Abstract
Background
The aim of this study was to evaluate the indications, safety, feasibility, and short- and long-term outcomes for elderly patients who underwent robot-assisted middle pancreatectomies (MPs).
Material/Methods
Ten patients (≥60 years) underwent robot-assisted middle pancreatectomies from 2012 to 2015. The perioperative data, including tumor size, operating time, rate of postoperative pancreatic fistula (POPF), postoperative morbidity, and other parameters, were analyzed. We collected and analyzed the follow-up information.
Results
The mean age of patients was 64.30 years (range, 60–73 years). The average tumor size was 2.61 cm. The 10 cases were all benign or low-grade malignant lesions. The mean operating time was 175.00 min. The mean blood loss was 113.00 ml with no blood transfusion needed. Postoperative fistulas developed in 5 patients; there were 2 Grade A fistulas and 3 grade B fistulas. There were 3 patients who underwent postoperative complications, including 2 Grade 1 or 2 complications and 1 Grade 3 complication. No reoperation and postoperative mortality occurred. The mean hospital stay was 19.91 days. After a median follow-up of 23 months, new onset of diabetes mellitus developed in 1 patient and none suffered from deterioration of previously diagnosed diabetes or exocrine insufficiency, and no tumor recurrence happened.
Conclusions
Robot-assisted middle pancreatectomy was safe and feasible for elderly people. It had low risk of exocrine or endocrine dysfunction and benefited patients’ long-term outcomes. Incidence of POPF was relatively high but we could prevent it from resulting in bad outcomes by scientific perioperative care and systemic treatment.
MeSH Keywords: Aged, Pancreatectomy, Robotics
Background
Pancreatic benign tumors are now found more frequently due to the improvement of radiological technology. Some kinds of pancreatic benign lesions, such as intraductal papillary mucinous neoplasm (IPMN), high-grade pancreatic intraepithelial neoplasia, and pancreatic endocrine neoplasm, have the potential for malignant transformation or can be accompanied with stubborn symptoms and are recommended to be resected by the surgeons.
But the choosing of surgical methods is still sometimes controversial, especially for lesions located in the neck or the proximal body of pancreas. Choosing the best surgical method is challenging for surgeons. Traditional distal pancreatectomies are most common, but always result in removal of a large piece of normal pancreatic tissue, and also increase the risk of exocrine and endocrine insufficiency. Enucleation is also another option, but it increases the risk of injury of the main pancreatic tube, long-term postoperative pancreatic fistula (POPF), readmission and reoperation, ad needing extend resections. Middle pancreatectomy is a parenchyma-sparing procedure for the lesions located in the neck or the proximal body of the pancreas, aiming to reduce the rate of postoperative exocrine and endocrine insufficiency. Elderly people have higher risk of postoperative pancreas dysfunction because of tissue or organ degeneration, so it is more important for them to reserve pancreas parenchyma.
The first laparoscopic MP was reported in 2003 [1], but it is still not widely used because of the technical complexity and high rate of postoperative fistula. The development of a robotic surgical system is a milestone for the application of minimally invasive technique for pancreatic surgeries. It complements some defects of traditional laparoscopic technique and brings patients more precise treatment, less harm, and faster recovery, especially for elderly people. In this article, we retrospectively assessed 10 robot-assisted middle pancreatectomies for patients who were over 60 years old, and we discuss the indications, safety, feasibility, and short- and long-term outcomes of robot-assisted MPs for elderly patients.
Material and Methods
Patients
In our center, from Aug 2012 to May 2015, a total of 10 patients who underwent robot-assisted middle pancreatectomy were retrospectively evaluated (study group). The same major surgeon performed all the operations. The mean age of the robotic group was 64.30±4.95 years. The mean BMI was 21.20±2.35. There were 7 males and 3 females. The baseline demographic characteristics of the patients are shown in Table 1. Eight patients had no symptoms and the other 2 were diagnosed because of unresolved epigastric pain. All patients received ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and other essential tests to locate the tumor and rule out surgery contradictions. All 10 lesions were located in the neck or proximal body of the pancreas (Figure 1B). The follow-up period was defined as the interval between the day of operation and the day of the last follow-up; the follow-up endpoint in this study was 1 May 2015. Outpatients were followed-up by telephone interviews. Exocrine deficiency was defined as steatorrhea or eating, combined with epigastrium pain and weight loss requiring pancreatic enzyme supplementation. Endocrine dysfunction means the postoperative new onset of Type 2 diabetes mellitus (T2DM) or worsening diabetes (defined as deterioration in the metabolic control of previously diagnosed diabetes, requiring modification of the medical treatment).
Table 1.
Baseline demographic characteristics of the patients.
| No. | Age (y) | Sex | BMI | ALB (g/L) | TB (μmol/L) | DM |
|---|---|---|---|---|---|---|
| 1 | 62 | M | 23.38 | 39 | 20.4 | 0 |
| 2 | 60 | M | 19.10 | 41 | 7.1 | 1 |
| 3 | 71 | M | 23.42 | 45 | 19.6 | 0 |
| 4 | 61 | F | 19.56 | 43 | 10.9 | 1 |
| 5 | 73 | M | 18.28 | 40 | 16.6 | 0 |
| 6 | 69 | M | 18.37 | 33 | 12.8 | 0 |
| 7 | 60 | M | 24.48 | 42 | 18 | 0 |
| 8 | 60 | F | 22.13 | 34 | 16.1 | 0 |
| 9 | 65 | F | 24.41 | 36 | 7.4 | 1 |
| 10 | 62 | M | 20.76 | 40 | 9 | 0 |
| Mean value | 64.30±4.95 | / | 21.20±2.35 | 39.30±3.89 | 13.80±5.10 | / |
BMI – body mass index; M – male; F – female; ALB – albumin; TB – total bilirubin DM – diabetes mellitus.
