Summary
Background
The purpose of the study is to identify the diagnostic value of adding diffusion weighted images (DWI) to routine MRI examinations of the scrotum.
Material/Methods
The study included 100 testes of 50 patients with a unilateral testicular disease. Fifty normal contralateral testes were used as a control group. All patients underwent conventional MRI and DWI examinations of the scrotum. The results of MRI and DWI of the group of patients treated surgically were correlated with histopathological findings. The MRI and DWI results of non-surgical cases were correlated with the results of clinical, laboratory and other imaging studies. Comparison of the ADC value of normal and pathological tissues was carried out followed by a statistical analysis.
Results
There was a significant difference between ADC values of malignant testicular lesions and normal testicular tissues as well as benign testicular lesions (P=0.000). At a cut-off ADC value of ≤0.99, it had a sensitivity of 93.3%, specificity of 90%, positive predictive value of 87.5%, and negative predictive value of 94.7% in the characterization of intratesticular masses.
Conclusions
Inclusion of DWI to routine MRI has a substantial value in improving diagnosis in patients with scrotal lesions and consequently can reduce unnecessary radical surgical procedures in these patients.
MeSH Keywords: Diagnosis, Diffusion Magnetic Resonance Imaging, Testicular Neoplasms, Testis
Background
Correct diagnosis and characterization of scrotal and testicular masses are important for their optimal treatment, including resection planning to avoid orchiectomy for some subtypes of benign tumors for which enucleation is an alternative. A confident preoperative characterization of the lesion in question is needed to spare the patient from the drastic procedure [1,2]. Imaging has an essential role in the investigation of testicular masses. However, this primary imaging technique does not always allow for confident characterization of the nature of a testicular mass [3–6]. Magnetic resonance imaging (MRI) of the scrotum provides valuable information in the detection and characterization of various scrotal disorders and in differentiating intratesticular and extratesticular lesions. The advantages of MRI are simultaneous imaging of both testicles and both inguinal regions, acquisition of adequate anatomic information, and satisfactory tissue contrast [7–14]. Recently, the diffusion weighted (DW) MR technique proved useful in the detection of malignant neoplasms and histological characterization of focal lesions in the abdomen [15,16]. The detection and characterization of the lesion generally depend on the extent of tissue cellularity. Increased cellularity is associated with restricted diffusion and reduced apparent diffusion coefficient (ADC) values [15,16]. The ADC values of malignancies are reported lower than those of benign lesions or normal tissues [15–28].
The aim of the study
The purpose of the study was to evaluate the benefit of adding DWI and ADC value measurements to routine MRI examinations in our imaging departments’ settings and to identify the accuracy of DWI and ADC values in the assessment of normal scrotal contents and in pathological conditions, trying to estimate a cut-off value for prediction of malignancy.
Material and Methods
The study was conducted in the Clinical Imaging Department of Hamad Medical Corporation in Doha, Qatar and in the Department of Diagnostic Imaging and Nuclear Medicine, Zagazig University, Cairo, Egypt in the period between January 2012 and January 2014. The study included 100 testes of 50 patients having a unilateral scrotal lesion, which were diagnosed by clinical and US examinations. The normal testes of each patient, total of 50 testes, were used as a control group for comparison with the pathological side and measurement of normal ADC values. The other 50 testes contained pathological lesions. Thirty-five patients showed intratesticular lesions; 15 of them were malignant and 20 were benign. The remaining 15 patients showed extra-testicular lesions, which were all benign. The age of the patients ranged from 14 to 65 years.
MR imaging protocol
The patients were scanned in a supine position on a 1.5-T MR system using a pelvic phased array coil. The MR protocol included: axial spin echo T1-weighted sequences (TR 500–650 [ms], TE 13–15 [ms]) and axial, sagittal and coronal fast spin echo T2-weighted images without and with fat suppression (FS) (TR 4000 [ms], TE 100–120 [ms]). Additionally, the axial fat-suppressed T1-weighted sequences were repeated when a lesion with high T1 signal intensity was noted. Gadolinium-DTPA (Omniscan) 0.2 mmol kg; Amersham Health, Oslo, Norway) was administered intravenously for contrast-enhanced images in the coronal, transverse and sagittal planes in spin echo, fat suppressed T1-weighted images. DWI was performed in the axial plane, using a single-shot, multislice spin echo planar diffusion pulse sequence (TR 3900 [ms], TE 115 [ms]) and b values of 400 and 800 s/mm. Similar parameters were used for all sequences, i.e. the slice thickness of 3–4 (mm), gap 0.5 (mm), matrix 180×256 (mm), FOV 24×27 (cm).
