Abstract
Food resources are often critical regulating factors affecting individual fitness and population densities. In the Himalayan Mountains, Bharal “blue sheep” (Pseudois nayaur) are the main food resource for the endangered snow leopard (Panthera uncia), as well as being preyed upon by other predators. Blue sheep, however, may face a number of challenges including food resource competition with other wild and domestic ungulates, and hunting pressure. Here, we characterized the diet of blue sheep in the Annapurna Conservation Area (ACA) of Nepal and conducted proximate nutritional analysis on a limited number of plants identified as foods. Furthermore, we investigated the macronutrient and fiber balance of these plants using nutritional geometry which is a state‐space approach to modeling multidimensional and interactive nutritional aspects of foraging. A total of 19 plant species/genera were identified in blue sheep pellets using microhistological analysis. On average, across seasons and regions of the study area, the two most frequently occurring plants in pellets were graminoids: Kobressia sp. and Carex spp. The macronutrient balance of Kobresia sp. was relatively high in carbohydrate and low in protein, while other plants in the diet were generally higher in protein and lipid content. Analysis of fiber balance showed that the two most consumed plants of blue sheep (i.e., Kobresia spp. and Carex spp.) contained the highest concentration of hemicellulose, which is likely digestible by blue sheep. The hemicellulose and lignin balance of plants ranged relatively widely, yet their cellulose contents showed less variation. Foraging by blue sheep may therefore be a balance between consuming highly digestible high‐carbohydrate plants and plants less‐digestible but higher in protein and/or lipid.
Keywords: Fiber, macronutrients, naur, nutritional ecology, nutritional geometry, right‐angled mixture triangle
Introduction
Food resources are often critical regulating factors affecting individual growth and population densities (Miyashita 1992; Raubenheimer and Simpson 1997; Carbone and Gittleman 2002; Simpson et al. 2004; Brasher et al. 2007). This includes large ungulate prey species, as ungulate biomass has been shown to depend upon regional food availability (Fritz and Duncan 1994). Herbivores, however, face numerous challenges related to food resources and nutrition, including nutritionally imbalanced foods (Wehi et al. 2013; Nie et al. 2014), plant toxins (Rosenthal and Berenbaum 1991), and incompletely digestible fiber (Milton 1979).
In the Himalayan mountains, native blue sheep (also known as naur and bharal; Pseudois nayaur) are the main prey species of the endangered snow leopard (Panthera uncia; >60% of their diet) and are also prey of other predators (Aryal et al. 2013, 2014a,b). The blue sheep is widely distributed, being found in mountainous areas of China, Nepal, Pakistan, and India (Aryal et al. 2013; Harris 2014), with highest abundance occurring in the Himalayan region of Nepal (Oli et al. 1993; Aryal et al. 2014a). Within Nepal, maintaining healthy blue sheep populations is considered important in reducing livestock depredation by snow leopard, thereby reducing human‐wildlife conflict (Oli et al. 1994; Aryal et al. 2014a,b,c). Factors influencing the distribution and abundance of blue sheep in Nepal include trophy hunting, which may be unsustainable (Aryal et al. 2010), and habitat preferences (Aryal et al. 2013). Blue sheep may also face competition with domestic sheep and goats due to dietary overlap, which will likely become more of a problem as pastoral use increases (Mishra et al. 2004; Shrestha et al. 2005; Raubenheimer 2011).
An increasing body of research has been devoted to blue sheep, including studies of: diet and ecology (Mishra et al. 2004, Shrestha et al. 2005; Shrestha and Wegge 2008); population dynamics (Oli and Rogers 1991; Aryal et al. 2014a); and general species information and habitat preferences (Cincotta et al. 1991; Schaller and Gu 1994; Harris and Miller 1995; Miller and Schaller 1998; Namgail et al. 2004; Namgail 2006). Yet despite these advances, there is a lack of information regarding the diet and nutritional ecology of blue sheep, especially the nutrient composition and balance of foods.
Studies utilizing nutritional geometry, a state‐space approach to modeling the multidimensional and interactive effects of nutrients, have demonstrated that the balance of macronutrients (protein, carbohydrate, and fat) in foods is a driving force behind animal foraging behavior across several taxa (Felton et al. 2009; Rothman et al. 2011; Simpson and Raubenheimer 2012; Johnson et al. 2013) rather than simply energy or single nutrient (e.g., protein) intake per se (Erlenbach et al. 2014; Solon‐Biet et al. 2014; Kohl et al. 2015; Simpson et al. 2015). These physiological and behavioral preferences have ecological effects, for example, the macronutrient balance of foods has been shown to strongly influence the body composition (e.g., lean vs. fat mass) of the consumer (Solon‐Biet et al. 2014), and predator body composition can be directly related to that of its prey (Hawlena and Schmitz 2010; Hawley et al. 2014). In fact, predation risk can influence the macronutrient selection of herbivores, which can result in changes in their body composition and ecosystem nutrient transfer (Hawlena and Schmitz 2010). Furthermore, macronutrient regulation can be used to inform the nutritional ecology of wild animals (Kearney et al. 2010; Coogan et al. 2014), and also their conservation (Raubenheimer and Simpson 2006; Raubenheimer et al. 2012). Studies incorporating the concept of macronutrient balance can, therefore, be extremely useful in understanding the habitat requirements, temporal nutrient dynamics, and nutritional constraints faced by wild animals by, for example, providing predictive models of foraging behavior based on the nutrient content of foods (Coogan et al. 2014). Such studies are of importance for large ungulate prey species that support populations of predators, such as the blue sheep.
The objective of this paper was to investigate the diet of blue sheep in the Annapurna Conservation Area (ACA) of Nepal. Our study focused on the diet of blue sheep in the Mustang and Manang regions of the ACA, with an emphasis on the remote Mustang region for which there is a lack of information due to its relatively isolated location (Aryal et al. 2014a). To that end, we identified plants found in blue sheep pellets using microhistological analysis. In addition, we conducted proximate nutritional analysis on a limited (due to logistical constraints) number of plants consumed by blue sheep and investigated the macronutrient and fiber balance of these plants using the right‐angled mixture triangle (RMT) which is a geometric analysis used to investigate the proportions of nutrients in foods or mixtures (Raubenheimer 2011; Raubenheimer et al. 2014).
Material and Methods
Study area
The study was carried out from January 2010 to June 2011 in the Yak Kharka region of the Manang district, as well as the Upper and Nammu regions of the Mustang districts of the ACA, which is located in the Trans‐Himalayan region of Nepal (Fig. 1). The Yak Kharka region experiences diverse climatic conditions due to a wide range in elevation (1600 m–8156 m), and a large portion (>1000 km2) of land is used for grazing by livestock (Aryal et al. 2014a). The upper Mustang region lies in the subalpine zone and experiences intense winds and solar radiation, and the entire area remains snow covered from approximately November to March (Aryal et al. 2014d). A more detailed description of the Yak Kharka and upper Mustang regions is given in Aryal et al. (2014a). The Nammu area of the Mustang district lies northeast of the district headquarters of Mustang district (i.e., approximate 20 km east from Jomsom; Fig. 1). In general, the study areas represent grassland habitat typical of the Trans‐Himalayan landscape. A human population of >30,000 resides in the study area and livestock farming was the main source of income in both districts (Manang and Mustang districts). Elevation in both study areas ranges from 2800 m to 6000 m and experiences low precipitation (<1000 mm/year; Aryal et al. 2014a,b,c). Predators in the study area include snow leopard, brown bear (Ursus arctos), wolf (Canis lupus), and jackal (Canis aureus; Aryal et al. 2014a,b). Other prey species in the study area include Tibetan argali (Ovis ammon hodgonii), Tibetan gazelle (Procapra picticaudata), and wild ass (Equus kiang) among others (Aryal et al. 2012b).