Figure 1.
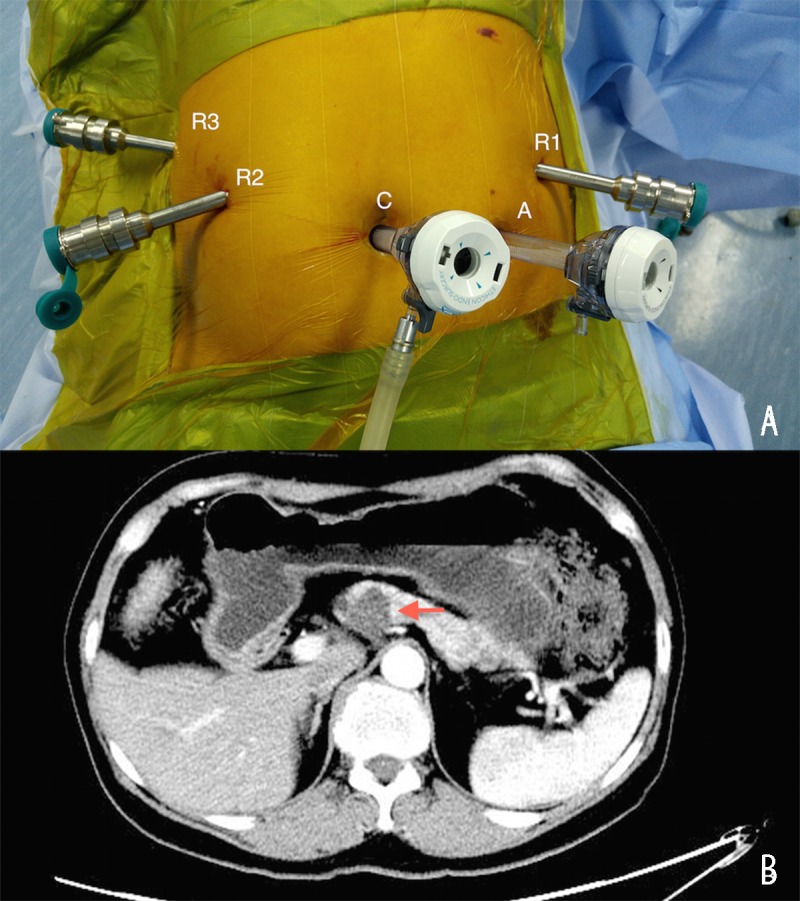
(A) Locations of trocars. C: camera port. R1: no. 1 machine arm. R2: no. 2 machine arm. R3: no. 3 machine arm. A: assistant port. (B) Typical tumor revealed by abdominal computed tomography. The arrow indicates the tumor.
We also reviewed another 55 patients who underwent robot-assisted middle pancreatectomies in the same period as a control group. We compared the perioperative parameters and follow-up data of both groups.
Definition of POPF
Amylase activity in the abdominal drain output was routinely measured every other day beginning on postoperative day 3 and continuing until drain removal. According to the International Study Group on Pancreatic Fistula (ISGPF) criteria, POPF was defined as “a drain output of any measurable volume of fluid on or after postoperative day 3 with amylase content greater than 3 times the serum amylase activity”. Grade A POPFs, also termed “transient fistulas”, do not require special treatment or prolonged hospital stays. Grade B POPFs require nothing by mouth (NPO), enteral or parenteral nutrition, and prolonged drain maintenance; CT scans may show peripancreatic collection(s), and grade B POPFs are always complicated by sepsis that requires antibiotics. Grade C POPFs are the most serious and are characterized by intra-abdominal collections, sepsis, and multiple organ failure and usually require admission to the intensive care unit (ICU) and reoperation.
Surgical procedures
Patient position and ports placement
Patients were was positioned in the supine position with legs apart and were placed in a 20° reverse Trendelenburg position. Pneumo-peritoneum was established using a Veress needle. The intra-abdominal pressure was usually 15 mmHg. Five ports were used, positioned along a semicircular arc facing the epigastrium. One 12-mm camera port, one 12-mm operating port for the assistant, and three 8-mm working ports were placed. The 12-mm camera port was placed approximately 0.5 cm up to the umbilicus. The scope was introduced to rule out any injury, and a 30° laparoscope was usually used. Under vision, the other three 8-mm robotic trocars and one 12-mm robotic trocar were placed (Figure 1A). The 12-mm assistant port was used for suction and instrument introduction. The major surgeon was seated at the robotic console, while one assistant surgeon was positioned at the left side of the patient.
