Abstract
Purpose
To investigate the effect of the complement activation product C5a on toll-like receptor (TLR) 4-induced responses in RPE cells.
Methods
Confluent cultures of human RPE cells (ARPE-19) were stimulated with C5a, lipopolysaccharide (LPS), or a combination of the two. The expression of TLR4 was determined by real-time PCR and flow cytometry. Cytokine profiles were determined by real-time PCR and enzyme-linked immunosorbent assay (ELISA). The phosphorylation of p38, ERK 1/2, and JNK was measured by flow cytometry.
Results
C5a stimulation enhanced the expression of TLR4 in a dose- and time-dependent manner. C5a was able to stimulate the production of TLR4-induced IL-6 and IL-8 by ARPE-19 cells. Blocking experiments showed that the effect of C5a on cytokine production was mediated via C5aR. ERK1/2, but not JNK or p38, were involved in the production of IL-6 and IL-8.
Conclusions
The results indicate that C5a can induce the TLR4 expression and enhance the production of TLR4-induced IL-6 and IL-8 by ARPE-19. The effect of C5a on cytokine production was mediated by C5aR and the phosphorylation of ERK1/2.
Introduction
The complement system and toll-like receptors (TLRs) are two major components of the innate immune system that recognize and respond rapidly to pathogens and serve as important mediators between innate and adaptive immunity [1]. The two systems have the ability to recognize pathogen-associated molecular patterns (PAMPs) and to destroy microbial invaders. It is becoming increasingly clear that there is crosstalk between TLRs and complement pathways, including the widespread regulation of TLR signaling by complements [2,3].
In ocular immunity, the complement system is implicated in the development and progression of several immune-mediated ocular conditions, including age-related macular degeneration (AMD) and uveitis [4]. Nozaki et al. showed that C5a is present in the drusen of patients with AMD, and it promotes choroidal neovascularisation (CNV), which is the hallmark of wet AMD, by increasing the expression of vascular endothelial growth factor (VEGF) [5,6]. Systemic and local anti-C5 therapies reduce the disease severity in experimental autoimmune uveoretinitis [7]. C5a induces the increased expressions of IL-1beta, IL-6, MCP-1, GM-CSF, and IL-8 in RPE cells [8]. Lipopolysaccharide (LPS), as a well-established ligand for TLR4 [9], can elicit acute ocular inflammation in animals and can lead to uveitis [10,11].
Many PAMPs activate both complements and TLRs, and recent studies have revealed a marked synergistic interaction between the two systems. Heiko and colleagues showed that C5a negatively regulates the TLR4-induced synthesis of IL-12 family cytokines (IL-12, IL-23, and IL-27) from inflammatory macrophages by extracellular signal-regulated kinase- and phosphoinositide 3 kinase-dependent pathways [2]. C5a controls TLR4-induced IL-10 and IL-12 production in mouse macrophages, and it was shown to depend on the extracellular signal-regulated kinase (ERK) 1/2 pathway [12]. A recent report demonstrated that C5a reduced the LPS-induced production of IL-12, IL-23, and IL-6 in immature dendritic cells (DCs), but the suppressive effect was time dependent [13].
Although it is reported that there is crosstalk between TLRs and complements in other cell types, whether and how these two systems when co-activated in the eye interact with each other has not been well studied. RPE cells have been shown to express both TLR4 [14] and C5a receptors [15], and they were therefore chosen in this study to investigate the interaction between these systems. It was observed that C5a leads to an enhanced TLR4 expression in RPE cells. C5a furthermore significantly increased the TLR4-induced synthesis of IL-6 and IL-8 by RPE cells, and evidence of the involvement of the C5aR signaling pathways and the activation of ERK was provided.
Methods
Cell culture
The human RPE (ARPE-19) cell line was obtained from the American Type Culture Collection (ATCC). Cells were cultured in a medium (Dulbecco’s modified Eagle’s medium: nutrient mixture F12 [DMEM/F12], 1:1; Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS; Invitrogen), 100 U/mL penicillin, and 100 ng/ml streptomycin. Cells were incubated in a humidified 5% CO2 atmosphere at 37 °C and they were passaged every 5 to 7 days. After reaching confluence, the cells were detached with a trypsin-EDTA solution, diluted at 1:3 to 1:4, and plated for subculture. The ARPE-19 cells used in the experiments were confluent. Before the experimental procedures, the ARPE-19 cells were kept under serum-free conditions for 24 h. Cells used in these experiments were validated as authentic ARPE-19 cells by STR analysis (Appendix 1).
