Abstract
Background
Contrast-induced nephropathy (CIN) refers to acute renal damage that occurs after the use of contrast agents. This study investigated the renal protective effect of probucol in a rat model of contrast-induced nephropathy and the mechanism of its effect.
Material/Methods
Twenty-eight Wistar rats were randomly divided into the control group, model group, N-acetylcysteine(NAC) group, and probucol group. We used a rat model of iopromide-induced CIN. One day prior to modeling, the rats received gavage. At 24 h after the modeling, blood biochemistry and urine protein were assessed. Malondialdehyde (MDA) and superoxide dismutase (SOD) were measured in renal tissue. Kidney sections were created for histopathological examination.
Results
The model group of rats showed significantly elevated levels of blood creatinine, urea nitrogen, 24-h urine protein, histopathological scores, and parameters of oxidative stress (P<0.05). Both the NAC and probucol groups demonstrated significantly lower Scr, BUN, and urine protein levels compared to the model group (P<0.05), with no significant difference between these 2 groups. The NAC group and the probucol group had significantly lower MDA and higher SOD than the model group at 24 h after modeling (P<0.05). The 8-OHdG-positive tubule of the probucol group and NAC group were significantly lower than those of the model group (p=0.046, P=0.0008), with significant difference between these 2 groups (P=0.024).
Conclusions
Probucol can effectively reduce kidney damage caused by contrast agent. The underlying mechanism may be that probucol accelerates the recovery of renal function and renal pathology by reducing local renal oxidative stress.
MeSH Keywords: Contrast Media, Nephrology, Oxidative Stress, Probucol
Background
Contrast-induced nephropathy (CIN) refers to acute renal damage that occurs after the use of contrast agents, and the general standard for CIN is a 25% increase in serum creatinine (Scr) compared to the basal level within 48 h or an increase of 5 mg/dl in the absolute level of Scr [1,2]. In recent years, with the rapid development of medical imaging technology and the rise of interventional radiology for the treatment of cardiovascular and cerebrovascular diseases and cancer therapy, large quantities of contrast agents have been used clinically, and the resulting form of acute kidney injury (AKI), together with surgery- and drug-induced AKI, constitute the 3 major causes of hospital-acquired acute renal failure [3] and have attracted significant attention from clinicians. Numerous studies indicate that the pathophysiology of CIN is closely related to renal hemodynamic changes and medullary ischemic injury, reactive oxygen species (ROS)-induced oxidative stress damage, indirect damage to the tubules, and tubular obstruction. Among these causes, ROS-induced oxidative stress damage is particularly important [4] and is currently an important drug intervention target for the prevention of CIN in clinical practice.
N-acetylcysteine (NAC) is the universally recognized CIN prevention drug, as it demonstrates strong antioxidant effects and is often used in combination with hydration to prevent the occurrence of CIN [5]. Probucol is a drug with both antioxidative and lipid-lowering effects and is commonly used in clinical practices for the prevention and treatment of atherosclerosis [6] and diabetic nephropathy [7]. Recent studies have shown that, prophylactic treatment with probucol in patients undergoing coronary angiography has a preventive role against CIN [8]. This study utilized the one of rat CIN model to investigate the renal protective effect of probucol and the underlying mechanism responsible for this protection.
Material and Methods
Experimental materials
A total of 28 clean-grade male Wistar (WT) rats at 6~8 weeks of age and a weight of 180~200 g were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. (Animal Certificate of Conformity: SCXK (Beijing) 2006–0008). The animals were adaptively fed in the clean animal room at the Experimental Animal Center of the Academy of Military Medical Sciences for 1 week. All procedures for animal experiments were performed in accordance with the protocols and related provisions of the Academy of Military Medical Sciences Laboratory Animal Committee.
Probucol was purchased from Qilu Pharmaceutical Co. Ltd. (China); NAC was purchased from Hainan Zambon Pharmaceutical Co. Ltd. (China), Mikara; and N-nitro and -L-arginine methyl ester (L-NAME) were purchased from Sigma Company (USA). The low-osmolar, nonionic contrast media agent (Iopromide) was obtained from Schering AG (Germany).