Middle pancreatectomy
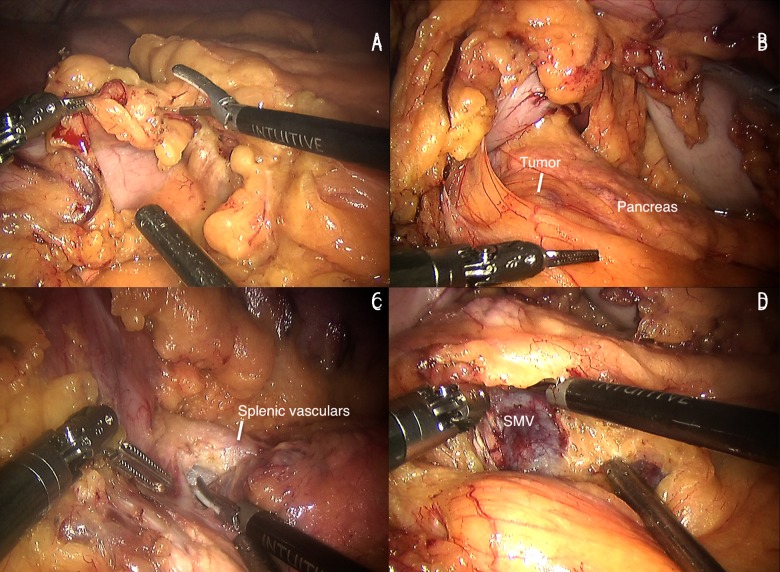
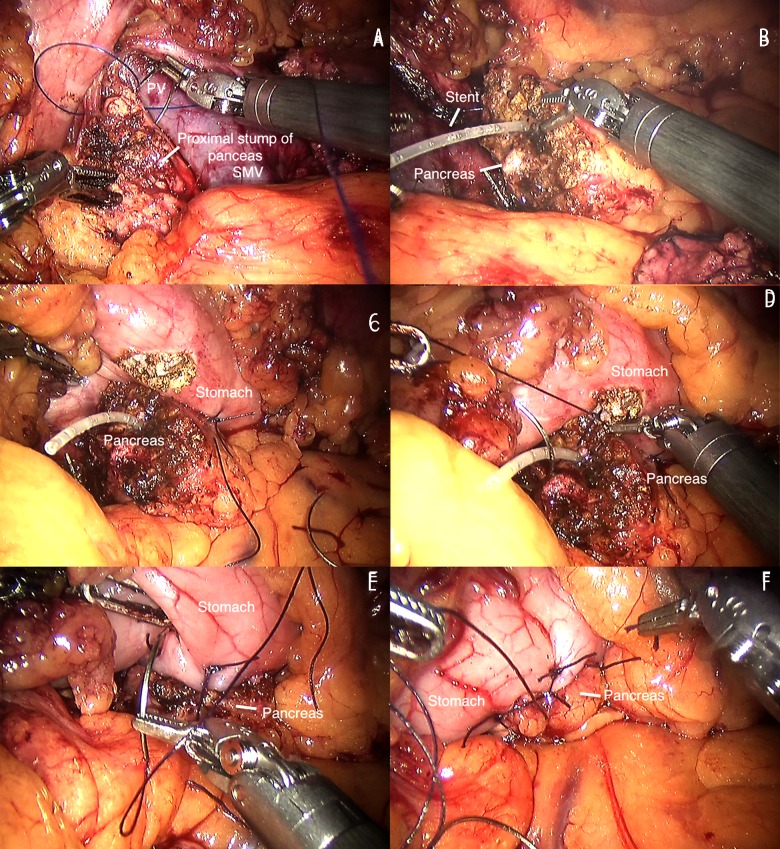
The ultrasonic scalpel was used to open the gastrocolic ligament (Figure 2A) and then we exposed the pancreas by lifting and retracting the posterior gastric wall by the fourth robotic arm (Figure 2B). Following this step, we generally could recognize the tumor and next exposed and dissected the portal vein (PV) at the superior edge of the pancreatic neck and the superior mesenteric vein (SMV) at the inferior edge of the neck of pancreas. Along with the vascular axis, the retropancreatic tunnel was carefully established (Figure 2C, 2D). Then, the pancreatic neck was transected using the ultrasonic scalpel. The proximal stump was intermittently sutured with polypropylene 4-0 for homeostasis and preventing POPF (Figure 3A). The distal pancreas was carefully dissected between the pancreas and the splenic vessels. Then, we transected the pancreas on the left side of the tumor with the ultrasonic scalpel. The distal pancreas was dissected about 2 or 3 cm for achieving pancreaticogastrostomy with no tension. We carried out 2-layer end-to-side pancreaticogastrostomy for reconstruction. First, we inserted a stent into the pancreatic tube for drainage, hoping to prevent the stenosis of anastomosis (Figure 3B) because it always increases the risk the POPF and pancreatitis [2]. Then the outer layer of posterior wall was performed with intermittent stitches of 4-0 Prolene sutures from the pancreatic parenchyma to the seromuscular layer (Figure 3C). After that, a 3–4 cm incision was made at the posterior wall of gastric body using the electric hook and the inner layer of the posterior wall was performed with intermittent stitches of 4-0 Prolene sutures from the stump of the pancreatic remnant to the full layer of the gastric body (Figure 3D). The anastomosis of the posterior wall was completed. Next, we inserted the stent into the gastric lumen and performed the anastomosis of the anterior wall in the same way (Figure 3E, 3F). Two drainage tubes were placed near the proximal pancreatic stump and the anastomosis.
Figure 2.
(A) Opening the gastrocolic ligament. (B) Pancreas exposure. (C) Vascular exposure at the superior edge of the pancreatic body. (D) SMV exposure at the inferior edge of the pancreatic body. SMV, superior mesenteric vein
Figure 3.
(A) Stent inserted. (B) The proximal stump of pancreas is sutured. (C) The outer layer of posterior wall is sutured. (D) The inner layer of posterior wall is sutured. (E) The inner layer of anterior wall is sutured. (F) The outer layer of anterior wall is sutured. SMV, superior mesenteric vein.
Literature review
We performed a literature search using PubMed, including data from 2010–2015. Key words of searching were “middle pancreatectomy”, “central pancreatectomy”, and “median pancreatectomy”. Only original research with at least 20 cases of MPs were included. Studies about minimally invasive middle pancreatotomies with more than 10 cases were reviewed considering the few reports and small sample sizes. For duplicated multiple publications of the same cohort of patients, only the most recent one was used. Two of the 10 authors (Tian Zhang and Xinjing Wang) reviewed all the retrieved studies meeting the inclusion criterion and extracted data on the following events: first author, year of publication, number of patients, mean age, patient sex, number, severity of pancreatic fistula, exocrine of endocrine insufficiency, and time of follow-up. Pancreatic fistula was defined and classified according to the ISGPF principle.