Stimulation of RPE
Cells were primed with LPS (100 ng/ml, Sigma, E. coli O127:B8) for 16 h in a total volume of 1 ml. Recombinant human C5a (50 ng/ml, Sigma) was added to the culture 10 min before LPS challenge. Inhibitors of JNK (SP600125, 10 uM), ERK1/2 (PD98059, 20 uM), and P38 (SB239063, 20 uM; all from Calbiochem, Darmstadt, Germany) were added 1 h before LPS stimulation. An antagonist for C5aR (W-54011, 10 ng/ml, Calbiochem) was added 4 h before LPS stimulation.
Real-time quantitative PCR analysis
Total RNA was extracted with TRIzol (Invitrogen) following the manufacturer’s instructions. RNA concentrations were determined with a Nano instrument (NanoDrop Technologies, Wilmington, DE). The first-strand cDNA was synthesized for each RNA sample using the Superscript III Reverse Transcriptase system (Invitrogen). Real-time quantitative PCR was performed on the iCycler (Biorad, UK) using the Quanti Tect SYBR Green PCR kit (Applied Biosystems). The forward and reverse primers for β-actin were designed using the Primer Premier software (Premier Biosoft International). The sequences of the PCR primer pairs were as follows: β-actin forward, 5′-GGA TGC AGA AGG AGA TCA CTG-3′ and reverse, 5′-CGA TCC ACA CGG AGT ACT TG-3′; TLR4 forward, 5′-AGT TTC CTG CAA TGG ATC AAGG-3′ and reverse 5′-CTG CTT ATC TGA AGG TGT TGC AC-3′; IL-6 forward, 5′-AGT GAG GAA CAA GCC AGA GC-3′ and reverse, 5′-CAG GGG TGG TTA TTG CAT CT-3′; IL-8 forward, 5′-GAC ATA CTC CAA ACC TTT CCA CCC-3′ and reverse, 5′-CCA GAC AGA GCT CTC TTC CAT CAG-3′. The human β-actin gene was used as an endogenous control for sample normalization. For each sample, the relative abundance of target mRNA was calculated from the obtained CΔt values for both the target and endogenous reference β-actin genes using the 2-ΔΔCt cycle threshold method.
Flow cytometry
To investigate the expression of TLR4, cells were incubated for 30 min at 4 °C with Anti-Human CD284 (TLR4) phycoerythrin (PE; eBioscience, San Diego, CA) or Mouse IgG2a K Isotype Control PE (eBioscience). Cells were then washed with a PBS solution and resuspended in a cold PBS solution for fluorescence assisted cell sorting (FACS) analysis.
Phosphospecific flow cytometry was used in the present study to detect the JNK, ERK1/2, and p38 protein phosphorylation levels. At the end of the cell treatments, the cells were rapidly detached and fixed immediately by adding 250 ul of a pre-warmed fixation buffer (eBioscience). The cells were then incubated at 37 °C for 15 min followed by washing twice with a cold PBS by centrifugation at 500 g for 5 min. Immediately after washing, the cells were permeabilized by adding a cold permeabilization buffer (eBioscience) and incubated for a minimum of 30 min at 4 °C. Finally, the cells were washed twice with PBS for 5 min by centrifugation at 500 g and resuspended in PBS at a concentration of 1×106 cells/ml. To each tube, 20 ul of anti-phospho-ERK1/2, 20 ul of anti-phospho-p38, and 5 ul of an anti-phospho-JNK antibody (BD Bioscience, San Diego, CA) were added. Flow cytometry was conducted on FACS Aria, and the data were analyzed with the FACSDiva Software (BD Bioscience).
Cytokine analysis
The concentrations of IL-6 and IL-8 were determined using human enzyme-linked immunosorbent assay (ELISA) development kits (R&D Systems) according to the manufacturer’s instructions, with detection limits of 9.4 pg/ml and 15.6 pg/ml, respectively.
Statistical analysis
Data were expressed as mean±standard deviation (SD) and a one-way analysis of variance (ANOVA) was applied using SPSS 17.0 software (SPSS Inc.). Significant differences are indicated for p<0.05 and p<0.01, respectively.
