Abstract
Background
Few studies have documented the possibility of treatment-induced improvements in language functions 12 months or longer after stroke. The purpose of the current study was to provide a preliminary estimate of efficacy of constraint-induced aphasia therapy (CIAT) when compared to no-intervention in patients with chronic (>1 year) post-stroke aphasia in order to provide the data needed to design an appropriately powered trial.
Material/Methods
This was a randomized, controlled, single-blinded, pilot trial. We identified 32 patients with chronic post-stroke aphasia. Of these, 27 were offered participation, and 24 were randomized (CONSORT diagram): 14 to CIAT and to 10 to no-intervention. CIAT groups received up to 4 hours/day of intervention for 10 consecutive business days (40 hours of therapy). Outcomes were assessed within 1 week of intervention and at 1 and 12 weeks after intervention and included several linguistic measures and a measure of overall subjective communication abilities (mini-Communicative Abilities Log (mini-CAL)). To maintain blinding, clinicians treating patients (CIAT group) did not communicate with other team members and the testing team members were blinded to treatment group assignment.
Results
Overall, the results of this pilot trial support the results of previous observational studies that CIAT may lead to improvements in linguistic abilities. At 12 weeks, the treatment group reported better subjective communication abilities (mini-CAL) than the no-intervention group (p=0.019). Other measures trended towards better performance in the CIAT group.
Conclusions
In this randomized, controlled, and blinded pilot study, intensive language therapy (CIAT) led to an improvement in subjective language abilities. The effects demonstrated allow the design of a definitive trial of CIAT in patients with a variety of post-stroke aphasia types. In addition, our experiences have identified important considerations for designing subsequent trial(s) of CIAT or other interventions for post-stroke aphasia.
MeSH Keywords: Aphasia, Language Disorders, Rehabilitation of Speech and Language Disorders, Stroke
Background
Aphasia is one of the most devastating sequelae of stroke. It typically improves in the weeks and months after stroke, yet about 50% of patients are left with long-term residual deficits [1]. After the first year, spontaneous recovery is thought to be unlikely. Therapies administered during the first 12 months after stroke accelerate recovery [2,3]. Few studies have documented the possibility of treatment-induced improvements in post-stroke language functions after 12 months [4].
Traditionally, aphasia interventions utilize compensatory communication strategies to assist the participant in immediate communication needs. Multi-modal strategies include gesturing, writing, drawing, and augmentative low- and high-technology systems, with a common expectation that use of the alternative communication techniques will decrease naturally as the language capabilities increase. These interventions improve overall communication abilities, but questions have been asked whether they contribute to the recovery of language function, or whether they actually contribute to a learned non-use phenomenon [5]. To counteract the possibility of learned non-use, therapies utilizing restraint have been developed to mirror constraint-induced motor therapies. While it is relatively easy to restrain an unaffected extremity in motor therapies [6,7], restraining attempts to communicate non-verbally can be more difficult. Constraint-induced aphasia therapy (CIAT) encourages intensive verbal practice with supported verbal cuing while excluding the use of previously habitual compensatory strategies [8,9].
In CIAT, the theoretical model of Taub’s motor system for use-dependent cortical re-organization has been applied to a language-based program [8]. In this model, it has been postulated that the behavior of attempting to speak without success leads to communication frustration. This then results in fewer speaking attempts, more reliance on compensatory strategies, and less cortical stimulation in the language areas. The CIAT framework provides a structured supportive environment, with clinician guidance and shaping, positive reinforcement from group members, and social interaction opportunities. The theory is that the supportive environment and speaking opportunities will encourage more verbal attempts and stimulate cortical reorganization [10]. Thus, the goal of the present study was to provide evidence for the potential efficacy of CIAT when compared to no-intervention in patients with chronic (>1 year) post-stroke aphasia. More specifically, this randomized, controlled, blinded pilot study was conducted in order to estimate effect sizes, allowing the design of an appropriately powered trial [11].