Figure 1.
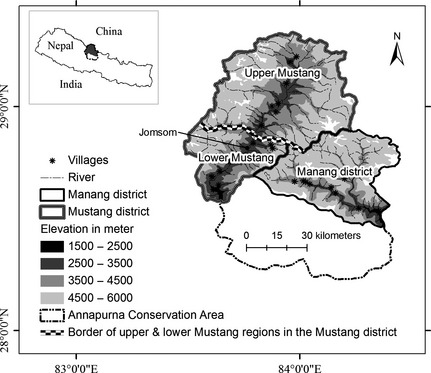
Map of the study area showing location within Nepal (upper) and the Manang and Mustang districts within Annapurna Conservation Area.
Diet composition of blue sheep
Due to logistical constraints and the patterns of distribution of the blue sheep, fecal pellets were collected in different regions of the study area at different times, including: January–February 2010 in the Yak Kharka, Manang (n = 48); February–March 2010 in the Upper Mustang (n = 61); May 2011 in the Upper Mustang (n = 58); and June–July 2011 in the Nammu area, Mustang (n = 43). Fecal pellets of blue sheep were collected after following blue sheep herds. Herds were located by direct observation, as they are relatively easy to sight in the study area. Once we located a herd, we followed the group and collected fresh pellet samples. Pellet samples were collected in plastic bags (one sample per bag), and later transferred to the Institute of Forestry, Pokhara, Nepal, for fecal laboratory analysis. Pellet samples were processed using microhistological diet analysis (Sparks and Malechek 1968; Shrestha et al. 2005; Aryal et al. 2012b,c). First, samples were oven‐dried at 40°C overnight (12 h) and then ground using a grind. Samples were then processed following established techniques for garments slide preparation (Sparks and Malechek 1968; Holechek and Gross 1982; Aryal et al. 2012c, 2014b). After grading the samples, fragments were washed with 2% ethanol (C2H6O) in order to keep them dry and sieved (1–0.3 mm). Samples were washed in 5% potassium hydroxide (KOH) to remove black colors from plant fragments and then passed through ethanol and finally xylene (C8H10) to remove moisture remains inside the fragments (Sparks and Malechek 1968; Holechek and Gross 1982; Aryal et al. 2012c, 2014b). We followed similar methods in preparing reference slides of plant species, which we used to identify plant fragments in fecal samples available. A total of 38 plant species were collected from the field and prepared as reference slides. Samples were selected based on a previous diet study of blue sheep (Shrestha et al. 2005) and availability of species in this region. For each permanent slide, 20 fecal plant fragments were randomly selected and identified to species using the reference species slides, and unidentified plant fragments were categorized as “unidentified” (Sparks and Malechek 1968; Holechek and Gross 1982; Aryal et al. 2012c, 2014b). After identifying plant species, we estimated the relative frequency (RF; %) of each species (Sparks and Malechek 1968; Aryal et al. 2012a; Panthi et al. 2012; Aryal et al. 2014b).
There are some limitations to microhistological analysis, as plants may not appear in scat in the proportion they were consumed depending upon digestibility; however, the method has been used successfully to rank plant species eaten by animals (Mcinnis et al. 1983). In order to correct for differential digestibly of plant material, we used conversion factors (CF) developed by Shrestha et al. (2005), which were based on bite counts and micro‐histological analysis of a similar species (domestic goat; Capra hircus), from the upper Mustang region and used to evaluate the diet of blue sheep and Tibetan argali. Specifically, we multiplied food items RF by the appropriate CF: 1.208 for graminoids; 5.311 for forbs; and 0.850 for woody browse (Shrestha et.al. 2005). We then summed the corrected RF estimates and calculated the % corrected RF of each food item. As unidentified species were not included in the correction, corrected food items were given as a percentage of the identified portion of the diet. We present both corrected and uncorrected RF data in order to facilitate comparison between studies where either approach has been used.
Plant sample collection and nutritional analysis
We collected a limited selection of plant samples from the Manang and Mustang districts of the ACA after following grazing herds of blue sheep. In grazing areas of blue sheep, we collected 200–400 g grass samples from available grasses for analysis. Samples were transferred to the Institute of Forestry, Pokhara, Nepal, where they were oven‐dried in the laboratory at 40°C for 24 h. The dry plant samples were analyzed for nutritional content following standard analysis methods used in Rothman et al. (2012). First, samples were ground in a Wiley Mill through a 1‐mm screen. Neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) content of food items were measured via sequential analysis using an A200 fiber analyzer (ANKOM, Macedon, NY). Samples were analyzed for NDF both with and without residual ash (with α‐amylase), then for ADF with residual ash, and finally for acid detergent lignin (Goering and Van Soest 1970; Van Soest et al. 1991). Total nitrogen (N) was estimated by combustion (AOAC 1990) using a Leco TruSpec (Leco, St. Joseph, MI). Crude fat (CF) was estimated using a XT15 Fat Analyzer (ANKOM, Macedon, NY), where samples were placed in filter bags and boiled in petroleum ether at 90°C for 120 min. Crude protein (CP) was estimated by multiplying %N by 6.25. Ash was measured by incinerating samples at 550°C. As blue sheep likely digest fiber as an energy source, the majority of which is likely hemicellulose, we estimated hemicellulose content of plants by subtracting ADF from NDF (NDF includes hemicellulose + cellulose + lignin, and ADF includes cellulose + lignin). We also estimated cellulose content of plants by subtracting ADL from ADF. Percent total nonstructural carbohydrates (TNC) were estimated by subtraction, where the sum of the percentage of ADF (for the reasons given above), EE, CP, and Ash were subtracted from 100%.
Geometric analysis of blue sheep forage
After performing nutritional analysis, we used right‐angled mixture triangle (RMT; Raubenheimer 2011) analysis to examine the balance of macronutrients and fiber in plant samples. The RMT is a geometric approach used to investigate multidimensional data on the ratios (or balance) of food components in individual foods or food mixtures and is especially relevant to field‐based nutritional ecology studies where proportional compositions (as opposed to accurate intake amounts) are the only metric available (Raubenheimer 2011; Raubenheimer et al. 2014). We used a 3‐dimensional RMT, where macronutrients were expressed as percentage of total macronutrients (i.e., crude protein + crude fat + TNC) on a dry matter basis, where protein was shown on the implicit z‐axis, the value of which is inversely related with distance from the origin. For fiber analyses, we modeled hemicellulose, cellulose, and lignin which were expressed as percentage of the sum of the three fiber types (i.e., hemicellulose + cellulose + lignin) on a dry matter basis. Cellulose was shown on the implicit z‐axis for the RMT analysis of fiber balance. We also used an RMT to examine the relationship between the macronutrient concentration and digestible fiber in plants sampled, where protein and nonprotein (fat + TNC) macronutrients were shown on the x‐ and y‐axes, hemicellulose on the implicit axis, and expressed as a percentage of the sum of macronutrients plus hemicellulose.