MR imaging data interpretation
MR images were studied by three radiologists who did not know the clinical, ultrasound, surgical or histopathological results, and any discrepancies were resolved by a consensus. The signal intensity of the lesion on conventional and contrast-enhanced MR images was recorded. The signal intensity of the lesion on DWI was compared with the normal control testicle of the same patient. ADC maps were created, the signal was recorded and a quantitative analysis was performed by positioning the circular region of interest (ROI) within the lesions, excluding areas of hemorrhage or necrosis revealed by other MR images. Three measurements were obtained and their mean value was calculated. Another similar ROI was placed in the normal contralateral testicle to measure the normal ADC values. Conventional MR imaging data were interpreted and the diagnosis was recorded. DWI and ADC map images were interpreted and the diagnosis depending only on these sequences was recorded. Lastly, the diagnosis including both conventional, DWI and ADC maps was recorded. The diagnosis of intratesticular malignancies was established using the MR criteria known from the previously published reports [7,29,30] and correlated with the pathological report. The statistical analysis of the accuracy of conventional MR imaging diagnosis, DWI diagnosis alone and combined conventional MR and DWI sequences in scrotal lesion characterization was carried out. The results of surgery and histopathological examinations were used as a standard in the operated patients. The clinical diagnosis and follow-up by clinical re-evaluation and repeated imaging evaluation were used as a standard for non-surgical cases.
Statistical analysis
Statistical analysis was performed with SPSS Base 16 for windows (SPSS Inc., Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant. The ADC values of normal testes and testicular pathology (malignant and benign lesions) were compared and a cut-off value was chosen. Comparison was carried out between conventional MRI result alone, DWI result alone and combined MRI with DWI results. The sensitivity, specificity and accuracy were calculated.
Results
The study included 100 testes of 50 patients with a unilateral testicular disease. Fifty normal contralateral testes (50%) were used as a control group. The normal testicular parenchyma appeared hyperintense on both T2-weighted and DW images, and slightly hypointense on the ADC maps. The mean ±s.d. of (ADC value 10−3mm2·s−1) of the normal testis was (1.12±0.67). The other 50 testes (50%) were abnormal with scrotal disorders. The study included 35 intratesticular lesions (70%), of which 15 (43%) were malignant and 20 (57%) benign. Extratesticular lesions were 15 cases, which all (100%) proved to be benign (Table 1). A total of 35 cases had benign-nature lesions of both intra- and extratesticular locations (70%) and 15 cases were histopathologically proved as malignant lesions (30%) all of intratesticular location. The conventional MRI features of 3 cases of tubular ectasia of rete testis and 3 cases of testicular cysts were typical in this series, in the form of the presence of testicular cyst or multicystic lesions involving the mediastinum of the testis, with fluid signal intensity, not enhancing after intravenous contrast material administration [31,32]. Seven cases of epididymo-orchitis were also correctly characterized by conventional MR images. Enlargement and contrast enhancement of the epididymis and the testis, combined with signal hyperintensity on T2-weighted images, were findings proved to correspond with acute inflammation on clinical and sonographic follow-up in this study. This study also included four cases of post-traumatic/post-infective heterogeneity which was seen by ultrasound and MRI, and the latter was aimed to rule out malignancy. The cases showed heterogeneous T2 signal with no mass effect or signs of pathological contrast enhancement. Three cases of pathologically proved testicular granulomas were included in this study, one case showed low signal in T2 and other two cases showed mixed hyperintense signals in T2 images; all three cases showed peripheral delayed enhancement. All benign 20 testicular lesions in this study (100%), were detected without causing significant restricted diffusion, therefore of low signal intensity on DW sequences. The mean ±s.d. of ADC values of benign intratesticular lesions was 1.58±0.63. This study included 15 cases of paratesticular lesions. MR imaging identified normal epididymis at T2-weighted and DW sequences as relatively hypointense, when compared to the normal testicular parenchyma. Three cases of acute epididymitis showed enlargement and post-contrast enhancement of the epididymis combined with heterogeneous low signal intensity. Two cases of chronic granulomatous epididymitis with enlarged heterogeneous epididymis, showed a T2 heterogeneous low signal