Material and Methods
Subjects
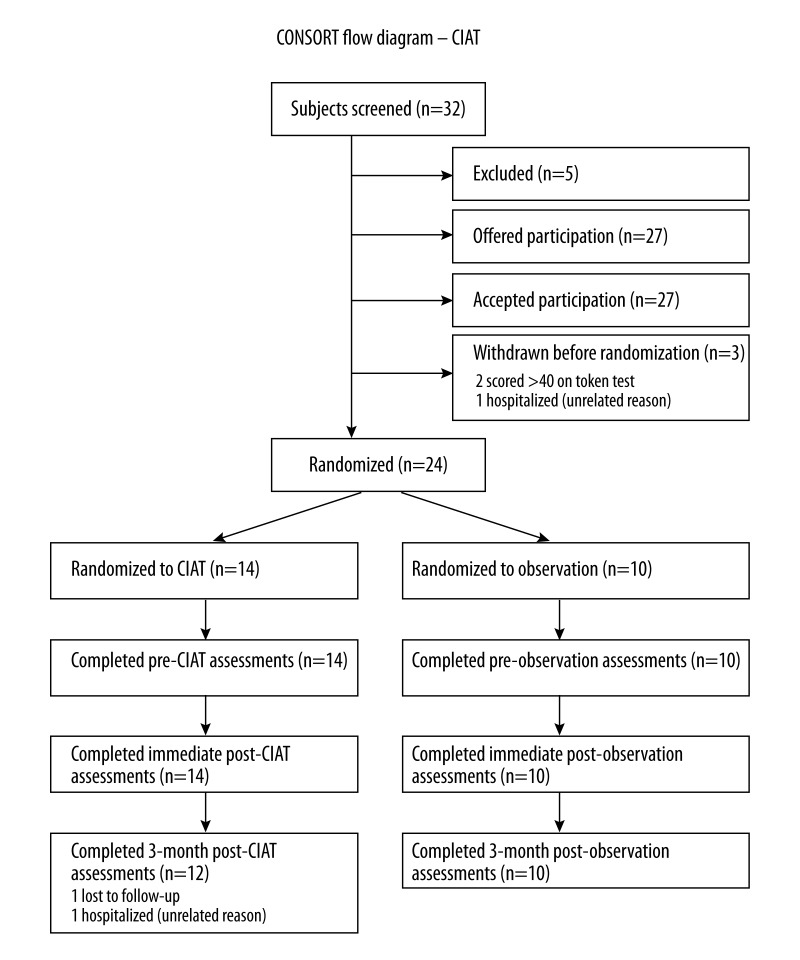
Subjects were recruited into this Institutional Review Board-approved study by word of mouth from among the stroke and aphasia clinics at the University of Cincinnati and University of Alabama at Birmingham, and from local aphasia support groups. We also listed the study on www.clinicaltrials.gov (registered NCT00843427; PI: Szaflarski), and several contacts were received directly from patients. After providing consent for screening, 32 individuals were interviewed. The inclusion criteria were chronic aphasia related to a single ischemic stroke in the left middle cerebral artery (LMCA) distribution (i.e., diagnosis of a single LMCA stroke was confirmed by medical record review including admission notes for the incident stroke and the results of brain imaging obtained prior to enrollment), Token Test in an impaired range (score ≤40), and pre-stroke fluency in English. The exclusion criteria were history of degenerative (e.g., dementia or Parkinson’s disease) or metabolic disorder (e.g., encephalopathy) or supervening illness (e.g., brain tumor or other cancer), history of severe depression or other mental illness, and positive pregnancy test in women of childbearing age. Patients with more than 1 stroke were not eligible. Five potential participants were excluded after interview. The format and the goals of the CIAT program were explained to all participants at the time of obtaining the informed consent. All patients indicated their understanding of the goals of the program prior to signing the informed consent; they also understood that they may be randomized to a no-intervention group, and that the follow-up testing will need to be performed. Of the 27 who were offered participation, 3 were excluded before randomization: 2 had a normal Token Test and 1 was hospitalized for reasons unrelated to the study. Fourteen subjects were randomized to receive CIAT (3–4 participants per group; 4 groups were assembled) and 10 to receive no-intervention (Figure 1). Demographic and clinical data of the participants are provided in Table 1.
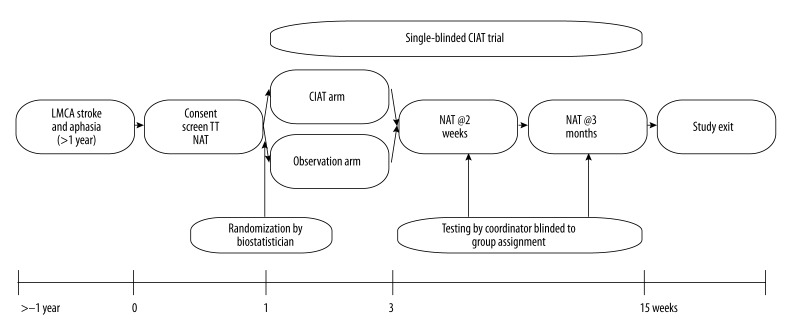
Figure 1.