Statistical analysis
We used an ANOVA to test for significant differences in the concentration and balance of macronutrients and fiber in plant samples between months of collection. We used a Kruskal‐Wallis test for data that were not normally distributed and/or heteroskedastic. We used a Shapiro–Wilk test to assess normality, and a Bartlett test to assess heterogeneity of variances. We used Pearson correlation test to examine the relationship between macronutrient and fiber concentration. All tests were conducted using the program R version 3.0.3 (R Core Team 2014).
Results
Diet composition of blue sheep
A total of 19 plant species/genera were recorded in blue sheep pellets, as well as unidentified fragments (Table 1). On average across seasons and study areas, the two most frequently occurring plants in blue sheep pellets (both with and without applying CF) were the graminoids Kobressia sp.(20.8% with CF) and Carex spp. (13.7% with CF; Table 1). Other species found at relatively high frequencies were Artemisia spp. (browse), and, after applying CF, Anaphalis sp., and Chesnaya sp. (Table 1). On average, graminoids made up 47%, forbs 13%, and browse 22% of identified fragments in blue sheep pellets, while 19% of plants fragments were unidentified. After applying CF, graminoids contributed 48%, forbs 37%, and browse 16% of the identified portion of diet. Kobresia sp. seemed to decrease in the diet from January to July, as well as other plants such as Oxytropis sp. (Table 1). Carex spp. seemed to decrease in the diet from January to May, but increased again during June–July in the Nammu area (Table 1). Conversely, Sedum sp. tended to increase in the diet from May to July. Some species occurred in the diet seemingly erratically, such as Chesneya sp., Anaphalis spp., and Ephedra spp. (Table 1).
Table 1.
Relative frequency (RF; %) of plants fragments found in blue sheep (Pseudois nayaur) pellets in the Manang and Mustang districts of the Annapurna Conservation Area of Nepal. RF is given both without and with applying the following correction factors (CF) from Shrestha et al. (2005): 1.208 for graminoids; 5.311 for forbs; and 0.850 for browse. Corrected estimates are given as a percentage of the identified portion of scats. Plants are listed in order of highest to lowest RF corrected
| Class | Yak Kharka, Manang | Upper Mustang | Upper Mustang | Nammu area, Mustang | Average | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study area and sampling period | Jan‐Feb 2010 (pellet, n = 48) | Feb ‐March 2010 (pellet, n = 61) | May 2011 (pellet, n = 58) | June‐July 2011 (pellet, n = 43) | |||||||
| Vegetation | % RF | Corrected %RF | % RF | Corrected %RF | % RF | Corrected %RF | % RF | Corrected %RF | % RF | Corrected %RF | |
| Kobresia sp. | Graminoid | 23.1 | 21.2 | 29.2 | 32.0 | 16.0 | 15.1 | 14.7 | 15.2 | 20.4 | 20.8 |
| Carex sp. | Graminoid | 17.6 | 16.2 | 14.2 | 15.5 | 7.5 | 7.1 | 16.4 | 16.9 | 13.5 | 13.7 |
| Anaphalis sp | Forb | 3.0 | 12.0 | 0.1 | 0.5 | 4.5 | 18.6 | 1.8 | 8.2 | 2.3 | 10.3 |
| Chesneya sp. | Forb | 3.5 | 14.2 | 0.0 | 0.0 | 2.8 | 11.5 | 2.0 | 8.9 | 2.0 | 9.0 |
| Oxytropis sp. | Forb | 2.5 | 10.2 | 3.0 | 14.6 | 1.0 | 4.0 | 0.0 | 0.0 | 1.6 | 7.3 |
| Artemisia spp. | Browse | 8.8 | 5.7 | 10.1 | 7.8 | 12.2 | 8.1 | 9.8 | 7.1 | 10.0 | 7.1 |
| Elymus spp. | Graminoid | 3.3 | 3.0 | 5.7 | 6.3 | 9.8 | 9.3 | 6.5 | 6.8 | 6.2 | 6.3 |
| Sedum sp. | Forb | 0.0 | 0.0 | 0.0 | 0.0 | 1.4 | 5.8 | 3.4 | 15.6 | 1.1 | 5.0 |
| Agrostis sp. | Graminoid | 4.4 | 4.0 | 3.5 | 3.8 | 2.3 | 2.1 | 3.4 | 3.6 | 3.3 | 3.4 |
| Lonicera spinosa | Browse | 3.1 | 2.0 | 1.2 | 1.0 | 8.7 | 5.7 | 6.1 | 4.4 | 4.6 | 3.3 |
| Caragana spp. | Browse | 4.4 | 2.8 | 10.1 | 7.8 | 2.3 | 1.5 | 0.2 | 0.1 | 4.2 | 3.0 |
| Potentilla fruticosa | Browse | 0.8 | 0.5 | 2.7 | 2.1 | 5.6 | 3.7 | 2.6 | 1.9 | 2.9 | 2.0 |
| Pennisetum flaccidium | Graminoid | 1.1 | 1.0 | 0.8 | 0.9 | 1.0 | 0.9 | 4.9 | 5.1 | 1.8 | 1.9 |
| Ephedra spp. | Browse | 0.8 | 0.5 | 4.7 | 3.6 | 0.0 | 0.0 | 5.1 | 3.7 | 2.5 | 1.8 |
| Astragalus spp. | Forb | 0.2 | 0.9 | 0.0 | 0.0 | 1.3 | 5.3 | 0.0 | 0.0 | 0.4 | 1.7 |
| Stipa sp. | Graminoid | 3.3 | 3.0 | 2.7 | 3.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.5 | 1.5 |
| Aster albescens | Browse | 1.8 | 1.1 | 0.3 | 0.3 | 1.3 | 0.8 | 3.4 | 2.5 | 1.6 | 1.2 |
| Clematis sp. | Browse | 2.3 | 1.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.4 |
| Salsola nepalensis | Browse | 0.0 | 0.0 | 1.0 | 0.8 | 0.6 | 0.4 | 0.0 | 0.0 | 0.4 | 0.3 |
| Unidentified | NA | 16.2 | 10.6 | 21.9 | 19.6 | 19.0 | |||||
Nutritional content, macronutrient, and fiber balance of plants
We performed nutritional analysis on a limited number of plants collected from the Mustang and Manang districts in January, March, June/July, and November (macronutrients in Table 2; and fiber in Table 3) and were thus limited in the ability to make comparisons with diet RF and between seasons and regions of the study area; however, patterns emerged in our RMT analysis of macronutrients (Fig. 2) and fiber (Fig. 3) despite these limitations. For example, the macronutrient balance of Kobresia spp., the most consumed (i.e., highest relative frequency) plant food, was relatively high in carbohydrate and low in protein content compared to other plants found in the diet of blue sheep, and the macronutrient balance changed little between November and January samples (Fig. 2A and B). The second most frequently consumed plant, Carex spp., was relatively similar to Kobresia spp. in protein content but lower in lipid during March, but a sample of Carex spp. from November showed a much higher protein balance (Figure 2a,b). The RMT analysis of fiber balance showed that the two most consumed plants of blue sheep, Kobresia spp. and Carex spp. (which together had a relative frequency of 34.5 with CF) contained the highest amounts of hemicellulose, which was relatively constant across sampling periods (Fig. 3A and B). The hemicellulose and lignin balance of plants ranged relatively widely, yet the cellulose content of plants showed less variation, being more tightly aligned along the z‐axis at approximately 40% cellulose content (Fig. 3A and B). The hemicellulose balance of plant samples varied inversely with macronutrient balance (Fig. 4): plants that were higher in macronutrients (protein, fat, and carbohydrates) balance had lower hemicellulose balance, while plants that were lower in macronutrient balance had higher hemicellulose balance.