Superoxide dismutase(SOD) and malondialdehyde (MDA) kit were purchased from Nanjing Jiancheng Company (China); 8-OHdG antibody was purchased from Abcam Company (England); A Mindary BS480 analyzer and a COBAS701 biochemical analyzer were used. The microscopic image acquisition and analysis system was from Olympus Co., Japan. The Hitachi H-600 transmission electron microscope was obtained from Hitachi.
Model and grouping
The rats were randomly divided into 4 groups: control group, model group, NAC group, and probucol group, with 7 rats in each group. CIN rats were subjected to CIN protocol as follows: [9,10] Rats in the model, NAC, and probucol group were anesthetized with 60 mg/kg pentobarbital. Pentobarbital sodium anesthesia was followed by CIN induction, which was performed with drug administration into a tail vein. Drugs administered consisted of indomethacin at a dose of 10 mg/kg, followed at 15 min and 30 min later with Nw-nitro-L-Arginine methyl ester (L-NAME) at dose of 10 mg/kg and with low-osmolar, non-ionic contrast medium agent (Iopromide) at a dose of 1600 mg iodine/kg. This quantity is the dose of contrast medium that is standard for clinical use and for other relevant experiments in rat models. Control rats were injected with an equivalent volume of saline at each time point. Rats in the NAC group received intragastric administration of NAC (150 mg/kg) 24 h prior to the CIN-inducing injections. Rats in the probucol group also received intragastric administration of probucol (500 mg/kg) 24 h prior to the CIN-inducing injections. The control group and the model group were given an equal volume of saline by intragastric administration.
Observation indicators
General condition
The mental state, activity, food intake, body weight, and mortality of rats were observed after the procedure. At 24 h after modeling, all rats were killed.
Renal function and urine protein examination
After the rats were anesthetized for the modeling procedure, 1 ml of blood was obtained from the inner canthus for blood biochemical examination prior to modeling. At 24 h after modeling, all rats were killed. In addition, 4 ml of blood was obtained from the abdominal aorta prior to removing the kidney tissue, and the upper layer of serum was collected after 15 min of centrifugation at 12 000 rpm to measure Scr and blood urea nitrogen (BUN). One day before modeling and after modeling, metabolic cages were used to collect 24-h urine protein measurements for each group of rats.
Detection of relevant renal oxidative stress indicators
After the rats were killed, the kidney tissues were quickly removed. Tissue blocks of the appropriate size were taken, placed in pre-cooled ice-cold normal saline, and made into tissue homogenate at a ratio of 1: 9. The supernatant was removed after 15 min of centrifugation at 3000 rpm, and the purchased kits were used to measure the levels of SOD and MDA in renal tissues. The kits were purchased from Nanjing Jiancheng Bioengineering Institute.
Renal pathological examination
Tissue blocks from rat kidney tissues were made, fixed in 10% formaldehyde, dehydrated with conventional methods, embedded in paraffin, and sectioned for Periodic acid-Schiff (PAS) staining. Renal pathological changes were observed using light microscopy. Histopathological scoring was performed by 2 pathologists using previously reported methods [11,12], based on the assessment of tubular expansion, cast formation, brush border loss, and epithelial cell necrosis caused by tissue damage. Then, the following 5-point scoring system was used to assess renal pathology: 0 points (normal without damage); 1 point (≤10%); 2 points (11~25%); 3 points (26~45%); 4 points (46~75%), and 5 points (≥76%). At least 10 random, non-overlapping fields (200× magnification) were observed for each slice. The pathologists were blinded to rat allocation group.
Renal cortex was fixed in phosphate buffer (pH 7.2) containing 3% glutaraldehyde and 0.22 mmol/L sucrose. After fixing in 1% osmium tetroxide, the samples were dehydrated in an ethanol gradient and embedded in epoxy resin. The pathology of the kidney ultrastructure was examined using a Hitachi H-600 transmission electron microscope.
