Abstract
Purpose
TH-302, a prodrug of the cytotoxic alkylating agent bromo-isophosphoramide mustard, is preferentially activated in hypoxic conditions. This phase II study investigated TH-302 in combination with doxorubicin, followed by single-agent TH-302 maintenance therapy in patients with first-line advanced soft tissue sarcoma (STS) to assess progression-free survival (PFS), response rate, overall survival, safety, and tolerability.
Patients and Methods
In this open-label phase II study, TH-302 300 mg/m2 was administered intravenously on days 1 and 8 with doxorubicin 75 mg/m2 on day 1 of each 21-day cycle. After six cycles, patients with stable and/or responding disease could receive maintenance monotherapy with TH-302.
Results
Ninety-one patients initiated TH-302 plus doxorubicin induction treatment. The PFS rate at 6 months (primary efficacy measure) was 58% (95% CI, 46% to 68%). Median PFS was 6.5 months (95% CI, 5.8 to 7.7 months); median overall survival was 21.5 months (95% CI, 16.0 to 26.2 months). Best tumor responses were complete response (n = 2 [2%]) and partial response (n = 30 [34%]). During TH-302 maintenance (n = 48), five patients improved from stable disease to partial response, and one patient improved from partial to complete response. The most common adverse events during induction were fatigue, nausea, and skin and/or mucosal toxicities as well as anemia, thrombocytopenia, and neutropenia. These were less severe and less frequent during maintenance. There was no evidence of TH-302–related hepatic, renal, or cardiac toxicity.
Conclusion
PFS, overall survival, and tumor response compared favorably with historical outcomes achieved with other first-line chemotherapies for advanced STS. A phase III study of TH-302 is ongoing (NCT01440088).
INTRODUCTION
Soft tissue sarcomas (STSs) comprise a highly diverse group of mesenchymal tumors (> 50 known histologic subtypes) with various natural histories, genetics, prognostic factors, and treatment sensitivities.1–4 Tumors may develop at any site, but manifest most often in the extremities and metastasize most often to the lungs and liver. STSs account for 15% of childhood cancers and less than 1% of adult cancers in the United States5; estimated mortality associated with these tumors is high (> 40%).6 Prognosis for patients with advanced higher-grade STS is poor: historical median overall survival (OS) is 8 to 12 months after developing metastatic disease.5
Chemotherapy is the standard treatment for metastatic or unresectable STS. First-line palliative chemotherapy may be beneficial to nearly 50% of patients with advanced STS.3 Doxorubicin and ifosfamide are considered the most active chemotherapeutic agents, yielding relatively consistent response rates of 10% and 25%, respectively, when administered as monotherapy.7,8 Higher response rates were achieved in some studies by using combination chemotherapy, but multidrug regimens have as yet failed to improve OS when compared with single agents administered sequentially.8,9 Moreover, hepatic metabolism of ifosfamide releases its active metabolite, isophosphoramide mustard (IPM) but also releases other metabolites associated with hemorrhagic cystitis, nephrotoxicity, and central neurotoxicity, which can be severe and occasionally fatal. Ifosfamide administration is also complicated because it requires administration of mesna to prevent hemorrhagic cystitis.10
Tumors often consist of large areas of highly hypoxic microenvironments that are associated with a poor prognosis and increased resistance to chemotherapy and radiotherapy.11–15 In human STS, tumor hypoxia is prominent15–17 and is associated with shorter survival.18 Doxorubicin may not adequately penetrate hypoxic regions of solid tumors.19 We hypothesize that tumor hypoxia may be a meaningful therapeutic target in patients with STS.
TH-302 (Threshold Pharmaceuticals, South San Francisco, CA, and Merck, Darmstatdt, Germany) is a hypoxia-activated nitroimidazole prodrug of the cytotoxic alkylating agent bromo-isophosphoramide mustard (Br-IPM). TH-302 is reduced at its nitroimidazole site by intracellular reductases and, under hypoxic conditions, it releases Br-IPM, the active metabolite. Br-IPM acts as a DNA cross-linking agent, rendering cells unable to replicate and divide. Findings from preclinical studies have also demonstrated a bystander effect of TH-302, which is likely associated with diffusion of the active moiety to adjacent normoxic regions of the tumor and possibly provides additional antitumor activity.20
Because TH-302 is preferentially activated in hypoxic tissues and does not have the same toxic metabolites (eg, acrolein and chloroacetaldehyde) as ifosfamide following hepatic metabolism, TH-302 should hypothetically display fewer hematologic, renal, bladder, and CNS toxicities than ifosfamide. This hypothesis could be supported by the specific reversible skin and mucosal toxicities found to be dose limiting in a phase I study of TH-302.21
A phase I/II trial of TH-302 in combination with doxorubicin in patients with advanced STS was conducted. The maximum-tolerated dose (MTD) of TH-302 was established at 300 mg/m2 when combined with full-dose doxorubicin (75 mg/m2) and prophylactic growth factor support (granulocyte colony-stimulating factor).22 Here, we present the results for the phase II trial of the combination therapy in first-line treatment of patients with advanced STS dosed at the MTD.
PATIENTS AND METHODS
Study Population
Patients (age ≥ 18 years) had locally advanced unresectable or metastatic, intermediate- or high-grade STS for which no standard curative therapy was available and for which single-agent doxorubicin was considered appropriate treatment. No central pathology review was conducted. Patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; at least one target lesion evaluable by RECIST 1.023; and adequate hepatic, renal, hematologic, and cardiac function. Patients who had received prior systemic therapy other than neoadjuvant or adjuvant therapy were excluded. Complete criteria are available in Ganjoo et al22 and the Appendix (online only). The study protocol was approved by the institutional review board for each study site, and all patients provided written informed consent for their participation.
Study Design
The primary objective of the single-arm, multicenter phase II segment of this open-label study was to perform a preliminary assessment of the efficacy of TH-302, administered at the MTD (300 mg/m2) established in the phase I segment, when combined with doxorubicin in patients with advanced STS. The study population included all patients enrolled and treated in the phase II (dose expansion) segment, as well as six patients treated at the MTD in the phase I dose-escalation segment who met the dose expansion eligibility criteria. No stopping rules were incorporated. The primary efficacy outcome measure was progression-free survival (PFS) rate at 6 months, in accordance with the suggestion of Van Glabbeke et al24 for phase II trials in STS. Six-month PFS rates between 30% and 56% have been reported for first-line chemotherapy, depending on STS histologic subtype.24 RECIST response rates of 10% to 25% have also been reported in first-line chemotherapy. These rates were used as a standard to assess the observed efficacy. A posteriori, the sample size enabled the PFS rate at 6 months to be estimated with an SE of 5%.