Table 2.
Proximate nutritional composition of plants consumed by blue sheep in the Mustang and Manang districts of the Annapurna Conservation Area of Nepal, including month of collection. All estimates are given as a percentage of dry matter, with the exception of dry matter (g/g wet weight). Total nonstructural carbohydrate (TNC) was estimated by subtraction [i.e., TNC = 100% − (acid detergent fiber + fat + crude protein + ash)]
| Name | Area | Month‐year | Dry matter | Crude protein | Fat | Ash | TNC |
|---|---|---|---|---|---|---|---|
| Anaphalis contorta | Manang | January‐10 | 0.94 | 6.56 | 1.31 | 9.74 | 34.08 |
| Anaphalis sp. | Mustang | June/July 10 | 0.95 | 12.05 | – | – | – |
| Anaphalis triplinervis | Mustang | June/July 10 | 0.93 | 9.96 | – | 22.31 | – |
| Artemisia biennis | Mustang | November‐09 | 0.93 | 11.18 | 1.64 | 14.64 | 40.41 |
| Artemisia sp. | Manang | January‐10 | 0.93 | 6.13 | 2.22 | – | – |
| Artemisia sp. | Mustang | November‐09 | 0.90 | 10.10 | 4.19 | 12.10 | 35.19 |
| Artemisia sp. | Mustang | November‐10 | 0.93 | 11.59 | 2.01 | – | – |
| Artemisia sp. | Mustang | March‐10 | 0.92 | 5.92 | 2.10 | 4.41 | 27.13 |
| Artemisia sp. | Mustang | November‐10 | 0.94 | 15.19 | 3.34 | 11.21 | 43.18 |
| Artemisia sp. | Mustang | June/July 10 | 0.94 | 15.63 | – | 8.35 | – |
| Artemisia sp. | Mustang | June/July 10 | 0.93 | 14.87 | – | 9.84 | – |
| Artemisia sp. | Mustang | June/July 10 | 0.93 | 15.23 | – | 11.43 | – |
| Artemisia sp. | Mustang | June/July 10 | 0.92 | 7.08 | – | 8.20 | – |
| Artemisia varnica | Mustang | March‐10 | 0.95 | 7.10 | 0.69 | 5.11 | 32.41 |
| Aster sp. | Mustang | November‐10 | 0.96 | 15.80 | 3.39 | 15.71 | 46.75 |
| Aster sp. | Manang | January‐10 | 0.93 | 6.08 | 3.27 | 6.46 | 50.26 |
| Astragalus sp. | Manang | January‐10 | 0.95 | 8.47 | 1.87 | 3.84 | 29.20 |
| Astragalus sp. | Mustang | March‐10 | 0.95 | 6.41 | 2.86 | 8.01 | 26.45 |
| Caragana gerardiana | Manang | January‐10 | 0.94 | 6.98 | 4.00 | 13.31 | 20.15 |
| Caragana sp. | Mustang | November‐09 | 0.92 | 10.66 | 2.01 | 19.33 | 25.27 |
| Caragana sp. | Mustang | March‐10 | 0.93 | 9.82 | 2.30 | 4.96 | 34.17 |
| Caragana sp. | Mustang | March‐10 | 0.94 | 8.80 | 2.13 | – | – |
| Caragana sp. | Mustang | June/July 10 | 0.91 | 10.00 | – | 18.63 | – |
| Caragana sp | Mustang | June/July 10 | 0.92 | 10.53 | – | 3.51 | – |
| Carex sp. | Mustang | November‐09 | 0.93 | 27.14 | 3.15 | 8.08 | 49.85 |
| Carex sp. | Mustang | March‐10 | 0.92 | 5.50 | 1.65 | 4.62 | 49.81 |
| Carex sp. | Mustang | June/July 10 | 0.94 | 11.85 | – | 10.27 | – |
| Carex sp. | Mustang | June/July 10 | 0.92 | 6.99 | – | 4.95 | – |
| Clematis tibetana | Mustang | November‐10 | 0.94 | 18.05 | 2.56 | 14.35 | 48.47 |
| Ephedra gerardiana | Mustang | March‐10 | 0.96 | 9.63 | 3.16 | 19.61 | 14.80 |
| Ephedra gerardiana | Manang | January‐10 | 0.95 | 8.36 | 3.89 | 10.52 | 36.46 |
| Ephedra sp. | Mustang | June/July 10 | 0.90 | 14.90 | – | 8.46 | – |
| Ephedra sp. | Mustang | June/July 10 | 0.91 | 14.61 | – | 10.43 | – |
| Kobresia sp. | Manang | January‐10 | 0.94 | 4.29 | 4.11 | 4.35 | 50.66 |
| Kobresia sp. | Mustang | November‐10 | 0.93 | 5.64 | 2.50 | 4.33 | 50.60 |
| Kobresia sp. | Mustang | June/July 10 | 0.92 | 12.67 | – | 10.49 | – |
| Kobresia sp. | Mustang | June/July 10 | 0.97 | 6.36 | – | – | – |
| Lonicera sp. | Mustang | March‐10 | 0.95 | 4.37 | 1.64 | 3.98 | 33.53 |
| Lonicera sp. | Manang | January‐10 | 0.94 | 4.80 | 5.22 | 3.62 | 28.49 |
| Lonicera sp. | Mustang | June/July 10 | 0.92 | 5.21 | – | 4.34 | – |
| Oxytropis sp. | Mustang | June/July 10 | 0.91 | 15.85 | – | 24.96 | – |
| Oxytropis williamsis | Manang | January‐10 | 0.93 | 11.66 | 3.43 | 11.67 | 26.04 |
| Potentilla fruticosa | Manang | January‐10 | 0.95 | 8.43 | 3.53 | 4.38 | 32.92 |
| Potentilla sp. | Mustang | November‐10 | 0.95 | 12.86 | 3.77 | 17.48 | 46.40 |
Table 3.