Immunohistochemistry of tubular 8-OHdG
Formaldehyde-fixed paraffin-embedded renal tissue sections were stained using the avidin-biotin complex. ABC method was used for 8-OHdG immunohistochemical examination. The OlympusIX51 biomedical image analysis system was used for manual analysis. Two pathologists continuously observed at least 10 high-power fields (×200) for each slice, counted the number of positive cells in each high-power field, and calculated the average number of positive cells to reflect the intensity of positive expression. The pathologists were blinded to rat allocation group.
Statistical methods
SPSS17.0 statistical software was used for data analysis. Measurement data are expressed as the mean ± standard deviation, and the Kolmogorov-Smirnov method was used to test the normality of each group. Comparisons among groups were performed using one-way analysis of variance (ANOVA), and the Levene method was used to test homogeneity of variance. Comparisons between 2 groups were performed using the SNK-q test, and P<0.05 was considered statistically significant.
Results
General condition of the animals
There were no deaths among the rats involved in this study. The rats in the control group did not show significant abnormalities in feeding or activity, although rats in the other groups showed various levels of malaise, lethargy, slowed mobility, loss of appetite, and weight loss, with the model group of rats showing the most significant symptoms.
Comparison of renal function among various treatment groups
Scr, BUN, and urine protein levels in the rats in each group were all at the same baseline level before model establishment (Table 1). After the procedure, the control group did not show significant changes in Scr, BUN, or urine protein levels in comparison to the pre-procedure levels. At 24 h after the procedure, the model group showed significantly higher levels of Scr, BUN, and urine protein than the control group (P<0.01,P=0.002, P<0.01). Both the NAC and probucol groups demonstrated significantly lower Scr, BUN, and urine protein levels compared to the model group (P<0.05), with no significant difference between these 2 groups (Figure 1).
Table 1.
Comparisons of baseline of SCr, BUN, and UPr for all groups.
| Control | Model | NAC | Probucol | |
|---|---|---|---|---|
| SCr (umol/L) | 21.4±3.51 | 22.9±4.34 | 20.0±3.64 | 23.2±4.89 |
| BUN (mmol/L) | 6.21±1.43 | 7.65±1.59 | 6.83±1.99 | 6.01±2.01 |
| UPr (mg/24 h) | 15.7±4.89 | 17.89±5.78 | 13.94±5.01 | 19.01±4.08 |
SCr – serum creatinine; BUN – blood urea nitrogen; UPr – urine protein.
Figure 1.
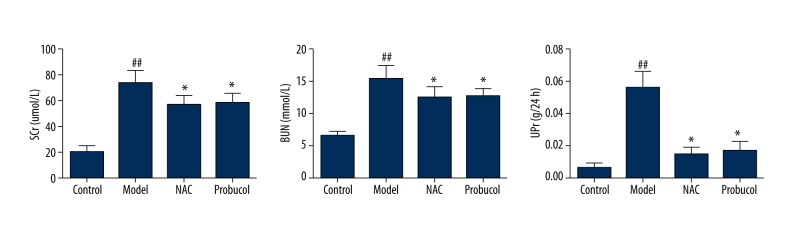
Comparisons of SCr, BUN, and UPr for all groups. ## P<0.01, compared with the control group. * P<0.05, compared with the model group.
Detection of relevant indicators of oxidative stress in renal tissue
After the procedure, the SOD and MDA levels in the renal tissues of the control group showed no significant changes in comparison to the baseline levels. However, at 24 h after the procedure, the SOD level of the model group was significantly decreased (P<0.01), whereas the MDA level was significantly increased (P<0.01). In addition, the NAC group and the probucol group showed significantly milder changes in the above indicators compared to the model group (P<0.05), with no significant difference between these 2 groups (Table 2).
Table 2.
Comparisons of SOD and MDA for all groups.
| Control | Model | NAC | Probucol | |
|---|---|---|---|---|
| SOD (U/mg protein) | 468.9±23.4 | 375.1±32.4## | 412.8±36.4* | 401.5±41.2* |
| MDA (nmol/mg protein) | 4.62±0.62 | 6.18±0.91## | 5.59±1.22* | 5.68±1.26* |
P<0.01 compared with the control group.
P<0.05, compared with the model group.
SOD – superoxide dismutase; MDA – malondialdehyde; NAC – N-acetylcysteine.