pattern with delayed heterogeneous enhancement. The study included four cases of adenomatoid tumors of the tunica and epididymis (Figure 1), which presented as an isointense-signal lesion in a T2-weighted image with early post-contrast enhancement. Also two cases of epididymal fibrous tumors showed a hypointense signal in both T1- and T2-weighted images and post- contrast enhancement. Two cases of spermatocele with a typical fluid signal and no post-contrast enhancement were detected (Figure 2). Both extratesticular benign neoplastic and inflammatory lesions showed signal hypointensity and hyperintensity on DW sequences and ADC maps, respectively. The mean ±s.d. of ADC values of normal epididymis was 1.08±0.033. The mean ±s.d. of ADC values of benign paratesticular lesions including both neoplastic and inflammatory ones was 1.17±0.48. The statistical difference between the ADC values of the normal epididymis and those of benign paratesticular lesions was noted (P=0.49), which was insignificant (Table 2). The mean ±s.d. of the ADC value of a benign paratesticular neoplasm was 0.99±1.53 and the mean ±s.d. of the ADC value of paratesticular inflammatory lesions was 1.29±0.59. Statistical difference between the ADC values of the benign neoplastic and inflammatory paratesticular lesions was noted (P=0.25), which was insignificant (Table 3). Two false positive cases for malignancy, based on the DW data alone, with acute post-traumatic epididymal hematoma were detected with restricted diffusion on DW images and very low ADC values. However, conventional MR sequences enabled the correct characterization in those patients. Both conventional and DW images proved accurate in characterizing the benign nature of all (100%) paratesticular lesions in this series. We had 15 malignant cases, all were intratesticular. There were 8 cases of seminoma (53%), (Figure 3) and 7 (47%) non-seminoma cases (Figure 4), (47%). Testicular malignancies were detected on T2-weighted images as either low signal in 9 cases or heterogeneous signal intensity in 6 cases, with areas of hemorrhage in 2 cases and necrosis in 4 cases, all heterogeneously enhancing after intravenous contrast material administration, except for three cases with mainly cystic components that showed peripheral enhancement. Invasion of the testicular tunica by a neoplasm in 5 cases, invasion of the epididymis in 2 cases and extension of the tumor to the spermatic cord in two cases were detected. When assessed by DW imaging, 12 cases (80%) of all testicular malignancies including seminoma (Figure 3) and non-seminoma (Figure 4), were depicted as areas of restricted diffusion, appearing hyperintense when compared to the normal testicular parenchyma and hypointense on the ADC maps, respectively. Three cases (20%) of malignancy with mainly cystic/necrotic components and a thin peripheral soft tissue rim showed no restriction in the cystic parts in DW images, appearing as a hyperintense signal in ADC maps. The mean ±s.d. of ADC values of intratesticular malignancies was 0.79±0.16. Conventional MR had a sensitivity of 100%, specificity of 80%, positive predictive value of 86.5%, negative predictive value of 100% and overall accuracy of 90% in differentiating malignant from benign testicular lesions. The ANOVA analysis between normal testis, benign and malignant intratesticular lesions showed that the mean ADC values were different (F23.0, P 0.00) (Table 4). The least significance difference test showed a difference between the ADC values of the normal testicular parenchyma and testicular malignancies (P=0.000), the ADC values of benign and malignant intratesticular lesions (P=0.000), and between the measurements of the normal testis and benign intratesticular lesions (P=0.004). DW images and ADC map using a cut-off value (≤0.99) had a sensitivity of 93.3%, specificity of 90%, positive predictive value of 87.5%, and negative predictive value of 94.7% in the characterization of intratesticular masses (Table 5). The combined evaluation of both conventional and DW images proved accurate in differentiating malignant from benign intratesticular mass lesions in all (100%) cases in this series.
Table 1.
Pathological diagnoses of intra- (n=35) and extratesticular diseases (n=15).
| Extratesticular lesions (n=15) | Intratesticular lesions (n =35) | ||||||
|---|---|---|---|---|---|---|---|
| Benign | Malignant | Benign | Malignant | ||||
| Epididymorchitis | 7 | Seminoma | 8 | Adenomatoid tumor of epididymis and tunica | 4 | – | – |
| Post infective/traumatic | 4 | Non seminoma* | 7 | Fibrous tumor of epididymis | 2 | ||
| Ectasia retitestis | 3 | Chronic granulomatous epididymitis | 2 | ||||
| Granuloma | 3 | Acute epididymitis | 3 | ||||
| Testicular cyst | 3 | Spermatocele | 2 | ||||
| Hematoma of epididymis | 2 | ||||||
| 20 | 15 | 15 | – | ||||
Non seminoma: included (Embryonal carcinoma, choriocarcinoma, yolk sac carcinoma and malignant teratoma).