TH-302 was administered by intravenous infusion over 30 minutes on days 1 and 8 of a 21-day cycle. The dose of doxorubicin was fixed at 75 mg/m2 (56 mg/m2 if bilirubin was higher than the upper limit of normal but ≤ 1.5× upper limit of normal) and was administered by bolus injection on day 1 of a 21-day cycle for up to six cycles (maximum cumulative dose, 450 mg/m2). Dexrazoxane (Zinecard; Pharmacia and Upjohn, New York, NY) was permitted at the discretion of the treating physician and used in only four patients. On days when both TH-302 and doxorubicin were administered, doxorubicin was injected 2 hours after completion of the TH-302 infusion. The Appendix contains information on dose modifications followed for hematologic, liver, and renal toxicities regardless of causality and for any other toxicity that was not clearly related to disease progression, intercurrent illness, concomitant medications, or other nondrug intervention (Appendix Tables A1–A3, online only). Prophylactic growth factor support (filgrastim or pegfilgrastim) was given from day 8 or 9 of cycles but could be discontinued at the investigator's discretion if neutropenia was minimal after the first two cycles. Prophylaxis against nausea and vomiting was implemented by using a regimen intended for moderately emetogenic chemotherapy. After six cycles, patients who had stable disease (SD) or responding disease after completing the combination therapy could continue on TH-302 monotherapy (maintenance) on days 1 and 8 of a 21-day cycle until disease progression or discontinuation for another reason.
Assessments and Outcome Measures
No patient was replaced, and analyses include all patients with available data. Tumor response was evaluated by the clinical site radiologist and medical oncologist after every even cycle using RECIST 1.0. SD required a minimum duration of 6 weeks. Survival follow-up continued for as long as 36 months. Efficacy outcome measures were PFS, best response, and confirmed response (requiring subsequent assessment at least 4 weeks after initial assessment of response) rates, duration of response, OS, and ECOG PS. PFS and OS end points, including rates at points in time, medians, and survival curves, were estimated by using the Kaplan-Meier method and associated 95% CIs. PFS included all deaths occurring within 9 weeks of the last tumor response assessment and all deaths occurring within 6 months of treatment initiation if not preceded by documented disease progression. Univariable and multivariable models were performed to compare PFS and OS across subgroups. The log-rank test was used to compare subgroups in the univariable models, and a stepwise Cox regression model was used for the multivariable analyses. The following covariates were analyzed: sex (male, female), age (≥ 60 years, < 60 years), ECOG PS (0, 1), metastatic disease (yes, no), time from initial diagnosis (≤ 1 year, > 1 year), time from advanced/metastatic disease (≤ 3 months, > 3 months), sites of disease (≤ four, > four), leiomyosarcoma (yes, no), liposarcoma (yes, no), unclassified and/or malignant fibrous histiocytoma (MFH; yes, no), site of primary (extremity, other), maximum tumor diameter (≤ 5 cm, > 5 cm), prior radiotherapy (yes, no), albumin (< 3.5 g/dL, ≥ 3.5 g/dL), and hemoglobin (< 12 g/dL, ≥ 12 g/dL). For the Cox model, the significant level to enter the model was 0.15, and the level to stay was 0.05; sex and age were fixed factors.
Clinical laboratory test results collected weekly, vital signs collected on dosing days, weight, and adverse events (AEs) were used to assess safety and/or tolerability and were summarized by using descriptive statistics. Severity of AEs or clinically significant laboratory test results were graded by using the Common Terminology Criteria for Adverse Events version 3.0.
RESULTS
Between August 2009 and June 2011, 91 patients received induction therapy with doxorubicin combined with the MTD of TH-302. Of these 91, 59 patients (65%) received six cycles of combination therapy, and 48 patients (53%) later received maintenance therapy with TH-302 alone. Baseline demographic and disease characteristics of the patients are summarized in Table 1. Of the 91 patients, 34 (37%) were age 60 years or older. The most common histopathologic diagnoses were leiomyosarcoma (31%), unclassified sarcoma and/or MFH (31%), and liposarcoma (21%). Most patients (82% on induction; 88% on maintenance) had distant metastases, particularly to the lung (69% on induction; 71% on maintenance). Approximately one fifth of patients had liver metastases (18% on induction; 21% on maintenance), including 9% with both liver and lung metastases. The median maximum tumor diameter was 6.1 cm, and 57% of patients had a target lesion larger than 5 cm in diameter.
Table 1.
Baseline Demographic and Disease Characteristics
| Characteristic | All Patients (N = 91) |
Maintenance Patients (n = 48) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Female sex | 53 | 58 | 25 | 52 |
| Age, years | ||||
| Median | 57 | 57 | ||
| Range | 23-78 | 28-78 | ||
| ECOG PS | ||||
| 0 | 39 | 43 | 21 | 44 |
| 1 | 52 | 57 | 27 | 56 |
| Prior adjuvant and/or neoadjuvant therapy | 15 | 16 | 10 | 21 |
| Sarcoma histology subtype | ||||
| Leiomyosarcoma | 28 | 31 | 13 | 27 |
| Unclassified and/or MFH | 28 | 31 | 17 | 35 |
| Liposarcoma | 19 | 21 | 8 | 17 |
| Angiosarcoma | 3 | 3 | 3 | 6 |
| Fibrosarcoma | 3 | 3 | 2 | 4 |
| Synovial sarcoma | 3 | 3 | 2 | 4 |
| Other subtype | 7* | 8 | 3† | 6 |
| Disease status | ||||
| Locally advanced, unresectable | 16 | 18 | 6 | 12 |
| Distant metastases | 75 | 82 | 42 | 88 |
| Site of metastatic disease | ||||
| Lung | 63 | 69 | 34 | 71 |
| Liver | 16 | 18 | 10 | 21 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; MFH, malignant fibrous histiocytoma.
Other: chondrosarcoma (n = 4), chordoma (n = 1), pleomorphic rhabdomyosarcoma (n = 1), and endometrial stromal cell sarcoma (n = 1).
Other: chondrosarcoma (n = 3).
Median drug exposure was seven cycles (range, one to 36 cycles). Median follow-up period was 19.4 months (range, 1.0 to 37.7 months). The 48 patients who continued on TH-302 maintenance had a median of 10 total cycles of TH-302 (range, seven to 36 cycles). Overall, 34% of patients had a dose delay and 41% had a dose modification, but this did not affect the overall dose intensity. Median dose intensity for induction therapy was 92% for TH-302 and 94% for doxorubicin.