Statistics
Results are presented as mean ±SD, including intraoperative parameters, postoperative parameters, postoperative complications and perioperative mortality. Data analysis was performed using SPSS version 19.0.
Results
Perioperative data
From Aug 2012 to May 2015, 10 consecutive robot-assisted middle pancreatectomies were performed in our center. The 10 lesions were all benign or low-grade malignant with no organ or vessel invasion and no metastasis. The mean operating time was 175.00±45.28 min (range, 120–240 min). No conversion occurred. The mean blood loss was 113.00±107.09 ml (range, 30–400 ml). No patient required blood transfusion. The mean tumor size was 2.61±1.51 cm (range, 1–6 cm, data not shown). The mean time of oral intake was 2.12±1.13 d (range, 1–4 days, data not shown). Five patients had POPFs, including 2 Grade A fistulas and 3 Grade B fistulas. Patients with grade B POPFs recovered by nothing by mouth (NPO), parenteral nutrition, and prolonged drain maintenance. Postoperative complications happened in 3 patients. Two patients had postoperative infections and recovered using antibiotics (Clavien Dindo Classification Grade 1 or 2). The other 1 had both postoperative infection and bile leak, and recovered with antibiotic therapy and endoscopic nose biliary drainage (ENBD) (Grade 3). The mean hospital stay was 19.91±8.85 d (range, 9–42 days). All patients’ symptoms resolved completely and no mortality occurred (Table 2).
Table 2.
Perioperative and postoperative parameters.
| No. | Blood loss (ml) | Operative time (min) | Blood transfusion | POPF | Complication | Hospital stay (d) | New onset or deterioration of DM | Exocrine insufficiency | Time of follow-up (m) | Reope-ration | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 100 | 220 | / | / | / | 15 | / | / | 33 | / | / |
| 2 | 150 | 240 | / | / | / | 17 | Yes | / | 32 | / | / |
| 3 | 400 | 200 | / | / | / | 21 | / | / | 28 | / | / |
| 4 | 50 | 120 | / | A | / | 26 | / | / | 24 | / | / |
| 5 | 100 | 150 | / | / | / | 13 | / | / | 22 | / | / |
| 6 | 50 | 170 | / | B | Infection | 24 | / | / | 19 | / | / |
| 7 | 100 | 200 | / | B | Infection | 21 | / | / | 18 | / | / |
| 8 | 50 | 210 | / | B | Infection and bile leak | 42 | / | / | 11 | / | / |
| 9 | 30 | 120 | / | A | / | 16 | / | / | 2 | / | / |
| 10 | 100 | 120 | / | / | / | 9 | / | / | 1 | / | / |
| Mean value | 113.00 ±107.09 | 175.00 ±45.28 | / | / | / | 19.91 ±8.85 | / | / | / | / | / |
POPF – post-operative pancreatic fistula; DM – diabetes mellitus.
Postoperative pathology presented 3 IPMNs, 4 serous cystadenomas, 2 pancreatic endocrine neoplasms (G1), and 1 mucinous cystadenoma.
At the same time, there was no significant difference in age, BMI, ALB, blood-loss, operating time, tumor size, rate of POPF, postoperative complications, reoperation, time of tumor removal, and post-operative hospital stay between the study group and control group, but the study group had more patients who had previously diagnosed T2DM (Table 3).
Table 3.
Intraoperative data and postoperative outcomes.
| Event | Study group (n=10) | Control group (n=55) | P value |
|---|---|---|---|
| Gender (M/F) | 7/3 | 15/40 | 0.024** |
| Age | 64.30±4.95 | 44.91±11.44 | <0.001* |
| BMI | 21.53±2.40 | 22.57±3.13 | 0.998* |
| ALB | 39.10±3.90 | 41.20±3.33 | 0.573* |
| Diabetes | 3/7 | 1/54 | 0.010** |
| Operating time (min) | 175.00±50.07 | 174.44±70.63 | 0.917* |
| Blood loss (ml) | 113.00±107.09 | 100.75±79.96 | 0.645* |
| Blood transfusion | 0 | 0 | 1.000** |
| Tumor size (cm) | 2.55±1.52 | 2.49±1.28 | 0.578* |
| POPF | 5 | 31 | 0.547** |
| Grade A | 2 | 11 | |
| Grade B | 3 | 18 | |
| Grade C | 0 | 4 | |
| Complication | 3 | 18 | 1.000** |
| Grade 1–2 | 2 | 13 | |
| Grade 3–5 | 1 | 5 | |
| Reoperation | 0 | 4 | 1.000** |
| Time of drain-tube-off (d) | 16.60±8.43 | 18.81±9.97 | 0.007* |
| Postoperative stay (d) | 19.91±9.16 | 22.86±11.89 | 0.358* |
| Exocrine insufficiency | 0 | 53 | 1.000** |
| Endocrine insufficiency | 1 | 4 | 0.579** |
| New onset of diabetes | 1 | 4 | |
| Worsening diabetes | 0 | 0 |
Male – male; F – female; BMI – body mass index; ALB – albumin; POPF – post-operative pancreatic fistula.
Unpaired t test;
Chi-square test.
In the control group, there were 28 serous cystadenomas, 11 solid pseudopapillary tumor, 5 pancreatic endocrine neoplasms (G1), 5 IPMNs, 4 mucinous cystadenomas, 1 PIN, and 1 lipoma (pancreas invasion).
Follow-up information
Up to 1 May 2015, the median follow-up time was 23 months (range, 2–35 m). No clear tumor recurrence was identified by CTA during the follow-up examinations. New onset of T2DM happened in 1 patient, requiring oral hypoglycemic drugs (OHAs) treatment but not insulin administration. No deterioration of previously diagnosed T2DM occurred. No patients developed new onset of exocrine insufficiency (Table 2).