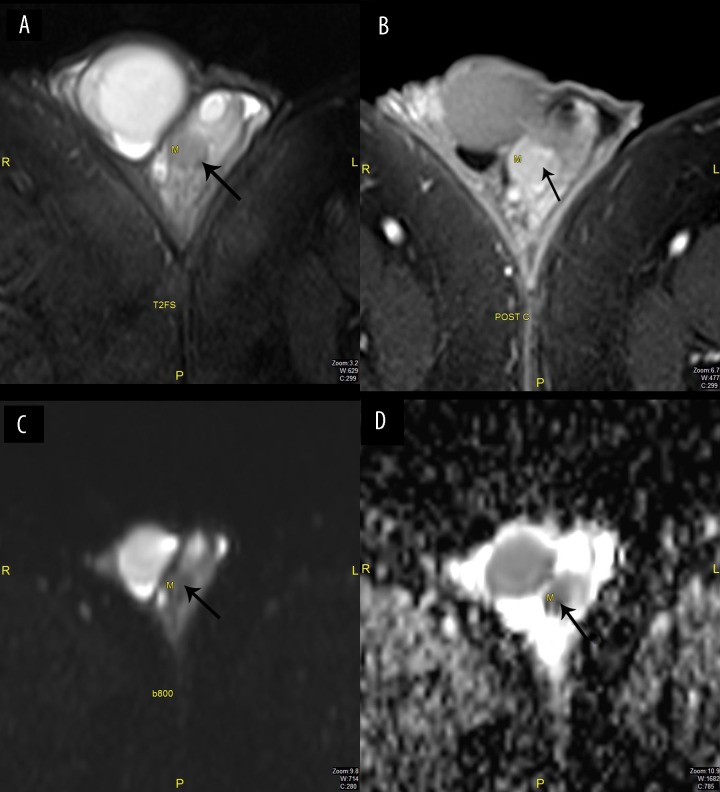
Figure 1.
Male, 32 years old, with adenomatoid tumor of the left epididymis. (A) T2-FSWI: Hypointense signal (arrow), (B) Post contrast: Enhancement of the lesion (arrow), (C) DWI b800: No restriction (arrow), (D) ADC map: Low signal (arrow).
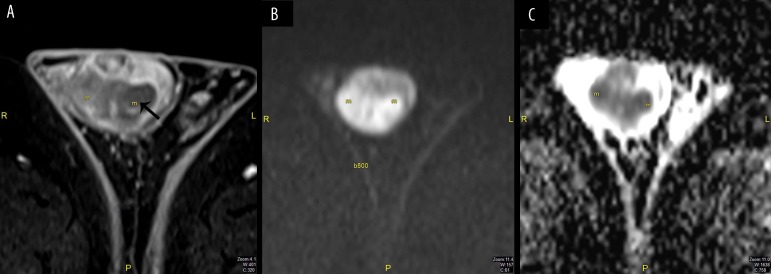
Figure 2.
Male, 24 years old, with a right epididymal cyst. (A) DWI b800: Hypointense signal with no restriction (arrow), (B) ADC map: Hyperintense signal (arrow).
Table 2.
ADC (Apparent diffusion coefficient) values in diagnosis of extratesticular lesions; normal epididymi (n=6) versus benign lesions (n=15), using paired t test.
| Normal epididymis (n=15) | Benign lesions (both neoplastic and non-neoplastic) (n=15) | P | |
|---|---|---|---|
| Mean ADC ±SD | 1.08±0.33×10−3 mm2·s−1 | 1.17±0.48×10−3 mm2·s−1 | 0.49 Non-significant |
| Range | 1.01–1.13×10−3 mm2·s−1 | 0.56–2.30×10−3 mm2·s−1 |
Table 3.
ADC (Apparent diffusion coefficient) values in diagnosis of extratesticular lesions; neoplastic (n=6) versus inflammatory (n=9)
| Neoplastic lesions (n=6) | Inflammatory lesions (n=9) | P | |
|---|---|---|---|
| Mean ADC value ±SD | 0.99±1.5×10−3 mm2·s−1 | 1.29±0.59×10−3 mm2·s−1 | 0.25 Non-significant |
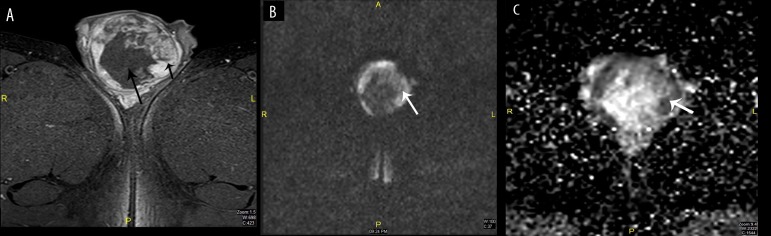
Figure 3.