Efficacy
Six-month PFS (primary efficacy measure) was 58% (95% CI, 46% to 68%), and 3-month PFS was 83% (95% CI, 74% to 89%). Median PFS was 6.5 months (95% CI, 5.8 to 7.7 months). The Kaplan-Meier curve for PFS is presented in Figure 1A. Median PFS after initiating maintenance (n = 48) was 3.7 months (95% CI, 2.4 to 5.5 months), as presented in Figure 1B. There were no significant differences in PFS among subgroups based on univariable (Table 2) or multivariable analyses. For example, the 6-month PFS rates were 65% for leiomyosarcoma, 51% for unclassified and/or MFH, 47% for liposarcoma, and 75% for other subtypes.
Fig 1.
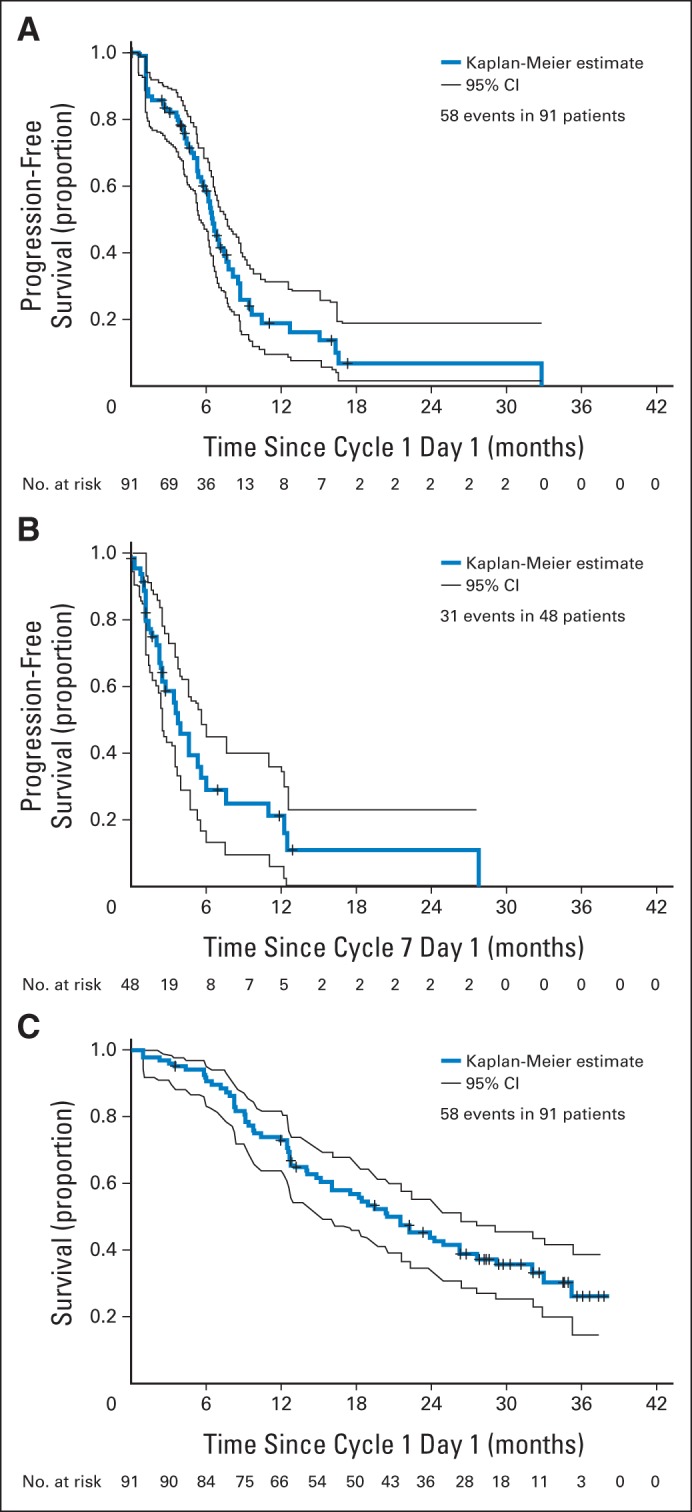
Kaplan-Meier plots of (A) progression-free survival on study (N = 91), (B) progression-free survival after initiating maintenance therapy (n = 48), and (C) overall survival on study (N = 91).
Table 2.
Efficacy Summary
| Characteristic | No. of Patients | 6-Month PFS Rate |
Median PFS |
Best Response Rate (PR or CR) |
Median OS |
||||
|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | Months | 95% CI | % | 95% CI | Months | 95% CI | ||
| Total | 91 | 58 | 46 to 68 | 6.5 | 5.8 to 7.7 | 36 | 26 to 47 | 21.5 | 16.0 to 26.2 |
| Sex | |||||||||
| Male | 38 | 60 | 42 to 74 | 6.6 | 5.8 to 8.7 | 35 | 20 to 53 | 21.5 | 12.7 to 29.1 |
| Female | 53 | 57 | 40 to 70 | 6.5 | 5.3 to 7.8 | 37 | 24 to 51 | 20.4 | 15.1 to 31.9 |
| Age, years | |||||||||
| < 60 | 57 | 52 | 37 to 66 | 6.2 | 5.3 to 7.4 | 30 | 19 to 44 | 19.5 | 12.7 to 27.6 |
| ≥ 60 | 34 | 66 | 47 to 80 | 6.7 | 5.0 to 9.4 | 45 | 28 to 64 | 21.5 | 16.0 to 32.9 |
| ECOG PS | |||||||||
| 0 | 39 | 61 | 43 to 75 | 7.1 | 5.3 to 8.7 | 39 | 24 to 57 | 26.2 | 14.1 to 32.9 |
| 1 | 52 | 56 | 39 to 70 | 6.5 | 5.6 to 7.8 | 33 | 21 to 48 | 18.3 | 14.0 to 24.9 |
| Sarcoma subtype | |||||||||
| Leiomyosarcoma | 28 | 65 | 43 to 80 | 6.9 | 5.8 to 7.8 | 46 | 28 to 66 | 31.9 | 20.4 to NR |
| Unclassified and/or MFH | 28 | 51 | 31 to 68 | 6.6 | 5.3 to 8.1 | 41 | 22 to 61 | 14.8 | 9.2 to 20.3 |
| Liposarcoma | 19 | 47 | 20 to 70 | 4.4 | 2.6 to NR | 22 | 6 to 48 | 32.9 | 9.8 to NR |
| Other* | 16 | 75 | 40 to 91 | 6.3 | 5.3 to 8.8 | 25 | 7 to 52 | 18.2 | 12.7 to 27.6 |
| Disease extent | |||||||||
| Locally advanced, unresectable | 16 | 55 | 17 to 82 | 6.2 | 5.4 to 32.9 | 13 | 2 to 38 | 32.9 | 22.2 to NR |
| Distant metastases | 75 | 59 | 46 to 70 | 6.6 | 5.8 to 7.8 | 41 | 30 to 53 | 18.3 | 14.0 to 24.2 |
| Largest tumor diameter, cm | |||||||||
| ≤ 5 | 39 | 60 | 43 to 74 | 6.6 | 5.3 to 8.6 | 49 | 32 to 65 | 27.6 | 20.3 to NR |
| > 5 | 52 | 57 | 39 to 71 | 6.4 | 5.4 to 7.8 | 26 | 15 to 40 | 14.1 | 10.4 to 22.2 |
| No. of disease sites | |||||||||
| ≤ 4 | 40 | 70 | 51 to 83 | 7.4 | 6.2 to 8.8 | 35 | 21 to 52 | 29.1 | 18.9 to NR |
| > 4 | 51 | 50 | 34 to 64 | 5.9 | 4.6 to 7.1 | 37 | 23 to 52 | 18.2 | 12.5 to 22.2 |
Abbreviations: CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; MFH, malignant fibrous histiocytoma; NR, not reached; OS, overall survival; PFS, progression-free survival; PR, partial response.
