft rejection (n=6 per group). (C) Representative skin graft pictures of IB eBMCs group (right 2 columns) and IB-cBMCs (left 2 columns). Every column represents pictures of an individual skin graft at 15, 35, and 70 days after skin transplantation. (D) At 70 days after skin transplantation, the recipient mice were sacrificed to assess the HSCs ratio in BMCs. The data were compared between IB eBMCs group and each control group (IB-cBMCs, IV-eBMCs, IV-cBMCs group respectively) (*, p<0.05; ns for not significant). Each dot is a recipient mouse result and bars are the mean for each group, n=6 for each group.
To explore whether hematopoietic cell reconstitution can improve donor-specific skin allotransplant survival, allogeneic skin transplantation was performed at week 12 after BMT. We found that only for the IB-eBMCs group, donor-derived skin graft survival time was prolonged (Figure 3B, 3C). The intravenous delivery of eBMCs did not prolong the skin allograft survival compared to the IV-cBMCs group (Figure 3B). All these results presented here show that intra-bone delivery of endosteal BMCs could better reconstitute donor hematopoietic and immune systems.
Intra-bone delivery of endosteal BMCs led to a higher level of donor stromal cell percentage and CXCL-12 gene expression
Previous studies by Short [15,17] have shown that endosteal BMCs have a higher percentage of stromal stem cells. To determine whether IBBMT of allogeneic endosteal BMCs can facilitate efficient donor stromal cell engraftment, we assessed donor stromal cell (H-2kb+ CD45-CD31-TER119-) percentage in recipient BMCs at 1 and 18 weeks after BMT. The gating protocol is shown in Figure 1A. We found that for IB-eBMCs group, an average of 36.18% (Figure 1B) and 61.68% (Figure 1C) recipient stromal cells were replaced by donor ones at 1 and 18 weeks, respectively. Donor stromal cell percentage for the IB-eBMCs group was significantly higher than that for the IB-cBMCs group at both evaluation points indicated (Figure 1B, 1C). Despite the harvested region of allogeneic BMCs (cBMCs or eBMCs), a small fraction of recipient stromal cells were replaced by the donor ones through IVBMT (Figure 1B, 1C). Therefore, IBBMT of endosteal BMCs could more successfully deliver donor stromal cells to recipient BM than IVBMT.
Allogeneic HSCs engraftment was closely related to CXCL-12 and n-cadherin, which is characteristically expressed in BM endosteum. To explore the homing related genetic expression change after BMT, we assessed the expression of CXCL-12 and n-cadherin at 1 and 18 weeks in recipient BM. At 1 week, the CXCL-12 expression for the IB-eBMCs group (183.3±62.63-fold increase) was significantly higher than in each control group (Figure 1D) but not at 18 weeks (Figure 1E). At 1 week, the n-cadherin expression for IB-eBMCs was significantly higher than those for IVBMT control groups (Figure 1F) but not at 18 weeks (Figure 1G).
AMD3100 blockade reduced the PBMCs chimerism level and BM stromal cell percentage
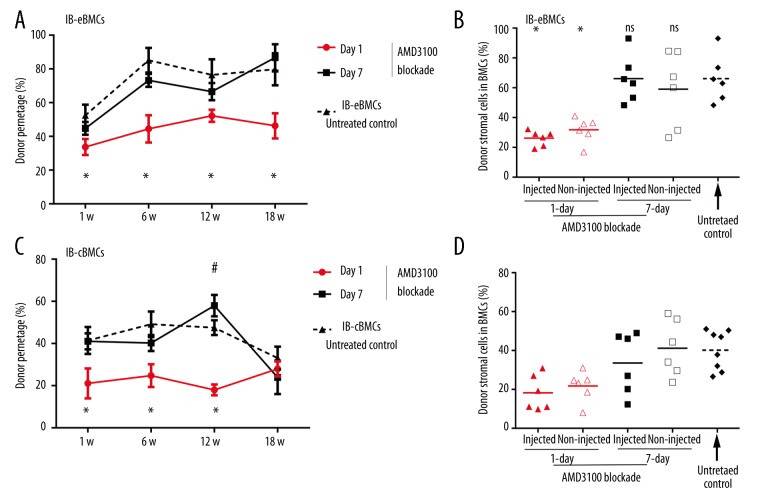
Data acquired so far suggested CXCL-12 may be related with the improved donor hematopoietic and stromal cells engraftment for the IB-eBMCs group. To determine whether the superiority of IBBMT of endosteal BMCs is dependent upon CXCL-12, we injected AMD3100 intraperitoneally at 1 or 7 days for IB-eBMCs and IB-cBMCs groups. As compared with the data from IB-eBMCs or IB-cBMCs groups without AMD3100 blockade (Figure 1A, 1C), AMD3100 injection at 1 day after BMT significantly reduced the PBMCs chimerism level (Figure 4A) assessed at 18 weeks. However, the AMD3100 blockade at 7 day did not reduce PBMCs chimerism level (Figure 4A, 4C).