For the control group, 4 patients suffered from new onset of T2DM, requiring OHAs treatment. No exocrine insufficiency developed (Table 3).
Literature review
Ten studies about open middle pancreatectomies involving 376 patients were included. An overview database of these studies is shown in Table 4. All these studies were retrospective studies. The sample sizes were all greater than 20. Of all the patients, 38% (range 14.2 to 63) suffered from POPFs, including 75 grade A fistulas and 98 grade B or C fistulas. Incidence of reoperation was 3.5%, and postoperative morbidity and mortality were 162 and 3, respectively. Thirty-two patients had exocrine or endocrine insufficiency (Table 4).
Table 4.
Literature review on the occurrence and severity of pancreatic fistula and pancreatic dysfunction after middle pancreatectomy.
| Reference | Year | No. of patients | Male/female | POPF | POPF Grade (A/B/C) | Reoperation | Morbidity | Mortality | Pancreatic insufficiency (Exo/Endo) |
|---|---|---|---|---|---|---|---|---|---|
| 1 Cataldegirmen et al. [33] | 2010 | 35 | 17/18 | 5 (14.2) | −/−/− | 0 | 9 (26) | 0 | 3/2 |
| 1 DiNorcia et al. [34] | 2010 | 73 | 20/53 | 15 (20.5) | 3/7/5 | 0 | 30 (41.1) | 0 | −/10 |
| 1 LaFemina et al. [35] | 2010 | 23 | 8/15 | 6 (26) | 5/1/0 | 0 | 8 (34.8) | 0 | 0/0 |
| 1 Shikano et al. [36] | 2010 | 26 | 14/12 | 8 (31) | 2/5/1 | 0 | 10 (38) | 0 | 1/0 |
| 1 Dumitrascu et al. [37] | 2012 | 24 | 8/16 | 10 (41.6) | 3/5/2 | 2 | 13 (54.2) | 0 | −/− |
| 1 Venara et al. [38] | 2012 | 25 | 18/7 | 12 (48) | 4/3/5 | 5 | 10 (40) | 0 | 0/3 |
| 1 Xiang et al. [39] | 2012 | 44 | 16/28 | 24 (54.5) | 12/11/1 | – | – | 0 | −/2 |
| 1 Yvain et al. [40] | 2014 | 100 | 33/67 | 63 (63) | 19/40/4 | 6 | 72 | 3 | 6/2 |
| 1 Song et al. [41] | 2014 | 26 | – | – | – | – | 10 | – | 0/3 |
| 1 Jasper et al. [42] | 2015 | 20 | – | 8 (40) | −/8 (B+C) | – | – | – | – |
| 2 Amer et al. [43] | 2013 | 13 | – | 12 (92.3) | 2/9/1 | 1 | 13 (100.0) | 0 | – |
| 2 Safi et al. [44] | 2013 | 13 | – | 9 (69.2) | 4/2/2 | 0 | 10 (76.9) | 0 | 0/0 |
| 2 Chen et al. [13] | 2014 | 15 | 2/13 | 2 (13.3) | 2/0/0 | 0 | 0 (0) | 0 | 0/0 |
| 2 Hong et al. [45] | 2015 | 10 | – | 3 (30.0) | 3/0/0 | 1 | 1 (10.0) | 0 | 0/0 |
| 2 Senthilnathan et al. [46] | 2015 | 14 | 6/8 | 4 (28.6) | 2/2/0 | 0 | 4 (28.6) | 0 | 2/0 |
Open middle pancreatectomy;
minimally invasive middle pancreatotomy.
POPF – post-operative pancreatic fistula; Exo – exocrine insufficiency; Endo – endocrine insufficiency.
Five studies about minimally invasive middle pancreatectomies involving 65 cases were reviewed. All studies were retrospective studies. POPFs occurred in 46.2% (range 13.3 to 92.3) of all patients, including 13 grade A fistulas and 17 grade B or C fistulas. Two patients suffered from reoperations, and postoperative morbidity and mortality were 38.5% and 0, respectively. No patients (data of one study was not available) developed exocrine or endocrine insufficiency (Table 4).
Discussion
The first segmental pancreatic resection was performed by Oskar Ehrhardt in 1908 [3]. In 1957, Guillemin and Bessot first reported a middle pancreatic (MP) resection with the pancreaticojejunostomy. It has been increasingly used for benign or low-grade malignant tumors located in the neck and proximal body of the pancreas. MP is still an uncommon surgical procedure because of the relatively high risk of postoperative fistula and procedural complexity. This may because: 1) the proximal and the distal pancreatic stump both have the risk of fistula and 2) the MPs are always applied to the benign pancreatic lesions located in the neck of the pancreas and the texture of such pancreas is always “soft”, thus contributing to the increased pancreatic fistula rate. However, MP is a parenchyma-sparing procedure and has the advantage of conserving exocrine and endocrine function. Some retrospective studies have shown MP is safe and POPF can be managed successfully by conservative measures or mini-invasive approaches despite the relatively high risk of fistulas, although there have been few RCTs about the perioperative or long-term outcome comparing MP and distal pancreatectomy.