Diagram of the CIAT study and associated testing (LMCA – left middle cerebral artery; TT – Token Test; NAT – Neuropsychological aphasia testing; CIAT – constraint-induced aphasia treatment).
Table 1.
Demographic characteristics for CIAT intervention and no-treatment (observation) groups.
| Control group (n=10) | CIAT group (n=14) | P value | |
|---|---|---|---|
| Age (years) – mean (SD) | 51 (13) | 57 (11) | 0.195 |
| White – n (%) | 9 (90.0) | 10 (71.4) | 0.358 |
| Non-Hispanic – n (%) | 10 (100.0) | 14 (100.0) | – |
| Male – n (%) | 5 (50.0) | 9 (64.3) | 0.678 |
| Past medical history – n (%) | |||
| History of HTN | 3 (30.0) | 7 (50.0) | 0.421 |
| History of DM | 1 (10.0) | 3 (21.4) | 0.615 |
| History of high cholesterol | 4 (40.0) | 8 (57.1) | 0.680 |
| History of CAD | 0 (0.0) | 2 (14.3) | 0.493 |
| History of MI | 1 (10.0) | 1 (7.1) | 1.000 |
| Smoking | 4 (40.0) | 7 (50.0) | 0.697 |
| Alcohol abuse | 0 (0.0) | 2 (16.6) | 0.493 |
| Drug abuse | 0 (0.0) | 0 (0.0) | – |
| Prior stroke | 2 (20.0) | 3 (21.4) | 1.000 |
| Severity – n (%) | |||
| Mild aphasia | 2 (20.0) | 6 (42.9) | 0.291 |
| Moderate aphasia | 4 (40.0) | 2 (14.3) | |
| Severe aphasia | 4 (40.0) | 6 (42.9) | |
| Time since stroke (months) – median (IQR) | 30 (58) | 38 (59) | 0.725 |
HTN – hypertension; DM – diabetes mellitus; CAD – coronary artery disease; MI myocardial infarction.
Definitions
After obtaining the informed consent, we initially screened the patients for the presence of aphasia with Token Test (TT) and categorized the severity of aphasia as mild (TT=40-37), moderate (TT=36-17) or severe (TT=16-0) [12]. Responders were defined as patients with at least a 20% relative improvement in at least 2 of the 5 primary scores in the 2-week period. Retainers were those patients whose 12-week score was not less than the 2-week score on at least 2 of the 5 outcome measures.
Description of the intervention
The 2-week CIAT protocol was a closely monitored, individualized program embedded within a larger group activity [8,9]. To ensure consistency of the intervention, all clinicians completed a training program. Training (approximately 4–6 hours) was conducted by ALB prior to initiating any intervention session including basic theory of learned non-use, and the procedures for the treatment. Clinicians also viewed sample videos that exemplified the nature and set-up of the CIAT intervention, and they were taught a hierarchy of cues that ranged from most to least supportive (e.g., imitation to verbal reminder).
During the first day of intervention, clinicians had no information on participants’ abilities as, by design, they did not participate in the initial evaluations. The measures were administered by coordinators who were blinded to group assignment (Figure 1). To enhance the chance for successful implementation of cues during the CIAT sessions, clinicians maintained a cuing tracking form that described the interaction between another clinician and their participant partners. The types of cues provided were tracked with a binary notation of whether the cuing resulted in a successful communication. This technique of behaviorally monitoring another clinician rather than self-monitoring was established in our previous study [9]. Clinicians and participants were rotated during each day’s session in order to provide a balance in communication partners. At the end of day 1, ALB studied the cuing tracking forms and the videos to create an individual treatment plan for the next day’s session. The treatment plan included identifying individual baseline linguistic strengths, suggested hierarchy of most beneficial cues, behaviors to constrain, and specific linguistic targets or goals [9]. The treatment plan was reviewed with the clinicians before the second day of therapy.
From the second day onwards, clinicians promoted individualized support with cues at a linguistic level suitable for each person and continued data tracking. The tracking provided constant reminders to only provide cues that resulted in successful communication. Each day, clinicians reviewed the individual program and levels and made adjustments as needed. At the beginning of each session day, the clinicians reminded each participant of the goals and constraints that had been established for them. Goals followed a linguistic complexity hierarchy. For example, a participant who was strong in producing nouns, but limited in verbs, had a goal to add a verb to create a 2-word phrase. A linguistic complexity chart was provided to the clinicians to support the progression. If a participant demonstrated a milder aphasia, then the D-level hierarchy of sentences was implemented [13].