Results
C5a upregulates the TLR4-Induced expressions of IL-6 and IL-8
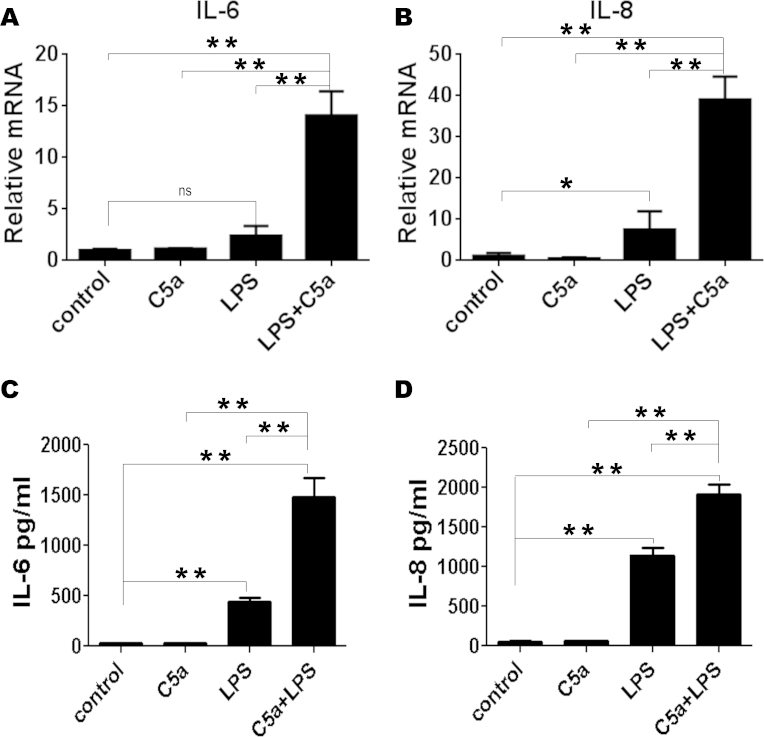
To evaluate the effects of C5a on the TLR4-induced expressions of IL-6 and IL-8, ARPE-19 cells were incubated with 100 ng/ml of LPS for 16 h in the presence or absence of recombinant C5a. Real-time PCR was performed to investigate the mRNA expressions of IL-6 and IL-8. As shown in Figure 1, 50 ng/ml of a C5a treatment of ARPE-19 cells has a minimal impact on IL-6 (Figure 1A) and IL-8 (Figure 1B) production following 16 h of in vitro stimulation, while it significantly increased IL-6 and IL-8 production induced by LPS (100 ng/ml). IL-6 and IL-8 levels increased by more than 4-fold in the co-presence of C5a and LPS in comparison to LPS and C5a alone, suggesting a strong enhancing effect of C5a on LPS-induced IL-6 and IL-8 production. Surprisingly, C5a did not enhance TLR3-induced IL-6 and IL-8 production after PolyI:C stimulation (Appendix 2a).
Figure 1.
C5a can increase TLR4-induced IL-6 and IL-8 production. ARPE-19 cells were stimulated with LPS (100 ng/ml) in the absence or presence of C5a (50 ng/ml) for 16 h. C5a was added to the culture 10 min before LPS challenge. A and B: The effect of C5a on TLR4-induced IL-6 (A) and IL-8 (B) mRNA expressions in ARPE-19 cells. C and D: Effect of C5a on TLR4-induced IL-6 (C) and IL-8 (D) protein expressions in ARPE-19 cells. The data are expressed as the mean±SD of three independent experiments. Statistical analysis was performed using a one-way ANOVA (* indicates p<0.05 and ** indicates p<0.01).
It was then tested whether the IL-6 and IL-8 mRNA expressions resulted in a difference in protein production. Consistent with the mRNA data of this study, the ELISA showed that C5a significantly increased TLR4-induced IL-6 (Figure 1C) and IL-8 (Figure 1D) secretions from ARPE-19 cells.