Since the first application of MP, the high POPF risk of this technique has always concerned surgeons, thus restraining the developing of MP [4,5]. The Mannheim Clinic series demonstrated that nearly 20% of postoperative deaths should be attributed to pancreatic fistula [6], which helps explain why MP has not become a regular procedure for partial pancreatectomy, unlike distal pancreatectomy. However, with the improvement of recognition to POPF, middle pancreatectomies are increasingly used all over the world, as are laparoscopic MPs, because surgeons care more about preserving postoperative pancreatic function, especially for patients with benign or low-grade malignant tumors. Enucleation is an ideal surgery for pancreatic benign tumors, but it has a high risk of bile tract or pancreatic duct injury and also has a high rate of POPF [7]. Distal pancreatectomy always sacrifices a large amount of normal pancreatic tissue and has risk of splenic vascular injury, as well as the high risk of POPF at the same time [8,9].
Laparoscopic pancreatic resection has been increasingly used since the 1990s [10], but there are still some barriers to the wide application of MP. Many patients needing MPs have been treated with open surgeries of traditional distal pancreatectomies [11]. Some surgeons are concerned about the high risk of POPFs. Another obstacle restraining the developing of MP is the complexity of the laparoscopic procedure, so laparoscopic pancreatectomy has rarely been reported [12–17]. The robotic surgical system overcomes some defects of traditional laparoscopic technique and has been used for pancreatic surgeries [18–21]. This is mainly because: 1) The 3-dimensional and magnified view allows surgeons to sense the depth of field better and observe subtle structures more clearly; 2) The 7 degrees of freedom machine arms ease suturing, stanching, and other procedures; thus, the learning curve considerably decreases; 3) It filters the natural hand quiver, which makes operations safer; and 4) Less trauma allows patients to recover faster [22–24]. Thus, minimally invasive pancreatic surgeries have become common, even some particularly complex procedures requiring vascular dissection or reconstruction [25,26]. Giulianotti reported the first case of robot-assisted MP in 2004 [27]. However, studies comparing robot-assisted MPs and open surgeries are rare. In 2011, our center published a retrospective study on robot-assisted MP. We demonstrated that robot-assisted MP was safe and feasible and that patients may benefit from quicker gastrointestinal tract recovery [21]. In another previous study of our center, we demonstrated that robot-assisted surgery had the advantage of preserving splenic vasculature [18].
Laugier reported that pancreatic exocrine changes with patient age. Aging alters pancreatic secretion. Pancreatic exocrine insufficiency due to ageing occurs through a decrease in flow rate, and bicarbonate and enzyme secretion [28]. In addition, the exocrine and endocrine functions of the pancreas have close anatomical and functional links between each other and any disease impairing one of them will inevitably affect the other [29]. Matteo determined that pancreatic exocrine insufficiency was common in diabetics, with a wide range in both type I diabetes mellitus (25–74%) and T2DM (28–54%) [30]. Therefore, it is important for elderly people to preserve the pancreatic parenchyma, preventing new onset of pancreatic dysfunction or deterioration of previously diagnosed pancreatic diseases.
From Aug 2012, our center did 10 consecutive robot-assisted middle pancreatectomies for elderly patients who suffered from pancreatic benign of low-grade malignant tumors locating in the neck of pancreas. Our perioperative database was comparable with reports of other centers. We had 5 patients with POPF, including 2 Grade A fistulas and 3 Grade B fistulas according the ISGPF criterion. Because Grade A fistula does not require intervention, the “true” postoperative fistula rate in our study was 30%, which is equal or less than those of other centers (Table 3). Postoperative complications occurred in 3 patients. Two patients had intra-abdominal infection and recovered by antibiotic treatment. The other 1 had intra-abdominal infection and bile leak, accepting ENBD. The overall rate of morbidity was 30%, also comparable with these of other reports (Table 3). The 3 patients all had Grade B fistulas. These results prove that Grade B or C fistula can result in relatively severe complications but poor outcomes can be prevented by a variety of appropriate therapies. Moreover, there was only 1 new onset of T2DM and no deterioration of previously diagnosed diabetes or exocrine insufficiency happened. Burkhart reported that 29% (23/78) of patients accepting distal pancreatectomies suffered from new onset of diabetes mellitus (NODM) [31]. Recently, De Bruijn reviewed 26 studies about distal pancreatectomy. The incidence of NODM after distal pancreatectomy performed for chronic pancreatitis was 39% and for benign or (potentially) malignant tumors it was 14%. Insulin treatment was accepted by 77% of patients with NODM [32]. In our study, we had a lower rate of NODM, but the difference was not statistically significant (data not shown). Patients can benefit from this kind of surgery, but given the small sample size, we require more practice to prove its feasibility.
In our study, the mean hospital stay was a little longer than that in other reports. This is mainly because our community health service system is still progressing, and patients are not discharged until they completely recover, including removal of the drainage tube and 1–2 days of observation. These factors seem to increase the hospital stay.
The present study has some defects. 1) It was not an RCT and the sample size was small, so bias is inevitable. 2) The same surgeon performed all of the operations, but different physicians provided postoperative care, and some aspects of the perioperative management may have varied slightly.
Conclusions
Robot-assisted middle pancreatectomy is safe and feasible for use in elderly people. It not only alleviates the surgical harm, but also effectively decreases exocrine or endocrine insufficiency of the pancreas. Although this technique has a relatively high risk of postoperative pancreatic fistula, poor outcomes could be prevented with suitable therapies. Larger series and RCTs are necessary to fully prove these potential advantages.