The treatment program for each of the 3–4 participant groups lasted 2 weeks, with direct therapy for about 4 hours per day for 10 consecutive weekdays. The sessions were organized into 4 45-minute periods, with a 10–15 minute break in between each session. Socialization between clients and clinicians was encouraged throughout the program, even during the break periods. At the beginning of each session, participants were dealt cards and they were instructed to play a go-fish type game. The cards provided the opportunity for the participants to interact with each other during an ongoing game that engaged participants’ visual, attention, and memory skills, although these were not specifically targeted. The cards provided visual stimuli of line drawings of nouns (singular, plural to elicit numbers, and with colors) and photos of action verbs [8,9].
The program included feedback given to the clinician, with guidance provided by ALB. Clinicians were encouraged to use redirecting phrases such as “try again” and “are you sure?” rather than using negative responses. If the other participants responded positively and the communicative exchange was successful, clinicians were instructed not to correct; communicative success is more important than the sentence accuracy. Whenever clinicians were observed to manage cards for the participant, this was discouraged; clinicians were trained to be supportive while encouraging independence.
Consistent with the current standards of care, the no-intervention group did not receive any specific treatment and the participants were asked to continue all previous activities as usual. All participants were asked not to take part in any other intervention during their involvement in the study and all complied.
Randomization
Patients were randomized by the study statistician (CJL) after the patients received all pre-requisite activities (consenting, clinical record review, neuropsychological aphasia testing (NAT)). Patients were assigned to receive either 2 weeks of CIAT, or no-intervention, and then to undergo NAT within one week and 3 months of CIAT completion (Figure 2 – CONSORT Diagram). We used a simple scheme that randomized each block of patients to either CIAT or control. Randomization occurred after consent, and with the statistician blinded to participant performance on screening and baseline testing. We did not replace subjects who do not complete the full 2 weeks of therapy.
Figure 2.
CONSORT Diagram.
Blinding
After randomization, sealed study charts containing all pre-intervention testing results (NAT) were funneled through the study biostatistician (CJL) to the therapists. Therapists set up the intervention groups so that the coordinators (CB, ANM) who collected all NAT data throughout the study remained blinded to group assignment. Participants were asked not to reveal their group assignment to coordinators during the post-treatment interactions.
Measures
The Token Test was used only for primary screening and study qualification. All participants received NAT which included: (1) the Boston Naming Test (BNT) [14], (2) the Controlled Oral Word Association Test [15], (3) the Semantic Fluency Test (SFT) [15,16], (4) the Complex Ideation subtest from the Boston Diagnostic Aphasia Examination (BDAE) [17], (5) the Peabody Picture Vocabulary Test III (PPVT III) [18], and (6) the Mini-Communicative Activities Log (Mini-CAL) which is a subjective measure of communicative abilities [8,9]. The study coordinators, who were extensively trained in the use of these measures and blinded to group assignment, administered the NAT measures within 1 week prior to CIAT and again during both the first week the twelfth week following CIAT completion. All data were entered into REDCap (Research Electronic Data Capture) for subsequent analysis [19].
Data analysis
Analysis was conducted on an intent-to-treat basis. One patient did not complete the 12 week visit or testing, and 7 additional patients were missing at least 1 NAT score. Missing scores were left missing, and are excluded from analysis. The primary analysis used Independent Samples T-Tests to examine the differences in NAT scores at each time point between the intervention and control groups, and effect sizes and 95% confidence intervals were calculated. The secondary analysis was an ad hoc analysis comparing the characteristics of patients who demonstrated a response or change in NAT scores to patients who did not. Independent T-Tests, The Mann-Whitney U test, and Fisher’s Exact Test were used to compare patient characteristics between patients who demonstrated a response or change and patients who did not. All statistical analyses were conducted using SPSS 22.0 (IBM Corporation, Armonk, NY) and R 2.15.3 (base package).
Results
Subjects
There were 24 patients enrolled in the study, 14 in the intervention group, and 10 in the control group (see CONSORT Diagram). Two patients in the intervention group did not complete the study, 1 due to lack of transportation and 1 due to hospitalization with an illness unrelated to the study. Overall, there were no significant differences in demographic characteristics and past medical history between the 2 groups (Table 1). The mean age was 57 (SD±11) years in the intervention group and 51 (SD±13) years in the control group. Most patients were Caucasian, 10/14 (71%) in the intervention group and 9/10 (90%) in the control group.