Male, 31 years old, with right testicular swelling due to seminoma. (A) T2WI with fat saturation: Hypointense signal (arrow), (B) DWI b800: Restriction, (C) ADC map: Low signal.
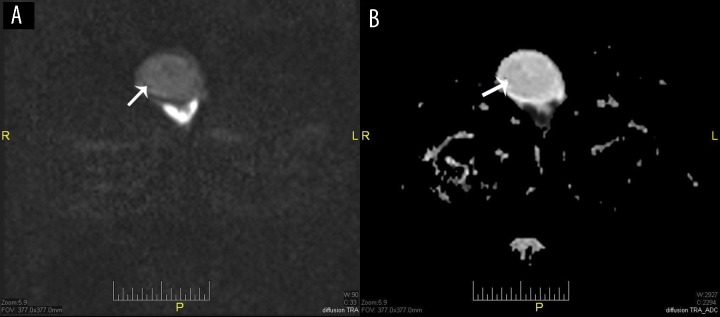
Figure 4.
Male, 22 years old, with left testicular rhabdomyosarcoma. (A) Post contrast study: Peripheral lesion enhancement (short black arrow) and central necrotic changes (long black arrow), (B) DWI b800: Restriction of the solid component (white arrow), (C) ADC map: Restricted part appears hypointense (white arrow).
Table 4.
Analysis of variance between the ADC (apparent diffusion coefficient) values of normal testicular parenchyma (n=35), benign (n=20) and malignant intratesticular lesions (n=15).
| P | Normal testes (n=35)a | Benign lesions (n=20)b | Malignant lesions (n= 15) | F | P |
|---|---|---|---|---|---|
| Mean ADC ±s.d. | 1.12±0.07×10−3 mm2·s−1 | 1.58±0.63×10−3 mm2·s−1 | 0.79±0.16×10−3 mm2·s−1 | 23.0 | 0.000 |
| ADC range | 1.02–1.29×10−3 mm2·s−1 | 0.82–2.88×10−3 mm2·s−1 | 0.40–1.02×10−3 mm2·s−1 |
Normal patients (control group) show significant difference from both benign and malignant lesions (P=0.004 and 0.000 respectively);
benign is significantly different from malignant lesions (P=0.000).
Table 5.
Validity of ADC in diagnosis of intratesticular lesions (n=35).
| ADC | Intratesticular lesions | P | |
|---|---|---|---|
| Malignant | Benign | Total | |
| ADC ≤0.99 | 14 | 2 | 16 |
| ADC >0.99 | 1 | 18 | 19 |
| Total | 15 | 20 | 35 |
AUC 0.96 (0.90–1.02), cut off value for prediction of malignancy 0.99, sensitivity= 93.3, specificity=90.0, PPV=87.5, NPV=94.7, Kappa 0.82, P=0.000.
Discussion
MRI of the scrotum provides valuable information in the detection and characterization of various scrotal disorders and differentiating intratesticular and extratesticular lesions. The advantages of MRI are simultaneous imaging of both testicles and inguinal regions due its wide field of view, multiplanar capabilities, high contrast and spatial resolution helping in the distinction between benign from malignant intratesticular lesions and evaluation of the local extent of malignant lesions [7–14]. MRI is used when sonographic findings are inconclusive or inconsistent with clinical findings [10,11]. As radical orchiectomy is the treatment of choice in the majority of intratesticular malignant masses, a confident preoperative characterization of the lesion in question is needed to spare the patient from the drastic procedure [8,14]. The MRI criteria which we used in characterization of testicular neoplasms were the presence of mainly hypointense or heterogeneous mass lesions on T2-weighted images and heterogeneous enhancement after contrast material administration [7,14]. The current study showed high accuracy of conventional MR sequences in characterization of both paratesticular and intratesticular lesions. The study showed a sensitivity of 100%, specificity of 80%, positive predictive value of 86.5%, negative predictive value of 100% and overall accuracy of 90%, which is in agreement with other research studies [7–14]. Paratesticular tumors are masses of slow and indolent growth and in most cases of benign nature, being that the case, the treatment of choice is a simple excision of the lesion and follow-up, based on observation only. In those identified as malignant (30%), treatment is more complex, consisting in radical orchiectomy with adjuvant chemotherapy or radiotherapy [33]. In this study, we had 15 cases of paratesticular lesions which were of benign nature (100%) according to MRI criteria and clinic-laboratory data as well as follow-up assessment. Absence of post-contrast enhancement is considered to be the most sensitive sign in predicting a benign intratesticular lesion. The presence of hemorrhagic changes, invasion of the tunicae, and extension of the tumor to the paratesticular regions and/or the spermatic cord constitute criteria used for the diagnosis of malignancy as proved by histopathological correlation [7–14]. In the current study, there were 35 cases of intratesticular lesions, 20 cases were benign (non-neoplastic) lesions (57%) and 15 cases were malignant (43%), according to MRI criteria, clinical-laboratory data, follow-up and histopathological findings in the operated cases. There were 3 cases of malignancy with large areas of necrotic non-enhancing changes. The lesions were associated with a peripheral rim of enhancement and other MRI sequences were helpful to identify these changes as of necrotic origin. Those three cases were proved to be of malignant nature in the histopathological results. However, we had no neoplastic benign lesions to include in this study.