Neutral detergent fiber (NDF), acid detergent fiber (ADF), acid detergent lignin (ADL), hemicellulose, and cellulose content of plants consumed by blue sheep in the Mustang and Manang districts of the Annapurna Conservation Area of Nepal, including month of collection. Estimates are given on a dry matter basis
| Name | Area | Month‐year | NDF | ADF | ADL | Hemicellulose | Cellulose |
|---|---|---|---|---|---|---|---|
| Anaphalis contorta | Manang | January‐10 | 60.78 | 48.31 | 19.49 | 12.48 | 28.82 |
| Anaphalis sp. | Mustang | June/July 10 | 59.90 | 48.43 | 30.91 | 11.47 | 17.52 |
| Anaphalis triplinervis | Mustang | June/July 10 | 58.99 | 43.41 | 16.64 | 15.59 | 26.76 |
| Artemisia biennis | Mustang | November‐09 | 45.57 | 32.12 | 14.07 | 13.45 | 18.04 |
| Artemisia sp. | Manang | January‐10 | 65.68 | 47.24 | 16.49 | 18.44 | 30.75 |
| Artemisia sp. | Mustang | November‐09 | 62.17 | 38.41 | 11.70 | 23.76 | 26.71 |
| Artemisia sp. | Mustang | November‐10 | 57.08 | 32.36 | 12.37 | 24.72 | 19.99 |
| Artemisia sp. | Mustang | March‐10 | 81.12 | 60.44 | 25.87 | 20.68 | 34.57 |
| Artemisia sp. | Mustang | November‐10 | 40.43 | 27.08 | 10.37 | 13.35 | 16.70 |
| Artemisia sp. | Mustang | June/July 10 | 46.74 | 36.73 | 19.41 | 10.00 | 17.32 |
| Artemisia sp. | Mustang | June/July 10 | 47.69 | 38.75 | 21.04 | 8.94 | 17.71 |
| Artemisia sp. | Mustang | June/July 10 | 65.89 | 36.88 | 10.05 | 29.00 | 26.83 |
| Artemisia sp. | Mustang | June/July 10 | 72.63 | 49.62 | 22.87 | 23.02 | 26.75 |
| Artemisia varnica | Mustang | March‐10 | 80.05 | 54.69 | 25.43 | 25.36 | 29.26 |
| Aster sp. | Mustang | November‐10 | 28.45 | 18.34 | 5.89 | 10.11 | 12.45 |
| Aster sp. | Manang | January‐10 | 49.55 | 33.92 | 10.87 | 15.63 | 23.06 |
| Astragalus sp. | Manang | January‐10 | 72.60 | 56.61 | 24.72 | 15.99 | 31.90 |
| Astragalus sp. | Mustang | March‐10 | 73.44 | 56.28 | 26.41 | 17.16 | 29.87 |
| Caragana gerardiana | Manang | January‐10 | 71.11 | 55.56 | 20.24 | 15.55 | 35.32 |
| Caragana sp. | Mustang | November‐09 | 54.35 | 42.73 | 25.23 | 11.62 | 17.50 |
| Caragana sp. | Mustang | March‐10 | 63.22 | 48.75 | 23.57 | 14.47 | 25.18 |
| Caragana sp. | Mustang | March‐10 | 57.65 | 44.11 | 29.19 | 13.54 | 14.92 |
| Caragana sp. | Mustang | June/July 10 | 60.04 | 49.33 | 29.78 | 10.72 | 19.54 |
| Caragana sp | Mustang | June/July 10 | 70.62 | 52.82 | 25.08 | 17.79 | 27.75 |
| Carex sp. | Mustang | November‐09 | 21.65 | 11.80 | 4.73 | 9.85 | 7.06 |
| Carex sp. | Mustang | March‐10 | 75.50 | 38.42 | 4.85 | 37.08 | 33.57 |
| Carex sp. | Mustang | June/July 10 | 76.68 | 39.33 | 13.45 | 37.35 | 25.88 |
| Carex sp. | Mustang | June/July 10 | 75.66 | 35.73 | 5.57 | 39.94 | 30.16 |
| Clematis tibetana | Mustang | November‐10 | 28.12 | 16.57 | 6.84 | 11.55 | 9.73 |
| Ephedra gerardiana | Mustang | March‐10 | 63.01 | 52.80 | 36.55 | 10.21 | 16.25 |
| Ephedra gerardiana | Manang | January‐10 | 48.96 | 40.78 | 22.20 | 8.18 | 18.58 |
| Ephedra sp. | Mustang | June/July 10 | 56.32 | 53.59 | 36.55 | 2.73 | 17.04 |
| Ephedra sp. | Mustang | June/July 10 | 55.31 | 50.27 | 32.60 | 5.04 | 17.67 |
| Kobresia sp. | Manang | January‐10 | 73.70 | 36.59 | 4.96 | 37.11 | 31.62 |
| Kobresia sp. | Mustang | November‐10 | 75.10 | 36.93 | 4.90 | 38.17 | 32.03 |
| Kobresia sp. | Mustang | June/July 10 | 64.79 | 31.13 | 10.39 | 33.66 | 20.74 |
| Kobresia sp. | Mustang | June/July 10 | 70.28 | 31.98 | 38.29 | ||
| Lonicera sp. | Mustang | March‐10 | 73.13 | 56.49 | 27.68 | 16.65 | 28.81 |
| Lonicera sp. | Manang | January‐10 | 75.11 | 57.87 | 26.96 | 17.24 | 30.91 |
| Lonicera sp. | Mustang | June/July 10 | 71.72 | 53.50 | 23.57 | 18.23 | 29.93 |
| Oxytropis sp. | Mustang | June/July 10 | 49.20 | 37.42 | 21.16 | 11.78 | 16.26 |
| Oxytropis williamsis | Manang | January‐10 | 61.47 | 47.20 | 14.32 | 14.27 | 32.88 |
| Potentilla fruticosa | Manang | January‐10 | 64.83 | 50.73 | 23.57 | 14.10 | 27.16 |
| Potentilla fruticosa | Mustang | March‐10 | 69.42 | 54.59 | 24.90 | 14.84 | 29.69 |
| Potentilla sp. | Mustang | November‐10 | 31.26 | 19.49 | 5.66 | 11.78 | 13.82 |
Figure 2.
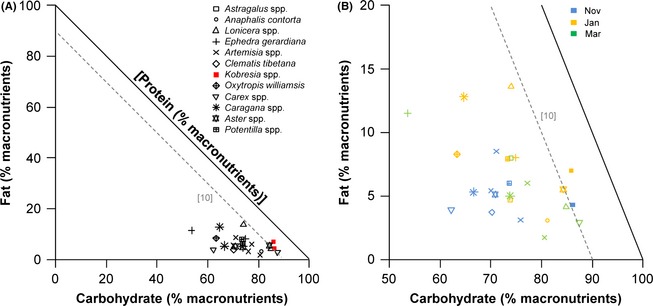
(A) Right‐angled mixture triangle (Raubenheimer 2011) showing the macronutrient balance of plants consumed by blue sheep (Pseudois nayaur). Macronutrients are expressed as percentage of total macronutrients (i.e.,. protein + fat + carbohydrate). Protein is shown on the implicit z‐axis, the value of which is inversely related with distance from the origin. A dashed gray line indicating 10% protein is shown for reference. The plant genus found most frequently in the diet of blue sheep (Kobresia spp.) is shown as a red symbol; (B) A close‐up of the region of nutrient space occupied by plants consumed by blue sheep [legend provided in panel (A)]. Macronutrient estimates are color‐coded to match the month in which the sample was collected. All data points represent a single sample.
Figure 3.