Figure 4.
The effects of AMD3100 blockade on peripheral blood chimerism and donor stromal percentage for IB-eBMCs and IB-cBMCs groups. AMD3100 was injected intraperitoneally at 1- or 7-days at 16 mg/kg per mouse for IB-eBMCs (A, B) and IB-cBMCs (C, D) groups. (A, C) The PBMCs chimerism level was assessed at 1-, 6-, 12-, and 18-weeks after BMT. PBMCs chimerism level data for the IB-eBMCs (A) or IB-cBMCs (C) without AMD3100 injection (dashed lines) was taken as the untreated control. All results were represented as Mean ±SD (%), n=6 per group. *, p value of day 1 injection versus untreated control; #, p value of day 7 injection versus untreated control, ns is for not significant. *, #, p<0.05. (B, D). Donor stromal cells percentage in injected and non-injected BM of IB-eBMCs (B) and IB-cBMCs (D) groups with AMD3100 injection at 1 (triangles) or 7 days (quadrilaterals) were analyzed at 18 weeks after BMT as compared with corresponding untreated controls. Each dot is a result of recipient mice and bars are the mean for each group (%).*, p<0.05.
To test whether AMD3100 blockade influence the allogeneic stromal cell engraftment, we assessed donor stromal cell percentage at 18 weeks after BMT. Consistent with the results of PBMCs mixed chimerism, only when the AMD3100 was injected at 1 day, the donor stromal cell percentage in BMCs was significantly reduced for the IB-eBMCs group (Figure 4B) and IB-cBMCs group (Figure 4D). However, no significant reduction was seen with AMD3100 injection at 7 days (Figure 4B, 4D).
Discussion
In the present study, we found that intra-bone delivery of BMCs harvested from endosteal region provided an efficient way of reconstituting allogeneic hematopoietic and stromal cells in recipient BMs. CXCL-12 pathway played a critical role immediately after IBBMT.
The advantage of endosteal BMCs was attributed to the higher capacity of proliferation and homing efficiency of endosteal BMCs [13]. Evidence also highlighted the importance of contact between stromal and donor HSCs [36]. In our study, we for the first time delivered allogeneic endosteal HSCs into recipient BM. Consistent with previous characterization of endosteal HSCs, more allogeneic HSCs harvested from endosteal region homed into recipient spleen and BMs, but not thymus. In addition, more HSCs homed into injected BM than contralateral BM through IBBMT, despite the source of HSCs. All these results suggest that HSCs from endosteal region have a better homing efficiency into lymphoid organs and BMs through the intra-bone approach.
IBBMT of central BMCs induced a higher and persistent mixed chimerism level and prolonged donor allograft survival than IVBMT [22,23]. In our study, we found IBBMT of endosteal BMCs induced a higher and more persistent mixed chimerism level in peripheral blood and prolonged skin allograft survival as compared to IBBMT and IVBMT of central BMCs. Our results suggest that IBBMT of allogeneic endosteal BMCs can be a useful method to induce donor hematopoietic reconstitution and skin allograft survival.
Intravenous transplantation of donor stromal or even stromal stem cells is of low efficiency, while IBBMT can induce a higher percentage of donor stromal cells in recipient BMs [37,38]. In this study, we found that IBBMT of endosteal BMCs more efficiently induce donor stromal cell engraftment than intravenous or intra-bone transplantation of central BMCs. One explanation is that our new method successfully delivered endosteal BMCs with a higher frequency of donor stromal stem cells [15,17] into recipient BMs. However, it remains to be determined exactly what cell population in these donor stromal cells may play a critical role in facilitating allogeneic hematopoietic cell engraftment.