Diffusion weighted MR imaging depends on the motion of water molecules. The extent of tissue cellularity and the presence of intact cell membranes define the impedance of water molecule diffusion [15,16]. A normal testis is hyperintense on DWI referring to restriction of water molecule movement within densely packed seminiferous tubules of normal testicular parenchyma, separated by thin fibrous septa [8]. The ADC value reflects the movement of water molecules. High-b-value DW imaging (b800 or b1000) reflects high signal intensity for malignant tumors in comparison to normal tissues and benign lesions, which correspond to low ADC values for the malignant tumors [15–28]. In this study, no benign neoplastic intratesticular tumors were included. Otherwise, all other benign intratesticular lesions show no restriction in DWI. The ADC values of the normal epididymis were lower than those of various benign paratesticular lesions, with values which proved as insignificant (P, 0.49). Malignant testicular carcinomas have higher signal intensity in comparison to normal testis; hence, assessment of the signal intensity on DW images was proved reliable in differentiating between normal testicular tissue and malignant tissues. In the present study, DWMR imaging proved valuable in differentiating testicular malignancy from various scrotal lesions. All, except for three cases of testicular malignancies, were hyperintense (restricted) on DW images at b value of 800. Those three malignant cases had large areas of necrotic changes, which presented no restriction at DWMR, however; they showed a restricted peripheral thin rim corresponding to a solid component, which was not conclusive. Conventional MRI was able to achieve diagnoses in those three cases and final diagnosis as malignancy was obtained. The ADC values of intratesticular malignancies were lower than those of normal testis and benign lesions (P, 0.000). There was no significant difference in the ADC value between normal testicular tissue and benign testicular lesions (P, 0.004). A cut-off value of the ADC value for prediction of malignancy for intratesticular lesions was calculated. Statistical analysis using this value had high sensitivity, specificity, positive predictive value and negative predictive value of 93.3%, 90.0%, 87.5%, and 94.7% respectively, suggesting that lesion with ADC value equal to or less than 0.99 is suggestive of a possibility of malignancy. This result agrees with the previous studies [27,28], which stated that the lower ADC values, the higher the potential for malignancies. The decrease in ADC values in testicular neoplasms is related to the histopathological characteristics of neoplastic tissue, that is, increased tissue cellularity and densely packed malignant cells, enlargement of the nuclei, and angulations of the nuclear contour, all causing reduced motility of water molecules. Therefore, both DW imaging characteristics and ADC calculations of the scrotal contents proved valuable in differentiating between a normal and abnormal scrotum, and more important, in differentiating normal from cancerous tissue in the testicles. In this study, we had two false-positive cases with paratesticular hematoma, related to the low ADC values of bloody products and three false-negatives in patients with multicystic testicular malignancies. Correct diagnosis was possible in those cases, which could be attempted using routine MR sequences. On a routine conventional MRI examination adenomatoid tumor of epididymis and tunica, and fibrous tumor of epididymis were displayed as enhancing paratesticular masses with no restriction in DWMR, which reflected the benign nature of those lesions. In the present study, we found that DW imaging data when interpreted in conjunction with conventional images improve the characterization of the lesion. The addition of DW sequences to routine scanning data improves the diagnostic accuracy with only minimal increase in examination time. There were some limitations in the current study as some pathological types of scrotal abnormalities were not included. Also, there is a wide individual variation of ADC measurements, so it was difficult to suggest the ADC cut-off value as an effective parameter for tissue characterization.