Primary analysis
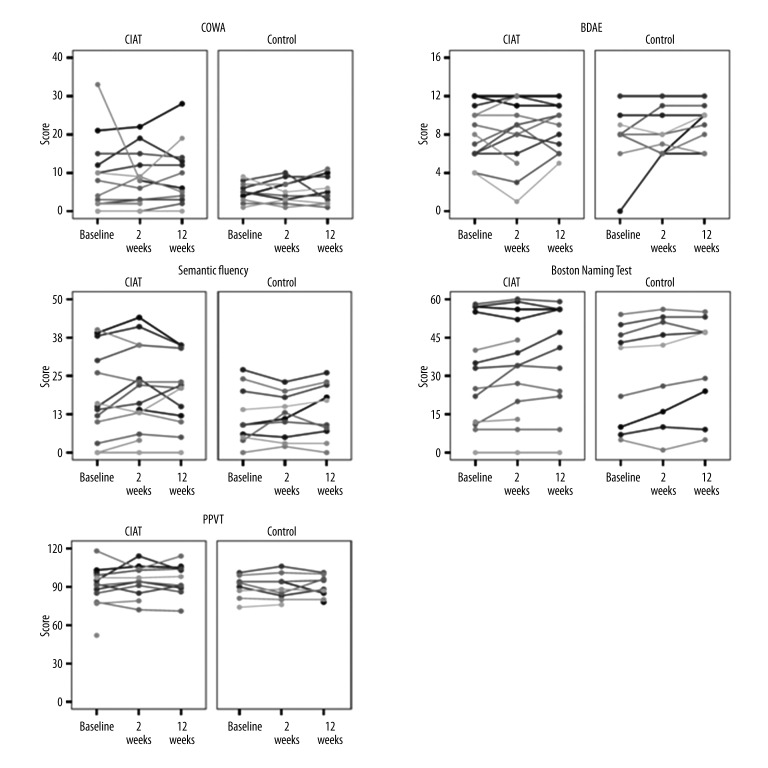
There were few statistically significant differences in NAT scores between the intervention and control groups (Table 2). Specifically, differences were observed in subjective communication abilities assessed with the mini-CAL. Patients who received CIAT scored higher on the mini-CAL at 12 weeks after intervention compared to the control group (Mean 31 vs. 23; difference=8, 95% CI 1.3 to 13.5, p=0.019). The SFT was marginally higher 2 weeks after intervention in CIAT patients than controls (Mean 21 vs. 12; difference 9, 95% CI −0.3 to 17.8, p=0.058). This difference was not sustained at twelve weeks after intervention. Scores for all NATs are presented in Table 2 and Figure 3.
Table 2.
Difference in NAT scores at 3 time points by treatment group.
| Control (n=10) | CIAT (n=14) | Diff. | 95% CI | P value* | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Lower | Upper | |||
| Mini Cal: Baseline | 22 | 9 | 26 | 9 | 3.671 | −4.291 | 11.633 | 0.347 |
| Mini Cal: Twelve weeks | 23 | 7 | 31 | 7 | 7.427 | 1.352 | 13.502 | 0.019 |
| Semantic Fluency Test: Baseline | 12 | 9 | 19 | 15 | 6.892 | −4.034 | 17.818 | 0.204 |
| Semantic Fluency Test: Two weeks | 12 | 7 | 21 | 14 | 8.714 | −0.332 | 17.761 | 0.058 |
| Semantic Fluency Test: Twelve weeks | 13 | 9 | 19 | 12 | 6.117 | −3.266 | 15.499 | 0.189 |
| Complex Ideation: Baseline | 8 | 3 | 8 | 3 | 0.410 | −2.325 | 3.146 | 0.758 |
| Complex Ideation: Two weeks | 8 | 2 | 8 | 4 | −0.068 | −2.865 | 2.729 | 0.960 |
| Complex Ideation: Twelve weeks | 9 | 2 | 9 | 2 | −0.475 | −2.603 | 1.654 | 0.645 |
| Controlled Oral Word Association Test: Baseline | 5 | 3 | 9 | 10 | 4.231 | −1.690 | 10.151 | 0.148 |
| Controlled Oral Word Association Test: Two weeks | 5 | 3 | 8 | 7 | 3.186 | −1.601 | 7.973 | 0.181 |
| Controlled Oral Word Association Test: Twelve weeks | 5 | 4 | 10 | 8 | 4.367 | −1.155 | 9.888 | 0.113 |
| Peabody Picture Vocabulary Test III: Baseline | 90 | 9 | 90 | 16 | 0.587 | −12.342 | 13.515 | 0.925 |
| Peabody Picture Vocabulary Test III: Two weeks | 90 | 10 | 94 | 12 | 4.788 | −5.916 | 15.492 | 0.360 |
| Peabody Picture Vocabulary Test III: Twelve weeks | 90 | 8 | 96 | 12 | 6.182 | −3.796 | 16.160 | 0.209 |
| Boston Naming Test (BNT): Baseline | 31 | 20 | 32 | 21 | 0.957 | −17.363 | 19.278 | 0.914 |
| Boston Naming Test (BNT): Two weeks | 33 | 21 | 34 | 20 | 0.940 | −17.253 | 19.134 | 0.915 |
| Boston Naming Test (BNT): Twelve weeks | 35 | 19 | 37 | 21 | 1.525 | −17.273 | 20.324 | 0.867 |
Unadjusted p values are provided. To correct for multiple comparisons the critical p value should be set to 0.003 (0.05/17 tests). All reported scores for all measures are reported unadjusted.