Other: angiosarcoma (n = 3), fibrosarcoma (n = 3), synovial sarcoma (n = 3), chondrosarcoma (n = 4), chordoma (n = 1), pleomorphic rhabdomyosarcoma (n = 1), and endometrial stromal cell sarcoma (n = 1).
Overall, there were two complete responses (CRs) and 30 partial responses (PRs), for an overall response rate of 36%, and confirmed responses were reported for 27 patients (30%). For the 32 patients whose best response was a PR or a CR, the median duration of response was 5.3 months (95% CI, 3.5 to 6.7 months) and ranged from 1.2 to 15.3 months. Forty-four patients (49%) had SD for at least 6 weeks. Response rates were similar across subgroups, except in patients with locally advanced unresectable tumors who had a 13% response rate (n = 16) compared with 41% in patients with distant metastases (n = 75; Table 2). The two CRs were reported in patients with angiosarcoma and unclassified undifferentiated sarcoma. Both patients progressed at the next visit at a new disease site. Among the 48 patients who received TH-302 maintenance therapy, the overall response rate was 44% after the combination therapy induction, increasing to 54% after maintenance (ie, including both induction and maintenance). During maintenance, five patients with SD during induction converted to a PR, and one patient with a PR during induction converted to a CR.
Figure 1C provides the Kaplan-Meier curve for OS. Median OS was 21.5 months (95% CI, 16.0 to 26.2 months). One- and 2-year survival rates were 73% (95% CI, 63% to 81%) and 44% (95% CI, 33% to 54%), respectively. In multivariable analyses, patients with leiomyosarcoma, higher levels of serum albumin, locally advanced disease, and fewer sites of disease had significantly longer survival.
Figure 2 presents the case of a 65-year-old woman with metastatic uterine leiomyosarcoma. She presented with large peritoneal metastases (including a 28-cm mass) with ascites. Comparison of computed tomography scans at baseline and at the end of four cycles of doxorubicin plus TH-302 showed resolution of ascites and a more than 40% decrease in the sum of longest diameters. Further reduction allowed complete resection.
Fig 2.
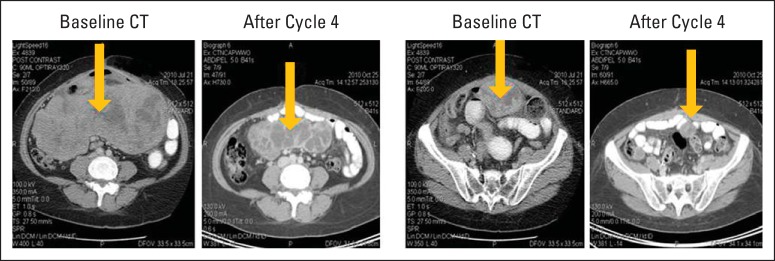
Case study of patient with metastatic uterine leiomyosarcoma exhibiting partial response to treatment with doxorubicin plus TH-302. Comparison of computed tomography (CT) scans at baseline and at the end of four cycles of doxorubicin plus TH-302. Images provided courtesy of Fritz Eilber, MD, University of California at Los Angeles.
Safety
Hematologic toxicity is summarized by grade (Table 3). Grade 3 or 4 neutropenia occurred in 28 patients (31%). Febrile neutropenia was rare, occurring in seven patients (8%), with no study discontinuations. Two cases of sepsis were reported with one case associated with grade 4 neutropenia. Grade 3 or 4 thrombocytopenia occurred in 29 patients (32%). Nine patients (10%) received at least one platelet transfusion. There were no thrombocytopenic-associated bleeding events and/or associated discontinuations. However, seven patients discontinued 4.4 to 6.5 months after initiating therapy when platelets did not recover to above 100,000/μL by the time requirement for initiating a dosing cycle. Grade 3 or 4 anemia occurred in 33 patients (36%). Seventeen patients (19%) received growth factors for anemia and no patients discontinued as a result of anemia. Grade 3 or 4 hematologic toxicity was seen primarily during induction.
Table 3.
Patients With Laboratory-Assessed Hematologic Toxicity
| Toxicity or Severity Grade | All Patients (N = 91) |
After Initiating TH-302 Maintenance (n = 48) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Hemoglobin | 91 | 100 | 47 | 97.9 |
| 1 | 15 | 16.5 | 30 | 62.5 |
| 2 | 43 | 47.3 | 14 | 29.2 |
| 3 | 32 | 35.2 | 2 | 4.2 |
| 4 | 1 | 1.1 | 1 | 2.1 |
| Platelets | 72 | 79.1 | 24 | 50.0 |
| 1 | 26 | 28.6 | 19 | 39.6 |
| 2 | 17 | 18.7 | 4 | 8.3 |
| 3 | 17 | 18.7 | 1 | 2.1 |
| 4 | 12 | 13.2 | 0 | 0 |
| Neutrophils | 47 | 52.6 | 16 | 33.3 |
| 1 | 11 | 12.1 | 8 | 16.7 |
| 2 | 8 | 8.8 | 7 | 14.6 |
| 3 | 7 | 7.7 | 1 | 2.1 |
| 4 | 21 | 23.1 | 0 | 0 |
| White blood cells | 75 | 82.4 | 22 | 45.8 |
| 1 | 17 | 18.7 | 12 | 25.0 |
| 2 | 23 | 25.3 | 10 | 20.8 |
| 3 | 19 | 20.9 | 0 | 0 |
| 4 | 16 | 17.6 | 0 | 0 |
| Lymphocytes | 62 | 68.1 | 24 | 50.0 |
| 1 | 7 | 7.7 | 6 | 12.5 |
| 2 | 13 | 14.3 | 11 | 22.9 |
| 3 | 34 | 37.4 | 7 | 14.6 |
| 4 | 8 | 8.8 | 0 | 0 |
Nonhematologic AEs are summarized in Table 4. Two AEs (subdural hemorrhage and postprocedural hemorrhage) were fatal. A total of 37 patients (41%) experienced 61 serious AEs (SAEs) regardless of relationship to study drug, primarily for hospitalization or prolongation of hospitalization. SAEs occurring in at least three patients were febrile neutropenia (7%), abdominal pain (4%), and fever (3%). Twenty-one SAEs (34% of all reported SAEs) in 17 patients were considered treatment related, including febrile neutropenia (6%) and fever (3%).