Renal morphological changes
PAS staining of kidney tissues showed that the renal tubular epithelial cells of the control group presented a normal morphology and structure, without any luminal expansion or urinary casts. However, at 24 h after the procedure, the model group showed obvious tubular epithelial vacuolar degeneration and disintegration and shedding of the brush border, as well as visible cell casts and protein casts in regions of the lumen. In comparison, the probucol group showed milder pathological changes than the model group, but still presented fairly obvious vacuolar degeneration and brush border loss, and the NAC group showed milder pathological changes than the probucol group (Figures 2, 3). Electron microscopy showed that the mitochondria of renal tubular epithelial cells in the model group were swollen and had ridge fractures. The NAC group and probucol mitochondrial group showed milder mitochondria swelling compared with the model group (Figure 4).
Figure 2.
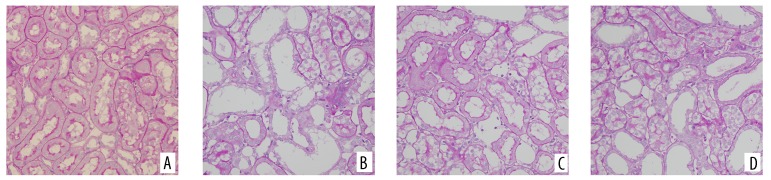
Pathological observations of kidney tissue in rats after modeling 24 h (PAS staining, ×200). (A) Control group; (B) model group; (C) NAC group; (D) probucol group.
Figure 3.
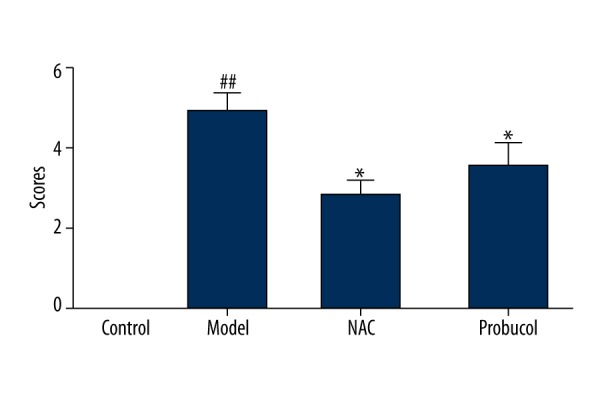
Tubular damage scores of rats after modeling 24 h. ## P<0.01, compared with the control group.* P<0.05, compared with the model group.
Figure 4.
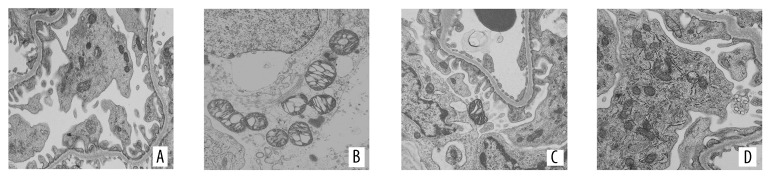
Morphological changes in kidney under electron microscopy after modeling 24 h. (A) Control group; (B) model group; (C) NAC group; (D) probucol group.
Observation of renal immunohistochemistry for each group
The conventional ABC method was used to perform 8-OHdG immunohistochemical staining on paraffin sections. As observed under a light microscope, the tubules in the control group presented a very low number of 8-OHdG-positive tubules, and the staining was light. At 24 h after model construction, the 8-OHdG-positive tubule area of the model group was significantly greater than that of the control group (P<0.01), and the positive tubule proportion of the probucol group and NAC group were significantly lower than those of the model group (p=0.046, P=0.0008). Differences were identified between the NAC and probucol groups (P=0.024). The positive tubule proportion of the NAC group was significantly lower than those of the probucol group, as shown in Figures 5 and 6.
Figure 5.