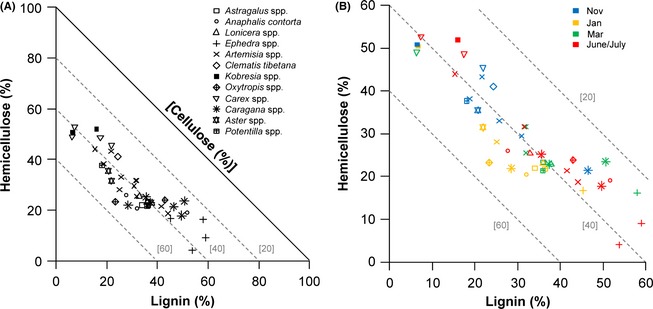
(A) Right‐angled mixture triangle (Raubenheimer 2011) showing the fiber (hemicellulose, cellulose, and lignin) balance of plants consumed by blue sheep (Pseudois nayaur) as a percentage of the sum of each (hemicellulose + cellulose + lignin). Cellulose is shown on the implicit z‐axis, the value of which is inversely related to the distance from the origin. Dashed gray lines indicating 20%, 40%, and 60% cellulose are shown for reference; (B) A close‐up of the region of the fiber nutrient space occupied by plants consumed by blue sheep. Fiber estimates are color‐coded to match the month in which the sample was collected.
Figure 4.
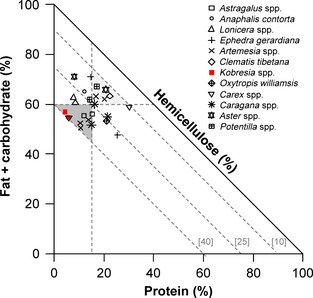
Right‐angled mixture triangle (Raubenheimer 2011) showing the balance of protein and nonprotein (fat + carbohydrate) macronutrients to digestible fiber (hemicellulose) of plants consumed by blue sheep (Pseudois nayaur) as a percentage of the sum of each (protein + nonprotein macronutrients + hemicellulose) on a dry matter basis. Hemicellulose is shown on the implicit z‐axis which is inversely related to the distance from the origin. Dashed gray lines indicating 40%, 25%, and 10% hemicellulose balance, as well as lines indicating 60% nonprotein macronutrient and 15% protein, are shown for reference. Light gray shading indicates plants that have higher macronutrient and lower hemicellulose balance, while dark gray shading indicated plants that have higher hemicellulose and lower macronutrient balance. The plants found most frequently in the blue sheep diet (Kobresia spp.) are shown as red squares.
The results of statistical tests indicated that the protein:nonprotein (ANOVA, P = 0.21) and the hemicellulose:cellulose + lignin (Kruskal‐Wallis, P = 0.07) balance of plant samples was not significantly different between monthly sampling periods; however, the concentration of total macronutrients (ANOVA, P = 0.002) and NDF (Kruskal‐Wallis, P = 0.003) on a dry matter basis were significantly different between months. Fiber (NDF) and total macronutrient concentration (% dry matter) of plants were negatively correlated (r = −0.73), yet hemicellulose concentration and total macronutrient content were not correlated (r = 0.06). Additional nutritional estimates for nonfood plants species are given in the Online Supplemental Information (Tables S1 and S2) to aid nutritional ecology studies of other species for which data may be limited.
Discussion
Our analysis indicated that Kobresia spp. and Carex spp. graminoids were the dominant foods of blue sheep within the Mustang and Manang districts of the ACA. In a previous study investigating the habitat use and resource availability of blue sheep (Aryal et al. 2013, 2014a), Kobresia pygmea was the most important plant species found in blue sheep habitat in both the Yak Kharka and upper Mustang regions, which in light of our results suggests the food resources are a strong determinant of habitat use. While a previous study found that graminoids were the main plant type consumed by blue sheep (followed by browse and forbs) in the Damodar Kunda area of the Mustang region during summer, Kobresia spp. were reportedly absent in blue sheep habitat, and were, accordingly, not found in their diet (Shrestha et al. 2005). Kobresia pygmea was, however, found in the diet of Argali in the Damodar Kunda region (Shrestha et al. 2005), suggesting that the two ungulate species may compete for these resources if present in regions where they are sympatric. Negali are rare in Nepal, however, and may only occur in the Damodar area (Shrestha et al. 2005). Other species of graminoids consumed by blue sheep were similar between the above‐mentioned studies, including Carex sp., Elymus spp., Stipa sp. and Agrostis sp. While Agrostis sp. was the most important species consumed by blue sheep in the Damodar Kunda (Shrestha et al. 2005), it was a relatively minor part of blue sheep diet in the Mustang and Manang. Among forbs, both Chesneya sp. and Oxytropis sp. were found in the diet of blue sheep in the Mustang/Manang and the Damodar Kunda; however, Sedum sp., which was not found in blue sheep habitat in the Damodar Kunda, was noticeable in the diet of blue sheep of the mustang region from May to July. Among browse, Potentilla fruticosa was an important browse species in the Damodar Kunda, yet was a relatively minor part of blue sheep diet in the Manang and Mustang regions of the study area. Overall, in the Damodar Kunda blue sheep were reported to consume 54% graminoids, 6% forbs, and 40% browse (uncorrected), and 51%, 22%, and 27%, respectively (corrected; Shrestha et al. 2005). Our analysis suggests that blue sheep in the Manang and Mustang districts similarly consumed a diet high in graminoids; however, forbs seemed to be consumed more by blue sheep, and browse consumed less, than in the Damodar Kunda.
Our RMT analysis of the macronutrient balance of blue sheep plant foods suggests that plants other than Kobresia spp. are complementary to high‐carbohydrate Kobresia spp. in the sense that they provide more protein and/or fat than Kobresia spp. alone. Our RMT analysis of the fiber balance of plants consumed by blue sheep, indicated that the two most consumed foods (Kobresia spp. and Carex spp.) were highest in hemicellulose content, which is likely digestible by blue sheep. Our results suggest that forage selection by blue sheep may be a balance between consuming easily digestible high‐carbohydrate foods and less‐digestible high protein and/or lipid forage. Blue sheep also seem to forage on plants either high in total macronutrient balance or high in digestible fiber (hemicellulose) balance. Total macronutrient concentration on a dry matter basis varied inversely with the NDF content of plants, but there was no correlation between the total dry matter concentration of macronutrients in plants and hemicellulose content due to the variability in the lignin and cellulose concentrations. Interestingly, however, the balance of the ratios of hemicellulose to protein and nonprotein macronutrients in plant foods of blue sheep indicated that there was an inverse relationship between the balance of macronutrients and digestible fiber.
As our analysis was based on a limited number of samples collected in different areas and seasons, we caution that future research is necessary to gain a complete picture of blue sheep nutritional ecology. One example would be an examination of seasonal changes in the nutritional content of foods and the relationship to the RF of plants in blue sheep diet. While our analysis detected differences in nutrient concentration between in plants sampled during different months, differences in species collected between periods confounds robust phenological comparisons and should be examined further. As well, differences in the nutritional content of plants across an elevation gradient likely influences the timing of plant nutrition (Coogan et al. 2012), and therefore diet of blue sheep across different areas. Furthermore, a more complete nutritional profile of available vs. consumed foods will help determine whether blue sheep are actively foraging for a balance of nutrients different from what they would consume if they simply foraged (proportional to availability). The toxic components of plants should also be included in future geometric analysis.