Table 4.
Patients Experiencing Nonhematologic Adverse Events (incidence ≥ 10%)
| Adverse Event (MedDRA preferred term) | Regardless of Relationship to Study Drug |
Related to Study Drug |
Grade 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Grade |
Grade 3 |
Grade 4 |
Any Grade |
Grade 3 |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Nausea | 68 | 74.7 | 2 | 2.2 | 0 | 57 | 62.6 | 1 | 1.1 | 0 | |
| Fatigue | 64 | 70.3 | 11 | 12.1 | 0 | 60 | 65.9 | 9 | 9.9 | 0 | |
| Alopecia | 49 | 53.8 | NA | 36 | 39.6 | NA | |||||
| Stomatitis | 47 | 51.6 | 1 | 1.1 | 0 | 45 | 49.5 | 1 | 1.1 | 0 | |
| Constipation | 42 | 46.2 | 0 | 0 | 22 | 24.2 | 0 | 0 | |||
| Rash/erythema* | 41 | 45.1 | 2 | 2.2 | 0 | 33 | 36.3 | 2 | 2.2 | 0 | |
| Anorexia | 37 | 40.7 | 0 | 0 | 32 | 35.2 | 0 | 0 | |||
| Diarrhea | 36 | 39.6 | 2 | 2.2 | 0 | 21 | 23.1 | 2 | 2.2 | 0 | |
| Vomiting | 35 | 38.5 | 1 | 1.1 | 0 | 28 | 30.8 | 0 | 0 | ||
| Hypoalbuminemia | 25 | 27.5 | 5 | 5.5 | 0 | 4 | 4.4 | 2 | 2.2 | 0 | |
| Pyrexia | 24 | 26.4 | 1 | 1.1 | 0 | 9 | 9.9 | 0 | 0 | ||
| Back pain | 24 | 26.4 | 2 | 2.2 | 0 | 3 | 3.3 | 0 | 0 | ||
| Skin hyperpigmentation | 22 | 24.2 | 0 | 0 | 20 | 22.0 | 0 | 0 | |||
| Headache | 22 | 24.2 | 2 | 2.2 | 0 | 6 | 6.6 | 1 | 1.1 | 0 | |
| Abdominal pain† | 21 | 23.1 | 4 | 4.4 | 0 | 5 | 5.5 | 0 | 0 | ||
| Dizziness | 20 | 22.0 | 1 | 1.1 | 0 | 7 | 7.7 | 1 | 1.1 | 0 | |
| Cough | 19 | 20.9 | 0 | 0 | 1 | 1.1 | 0 | 0 | |||
| Pain in extremity | 18 | 19.8 | 4 | 4.4 | 0 | 4 | 4.4 | 0 | 0 | ||
| Palmar-plantar erythrodysesthesia syndrome | 18 | 19.8 | 1 | 1.1 | 0 | 18 | 19.8 | 1 | 1.1 | 0 | |
| Decreased weight | 18 | 19.8 | 1 | 1.1 | 0 | 6 | 6.6 | 1 | 1.1 | 0 | |
| Hemorrhoids | 17 | 18.7 | 0 | 0 | 7 | 7.7 | 0 | 0 | |||
| Dysgeusia | 16 | 17.6 | 0 | 0 | 14 | 15.4 | 0 | 0 | |||
| Dyspnea | 15 | 16.5 | 2 | 2.2 | 0 | 4 | 4.4 | 0 | 0 | ||
| Edema peripheral | 15 | 16.5 | 1 | 1.1 | 0 | 3 | 3.3 | 0 | 0 | ||
| Hypokalemia | 15 | 16.5 | 4 | 4.4 | 1 | 1.1 | 3 | 3.3 | 2 | 2.2 | 0 |
| Dysphagia | 13 | 14.3 | 0 | 0 | 11 | 12.1 | 0 | 0 | |||
| Urinary tract infection | 13 | 14.3 | 2 | 2.2 | 0 | 0 | 0.0 | 0 | 0 | ||
| Arthralgia | 12 | 13.2 | 0 | 0 | 1 | 1.1 | 0 | 0 | |||
| Dehydration | 12 | 13.2 | 1 | 1.1 | 0 | 6 | 6.6 | 1 | 1.1 | 0 | |
| Hyponatremia | 12 | 13.2 | 5 | 5.5 | 0 | 2 | 2.2 | 1 | 1.1 | 0 | |
| Insomnia | 12 | 13.2 | 0 | 0 | 1 | 1.1 | 0 | 0 | |||
| Increased ALP | 11 | 12.1 | 0 | 0 | 1 | 1.1 | 0 | 0 | |||
| Increased ALT | 10 | 11.0 | 2 | 2.2 | 0 | 2 | 2.2 | 0 | 0 | ||
| Anxiety | 10 | 11.0 | 0 | 0 | 0 | 0.0 | 0 | 0 | |||
| Bone pain | 10 | 11.0 | 0 | 0 | 0 | 0.0 | 0 | 0 | |||
| Decreased appetite | 10 | 11.0 | 0 | 0 | 3 | 3.3 | 0 | 0 | |||
| Dyspepsia | 10 | 11.0 | 0 | 0 | 7 | 7.7 | 0 | 0 | |||
| Hyperglycemia | 10 | 11.0 | 2 | 2.2 | 0 | 2 | 2.2 | 1 | 1.1 | 0 | |
| Oropharyngeal pain | 10 | 11.0 | 0 | 0 | 7 | 7.7 | 0 | 0 | |||
| Peripheral sensory neuropathy | 10 | 11.0 | 0 | 0 | 7 | 7.7 | 0 | 0 | |||
| Upper respiratory tract infection | 10 | 11.0 | 1 | 1.1 | 0 | 0 | 0.0 | 0 | 0 | ||
Abbreviations: MedDRA, Medical Dictionary for Regulatory Activities; NA, not applicable.
Includes all MedDRA preferred terms containing the words “rash” or “erythema.”
Includes all MedDRA preferred terms containing the words “abdominal pain.”