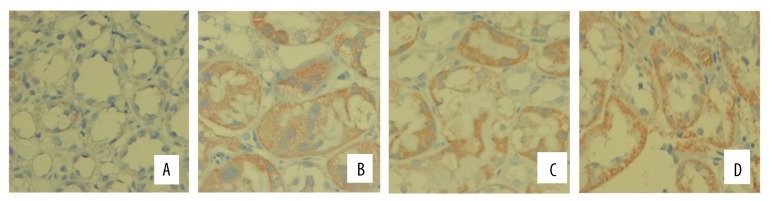
Immunohistochemistry of 8-OHdG in rat kidney section after modeling 24 h (×200). (A) Control group; (B) model group; (C) NAC group; (D) probucol group.
Figure 6.
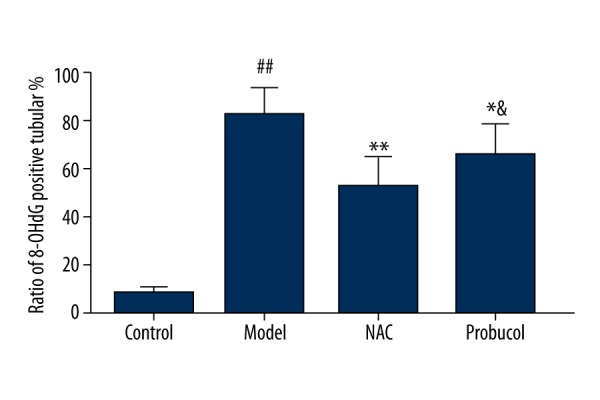
Ratio of 8-OHdG positive tubular with immunolocalization staining in rats after modeling. ## P<0.01, compared with the control group; * P<0.05, ** P<0.01, compared with the model group; & P<0.05, compared with the NAC group.
Discussion
CIN is the third most commont cause of hospital-acquired acute renal failure and accounts for approximately 12% of cases of hospital-acquired acute renal failure [13]. With the steady increase in the use of a variety of contrast-related technologies in recent years, CIN has received significant attention from clinicians.
The mechanism by which contrast agents cause CIN is complex, and oxidative stress is an important factor [14]. After entering the body, contrast agents directly or indirectly generate oxygen radicals through a variety of pathophysiological effects. First, calcium ions and adenosyl fragments mediate vasoconstriction, which is directly involved in the generation of oxygen radicals. Second, the glomerular basement membrane and mesangial cells are damaged and leukocyte chemotaxis is enhanced, further leading to oxygen radical production. Third, the xanthine oxidase activity in renal tissue is increased, which also increases the production of oxygen radicals. In addition, iodine-containing contrast agents can also provide iodine atoms, which are directly involved in the generation of oxygen radicals. Oxygen radicals are thought to be the main mediator of the direct nephrotoxicity of contrast agents, and these molecules can cause toxic ischemia and immune-mediated tissue damage. In the present study, the model group showed significantly decreased SOD and significantly increased MDA levels after modeling, consistent with the findings of Duan et al. [15].
8-OHdG is produced as a result of ROS-induced DNA oxidative damage and is a commonly used marker of oxidative damage and DNA mutation [16]. Serdar et al. [17] previously showed that the urinary 8-OHdG levels of patients with diabetic nephropathy were significantly higher than those of the control group, and this observation was closely related to the long-term presence of oxidative stress in patients with diabetic nephropathy. Some scholars have also proposed to use urinary 8-OHdG as a marker of in vivo oxidative DNA damage [18].
Among patients with acute renal failure caused by contrast agent administration, only 57.2% of these patients achieve fully restored renal function, while 19.0% show partial alleviation, 23.8% experience irreversible kidney damage, and 24.0% eventually develop end-stage renal disease [19]. The clinical prevention of CIN is generally based on adequate hydration and antioxidant therapy [20]. N-acetylcysteine is the first tested antioxidant, considering also its double properties, as a free-radical scavenger, as well as being a drug able to increase the vasodilating effect of NO [4]. Although the precise mechanism by which NAC protects against contrast agent-induced renal damage currently remains controversial, there is no doubt that its function in anti-oxidative stress is an important part of its protective mechanism. In particular, NAC can effectively reduce contrast agent-induced oxidative stress and protect the kidneys via multiple mechanisms, such as removing ROS, inducing the synthesis of glutathione (GSH), and stabilizing NO [21]. In addition to NAC, other antioxidants, such as vitamin C [22], have also been shown to ameliorate CIN; therefore, the current study used NAC as a positive control drug for comparisons with probucol.