The HSCs engraftment involves complex and ordered procedures and CXCL-12 was proved to play a critical role in the first step of HSCs engraftment [12,39]. AMD3100 is a special antagonist to disentangle HSCs from stromal cells [35,40]. In our study, we found that intra-bone delivery of endosteal BMCs led to a higher CXCL-12 expression. AMD3100 blockade at 1 day reduced the otherwise higher peripheral mixed chimerism and donor stromal cell percentage for IB-eBMCs and IB-cBMCs groups. The timing of blockade was so critical that 7-day blockade did not affect the results at all. All these results suggest that the early CXCL-12 dependent contact of endosteal BMCs with recipient stroma is critical in facilitating both hematopoietic and stromal engraftment. It remained to be seen whether this decrease of donor mixed chimerism was due to the absence of HSCs contact with donor stromal cells or the reduced frequency of donor HSCs in recipient BM.
Conclusions
In this study, we performed a new approach of HSCT by delivering allogeneic endosteal BMCs into recipient BMs. We found that this new method could facilitate the homing efficiency of allogenic HSCs and induced a higher mixed chimerism and donor stromal chimerism. This advantage could be attributed to an early expression of CXCL-12. This method provides new insights into finding new HSCs sources. In clinical settings, the endosteal BMCs could be mobilized to provide a robust source of donor HSCs and the intra-bone approach offers an efficient transplantation channel to induce donor hematopoietic and stromal cell reconstitution. Further profiling of endosteal hematopoietic and stromal composition is required to further explore this new method.
Footnotes
Source of support: This research is supported by National Natural Science Foundation of China #30830102, #30801003, Xijing Hospital Basic Research Grant #XJZT12M
References
- 1.Eaves CJ. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood. 2015;125:2605–13. doi: 10.1182/blood-2014-12-570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granados JM, Benichou G, Kawai T. Hematopoietic stem cell infusion/transplantation for induction of allograft tolerance. Curr Opin Organ Transplant. 2015;20:49–56. doi: 10.1097/MOT.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallardo D, de la Camara R, Nieto JB, et al. Is mobilized peripheral blood comparable with bone marrow as a source of hematopoietic stem cells for allogeneic transplantation from HLA-identical sibling donors? A case-control study. Haematologica. 2009;94:1282–88. doi: 10.3324/haematol.2009.006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haspel RL, Miller KB. Hematopoietic stem cells: source matters. Curr Stem Cell Res Ther. 2008;3:229–36. doi: 10.2174/157488808786734033. [DOI] [PubMed] [Google Scholar]
- 5.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azizidoost S, Babashah S, Rahim F, et al. Bone marrow neoplastic niche in leukemia. Hematology. 2014;19:232–38. doi: 10.1179/1607845413Y.0000000111. [DOI] [PubMed] [Google Scholar]
- 7.Calvi LM, Link DC. Cellular complexity of the bone marrow hematopoietic stem cell niche. Calcif Tissue Int. 2014;94:112–24. doi: 10.1007/s00223-013-9805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He N, Zhang L, Cui J, Li Z. Bone marrow vascular niche: home for hematopoietic stem cells. Bone Marrow Res. 2014;2014:128436. doi: 10.1155/2014/128436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliopoulos N, Stagg J, Lejeune L, et al. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–65. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 10.Asada N, Katayama Y. Regulation of hematopoiesis in endosteal microenvironments. Int J Hematol. 2014;99:679–84. doi: 10.1007/s12185-014-1583-1. [DOI] [PubMed] [Google Scholar]
- 11.Levesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–92. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–99. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 13.Haylock DN, Williams B, Johnston HM, et al. Hemopoietic stem cells with higher hemopoietic potential reside at the bone marrow endosteum. Stem Cells. 2007;25:1062–69. doi: 10.1634/stemcells.2006-0528. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Arai F, Iwasaki H, et al. Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood. 2010;116:1422–32. doi: 10.1182/blood-2009-08-239194. [DOI] [PubMed] [Google Scholar]
- 15.Short BJ, Brouard N, Simmons PJ. Prospective isolation of mesenchymal stem cells from mouse compact bone. Methods Mol Biol. 2009;482:259–68. doi: 10.1007/978-1-59745-060-7_16. [DOI] [PubMed] [Google Scholar]