The most common nonhematologic AEs initiating in TH-302 maintenance, regardless of relationship to study drug, were pain in extremities (25%), skin rash (21%), diarrhea (21%), fatigue (17%), constipation (17%), and stomatitis (17%). Five patients had a grade 3 AE assessed by the clinical investigator as at least possibly related to TH-302 after initiating maintenance: pruritus and urticaria (two patients), plantar erythema (one), hypoalbuminemia (one), and hypokalemia (one). There were single reports of grade 3 to 4 thrombocytopenia and neutropenia and no reports of grade 3 to 4 leukopenia. One patient was reported with bone marrow hypoplasia after 25 cycles.
No study drug–related deaths occurred during induction or maintenance therapy. There was no evidence of hepatic, renal, or cardiac toxicity related to TH-302 and no other consistent pattern of nonhematologic laboratory abnormalities.
Grade 3 decreases in left ventricular ejection fractions were reported in three patients. One patient had a subsequent assessment in the normal range. The other two decreases were at the last ejection fraction assessment after completing doxorubicin and were considered to be related to doxorubicin.
Seventy-one patients (78%) went on to receive subsequent chemotherapy after discontinuing from the study. The most common chemotherapies were gemcitabine-containing regimens (38%), dacarbazine-containing regimens (21%), or pazopanib-containing regimens (20%). Thirteen (14%) and 16 patients (18%) received a doxorubicin-containing or ifosfamide-containing regimen, respectively. Twenty-two patients (24%) received subsequent radiotherapy.
DISCUSSION
There is an unmet need for a therapeutic agent that can increase the efficacy of first-line doxorubicin treatment of advanced STS without adding unacceptable toxicity. This phase II study used investigator-assessed 6-month PFS rate as the primary variable to assess efficacy of TH-302 administered in combination with doxorubicin in patients who had unresectable localized or metastatic STS and had not previously received first-line systemic chemotherapy. The 6-month PFS rate was 58%, which is higher than historical rates of 30% to 56% reported for first-line chemotherapy for advanced STS.24 Historically, patients with STS who receive first-line treatment with single-agent doxorubicin survive for a median of 8 to 12 months. To date, results of randomized trials of combination chemotherapy have failed to demonstrate a benefit over single-agent doxorubicin, including the findings from the two most recently reported phase III trials that compared doxorubicin with combination therapy with ifosfamide25 or palifosfamide.26 In this single-arm study, postprogression survival was prolonged, with a median OS of 21.5 months, which was 15 months longer than the median PFS.
Prolonged survival can be associated with lower-grade tumors. In this study, only five patients were enrolled with sarcomas generally considered to be low grade: four patients with chondrosarcoma and one patient with chordoma. All other patients met eligibility criteria excluding low-grade tumors, but no central pathology review was conducted. Subsequent therapies after discontinuing TH-302 could also have contributed to the observed prolonged survival. After discontinuing TH-302, most patients received subsequent chemotherapy; some patients received doxorubicin and/or ifosfamide. Pazopanib was recently approved for extended PFS but did not show a significant survival benefit. No other therapy has been approved as second-line therapy for non-GI stromal tumor STS.
Implementation of maintenance treatment to prolong response duration and delay disease progression in patients with STS who responded to their first-line chemotherapy regimen is being investigated with various anticancer agents.5 The data from this study indicate that continued administration of TH-302 as single-agent maintenance therapy is tolerated with limited hematologic toxicity, manageable skin and mucosal toxicity, no cardiac toxicity (a concern with doxorubicin), and no cumulative toxicity. Improved tumor response classifications were observed in six patients during maintenance, and the median PFS after maintenance initiation was 3.7 months.
We hypothesize that therapeutic targeting of hypoxic regions of tumors may prolong survival. Hypoxic areas of tumors are believed to serve as sanctuaries for neoplastic cells by shielding them from cytotoxic effects of many conventional chemotherapies.27–29 TH-302 may suppress or eliminate cells sheltered in these hypoxic areas, such that subsequent disease progression may represent progression in nonhypoxic areas in which conventional cytotoxic salvage therapies may be more effective. Hypoxia may also influence metastatic dissemination of tumor cells from the primary site.30 TH-302 treatment in the maintenance setting may prevent further metastatic dissemination of cells, thereby delaying development of additional metastatic sites.
Given the association of tumor hypoxia with chemotherapy/radiation resistance, metastasis, and poor clinical outcomes, the targeting of hypoxic tumor cells is an important and active area of cancer research. Our preliminary efficacy and safety data with a TH-302 hypoxia-targeting strategy in this phase II study supported the initiation of a randomized, controlled phase III study of doxorubicin and TH-302 in STS (NCT01440088).
Glossary Terms
- bystander effect:
the biologically positive response observed in untreated cells when neighboring cells are treated. Because of the bystander effect, the magnitude of the therapeutic response exceeds the effect expected from direct target-cell treatment, illustrating that the treatment not only induces direct cytotoxic effects against the individual target cell but also causes the growth suppression of bystander, untreated cells via other mechanisms.
- hypoxia:
oxygen concentration below normal physiological limits in a specific tissue.
- prodrug:
a drug that is given in an inactive form and is bioactivated to a pharmacologic drug by one or more metabolic processes.
Appendix
Complete Eligibility Criteria
Inclusion criteria.
Patients who met the following criteria were eligible for the study. Each patient must:
Be at least 18 years of age
Have the ability to understand the purposes and risks of the study and must have signed a written informed consent form approved by the investigator's institutional review board
- Have a pathologically confirmed diagnosis of soft tissue sarcoma of one of the following subtypes:
- Synovial sarcoma
- High-grade fibrosarcoma
- Unclassified, undifferentiated sarcoma
- Liposarcoma
- Leiomyosarcoma (excluding GI soft tissue sarcoma)
- Angiosarcoma (excluding Kaposi's sarcoma)
- Pleomorphic sarcoma/malignant fibrous histiocytoma
Have locally advanced unresectable or metastatic disease with no standard curative therapy available and be a patient for whom treatment with single-agent doxorubicin is considered appropriate; patients in the dose escalation cohorts must have progressed since their most recent systemic therapy
Have recovered from reversible toxicities of prior therapy
Have evaluable disease by RECIST (≥ one target or nontarget lesion for phase I; ≥ one target lesion for phase II)
Have an Eastern Cooperative Oncology Group performance status of 0 or 1
Have a life expectancy of ≥ 3 months
- Have acceptable liver function with the following parameters:
- Bilirubin ≤ 1.5× upper limit of normal (ULN)
- ALT and AST ≤ 2.5× ULN
- Have acceptable renal function with the following parameter:
- Serum creatinine ≤ ULN
- Have acceptable hematologic status (without hematologic support) with the following parameters:
- Absolute neutrophil count (ANC) ≥ 1,500/μL
- Platelet count ≥ 100,000/μL
- Hemoglobin ≥ 9.0 g/dL
- Have acceptable cardiac function with the following parameters:
- Normal 12-lead electrocardiogram (clinically insignificant abnormalities were permitted)
- Left ventricular ejection fraction normal by multigated radionuclide angiography or echocardiogram
Have a urinalysis with no clinically significant abnormalities
Have had a negative serum pregnancy test (for all women with childbearing potential) and have agreed to use effective means of contraception (surgical sterilization or the use of barrier contraception with either a condom or diaphragm in conjunction with spermicidal gel or an intrauterine device with their partner from entry onto the study through 6 months after the last dose)
Exclusion criteria.