Despite the limitations, our study contributes important information on the nutritional ecology of blue sheep for which relatively limited information is available, and which may be used to inform conservation and management strategies of blue sheep in the wild. For example, habitat conserved for blue sheep should include some of the key species (Kobresia spp. and Carex spp.) identified in the study; however, if not available plants with similar macronutrient and fiber balance may provide a suitable alternative. It might also be that agricultural plants with similar nutrient balance to Kobresia spp. and Carex spp. may be more prone to crop depredation by blue sheep. Further understanding the nutritional preferences of blue sheep and the nutritional characteristics of available foods will provide information that may help reduce human–wildlife conflict and aid the management and conservation of both prey and predator alike.
Conflict of Interest
None declared.
Supporting information
Table S1. Proximate analysis of plants not found in blue sheep diet.
Table S2. Fiber content of plants not found in blue sheep diet.
Acknowledgments
We thank the Department of National Park and Wildlife Conservation (DNPWC) and Annapurna Conservation Area Project (ACAP) for permission to conduct this research; Ocean Park Conservation Foundation (OPCF), Hong Kong; Keidanren Nature Conservation Fund (KNCF), Japan, and the Massey University Research Fund (Massey University, New Zealand) for funding the study; and B. Adhikari, S. Paudel, F. Gurung. P. Gurung, S. Gurung, N. Lama and N. Dhungana for their support in field for data collection. SCPC was funded by the International Postgraduate Research Scholarship (IPRS) and Australian Postgraduate Award (APA), and the Natural Sciences and Engineering Council (NSERC) of Canada.
Ecology and Evolution 2015; 5(18): 4007–4017
References
- AOAC . 1990. Official methods of analysis. 74 p. Association of Official Analytical Chemists, Arlington, VA. [Google Scholar]
- Aryal, A. , Brunton D., Ji W., Yadav H., Adhikari B., and Raubenheimer D.. 2012a. Diet and habitat use of hispid hare Caprolagus hispidus in Shuklaphanta Wildlife Reserve Nepal. Mammal Study 37:147–154. [Google Scholar]
- Aryal, A. , Raubenheimer D., Sathyakumar S., Poudel B. S., Ji W., Kunwar K. J., et al. 2012b. Conservation strategy for brown bear and its habitat in Nepal. Diversity 4:301–317. [Google Scholar]
- Aryal, A. , Brunton D., Ji W., Yadav H., Adhikari B., and Raubenheimer D.. 2012c. Diet and habitat use of hispid hare Caprolagus hispidus in Shuklaphanta Wildlife Reserve Nepal. Mamm. Study 37:147–154. [Google Scholar]
- Aryal, A. , Raubenheimer D., and Brunton D.. 2013. Habitat assessment for the translocation of blue sheep to maintains a viable snow leopard population in the Mt Everest Region, Nepal. Zool. Ecol. 23:66–82. [Google Scholar]
- Aryal, A. , Brunton D., Ji W., and Raubenheimer D.. 2014a. Blue sheep in the Annapurna Conservation Area, Nepal: habitat use, population biomass and their contribution to the carrying capacity of snow leopards. Integr. Zool. 9:34–45. [DOI] [PubMed] [Google Scholar]
- Aryal, A. , Brunton D., McCarthy T., Karmachharya D., Ji W., Bencini R., et al. 2014b. Multipronged strategy including genetic analysis for assessing conservation options for the snow leopard in the central Himalaya. J. Mammal. 95:871–881. [Google Scholar]
- Aryal, A. , Brunton D., Ji W., Barraclough R. K., and Raubenheimer D.. 2014c. Human‐Carnivore Conflict: Ecological and economical sustainability of predation on livestock by snow leopard and other carnivores in the Himalaya. Sustainability Sci. 9:321–329. [Google Scholar]
- Aryal, A. , Brunton D., and Raubenheimer D.. 2014d. Impacts of climate change on human‐wildlife‐ecosystem interactions in the Trans‐Himalayan region of Nepal. Theoret. Appl. Climatol. 115:517–529. [Google Scholar]
- Brasher, M. G. , Steckel J. D., and Gates R. J.. 2007. Energetic carrying capacity of actively and passively managed wetlands for migrating ducks in Ohio. J. Wild. Manag. 71:2532–2541. [Google Scholar]
- Carbone, C. , and Gittleman J. L.. 2002. A common rule for the scaling of carnivore density. Science 295:2273–2276. [DOI] [PubMed] [Google Scholar]
- Cincotta, R. P. , Van Soest P. J., Robertson J. B., Beall C. M., and Goldstein M. C.. 1991. Foraging ecology of livestock on the TibetanChangtang: a comparison of three adjacent grazing areas. Arctic Alpine Res. 23:149–161. [Google Scholar]
- Coogan, S. C. P. , Nielsen S. E., and Stenhouse G. B.. 2012. Spatial and temporal heterogeneity creates a “brown tide” in root phenology and nutrition. ISRN Ecol. 2012:10. [Google Scholar]
- Coogan, S. C. P. , Raubenheimer D., Stenhouse G. B., and Nielsen S. E.. 2014. Macronutrient optimization and seasonal diet mixing in a large omnivore, the grizzly bear: a geometric analysis. PLoS ONE 9:e97968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenbach, J. A. , Rode K. D., Raubenheimer D., and Robbins C. T.. 2014. Macronutrient optimization and energy maximization determine diets of brown bears. J. Mammal. 95:160–168. [Google Scholar]
- Felton, A. M. , Felton A., Raubenheimer D., Simpson S. J., Foley W. J., Wood J. T., et al. 2009. Protein content of diets dictates the daily energy intake of a free‐ranging primate. Behav. Ecol. 20:685–690. [Google Scholar]
- Fritz, H. , and Duncan P.. 1994. On the carrying capacity for large ungulates of African Savanna ecosystems. Proc. Biol. Sci. 256:77–82. [DOI] [PubMed] [Google Scholar]
- Goering, H. K. , and P. J., Van Soest . 1970. Forage fiber analysis. Pp 1–20. United States Department of Agriculture, USA. [Google Scholar]
- Harris, R. B. 2014. Pseudois nayaur. The IUCN Red List of Threatened Species. Version 2014.2. www.iucnredlist.org. Downloaded on 23 August 2014.