C5a induces changes in the expression levels of TLR4
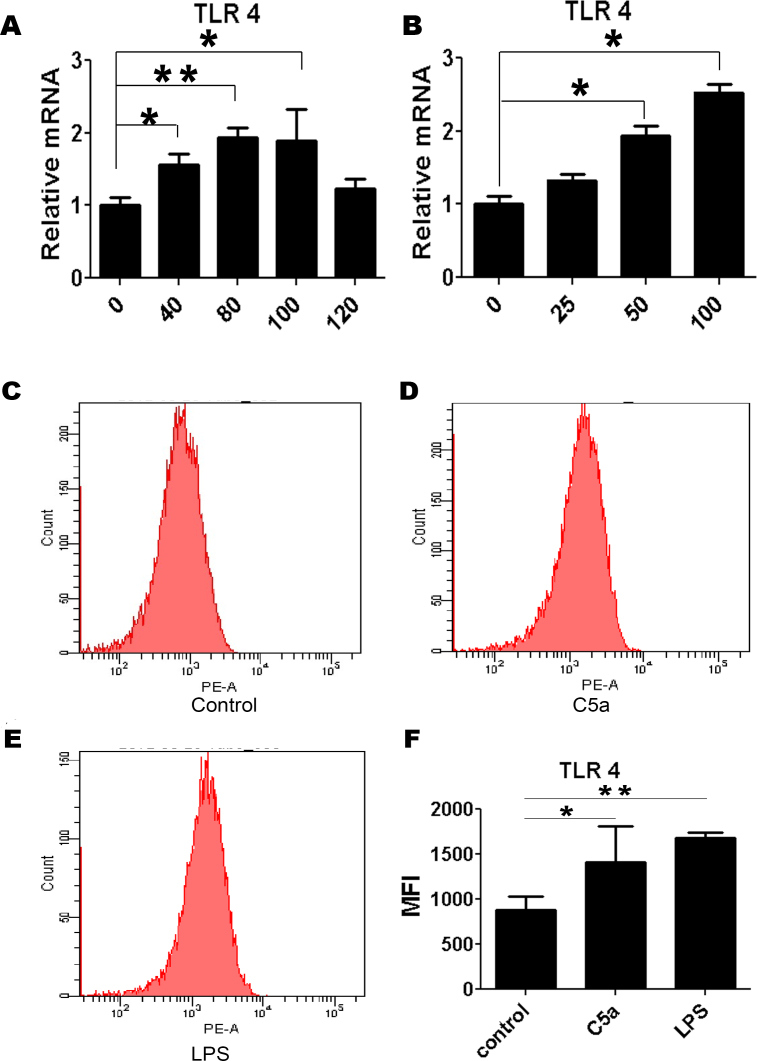
The experiments shown above could be explained by the fact that C5a upregulated the TLR expression on RPE cells, and this hypothesis was therefore tested in the following series of experiments. It was found that C5a did not seem to change the TLR4 expression after stimulation for 16 h (Appendix 3). RPE cells were then stimulated with C5a for shorter time periods ranging from 0 to 120 min (Figure 2A). TLR4 gene transcripts were significantly upregulated at 40 min and peaked at 80 min. At 120 min, the expression of TLR4 reached preincubation levels (Figure 2A).
Figure 2.
C5a induces changes in the expression level of TLR4. A and B: Time- (A) and dose-dependent (B) regulation of the TLR4 mRNA expression. ARPE-19 cells were stimulated with C5a (50 ng/ml) for 40, 80, 100, and 120 min, or they were stimulated with different concentrations of C5a (25, 50, and 100 ng/ml) for 80 min. C, D, E, and F: Flow cytometric analysis of the TLR4 protein expression. Cells were stimulated with C5a (50 ng/ml) or LPS (100 ng/ml) for 80 min. The data are expressed as the mean±SD of three independent experiments. Statistical analysis was performed using a one-way ANOVA (* indicates p<0.05 and ** indicates p<0.01).
Incubation of ARPE-19 cells with varying concentrations showed that the addition of 50 ng/ml and 100 ng/ml of C5a enhanced the mRNA expression of TLR4 (Figure 2B). No significant upregulation of the mRNA levels of TLR4 was detected when cells were treated with a concentration of 25 ng/ml C5a.
The study data thus demonstrated that the TLR4 mRNA expression in RPE cells was significantly elevated by C5a in a time- (Figure 2A) and dose-dependent (Figure 2B) manner. In the following experiments, 50 ng/ml of C5a and 80 min of incubation were chosen to investigate whether C5a can affect the TLR4 protein expression.
Flow cytometry of RPE cells showed that the cell-surface TLR4 expression was significantly increased at 80 min after C5a or LPS treatment (Figure 2F). The TLR4 expression after LPS treatment was higher than that seen after C5a treatment (Figure 2F).
C5a-enhanced TLR4-induced IL-6 and IL-8 production is mediated via C5aR
C5a binds to two trans-membrane receptors: C5aR/CD88 and C5L2 (GPR77). Previous studies suggest that C5aR is the predominant receptor facilitating the ability of C5a to induce cytokine release in LPS-stimulated macrophages [16]. Pre-treatment of ARPE-19 cells with the C5aR antagonist W-54011 (10 ng/ml) for 4 h before LPS stimulation showed that the enhanced effect of C5a was abrogated (Figure 3), which is in agreement with a previous study using macrophages [11]. These results indicated that the effect of C5a on IL-6 and IL-8 production was mainly mediated by C5aR.
Figure 3.