Footnotes
Source of support: Departmental surces
References
- 1.Baca I, Bokan I. Laparoscopic segmental pancreas resection and pancreatic cystadenoma. Chirurg. 2003;74(10):961–65. doi: 10.1007/s00104-003-0690-y. [in German] [DOI] [PubMed] [Google Scholar]
- 2.Tsalis K, Antoniou N, Koukouritaki Z, et al. Successful treatment of recurrent cholangitis by constructing a hepaticojejunostomy with long Roux-en-Y limb in a long-term surviving patient after a Whipple procedure for pancreatic adenocarcinoma. Am J Case Rep. 2014;15:348–51. doi: 10.12659/AJCR.890436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iacono C, Ruzzenente A, Bortolasi L, Guglielmi A. Central pancreatectomy: The Dagradi Serio Iacono operation. Evolution of a surgical technique from the pioneers to the robotic approach. World J Gastroenterol. 2014;20(42):15674–81. doi: 10.3748/wjg.v20.i42.15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iacono C, Verlato G, Ruzzenente A, et al. Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br J Surg. 2013;100(7):873–85. doi: 10.1002/bjs.9136. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y-M, Zhang X-F, Wu L-P, et al. Pancreatic fistula after central pancreatectomy: case series and review of the literature. Hepatobiliary Pancreat Dis Int. 2014;13(2):203–8. doi: 10.1016/s1499-3872(14)60032-1. [DOI] [PubMed] [Google Scholar]
- 6.Gouma DJ, van Geenen RC, van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232(6):786–95. doi: 10.1097/00000658-200012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strobel O, Cherrez A, Hinz U, et al. Risk of pancreatic fistula after enucleation of pancreatic tumours. Br J Surg. 2015;102(10):1258–66. doi: 10.1002/bjs.9843. [DOI] [PubMed] [Google Scholar]
- 8.Ntourakis D, Marescaux J, Pessaux P. Robotic spleen preserving distal pancreatectomy: how I do it (with video) World J Surg. 2015;39(1):292–96. doi: 10.1007/s00268-014-2784-8. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Wang X, Shen B, et al. Preliminary experience of the robot-assisted laparoscopic excision of a retroperitoneal mass: A case report. Oncol Lett. 2014;8(6):2399–402. doi: 10.3892/ol.2014.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Cruz L, Cosa R, Blanco L, et al. Curative laparoscopic resection for pancreatic neoplasms: a critical analysis from a single institution. J Gastrointest Surg. 2007;11(12):1607–21. doi: 10.1007/s11605-007-0266-0. discussion 1621–22. [DOI] [PubMed] [Google Scholar]
- 11.Crippa S, Bassi C, Warshaw AL, et al. Middle pancreatectomy: indications, short- and long-term operative outcomes. Ann Surg. 2007;246(1):69–76. doi: 10.1097/01.sla.0000262790.51512.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang CM, Lee JH, Lee WJ. Minimally invasive central pancreatectomy: current status and future directions. J Hepatobiliary Pancreat Sci. 2014;21(12):831–40. doi: 10.1002/jhbp.143. [DOI] [PubMed] [Google Scholar]
- 13.Chen XM, Zhang Y, Sun DL. Laparoscopic central pancreatectomy for solid pseudopapillary tumors of the pancreas: our experience with ten cases. World J Surg Oncol. 2014;12:312. doi: 10.1186/1477-7819-12-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machado MA, Surjan RC, Epstein MG, Makdissi FF. Laparoscopic central pancreatectomy: a review of 51 cases. Surg Laparosc Endosc Percutan Tech. 2013;23(6):486–90. doi: 10.1097/SLE.0b013e3182a4bf69. [DOI] [PubMed] [Google Scholar]
- 15.Stauffer JA, Asbun HJ. Minimally invasive pancreatic surgery. Semin Oncol. 2015;42(1):123–33. doi: 10.1053/j.seminoncol.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Sucandy I, Pfeifer CC, Sheldon DG. Laparoscopic assisted central pancreatectomy with pancreaticogastrostomy reconstruction – An alternative surgical technique for central pancreatic mass resection. N Am J Med Sci. 2010;2(9):438–41. doi: 10.4297/najms.2010.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machado MA, Surjan RC, Goldman SM, et al. Laparoscopic pancreatic resection. From enucleation to pancreatoduodenectomy. 11-year experience. Arq Gastroenterol. 2013;50(3):214–18. doi: 10.1590/S0004-28032013000200038. [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Zhan Q, Chen JZ, et al. Robotic approach improves spleen-preserving rate and shortens postoperative hospital stay of laparoscopic distal pancreatectomy: a matched cohort study. Surg Endosc. 2015 doi: 10.1007/s00464-015-4101-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Addeo P, Marzano E, Nobili C, et al. Robotic central pancreatectomy with stented pancreaticogastrostomy: operative details. Int J Med Robot. 2011;7(3):293–97. doi: 10.1002/rcs.397. [DOI] [PubMed] [Google Scholar]
- 20.Abood GJ, Can MF, Daouadi M, et al. Robotic-assisted minimally invasive central pancreatectomy: technique and outcomes. J Gastrointest Surg. 2013;17(5):1002–8. doi: 10.1007/s11605-012-2137-6. [DOI] [PubMed] [Google Scholar]
- 21.Cheng K, Shen B, Peng C, et al. Initial experiences in robot-assisted middle pancreatectomy. HPB. 2013;15(4):315–21. doi: 10.1111/j.1477-2574.2012.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moskowitz RM, Young JL, Box GN, et al. Retroperitoneal transdiaphragmatic robotic-assisted laparoscopic resection of a left thoracolumbar neurofibroma. JSLS. 2009;13(1):64–68. [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi T, Kinugasa Y, Shiomi A, et al. Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc. 2015;29(7):1679–85. doi: 10.1007/s00464-014-3855-5. [DOI] [PubMed] [Google Scholar]