Conclusions
MRI is a reliable method in diagnostics of patients with scrotal lesions. Inclusion of DW imaging and ADC values in routine scrotal MR imaging is considered valuable in differentiation between normal, benign and malignant scrotal lesions, and consequently can reduce unnecessary radical surgical procedures in these patients.
References
- 1.Sloan JC, Beck SD, Bihrle R, Foster RS. Bilateral testicular epidermoid cysts managed by partial orchiectomy. J Urol. 2002;167:255–56. [PubMed] [Google Scholar]
- 2.Heidenreich A, Engelmann UH, Vietsch HV, Derschum W. Organ preserving surgery in testicular epidermoid cysts. J Urol. 1995;153:1147–50. [PubMed] [Google Scholar]
- 3.Benson CB, Doubilet PM, Richie JP. Sonography of the male genital tract. Am J Roentgenol. 1989;153:705–13. doi: 10.2214/ajr.153.4.705. [DOI] [PubMed] [Google Scholar]
- 4.Sohaib SA, Koh DW, Husband JE. The role of imaging in the diagnosis, staging and management of testicular cancer. Am J Roentgenol. 2008;191:387–95. doi: 10.2214/AJR.07.2758. [DOI] [PubMed] [Google Scholar]
- 5.Dogra VS, Gottlieb RH, Oka M, Rubens DJ. Sonography of the scrotum. Radiology. 2003;227:18–36. doi: 10.1148/radiol.2271001744. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh ML, Huang SI, Huang HC, et al. The reliability of ultrasonographic measurements of testicular volume assessment: comparison of three common formulas with true testicular volume. Asian J Androl. 2009;11:261–65. doi: 10.1038/aja.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsili AC, Argyropoulou MI, Giannakis D, et al. Magnetic resonance imaging in the characterization and local staging of testicular neoplasms. Am J Roentgenol. 2010;194:682–89. doi: 10.2214/AJR.09.3256. [DOI] [PubMed] [Google Scholar]
- 8.Woodward PJ, Sohaey R, O’Donoghue MJ, Green DE. Tumors and tumor like lesions of the testis: radiologic – pathologic correlation. Radiographics. 2002;22:189–216. doi: 10.1148/radiographics.22.1.g02ja14189. [DOI] [PubMed] [Google Scholar]
- 9.Akbar SA, Sayyed TA, Jafri SZ, et al. Multimodality imaging of paratesticular neoplasms and their rare mimics. Radiographics. 2003;23:1461–76. doi: 10.1148/rg.236025174. [DOI] [PubMed] [Google Scholar]
- 10.Muglia V, Tucci S, Elias J, et al. Magnetic resonance imaging of scrotal diseases: when it makes the difference. Urology. 2002;59:419–23. doi: 10.1016/s0090-4295(01)01579-5. [DOI] [PubMed] [Google Scholar]
- 11.Serra AD, Hricak H, Coakley FV, et al. Inconclusive clinical and ultrasound evaluation of the scrotum: impact on magnetic resonance imaging on patient management and cost. Urology. 1998;51:1018–21. doi: 10.1016/s0090-4295(98)00097-1. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Perez GC, Tardaguila FM, Velasco M. Radiologic findings of segmental testicular infarction. Am J Roentgenol. 2005;184:1587–93. doi: 10.2214/ajr.184.5.01841587. [DOI] [PubMed] [Google Scholar]
- 13.Liu HY, Fu YT, Wu CJ, Sun GH. Tuberculous epididymitis: a case report and literature review. Asian J Androl. 2005;7:329–32. doi: 10.1111/j.1745-7262.2005.00038.x. [DOI] [PubMed] [Google Scholar]
- 14.Cassidy FH, Ishioka KM, McMahon CJ, et al. MR imaging of scrotal tumors and pseudotumors. Radiographics. 2010;30:665–83. doi: 10.1148/rg.303095049. [DOI] [PubMed] [Google Scholar]
- 15.Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009;29:1797–810. doi: 10.1148/rg.296095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saremi F, Knoll AN, Bendavid OJ, et al. Characterization of genitourinary lesions with diffusion-weighted imaging. Radiographics. 2009;29:1295–317. doi: 10.1148/rg.295095003. [DOI] [PubMed] [Google Scholar]