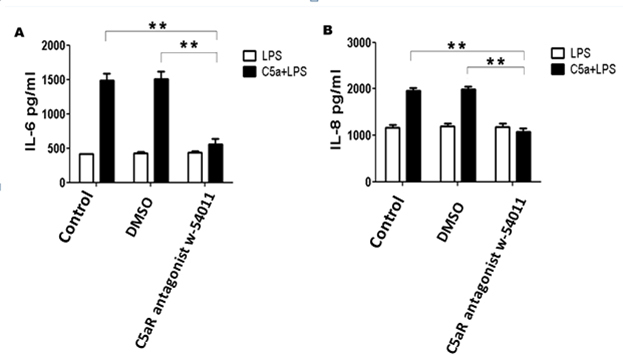
C5a enhanced LPS-induced IL-6 and IL-8 production, which may be mediated via C5aR. ARPE-19 cells were stimulated with LPS (100 ng/ml) in the absence or presence of C5a (50 ng/ml) for 16 h. C5a was added to the culture 10 min before LPS challenge, and the C5aR antagonist W-54011 (10 ng/ml) was added 4 h before C5a stimulation. The increased effect of C5a was abrogated by the addition of W-54011. The data are expressed as the mean±SD of three independent experiments. Statistical analysis was performed using a one-way ANOVA (* indicates p<0.05 and ** indicates p<0.01).
C5a actions are dependent on the ERK1/2 pathway
As it was demonstrated previously that C5a is engaged in the ERK1/2, p38, and JNK pathways for cytokine production [2], it was investigated whether the same pathways are also responsible for the cytokine production observed upon the C5a stimulation of ARPE-19 cells. RPE cells were stimulated with LPS, C5a, or a combination of both for 30 min, and flow cytometry was used to measure the phosphorylation of ERK1/2, p38, and JNK. As shown in Figure 4B, the phosphorylation of ERK1/2, but not JNK or p38, was higher when LPS and C5a were used in combination as compared to LPS or C5a alone.
Figure 4.
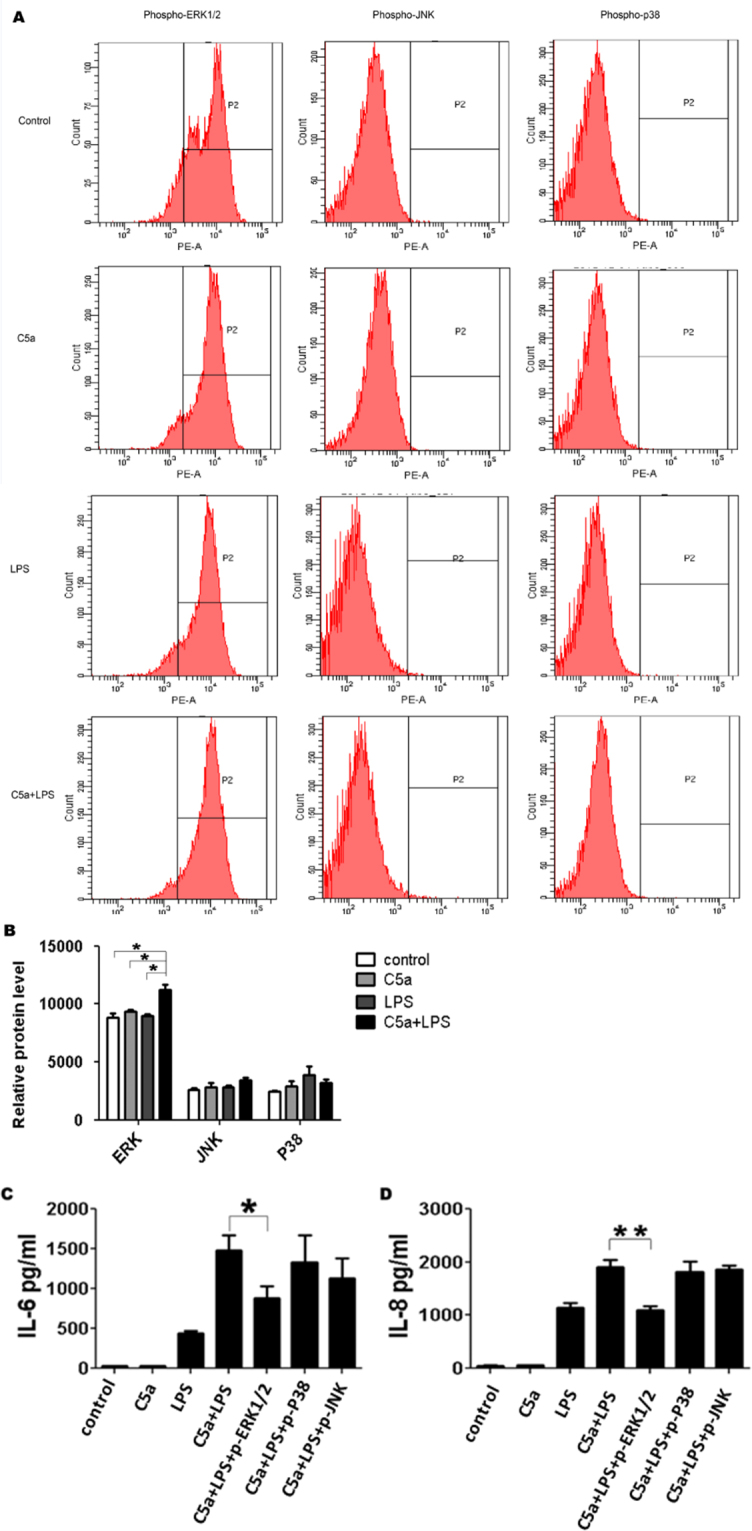
C5a regulates TLR4-induced IL-6 and IL-8 expressions through the activation of ERK1/2. ARPE-19 cells were stimulated with LPS (100 ng/ml) in the absence or presence of C5a (50 ng/ml) for 30 min. A: Representative histograms for the phosphorylation of MAPK are shown at 30 min. B: The MFI of phosphor-ERK1/2, JNK, and P38. C5a was added to the culture 10 min before LPS challenge. Inhibitors of JNK (SP = SP600125, 10 uM), ERK1/2 (PD = PD98059, 20 uM), and P38 (SB = SB239063, 20 uM) were added 1 h before LPS stimulation. C and D: Contribution of ERK1/2, JNK, and P38 to the effect of C5a on TLR4-induced IL-6 (C) and IL-8 (D) expressions. The data are expressed as the mean±SD of three independent experiments. Statistical analysis was performed using a one-way ANOVA (* indicates p<0.05 and ** indicates p<0.01).