Figure 3.
Stick plots for each aphasia test at each time point split by treatment group. Each line represents a single case.
Secondary analysis
Overall, 5/24 (21%) of participants could be classified as a “responders”, as defined in the methods section. Of these, 4/5 (80%) were in the CIAT group. Complete characteristics of responders and non-responders are presented in Table 3. Responders were less likely to have a history of hypertension 0/5 (0%), compared to non-responders 10/14 (53%), p=0.053. Of the 5 patients who demonstrated a change in NAT scores during the course of the study, only 1 (20%) retained the change at the twelve-week visit.
Table 3.
Patient characteristics by responder.
| Non-Responder (n=19) | Responder (n=5) | P Value | |
|---|---|---|---|
| CIAT – n (%) | 10 (52.6) | 4 (80.0) | 0.358 |
| Age – mean (SD) | 54 (12) | 58 (11) | 0.547 |
| Caucasian – n (%) | 14 (73.7) | 5 (100.0) | 0.544 |
| Male – n (%) | 12 (63.2) | 2 (40.0) | 0.615 |
| Months since stroke – median (IQR) | 28 (62) | 41 (35) | 0.406 |
| Past medical history – n (%) | |||
| Hypertension | 10 (52.6) | 0 (0.0) | 0.053 |
| High cholesterol | 11 (57.9) | 1 (20.0) | 0.317 |
| Diabetes | 4 (21.1) | 0 (0.0) | 0.544 |
| MI | 2 (10.5) | 0 (0.0) | 1.000 |
| CAD | 2 (10.5) | 0 (0.0) | 1.000 |
| Non-smoker | 9 (47.4) | 4 (80.0) | 1.000 |
| Severe aphasia – n (%) | 6 (31.6) | 4 (80.0) | 0.122 |
| Motor impairment – n (%)* | 5 (50.0) | 3 (100.0) | 0.231 |
CIAT group only.
Discussion
In this prospective, preliminary, randomized, blinded study of patients with chronic post-stroke aphasia, we estimated the effect of CIAT on linguistic performance. While the results are largely not statistically significant, a few points need to be discussed. First, the study included patients with highly variable levels of post-stroke aphasia, making the comparisons between the groups somewhat difficult. Second, the observed effects are consistent with improvement in the CIAT group beyond that in the control group, with trends towards statistical significance for some of the variables even in this small sample. Defining a responder as a participant with 20% improvement on at least 2 of the 5 tests, then the number needed to show significant between-group differences would be 62 and allows for the design of a more comprehensive trial. Finally, a significant improvement was noted in the mini-CAL results for the CIAT group compared to controls. This indicates that, at least subjectively, the patients in CIAT group perceived more improvement over time than participants in the control group.
Reasons for the participants’ perception of improvement, as measured by the mini-CAL, may be related to the social activity of the therapy sessions. In order to isolate the variable of social interaction from CIAT theory, future studies should consider including a study arm involving exposure to social interaction with the same duration and frequency as the CIAT group, but without the CIAT intervention. This could also assess for possible placebo effects [20]. A preliminary qualitative investigation suggested a relationship with social factors [21]. Social factors may be related to both clinicians and other participants in the group.
To date, few reports on the potential efficacy of CIAT have been published. Five studies in patients with chronic aphasia have included both a treatment group and a control group [8,22–25]. Only in the original study were participants randomized to treatment group [8]; however, unlike the current study, the personnel collecting linguistic data were not blinded to treatment assignment in any of these studies. In the original study, treatment intensity was much higher in the CIAT group [8]. In the other 4 studies CIAT was compared to other treatment approaches – either model-based aphasia therapy (training based on functional deficit) [25], CIAT plus written module [24], PACE [23], or model-oriented aphasia therapy (MOAT) [22]. In addition to the above studies, a recent study compared modified CIAT to conventional aphasia therapy in patients with less than 4 months (subacute) since the incident ischemic or hemorrhagic stroke [26]. All these studies showed that while participants receiving treatment improved linguistic performance, between-group differences were minimal, if any. Thus, when comparing the results of the original study by Pulvermuller et al. and the results of the subsequent studies, including ours, the questions of training intensity and social interactions between participants, and participants and clinicians need to be considered; both can contribute to improved communicative abilities via increased verbal communication practice [8,21,24]. This demonstrates the importance of comparing treatment approaches against a non-active control.