Probucol was originally used as a cholesterol-lowering drug, but it was later shown that this drug was effective at reducing urine protein levels. In addition, probucol was shown to possess anti-inflammatory, anti-fibrotic, and anti-oxidative effects [23,24] to delay the progress of early diabetic nephropathy [23,25]. Jiang et al. [26] showed that probucol markedly increased the ROS production and inhibited lipoprotein lipid oxidation. Moreover, in a diabetic nephropathy model, probucol significantly reduced MDA levels, enhanced GSH-Px activity, and reduced glomerular pathological changes [15]. The current study applied probucol to a rat model of CIN and found that it could effectively protect renal function, reduce 24-h urine protein levels, increase SOD levels in renal tissue, decrease MDA content, and reduce the proportion of 8-OHdG-positive tubules, while also reducing the typical pathological changes associated with CIN, such as tubular epithelial vacuolar degeneration and brush border disintegration and shedding and mitochondria swelling.
Conclusions
Probucol effectively reduced kidney damage caused by contrast agent administration, and the underlying mechanism may be associated with the accelerated recovery of renal function and renal pathological changes via the reduction of local renal oxidative stress. Therefore, these results provide an experimental basis for the use of probucol in the clinical prevention and treatment of CIN.
Footnotes
Declaration of conflicting interests
The authors declare no conflict of interest.
Source of support: This work was supported by grants from the National Sciences Foundation of China (81273968 and 81471027), Ministerial projects of the National Working Commission on Aging (QLB2014W002),and The Four hundred project of 301 (YS201408)
References
- 1.Mehran R, Nikolsky E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl. 2006;100:S11–15. doi: 10.1038/sj.ki.5000368. [DOI] [PubMed] [Google Scholar]
- 2.Asif A, Preston RA, Roth D. Radiocontrast-induced nephropathy. Am J Ther. 2003;10(2):137–47. doi: 10.1097/00045391-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Jabara R, Gadesam RR, Pendyala LK, et al. Impact of the definition utilized on the rate of contrast-induced nephropathy in percutaneous coronary intervention. Am J Cardiol. 2009;103(12):1657–62. doi: 10.1016/j.amjcard.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 4.Heyman SN, Rosen S, Khamaisi M, et al. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol. 2010;45(4):188–95. doi: 10.1097/RLI.0b013e3181d2eed8. [DOI] [PubMed] [Google Scholar]
- 5.Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354(26):2773–82. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 6.Adameova A, Xu YJ, Duhamel TA, et al. Anti-atherosclerotic molecules targeting oxidative stress and inflammation. Curr Pharm Des. 2009;15(27):3094–107. doi: 10.2174/138161209789058048. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida M, Kimura H, Kyuki K, Ito M. Combined effect of probucol and insulin on renal damage in diabetic rats fed a high cholesterol diet. Eur J Pharmacol. 2006;548(1–3):174–80. doi: 10.1016/j.ejphar.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 8.Li G, Yin L, Liu T, et al. Role of probucol in preventing contrast-induced acute kidney injury after coronary interventional procedure. Am J Cardiol. 2009;103(4):512–14. doi: 10.1016/j.amjcard.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Kongkham S, Sriwong S, Tasanarong A. Protective effect of alpha tocopherol on contrast-induced nephropathy in rats. Nefrologia. 2013;33(1):116–23. doi: 10.3265/Nefrologia.pre2012.Nov.11736. [DOI] [PubMed] [Google Scholar]