To investigate further the contribution of these pathways to IL-6 and IL-8 production, experiments were conducted in which the RPE cells were pre-incubated with ERK1/2 (PD98059), P38 (SB239063), and JNK (SP600125) inhibitors for 1 h before C5a/LPS stimulation. IL-6 and IL-8 production was significantly inhibited by the ERK1/2 inhibitor PD98059, while the JNK inhibitor SP600125 and p38 inhibitor SB239063 had no inhibitory effect on IL-8 and IL-6 production (Figure 4C,D). These results suggested that the enhancing effect of C5a on LPS-induced IL-6 and IL-8 production by RPE cells might be mediated by the phosphorylation of ERK1/2, but not of the JNK or p38 signaling pathways.
Discussion
This study provided evidence that C5a can induce the TLR4 expression, and it enhances the production of TLR4-induced IL-6 and IL-8 by ARPE-19 cells. The effect of C5a on cytokine production was mediated by C5aR and the phosphorylation of ERK1/2.
This study is in agreement with earlier findings that have suggested a regulatory role of C5a in the expression of TLR4-induced inflammatory mediators in a variety of cells [2,3]. To the best of the authors’ knowledge, crosstalk between C5a and TLR4 in RPE cells has not yet been reported.
It was found that C5a could significantly enhance the expression of TLR4-induced IL-6 and IL-8 by RPE cells, whereas a recent report demonstrated that C5a reduced the LPS-induced production of IL-12, IL-23, and IL-6 in immature DCs [13]. In this latter study, the suppressive effect was time dependent, which implies that signaling via C5aR can only affect TLR-induced signaling when initiated around the same time [13]. Another study showed that C5a could strongly amplify the IL-8 expression from human whole blood cells induced by LPS [17]. Complements can enhance LPS-induced IL-6 and TNF-alpha production in wild type (WT) mice [18]. In some studies, however, C5a treatment enhanced IL-6 production by LPS-stimulated mouse neutrophils but suppressed LPS-induced IL-6 production by macrophages [19]. These experiments point out an interesting perspective concerning the cell-type-specific regulatory effects of complements on TLR responses.
It was recently reported that the incubation of bovine neutrophils with a high concentration of C5a resulted in changes in the transcription of selected genes of the TLR4 pathway and resulted in a higher expression of the LPS receptor CD14 [20]. Similarly, a clear increase was found in mRNA and the protein level of TLR4 upon C5a incubation in a time- and dose-dependent manner.