Patients who met any of the following criteria were excluded from the study. Each patient must:
- Have had prior therapy of the following type:
- Phase I: prior treatment with more than two myelosuppressive cytotoxic chemotherapy regimens
- Phase II: prior systemic therapy for advanced disease (neoadjuvant and adjuvant therapy were permitted)
Have low-grade tumors according to standard grading systems (eg, American Joint Committee on Cancer grade 1 or 2)
Have had prior therapy with ifosfamide or cyclophosphamide
Have had prior therapy with an anthracycline or anthracenedione
Have had prior mediastinal and/or cardiac radiotherapy
Currently be using drugs with known cardiotoxicity or known interactions with doxorubicin (see product label)
Have had anticancer treatment with radiation therapy, chemotherapy, targeted therapies (eg, erlotinib, lapatinib), immunotherapy, hormones, or other antitumor therapies within 4 weeks before study entry (6 weeks for nitrosoureas or mitomycin)
- Have had significant cardiac dysfunction of at least one of the following types:
- Any history of congestive heart failure
- Any history of transmural myocardial infarction
- Uncontrolled arrhythmias within the past 6 months
- Angina pectoris requiring antianginal medication within the past 6 months
- Clinically significant valvular heart disease
- Poorly controlled hypertension within the last 6 months
Have had seizure disorders requiring anticonvulsant therapy
Have known brain metastases (unless previously treated and well controlled for a period of ≥ 3 months)
Have had previously treated malignancies, except for adequately treated nonmelanoma skin cancer, in situ cancer, or other cancer from which the patient has been disease-free for at least 5 years
Have had severe chronic obstructive or other pulmonary disease with hypoxemia (required supplementary oxygen or had symptoms resulting from hypoxemia or oxygen saturation < 90% by pulse oximetry after a 2-minute walk) or, in the opinion of the investigator, any physiologic state likely to cause normal tissue hypoxia
Have had major surgery, other than diagnostic surgery, within 4 weeks before day 1 without complete recovery
Have active, uncontrolled bacterial, viral, or fungal infections requiring systemic therapy
Have had prior therapy with a hypoxic cytotoxin
Have participated in an investigational drug or device study within 28 days before study entry
Have a known active infection with HIV, hepatitis B, or hepatitis C
Have exhibited allergic reactions to a structural compound, biologic agent, or formulation (containing solutol and/or propylene glycol) similar to TH-302
Be pregnant or breast-feeding (females)
Have a concomitant disease or condition that could interfere with the conduct of the study or that would have, in the opinion of the investigator, posed an unacceptable risk to the patient in this study
Be unwilling or unable to comply with the study protocol for any reason
Dose Modifications
Blood for CBC and serum creatinine was drawn within 5 days of cycle 1 day 1, within 3 days before day 1 of subsequent cycles, and within 1 day of dosing on day 8 of all cycles.
Dose modification rules were followed for all hematologic, liver, and renal toxicities regardless of causality and for any other toxicity that was not clearly related to disease progression, intercurrent illness, concomitant medications, or other nondrug intervention. • If a patient required more than two dose reductions for toxicity, he or she discontinued from the study • Any patient who missed more than one cycle (> 3 weeks of day 1 of a cycle) for treatment-related toxicity discontinued from the study
TH-302 Plus Doxorubicin Dose Modifications
If day 1 of a cycle for either drug was withheld, the whole cycle was delayed. If day 8 was withheld, the dose was skipped rather than delayed.
If study drug was held at day 1 of any cycle, counts were repeated weekly, and therapy was reinstituted when the ANC was ≥ 1,500/μL and platelets were ≥ 100,000/μL.
For febrile neutropenia, defined as ANC less than 500/μL and temperature ≥ 38.2°C (100.8°F), doxorubicin was reduced 25%, and TH-302 was reduced one dose level in all subsequent cycles.
Table A1.
Doxorubicin Dose Modifications for Hematologic Toxicity
| Nadir ANC (per microliter) | Nadir Platelet Count (per microliter) | % of Full Dose After Recovery to Grade 0 to 1 | |
|---|---|---|---|
| < 500* | And/or | < 50,000* | 75 |
Abbreviation: ANC, absolute neutrophil count.
ANC must be ≥ 1,500/μL and platelet count ≥ 100,000/μL to continue dosing.
Table A2.
TH-302 Dose Modifications for Hematologic Toxicity
| ANC (per microliter) | Platelets (per microliter) | % of Full Dose for Any Cycle |
||
|---|---|---|---|---|
| Day 1 | Day 8 | |||
| ≥ 1,500 | And | ≥ 100,000 | 100 | 100 |
| 500-1,499 | And/or | 50,000-99,999 | Hold | 100 |
| < 500 | And/or | < 50,000 | Hold | Hold |
Abbreviation: ANC, absolute neutrophil count.
Table A3.
TH-302 and Doxorubicin Dose Modifications for Nonhematologic Toxicity
| Toxicity | Details | Hold Dose | % of Full Dose After Recovery to Grade 0 to 1 |
|---|---|---|---|
| Increased serum creatinine | Regardless of causality | Hold dose and repeat serum creatinine at least 3 days later | 100 Discontinue from study if serum creatinine remains increased |
| Grade 1, 2, or 3 | Serum bilirubin | Grade 1: no | 75% (doxorubicin) |
| Grade 2 or higher: hold dose until resolution to grade 0 or 1 | 100% (TH-302) | ||
| Grade 2* | Except for nausea, vomiting, diarrhea, alopecia, and fatigue | Hold dose until resolution to grade 0 or 1 | 100 |
| Intolerable skin toxicity | Hold dose until resolution to grade 0 or 1 | 75 (TH-302 only) | |
| Grade 3† | Except nausea and vomiting | Hold dose until resolution to grade 0 or 1 | 75 |
| Grade 4 | Life-threatening conditions (study drug related) | Treatment should be discontinued | NA |
| Grade 4 | Other grade 4 events such as fatigue and non–life-threatening pulmonary embolism that are adequately treated | Hold dose until resolution to grade 0 or 1 | 50 |
Abbreviation: NA, not applicable.
Grade 2 ALT/AST at baseline; treatment should not be held if it remains grade 2.
Grade 2 ALT/AST at baseline; treatment resumes when value returns to grade 2.