- 10.Reichman J, Cohen S, Goldfarb M, et al. Renal effects of nabumetone, a COX-2 antagonist: impairment of function in isolated perfused rat kidneys contrasts with preserved renal function in vivo. Exp Nephrol. 2001;9(6):387–96. doi: 10.1159/000052637. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Chen G, Wyburn KR, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117(10):2847–59. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada K, Miwa T, Liu J, et al. Critical protection from renal ischemia reperfusion injury by CD55 and CD59. J Immunol. 2004;172(6):3869–75. doi: 10.4049/jimmunol.172.6.3869. [DOI] [PubMed] [Google Scholar]
- 13.Pannu N, Wiebe N, Tonelli M. Prophylaxis strategies for contrast-induced nephropathy. JAMA. 2006;295(23):2765–79. doi: 10.1001/jama.295.23.2765. [DOI] [PubMed] [Google Scholar]
- 14.Persson PB, Tepel M. Contrast medium-induced nephropathy: the pathophysiology. Kidney Int Suppl. 2006;100:S8–10. doi: 10.1038/sj.ki.5000367. [DOI] [PubMed] [Google Scholar]
- 15.Duan SB, Liu GL, Chen GC, et al. Aged rats are susceptible to nephrotoxicity induced by iodinated contrast media. Ren Fail. 2013;35(1):150–54. doi: 10.3109/0886022X.2012.741650. [DOI] [PubMed] [Google Scholar]
- 16.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(2):120–39. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 17.Serdar M, Sertoglu E, Uyanik M, et al. Comparison of 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels using mass spectrometer and urine albumin creatinine ratio as a predictor of development of diabetic nephropathy. Free Radic Res. 2012;46(10):1291–95. doi: 10.3109/10715762.2012.710902. [DOI] [PubMed] [Google Scholar]
- 18.Leinonen J, Lehtimaki T, Toyokuni S, et al. New biomarker evidence of oxidative DNA damage in patients with non-insulin-dependent diabetes mellitus. FEBS Lett. 1997;417(1):150–52. doi: 10.1016/s0014-5793(97)01273-8. [DOI] [PubMed] [Google Scholar]
- 19.Huber W, Jeschke B, Kreymann B, et al. Haemodialysis for the prevention of contrast-induced nephropathy: outcome of 31 patients with severely impaired renal function, comparison with patients at similar risk and review. Invest Radiol. 2002;37(9):471–81. doi: 10.1097/01.RLI.0000023572.58117.55. [DOI] [PubMed] [Google Scholar]
- 20.Wong PC, Li Z, Guo J, Zhang A. Pathophysiology of contrast-induced nephropathy. Int J Cardiol. 2012;158(2):186–92. doi: 10.1016/j.ijcard.2011.06.115. [DOI] [PubMed] [Google Scholar]
- 21.Pflueger A, Abramowitz D, Calvin AD. Role of oxidative stress in contrast-induced acute kidney injury in diabetes mellitus. Med Sci Monit. 2009;15(6):RA125–36. [PubMed] [Google Scholar]
- 22.Cetin M, Devrim E, Serin Kilicoglu S, et al. Ionic high-osmolar contrast medium causes oxidant stress in kidney tissue: partial protective role of ascorbic acid. Ren Fail. 2008;30(5):567–72. doi: 10.1080/08860220802064739. [DOI] [PubMed] [Google Scholar]
- 23.Du J, Wang L, Liu X, et al. Janus kinase 2/signal transducers and activators of transcription signal inhibition regulates protective effects of probucol on mesangial cells treated with high glucose. Biol Pharm Bull. 2010;33(5):768–72. doi: 10.1248/bpb.33.768. [DOI] [PubMed] [Google Scholar]
- 24.Koya D, Hayashi K, Kitada M, et al. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol. 2003;14(8 Suppl 3):S250–53. doi: 10.1097/01.asn.0000077412.07578.44. [DOI] [PubMed] [Google Scholar]
- 25.Atsumi H, Kitada M, Kanasaki K, Koya D. Reversal of redox-dependent inhibition of diacylglycerol kinase by antioxidants in mesangial cells exposed to high glucose. Mol Med Rep. 2011;4(5):923–27. doi: 10.3892/mmr.2011.524. [DOI] [PubMed] [Google Scholar]
- 26.Jiang YS, Lei JA, Feng F, et al. Probucol suppresses human glioma cell proliferation in vitro via ROS production and LKB1-AMPK activation. Acta Pharmacol Sin. 2014;35(12):1556–65. doi: 10.1038/aps.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