C5a stimulates cells via an interaction with the C5a receptors (C5aR), which belong to a family of G-protein-coupled receptors with seven transmembrane segments [21]. Another receptor for C5a, C5L2, was originally termed a ‘default receptor,’ but its exact function is not yet clear. Recently, C5L2 has been reported as a negative modulator of C5aR activity, thereby ensuring the regulatory control of the biologic activities of C5a [22]. C5aR is expressed in RPE cells [15], but the expression of C5L2 on RPE cells has not yet been reported. The two receptors are expressed on various immune and non-immune cells, such as granulocytes and dendritic cells [22,23]. Consistent with previous studies that showed that C5a induces cytokine release mainly via C5aR in LPS-stimulated macrophages, it was found that the C5a-mediated increase of LPS-induced IL-6 and IL-8 production by ARPE cells was susceptible to the C5aR antagonist W-54011, which indicates that the observed effect of C5a on TLR-induced cytokine production is mediated via the C5aR. In a recent study, pre-exposure of PBMCs to LPS enhanced the expression of C5a-induced IL-6 and IL-8.The effect of TLR4 on C5a was not mediated via the C5aR, but this was caused by a reduced expression of C5L2 [24]. The authors suggested that the reduction of the competing C5L2 receptor for C5a enhanced the interaction of the available C5a for C5aR. Whether a similar mechanism might be operative in RPE cells deserves further study. It should also be noted that the regulation of C5a on TLR4-induced immune responses might be amplified by TLR4-induced cytokines, such as IFN-γ [25], which may promote the expression of C5aR in RPE cells [15].
It has been previously shown that the enhancing effect of C5a on LPS-induced cytokine production is dependent on the activation of the ERK1/2, JNK, and p38 signaling pathways [2]. These findings prompted the investigation of whether C5a could regulate TLR4-reduced responses by RPE cells through these three signaling pathways. ERK1/2 phosphorylation, but not JNK or p38 phosphorylation, was higher when LPS and C5a were used in combination, as compared to LPS or C5a alone, which is consistent with the finding that LPS plus C5a resulted in higher levels of IL-6 and IL-8 released from RPE cells. More importantly, the blockade of the ERK1/2 signaling pathway with the specific inhibitor PD98059 resulted in a significant inhibition of IL-6 and IL-8 production by RPE cells.
In conclusion, this study revealed that the effect of C5a on the TLR4 expression, which may lead to an increased production of TLR4-induced IL-6 and IL-8, was mediated by C5aR and it was involved in the phosphorylation of ERK1/2. Manipulation of the crosstalk pathways between the TLR and complement system may offer future opportunities to control intraocular inflammation.
Acknowledgments
This work was supported in part by National Basic Research Program of China (2011CB510200, 2011ZX09302007002), Chongqing Municipal Health Bureau (2010–1-13, 2012–1-026) and Chongqing Key Laboratory of Ophthalmology (CSTC, 2008 CA5003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Appendix 1. STR analysis.
To access these data, click or select the words "Appendix 1".
Appendix 2. C5a could not enhance TLR-3 induced IL-6 and IL-8 production.
ARPE-19 cells were stimulated with poly(I:C) (1 μg/ml) in the absence or presence of C5a (50 ng/ml) for 16h. C5a was added to the culture 10 min prior to poly(I:C) challenge. Effect of C5a on TLR3-indcuced IL-6 (A) and IL-8 (B) mRNA expression in ARPE-19 cells. The data are expressed as mean±SEM of three independent experiments. Statistical analysis was performed using one-way ANOVA. (* indicates p < 0.05 and ** indicates p < 0.01). To access these data, click or select the words "Appendix 2".
Appendix 3. C5a did not change TLR4 expression after stimulation for 16 h.
ARPE-19 cells were stimulated with C5a (50ng/ml) for 16 h. TLR-4 mRNA expression in ARPE-19 cells. The data are expressed as mean±SEM of three independent experiments. Statistical analysis was performed using one-way ANOVA. (* indicates p < 0.05 and ** indicates p< 0.01). To access these data, click or select the words "Appendix 3".