- 24.Yang MS, Kim KN, Yoon DH, et al. Robot-assisted resection of paraspinal Schwannoma. J Korean Med Sci. 2011;26(1):150–53. doi: 10.3346/jkms.2011.26.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joyce D, Morris-Stiff G, Falk GA, et al. Robotic surgery of the pancreas. World J Gastroenterol. 2014;20(40):14726–32. doi: 10.3748/wjg.v20.i40.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giulianotti PC, Addeo P, Buchs NC, et al. Robotic extended pancreatectomy with vascular resection for locally advanced pancreatic tumors. Pancreas. 2011;40(8):1264–70. doi: 10.1097/MPA.0b013e318220e3a4. [DOI] [PubMed] [Google Scholar]
- 27.Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. 2010;24(7):1646–57. doi: 10.1007/s00464-009-0825-4. [DOI] [PubMed] [Google Scholar]
- 28.Laugier R, Bernard JP, Berthezene P, Dupuy P. Changes in pancreatic exocrine secretion with age: pancreatic exocrine secretion does decrease in the elderly. Digestion. 1991;50(3–4):202–11. doi: 10.1159/000200762. [DOI] [PubMed] [Google Scholar]
- 29.Czako L, Hegyi P, Rakonczay Z, Jr, et al. Interactions between the endocrine and exocrine pancreas and their clinical relevance. Pancreatology. 2009;9(4):351–59. doi: 10.1159/000181169. [DOI] [PubMed] [Google Scholar]
- 30.Piciucchi M, Capurso G, Archibugi L, et al. Exocrine pancreatic insufficiency in diabetic patients: prevalence, mechanisms, and treatment. Int J Endocrinol. 2015;2015:595649. doi: 10.1155/2015/595649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burkhart RA, Gerber SM, Tholey RM, et al. Incidence and severity of pancreatogenic diabetes after pancreatic resection. J Gastrointest Surg. 2015;19(2):217–25. doi: 10.1007/s11605-014-2669-z. [DOI] [PubMed] [Google Scholar]
- 32.De Bruijn KM, van Eijck CH. New-onset diabetes after distal pancreatectomy: a systematic review. Ann Surg. 2015;261(5):854–61. doi: 10.1097/SLA.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 33.Cataldegirmen G, Schneider CG, Bogoevski D, et al. Extended central pancreatic resection as an alternative for extended left or extended right resection for appropriate pancreatic neoplasms. Surgery. 2010;147(3):331–38. doi: 10.1016/j.surg.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 34.DiNorcia J, Ahmed L, Lee MK, et al. Better preservation of endocrine function after central versus distal pancreatectomy for mid-gland lesions. Surgery. 2010;148(6):1247–54. doi: 10.1016/j.surg.2010.09.003. discussion 1254–56. [DOI] [PubMed] [Google Scholar]
- 35.LaFemina J, Vagefi PA, Warshaw AL, Fernandez-del Castillo C. Transgastric pancreaticogastric anastomosis: an alternative operative approach for middle pancreatectomy. Arch Surg. 2010;145(5):476–81. doi: 10.1001/archsurg.2010.61. [DOI] [PubMed] [Google Scholar]
- 36.Shikano T, Nakao A, Kodera Y, et al. Middle pancreatectomy: safety and long-term results. Surgery. 2010;147(1):21–29. doi: 10.1016/j.surg.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 37.Dumitrascu T, Barbu ST, Purnichescu-Purtan R, et al. Risk factors for surgical complications after central pancreatectomy. Hepatogastroenterology. 2012;59(114):592–98. doi: 10.5754/hge11758. [DOI] [PubMed] [Google Scholar]
- 38.Venara A, de Franco V, Mucci S, et al. Central pancreatectomy: comparison of results according to the type of anastomosis. J Visc Surg. 2012;149(2):e153–58. doi: 10.1016/j.jviscsurg.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Xiang GM, Tan CL, Zhang H, et al. Central pancreatectomy for benign or borderline lesions of the pancreatic neck: a single centre experience and literature review. Hepatogastroenterology. 2012;59(116):1286–89. doi: 10.5754/hge11937. [DOI] [PubMed] [Google Scholar]
- 40.Goudard Y, Gaujoux S, Dokmak S, et al. Reappraisal of central pancreatectomy a 12-year single-center experience. JAMA Surg. 2014;149(4):356–63. doi: 10.1001/jamasurg.2013.4146. [DOI] [PubMed] [Google Scholar]
- 41.Song KB, Kim SC, Park KM, et al. Laparoscopic central pancreatectomy for benign or low-grade malignant lesions in the pancreatic neck and proximal body. Surg Endosc. 2015;29(4):937–46. doi: 10.1007/s00464-014-3756-7. [DOI] [PubMed] [Google Scholar]
- 42.Atema JJ, Jilesen AP, Busch OR, et al. Pancreatic fistulae after pancreatic resections for neuroendocrine tumours compared with resections for other lesions. HPB. 2015;17(1):38–45. doi: 10.1111/hpb.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zureikat AH, Moser AJ, Boone BA, et al. 250 robotic pancreatic resections: safety and feasibility. Ann Surg. 2013;258(4):554–59. doi: 10.1097/SLA.0b013e3182a4e87c. discussion 559–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dokmak S, Aussilhou B, Fteriche FS, et al. Pure laparoscopic middle pancreatectomy: single-center experience with 13 cases. Surg Endosc. 2014;28(5):1601–6. doi: 10.1007/s00464-013-3357-x. [DOI] [PubMed] [Google Scholar]
- 45.Hong D, Liu Y, Peng S, et al. Binding pancreaticogastrostomy in laparoscopic central pancreatectomy: a novel technique in laparoscopic pancreatic surgery. Surg Endosc. 2015 doi: 10.1007/s00464-015-4265-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Senthilnathan P, Gul SI, Gurumurthy SS, et al. Laparoscopic central pancreatectomy: Our technique and long-term results in 14 patients. J Minim Access Surg. 2015;11(3):167–71. doi: 10.4103/0972-9941.158967. [DOI] [PMC free article] [PubMed] [Google Scholar]