We did not show any statistically significant improvement in objective tests of linguistic performance from before to after treatment. One of the reasons for this lack of statistical significance in our small sample size is the high variability in language scores observed in both groups (Figure 3). A future study enrolling a highly-variable group of participants would require a sample size of only 62 per treatment group to show a significant advantage of CIAT over passive observation. But, the effects observed by us are in line with the results of the original study by Pulvermuller et al. (2001).
We identified important considerations for study design that will minimize unnecessary variability and maximize the potential for observing treatment benefit. Treatment fidelity needs to be monitored, either via independent reviewers performing video or by direct review of the conducted sessions. A transfer package similar to that offered in motor rehabilitation studies will need to be developed and applied to this group [27]. Also, the outcome measures should include measures of pre- and post-intervention discourse and assess changes in the severity of aphasia using, e.g., the Western Aphasia Battery-Revised [28]; assessing linguistic complexity using mean length of utterances [29]; or indices of syntactic form [30]. In addition to the above, stratification by severity, education, and socioeconomic status might emphasize the magnitude of improvement relative to the degree of aphasia [31]. Finally, other factors, including the effects of lesion size and location on changes in aphasia diagnosis (type) during the therapy session, should be assessed [32,33].
Conclusions
Modest improvements were noted in the CIAT group on the collected objective tests that were not noted in the control group, forming the basis for estimating effect sizes needed for a comprehensive study. Moreover, the CIAT group reported significant subjective improvement that confirms the importance of this approach. Larger randomized controlled trials that adopt the measures suggested to reduce variability and maximize observable benefit are warranted.
Acknowledgements
The authors acknowledge Christi Banks, CCRC for her help with data collection.
Footnotes
This study was presented in part at the Annual Meeting of the American Academy of Neurology in Philadelphia, PA in 2014
Source of support: This study was supported by NINDS R01 NS 048281 and by NIH/NCRR UL1-RR026314 (REDCap Database)
References
- 1.Pedersen PM, Jorgensen HS, Nakayama H, et al. Aphasia in acute stroke: incidence, determinants, and recovery. Ann Neurol. 1995;38(4):659–66. doi: 10.1002/ana.410380416. [DOI] [PubMed] [Google Scholar]
- 2.Carlomagno S, Pandolfi M, Labruna L, et al. Recovery from moderate aphasia in the first year poststroke: effect of type of therapy. Arch Phys Med Rehabil. 2001;82(8):1073–80. doi: 10.1053/apmr.2001.25155. [DOI] [PubMed] [Google Scholar]
- 3.Robey RR. A meta-analysis of clinical outcomes in the treatment of aphasia. J Speech Lang Hear Res. 1998;41(1):172–87. doi: 10.1044/jslhr.4101.172. [DOI] [PubMed] [Google Scholar]
- 4.Berthier ML, Pulvermuller F. Neuroscience insights improve neurorehabilitation of poststroke aphasia. Nat Rev Neurol. 2011;7(2):86–97. doi: 10.1038/nrneurol.2010.201. [DOI] [PubMed] [Google Scholar]
- 5.Andre JM, Didier JP, Paysant J. “Functional motor amnesia” in stroke (1904) and “learned non-use phenomenon” (1966)”. J Rehabil Med. 2004;36(3):138–40. doi: 10.1080/16501970410026107. [DOI] [PubMed] [Google Scholar]
- 6.Page SJ, Levine P, Leonard A, et al. Modified constraint-induced therapy in chronic stroke: results of a single-blinded randomized controlled trial. Phys Ther. 2008;88(3):333–40. doi: 10.2522/ptj.20060029. [DOI] [PubMed] [Google Scholar]
- 7.Taub E, Uswatte G, Pidikiti R. Constraint-induced movement therapy: A new family of techniques with broad application to physical rehabilitation – a clinical review. J Rehabil Res Dev. 1999;36:237–51. [PubMed] [Google Scholar]
- 8.Pulvermuller F, Neininger B, Elbert T, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32(7):1621–26. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- 9.Szaflarski JP, Ball A, Grether S, et al. Constraint-induced aphasia therapy stimulates language recovery in patients with chronic aphasia after ischemic stroke. Med Sci Monit. 2008;14(5):CR243–50. [PMC free article] [PubMed] [Google Scholar]