- 17.Fujii S, Matsusue E, Kigawa J, et al. Diagnostic accuracy of the apparent diffusion coefficient in differentiating benign from malignant uterine endometrial cavity lesions: initial results. Eur Radiol. 2008;18:384–89. doi: 10.1007/s00330-007-0769-9. [DOI] [PubMed] [Google Scholar]
- 18.Ren J, Huan Y, Wang H, et al. Seminal vesicle invasion in prostate cancer: prediction with combined T2-weighted and diffusion-weighted MR imaging. Eur Radiol. 2009;19:2481–86. doi: 10.1007/s00330-009-1428-0. [DOI] [PubMed] [Google Scholar]
- 19.Zelhof B, Pickles M, Liney G, et al. Correlation of diffusion weighted magnetic resonance data with cellularity in prostate cancer. BJU Int. 2009;103:883–88. doi: 10.1111/j.1464-410X.2008.08130.x. [DOI] [PubMed] [Google Scholar]
- 20.Cova M, Squillaci E, Stacul F, et al. Diffusion-weighted MRI in the evaluation of renal lesions: preliminary results. Br J Radiol. 2004;77:851–57. doi: 10.1259/bjr/26525081. [DOI] [PubMed] [Google Scholar]
- 21.El-Assmy A, Abou-El-Ghar ME, Mosbah A, et al. Bladder tumour staging: comparison of diffusion and T2-weighted MR imaging. Eur Radiol. 2009;19:1575–81. doi: 10.1007/s00330-009-1340-7. [DOI] [PubMed] [Google Scholar]
- 22.Namimoto T, Awai K, Nakaura T, et al. Role of diffusion-weighted imaging in the diagnosis of gynecologic diseases. Eur Radiol. 2009;19:745–60. doi: 10.1007/s00330-008-1185-5. [DOI] [PubMed] [Google Scholar]
- 23.Lim HK, Kim JK, Kim KA, Cho KS. Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection – a multireader study. Radiology. 2009;250:145–51. doi: 10.1148/radiol.2501080207. [DOI] [PubMed] [Google Scholar]
- 24.Whittaker CS, Coady A, Culver L, et al. Diffusion-weighted MR imaging of female pelvic tumors: a pictorial review. Radiographics. 2009;29:759–78. doi: 10.1148/rg.293085130. [DOI] [PubMed] [Google Scholar]
- 25.Inada Y, Matsuki M, Nakai G, et al. Body diffusion-weighted MR imaging of uterine endometrial cancer: is it helpful in the detection of cancer in nonenhanced MR imaging? Eur J Radiol. 2009;70:122–27. doi: 10.1016/j.ejrad.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Zhang V, Liang B, Yang Z. The utility of diffusion weighted MR imaging in cervical cancer. Eur J Radiol. 2010;74:101–16. doi: 10.1016/j.ejrad.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Matsuki M, Inada Y, Tatsugami F, et al. Diffusion-weighted MR imaging for urinary bladder carcinoma: initial results. Eur Radiol. 2007;17:201–4. doi: 10.1007/s00330-006-0281-7. [DOI] [PubMed] [Google Scholar]
- 28.Fujii S, Matsusue E, Kanasaki Y, et al. Detection of peritoneal dissemination in gynaecological malignancy: evaluation by diffusion-weighted MR imaging. Eur Radiol. 2008;18:18–23. doi: 10.1007/s00330-007-0732-9. [DOI] [PubMed] [Google Scholar]
- 29.Johnson JO, Mattrey RF, Phillipson J. Differentiation of seminomatous from non-seminomatous testicular tumors with MR imaging. Am J Roentgenol. 1990;154:539–43. doi: 10.2214/ajr.154.3.2106218. [DOI] [PubMed] [Google Scholar]
- 30.Tsili ACI, Tsampoulas C, Giannakopoulos X, et al. MRI in the histologic characterization of testicular neoplasms. Am J Roentgenol. 2007;189(6):W331–37. doi: 10.2214/AJR.07.2267. [DOI] [PubMed] [Google Scholar]
- 31.Rouviere O, Bouvier R, Pangaud C, et al. Tubular ectasia of the rete testis: a potential pitfall in scrotal imaging. Eur Radiol. 1999;9:1862–68. doi: 10.1007/s003300050936. [DOI] [PubMed] [Google Scholar]
- 32.Tartar VM, Trambert MA, Balsara ZN, Mattrey RF. Tubular ectasia of the testicle: sonographic and MR imaging appearance. Am J Roentgenol. 1993;160:539–42. doi: 10.2214/ajr.160.3.8430548. [DOI] [PubMed] [Google Scholar]
- 33.Roman Birmingham PI, Navarro Sebastian FJ, Garcia Gonzalez J, Romero Barriuso G, Guijarro Espadas A. Paratesticular tumors. Description of our case series through a period of 25 years. Arch Esp Urol. 2012;65(6):609–15. [PubMed] [Google Scholar]