- 16.Zhu H, Guo ZK, Jiang XX, et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550–60. doi: 10.1038/nprot.2009.238. [DOI] [PubMed] [Google Scholar]
- 17.Short B, Wagey R. Isolation and culture of mesenchymal stem cells from mouse compact bone. Methods Mol Biol. 2013;946:335–47. doi: 10.1007/978-1-62703-128-8_21. [DOI] [PubMed] [Google Scholar]
- 18.Grassinger J, Haylock DN, Williams B, et al. Phenotypically identical hemopoietic stem cells isolated from different regions of bone marrow have different biologic potential. Blood. 2010;116:3185–96. doi: 10.1182/blood-2009-12-260703. [DOI] [PubMed] [Google Scholar]
- 19.Suire C, Brouard N, Hirschi K, Simmons PJ. Isolation of the stromal-vascular fraction of mouse bone marrow markedly enhances the yield of clonogenic stromal progenitors. Blood. 2012;119:e86–e95. doi: 10.1182/blood-2011-08-372334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siemionow M, Zielinski M, Ozmen S, Izycki D. Intraosseus transplantation of donor-derived hematopoietic stem and progenitor cells induces donor-specific chimerism and extends composite tissue allograft survival. Transplant Proc. 2005;37:2303–8. doi: 10.1016/j.transproceed.2005.03.127. [DOI] [PubMed] [Google Scholar]
- 21.Baba S, Inaba M, Iwai H, et al. Intra-bone marrow-bone marrow transplantation facilitates hemopoietic recovery including dendritic cells. Immunobiology. 2005;210:33–42. doi: 10.1016/j.imbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Kushida T, Inaba M, Hisha H, et al. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood. 2001;97:3292–99. doi: 10.1182/blood.v97.10.3292. [DOI] [PubMed] [Google Scholar]
- 23.Guo K, Inaba M, Li M, et al. Long-term donor-specific tolerance in rat cardiac allografts by intrabone marrow injection of donor bone marrow cells. Transplantation. 2008;85:93–101. doi: 10.1097/01.tp.0000296061.71662.76. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki S, Hisha H, Mizokami T, et al. Successful acceptance of adult liver allografts by intra-bone marrow-bone marrow transplantation. Stem Cells Dev. 2008;17:629–39. doi: 10.1089/scd.2007.0218. [DOI] [PubMed] [Google Scholar]
- 25.Koide Y, Morikawa S, Mabuchi Y, et al. Two distinct stem cell lineages in murine bone marrow. Stem Cells. 2007;25:1213–21. doi: 10.1634/stemcells.2006-0325. [DOI] [PubMed] [Google Scholar]
- 26.Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–70. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Liu Y, Kalajzic Z, et al. Heterogeneity of engrafted bone-lining cells after systemic and local transplantation. Blood. 2005;106:3650–57. doi: 10.1182/blood-2005-02-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn JY, Park G, Shim JS, et al. Intramarrow injection of beta-catenin-activated, but not naive mesenchymal stromal cells stimulates self-renewal of hematopoietic stem cells in bone marrow. Exp Mol Med. 2010;42:122–31. doi: 10.3858/emm.2010.42.2.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujisaki J, Wu J, Carlson AL, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–19. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingulli E. Tracing tolerance and immunity in vivo by CFSE-labeling of administered cells. Methods Mol Biol. 2007;380:365–76. doi: 10.1007/978-1-59745-395-0_23. [DOI] [PubMed] [Google Scholar]
- 31.Krause DS, Delelys ME, Preffer FI. Flow cytometry for hematopoietic cells. Methods Mol Biol. 2014;1109:23–46. doi: 10.1007/978-1-4614-9437-9_2. [DOI] [PubMed] [Google Scholar]
- 32.Markees TG, Phillips NE, Gordon EJ, et al. Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4(+) T cells, interferon-gamma, and CTLA4. J Clin Invest. 1998;101:2446–55. doi: 10.1172/JCI2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkler IG, Pettit AR, Raggatt LJ, et al. Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 2012;26:1594–601. doi: 10.1038/leu.2012.17. [DOI] [PubMed] [Google Scholar]
- 36.Massollo M, Podesta M, Marini C, et al. Contact with the bone marrow microenvironment readdresses the fate of transplanted hematopoietic stem cells. Exp Hematol. 2010;38:968–77. doi: 10.1016/j.exphem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Pereira RF, O’Hara MD, Laptev AV, et al. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA. 1998;95:1142–47. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muguruma Y, Yahata T, Miyatake H, et al. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107:1878–87. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- 39.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–30. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]