References
- 1.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–63. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Kohl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–26. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Fang C, Zhang X, Miwa T, Song WC. Complement promotes the development of inflammatory T-helper 17 cells through synergistic interaction with Toll-like receptor signaling and interleukin-6 production. Blood. 2009;114:1005–15. doi: 10.1182/blood-2009-01-198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jha P, Bora PS, Bora NS. The role of complement system in ocular diseases including uveitis and macular degeneration. Mol Immunol. 2007;44:3901–8. doi: 10.1016/j.molimm.2007.06.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, Ambati J. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci USA. 2006;103:2328–33. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortright DN, Meade R, Waters SM, Chenard BL, Krause JE. C5a, but not C3a, increases VEGF secretion in ARPE-19 human retinal pigment epithelial cells. Curr Eye Res. 2009;34:57–61. doi: 10.1080/02713680802546658. [DOI] [PubMed] [Google Scholar]
- 7.Copland DA, Hussain K, Baalasubramanian S, Hughes TR, Morgan BP, Xu H, Dick AD, Nicholson LB. Systemic and local anti-C5 therapy reduces the disease severity in experimental autoimmune uveoretinitis. Clin Exp Immunol. 2010;159:303–14. doi: 10.1111/j.1365-2249.2009.04070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuoka Y, Strainic M, Medof ME. Differential cytokine expression of human retinal pigment epithelial cells in response to stimulation by C5a. Clin Exp Immunol. 2003;131:248–53. doi: 10.1046/j.1365-2249.2003.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akira S. Uematsu, S.Takeuchi, O. Pathogen recognition and innate immunity. Cell. 2006;124:4. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Shen DF, Chang MA, Matteson DM, Buggage R, Kozhich AT, Chan CC. Biphasic ocular inflammatory response to endotoxin-induced uveitis in the mouse. Arch Ophthalmol. 2000;118:521–7. doi: 10.1001/archopht.118.4.521. [DOI] [PubMed] [Google Scholar]
- 11.Cousins SW, Guss RB, Howes EL, Jr, Rosenbaum JT. Endotoxin-induced uveitis in the rat: observations on altered vascular permeability, clinical findings, and histology. Exp Eye Res. 1984;39:665–76. doi: 10.1016/0014-4835(84)90065-4. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki N, Hazeki K, Izumi T, Nigorikawa K, Hazeki O. C5a controls TLR-induced IL-10 and IL-12 production independent of phosphoinositide 3-kinase. J Biochem. 2011;149:265–74. doi: 10.1093/jb/mvq136. [DOI] [PubMed] [Google Scholar]
- 13.Zaal A, Lissenberg-Thunnissen SN. van SG, Wouters D, van HSM, ten BA. Crosstalk between Toll like receptors and C5a receptor in human monocyte derived DCs suppress inflammatory cytokine production. Immunobiology. 2013;218:175–80. doi: 10.1016/j.imbio.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu M, Liu B, Jawad S, Ling D, Casady M, Wei L, Nussenblatt RB. C5a contributes to intraocular inflammation by affecting retinal pigment epithelial cells and immune cells. Br J Ophthalmol. 2011;95:1738–44. doi: 10.1136/bjophthalmol-2011-300235. [DOI] [PubMed] [Google Scholar]
- 16.Bosmann M, Patel VR, Russkamp NF, Pache F, Zetoune FS, Sarma JV, Ward PA. MyD88-dependent production of IL-17F is modulated by the anaphylatoxin C5a via the Akt signaling pathway. FASEB J. 2011;25:4222–32. doi: 10.1096/fj.11-191205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Han G, Wang R, Chen G, Xu R, Xiao H, Li X, Geng S, Li Y, Wang J, Feng J, Riedemann NC, Guo R, Shen B. Regulation of IL-8 production by complement-activated product, C5a, in vitro and in vivo during sepsis. Clin Immunol. 2010;137:157–65. doi: 10.1016/j.clim.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–36. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen NJ, Mirtsos C, Suh D, Lu YC, Lin WJ, McKerlie C, Lee T, Baribault H, Tian H, Yeh WC. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature. 2007;446:203–7. doi: 10.1038/nature05559. [DOI] [PubMed] [Google Scholar]
- 20.Stevens MG, Van Poucke M, Peelman LJ, Rainard P, De Spiegeleer B, Rogiers C, Van de Walle GR, Duchateau L, Burvenich C. Anaphylatoxin C5a-induced toll-like receptor 4 signaling in bovine neutrophils. J Dairy Sci. 2011;94:152–64. doi: 10.3168/jds.2010-3358. [DOI] [PubMed] [Google Scholar]
- 21.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Kohl J, Gerard C, Sarma JV, Ward PA. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–7. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C, Gerard NP. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem. 2010;285:7633–44. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabiet MJ, Huet E, Boulay F. The N-formyl peptide receptors and the anaphylatoxin C5a receptors: an overview. Biochimie. 2007;89:1089–106. doi: 10.1016/j.biochi.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raby AC, Holst B, Davies J, Colmont C, Laumonnier Y, Coles B, Shah S, Hall J, Topley N, Kohl J, Morgan BP, Labeta MO. TLR activation enhances C5a-induced pro-inflammatory responses by negatively modulating the second C5a receptor, C5L2. Eur J Immunol. 2011;41:2741–52. doi: 10.1002/eji.201041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung KW, Barnstable CJ, Tombran-Tink J. Bacterial endotoxin activates retinal pigment epithelial cells and induces their degeneration through IL-6 and IL-8 autocrine signaling. Mol Immunol. 2009;46:1374–86. doi: 10.1016/j.molimm.2008.12.001. [DOI] [PubMed] [Google Scholar]