- 10.Meinzer M, Flaisch T, Breitenstein C, et al. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. Neuroimage. 2008;39(4):2038–46. doi: 10.1016/j.neuroimage.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Katz RC, Hallowell B, Code C, et al. A multinational comparison of aphasia management practices. Int J Lang Commun Disord. 2000;35(2):303–14. doi: 10.1080/136828200247205. [DOI] [PubMed] [Google Scholar]
- 12.De Renzi E, Vignolo LA. The token test: A sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–78. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg S, Abbeduto L. Indicators of linguistic competence in the peer group conversational behavior of mildly retarded adults. Appl Psycholinguistics. 1987;8:19–32. [Google Scholar]
- 14.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 15.Lezak M. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- 16.Kozora E, Cullum C. Generative naming in normal aging: Total output and qualitative changes using phonemic and semantic constraints. Clin Neuropsychologist. 1995;9(4):313–20. [Google Scholar]
- 17.Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Philadelphia: Lea & Febiger; 1972. [Google Scholar]
- 18.Dunn L, Dunn L. Peabody Picture Vocabulary Test. 3rd Edition. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espay AJ, Norris MM, Eliassen JC, et al. Placebo effect of medication cost in Parkinson disease: A randomized double-blind study. Neurology. 2015;84(8):794–802. doi: 10.1212/WNL.0000000000001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ball A, Griffith J, Vannest J, et al., editors. The social dynamics of CIAT without barriers. ASHA Annual Meeting; Chicago. 2013. [Google Scholar]
- 22.Barthel G, Meinzer M, Djundja D, Rockstroh B. Intensive language therapy in chronic aphasia: Which aspects contribute most. Aphasiology. 2008;22(4):408–21. [Google Scholar]
- 23.Maher LM, Kendall D, Swearengin JA, et al. A pilot study of use-dependent learning in the context of Constraint Induced Language Therapy. J Int Neuropsychol Soc. 2006;12(6):843–52. doi: 10.1017/S1355617706061029. [DOI] [PubMed] [Google Scholar]
- 24.Meinzer M, Djundja D, Barthel G, et al. Long-term stability of improved language functions in chronic aphasia after constraint-induced aphasia therapy. Stroke. 2005;36(7):1462–66. doi: 10.1161/01.STR.0000169941.29831.2a. [DOI] [PubMed] [Google Scholar]
- 25.Meinzer M, Elbert T, Wienbruch C, et al. Intensive language training enhances brain plasticity in chronic aphasia. BMC Biol. 2004;2(1):20. doi: 10.1186/1741-7007-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sickert A, Anders LC, Munte TF, Sailer M. Constraint-induced aphasia therapy following sub-acute stroke: a single-blind, randomised clinical trial of a modified therapy schedule. J Neurol Neurosurg Psychiatry. 2014;85(1):51–55. doi: 10.1136/jnnp-2012-304297. [DOI] [PubMed] [Google Scholar]
- 27.Johnson ML, Taub E, Harper LH, et al. An enhanced protocol for constraint-induced aphasia therapy II: a case series. Am J Speech Lang Pathol. 2014;23(1):60–72. doi: 10.1044/1058-0360(2013/12-0168). [DOI] [PubMed] [Google Scholar]
- 28.Kertesz A. Western Aphasia Battery-Revised. San Antonio, TX: Psychological Corporation; 2006. [Google Scholar]
- 29.Miller JF, Andriacchi K, Knockerts A. Assessing language production using salt software: A clinician’s guide to language sample analysis. Middletown, WI: SALT Software LLC; 2011. [Google Scholar]
- 30.Boyle M. Test-retest stability of word retrieval in aphasic discourse. J Speech Lang Hear Res. 2014;57(3):966–78. doi: 10.1044/2014_JSLHR-L-13-0171. [DOI] [PubMed] [Google Scholar]
- 31.Kissela B, Schneider A, Kleindorfer D, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35(2):426–31. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 32.Szaflarski JP, Allendorfer JB, Banks C, et al. Recovered vs. not-recovered from post-stroke aphasia: The contributions from the dominant and non-dominant hemispheres. Restor Neurol Neurosci. 2013;31(4):347–60. doi: 10.3233/RNN-120267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szaflarski JP, Allendorfer JB, Byars AW, et al. Age at stroke determines post-stroke language lateralization. Restor Neurol Neurosci. 2014;32(6):733–42. doi: 10.3233/RNN-140402. [DOI] [PMC free article] [PubMed] [Google Scholar]