- Harris, R. B. , and Miller D. J.. 1995. Overlap in summer habitats and diets of Tibetan Plateau ungulates. Mammalia 59:197–212. [Google Scholar]
- Hawlena, D. , and Schmitz O. J.. 2010. Herbivore physiological response to predation risk and implications for ecosystem nutrient dynamics. Proc. Natl. Acad. Sci. USA 107:15503–15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley, J. , Simpson S. J., and Wilder S. M.. 2014. Effects of prey macronutrient content on body composition and nutrient intake in a web‐building spider. PLoS ONE 9:e99165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holechek, J. L. , and Gross B. D.. 1982. Training needed for quantifying simulated diets from fragmented range plants. J. Range Manage. 35:644–647. [Google Scholar]
- Johnson, C. A. , Raubenheimer D., Rothman J. M., Clarke D., and Swedell L.. 2013. 30 days in the life: daily nutrient balancing in a wild chacma baboon. PLoS ONE 8:e70438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney, M. , Simpson S. J., Raubenheimer D., and Helmuth B.. 2010. Modelling the ecological niche from functional traits. Philos. Trans. R Soc. Lond. B Biol. Sci. 365:3469–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl, K. D. , Coogan S. C. P., and Raubenheimer D.. 2015. Do wild predators forage for prey or for nutrients? BioEssays 37:701–709. [DOI] [PubMed] [Google Scholar]
- Mcinnis, M. L. , Vavra M., and Krueger W. C.. 1983. A comparison of four methods used to determine the diets of large herbivores. J. Range Manag. 36:302–306. [Google Scholar]
- Miller, D. J. , and Schaller G. B.. 1998. Rangeland dynamics in the Chang TangWildlife Reserve, Tibet Pp. 125–147 in Stellrecht I., ed. Karakorum Hindukush–Himalaya: dynamics of change. Rudiger Koppe Verlag, Koln. [Google Scholar]
- Milton, K. 1979. Factors influencing leaf choice by howler monkeys: a test of some hypotheses of food selection by generalist herbivores. Am. Nat. 114:363–378. [Google Scholar]
- Mishra, C. , Van Wieren S. E., Ketner P., et al. 2004. Competition between domestic livestock and wild bharal Pseudois nayaur in the Indian Trans‐ Himalaya. J. Appl. Ecol. 41:344–354. [Google Scholar]
- Miyashita, T. 1992. Food limitation of population‐density in the orb‐web spider, Nephila clavata. Res. Popul. Ecol. 34:143–153. [Google Scholar]
- Namgail, T. 2006. Winter habitat partitioning between Asiatic Ibex and blue sheep in Ladakh, Northern India. J. Mt. Ecol. 8:7–13. [Google Scholar]
- Namgail, T. , Fox J. L., and Bhatnagar Y. C.. 2004. Habitat segregation between sympatric Tibetan argali (Ovis ammon hodgsoni) and Blue sheep (Pseduois nayaur) in the Indian Trans‐Himalaya. J. Zool. 262:57–63. [Google Scholar]
- Nie, Y. , Zhang Z., Raubenheimer D., Elser J. J., Wei W., and Wei F.. 2014. Obligate herbivory in an ancestrally carnivorous lineage: the giant panda and bamboo from the perspective of nutritional geometry. Funct. Ecol. 29:26–34. [Google Scholar]
- Oli, M. , and Rogers M. E.. 1991. Seasonal pattern in group size and population composition of blue sheep in Manang, Nepal. J. Wildl. Manag. 60:797–801. [Google Scholar]
- Oli, M. K. , Taylor I. R., and Rogers M. E.. 1993. Diet of the snow leopard (Panthera unica) in the Annapurna Conservation Area, Nepal. J. Zool. 231:365–370. [Google Scholar]
- Oli, M. K. , Taylor I. R., and Rogers M. E.. 1994. Snow leopard Panthera uncial predation of livestock: an assessment of local perceptions in the Annapurna Conservation Area, Nepal. Biol. Conserv. 68:63–68. [Google Scholar]
- Panthi, S. , Aryal A., Lord J., Adhikari B., and Raubenheimer D.. 2012. Summer diet and distribution of red panda (Ailurus fulgens fulgens) in dhopatan hunting reserve, Nepal. Zool. Stud. 51:701–709. [Google Scholar]
- R Core Team . 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/ [Google Scholar]
- Raubenheimer, D. 2011. Toward a quantitative nutritional ecology: the right‐angled mixture triangle. Ecol. Monogr. 81:407–427. [Google Scholar]
- Raubenheimer, D. , and Simpson S. J.. 1997. Integrative models of nutrient balancing: applications to insects and vertebrates. Nut. Res. Rev. 10:151–179. [DOI] [PubMed] [Google Scholar]
- Raubenheimer, D. , and Simpson S. J.. 2006. The challenge of supplementary feeding: can geometric analysis help save the kakapo? Notornis 53:100–111. [Google Scholar]
- Raubenheimer, D. , Simpson S. J., and Tait A.. 2012. Match and mismatch: conservation, physiology, nutritional ecology and the timescales of biological adaptation. Philos. Trans. R Soc. Lond. B Biol. Sci. 367:1628–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubenheimer, D. , Machovsky‐Capuska G. E., Chapman C. A., and Rothman J. M.. 2014. Geometry of nutrition in field studies: an illustration using wild primates. Oecologia 177:223–234. [DOI] [PubMed] [Google Scholar]
- Rosenthal, G. A., and Berenbaum M. R., eds. 1991. Herbivores: their interaction with secondary plant metabolites Vol. 2. Ecological and evolutionary processes. Academic Press, London. [Google Scholar]
- Rothman, J. M. , Raubenheimer D., and Chapman C. A.. 2011. Nutritional geometry: gorillas prioritize non‐protein energy while consuming surplus protein. Biol. Lett. 7:847–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman, J. M. , Chapman C. A., and van Soest P. J.. 2012. Methods in primate nutritional ecology: a user's guide. Int. J. Primatol. 33:542–566. [Google Scholar]
- Schaller, G. B. , and Gu B.. 1994. Comparative ecology of ungulates in the Aru Basin of Northwest Tibet. Res. Explor. 10:266–293. [Google Scholar]
- Shrestha, R. , and Wegge P.. 2008. Wild sheep and livestock in Nepal Trans‐Himalaya: Coexistence or competition? Environ. Conserv. 35:125–36. [Google Scholar]
- Shrestha, R. , Wegge P., and Koirala R. A.. 2005. Summer diets of wild and domestic ungulates in Nepal Himalaya. J. Zool. 266:111–119. [Google Scholar]
- Simpson, S. J. , and Raubenheimer D.. 2012. The nature of nutrition: a unifying framework from animal adaptation to human obesity. Princeton University Press, Princeton. [Google Scholar]
- Simpson, S. J. , Sibley R. M., Lee K. P., Behmer S. T., and Raubenheimer D.. 2004. Optimal foraging when regulating intake of multiple nutrients. Anim. Behav. 68:1299–1311. [Google Scholar]
- Simpson, S. J. , Le Couteur D. G., and Raubenheimer D.. 2015. Putting the balance back in diet. Cell 161:18–23. [DOI] [PubMed] [Google Scholar]
- Solon‐Biet, M. , McMahon A. C., Ballard J. W. O., Ruohonen K., Wu L. E., Cogger V. C., et al. 2014. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum‐fed mice. Cell Metab. 19:418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks, D. R. , and Malechek J. C.. 1968. Estimating percentage dry weight indiets using a microscopic technique. J. Range Manag. 21:264–265. [Google Scholar]
- Van Soest, P. J. , Robertson J. B., and Lewis B. A.. 1991. Methods for dietary fiber, neutral detergent fiber, and non‐starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. [DOI] [PubMed] [Google Scholar]
- Wehi, P. M. , Raubenheimer D., and Morgan‐Richards M.. 2013. Tolerance for nutrient imbalance in an intermittently feeding herbivorous cricket, the Wellington Tree Weta. PLoS ONE 8:e84641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Proximate analysis of plants not found in blue sheep diet.
Table S2. Fiber content of plants not found in blue sheep diet.