Footnotes
Supported by Threshold Pharmaceuticals, South San Francisco, CA, and Merck KGaA, Darmstadt, Germany. Editorial assistance and submission were supported by Scientific Connections, Threshold Pharmaceuticals, and Merck KGaA.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Presented in part at the 17th Annual Meeting of the Connective Tissue Oncology Society, Prague, Czech Republic, November 14-17, 2012, and at the 16th Annual Meeting of the Connective Tissue Oncology Society, Chicago, IL, October 26-29, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00742963.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Stew Kroll, Threshold Pharmaceuticals (C) Consultant or Advisory Role: Sant P. Chawla, Amgen (C), GlaxoSmithKline (C), Threshold Pharmaceuticals (C), Berg Pharma (C); Brian A. Van Tine, EMD Serono (C) Stock Ownership: Stew Kroll, Threshold Pharmaceuticals Honoraria: Sant P. Chawla, Amgen, GlaxoSmithKline, Threshold Pharmaceuticals, Berg Pharma Research Funding: Sant P. Chawla, Amgen, GlaxoSmithKline, Threshold Pharmaceuticals, Berg Pharma; Lee D. Cranmer, Threshold Pharmaceuticals; Douglas R. Adkins, Threshold Pharmaceuticals Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Sant P. Chawla, Lee D. Cranmer, Stew Kroll, Kristen N. Ganjoo
Provision of study materials or patients: Sant P. Chawla, Lee D. Cranmer, Brian A. Van Tine, Damon R. Reed, Scott H. Okuno, James E. Butrynski, Douglas R. Adkins, Andrew E. Hendifar, Kristen N. Ganjoo
Collection and assembly of data: Sant P. Chawla, Lee D. Cranmer, Brian A. Van Tine, Damon R. Reed, James E. Butrynski, Douglas R. Adkins, Andrew E. Hendifar, Stew Kroll, Kristen N. Ganjoo
Data analysis and interpretation: Sant P. Chawla, Lee D. Cranmer, Brian A. Van Tine, Damon R. Reed, Scott H. Okuno, James E. Butrynski, Douglas R. Adkins, Stew Kroll, Kristen N. Ganjoo
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Eriksson M. Histology-driven chemotherapy of soft-tissue sarcoma. Ann Oncol. 2010;21:vii270–vii276. doi: 10.1093/annonc/mdq285. [DOI] [PubMed] [Google Scholar]
- 2.Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2,185 patients treated with anthracycline-containing first-line regimens—A European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 3.Karavasilis V, Seddon BM, Ashley S, et al. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: Retrospective analysis and identification of prognostic factors in 488 patients. Cancer. 2008;112:1585–1591. doi: 10.1002/cncr.23332. [DOI] [PubMed] [Google Scholar]
- 4.Sleijfer S, Ouali M, van Glabbeke M, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: An exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) Eur J Cancer. 2010;46:72–83. doi: 10.1016/j.ejca.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Riedel RF. Systemic therapy for advanced soft tissue sarcomas: Highlighting novel therapies and treatment approaches. Cancer. 2012;118:1474–1485. doi: 10.1002/cncr.26415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Cancer Society. Cancer Facts & Figures 2008. Atlanta, GA: American Cancer Society; 2008. [Google Scholar]
- 7.Lorigan P, Verweij J, Papai Z, et al. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 2007;25:3144–3150. doi: 10.1200/JCO.2006.09.7717. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen OS, Blay JY, Judson IR, et al. Metastatic soft tissue sarcoma in adults: Prognosis and treatment options. Am J Cancer. 2003;2:211–221. [Google Scholar]
- 9.Casali PG, Blay JY. ESMO/CONTICANET/EUROBONET Consensus Panel of experts: Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v198–v203. doi: 10.1093/annonc/mdq209. [DOI] [PubMed] [Google Scholar]
- 10.Kerbusch T, de Kraker J, Keizer HJ, et al. Clinical pharmacokinetics and pharmacodynamics of ifosfamide and its metabolites. Clin Pharmacokinet. 2001;40:41–62. doi: 10.2165/00003088-200140010-00004. [DOI] [PubMed] [Google Scholar]
- 11.Brizel DM, Rosner GL, Harrelson J, et al. Pretreatment oxygenation profiles of human soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 1994;30:635–642. doi: 10.1016/0360-3016(92)90950-m. [DOI] [PubMed] [Google Scholar]
- 12.Kaanders JH, Wijffels KI, Marres HA, et al. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res. 2002;62:7066–7074. [PubMed] [Google Scholar]
- 13.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 14.Shannon AM, Bouchier-Hayes DJ, Condron CM, et al. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29:297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 15.Vaupel P, Höckel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9:1221–1235. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 16.Francis P, Namløs HM, Müller C, et al. Diagnostic and prognostic gene expression signatures in 177 soft tissue sarcomas: Hypoxia-induced transcription profile signifies metastatic potential. BMC Genomics. 2007;8:73. doi: 10.1186/1471-2164-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajendran JG, Wilson DC, Conrad EU, et al. [(18)F]FMISO and [(18)F]FDG PET imaging in soft tissue sarcomas: Correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging. 2003;30:695–704. doi: 10.1007/s00259-002-1096-7. [DOI] [PubMed] [Google Scholar]
- 18.Nordsmark M, Alsner J, Keller J, et al. Hypoxia in human soft tissue sarcomas: Adverse impact on survival and no association with p53 mutations. Br J Cancer. 2001;84:1070–1075. doi: 10.1054/bjoc.2001.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Primeau AJ, Rendon A, Hedley D, et al. The distribution of the anticancer drug doxorubicin in relation to blood vessels in solid tumors. Clin Cancer Res. 2005;11:8782–8788. doi: 10.1158/1078-0432.CCR-05-1664. [DOI] [PubMed] [Google Scholar]
- 20.Meng F, Evans JW, Bhupathi D, et al. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol Cancer Ther. 2012;11:740–751. doi: 10.1158/1535-7163.MCT-11-0634. [DOI] [PubMed] [Google Scholar]
- 21.Weiss GJ, Infante JR, Chiorean EG, et al. Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin Cancer Res. 2011;17:2997–3004. doi: 10.1158/1078-0432.CCR-10-3425. [DOI] [PubMed] [Google Scholar]
- 22.Ganjoo KN, Cranmer LD, Butrynski JE, et al. A phase I study of the safety and pharmacokinetics of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. Oncology. 2011;80:50–56. doi: 10.1159/000327739. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Van Glabbeke M, Verweij J, Judson I, et al. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543–549. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 25.Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 26.Ryan CW, Schoffski P, Merimsky O, et al. PICASSO 3: A phase 3 international, randomized, double-blind, placebo-controlled study of doxorubicin (dox) plus palifosfamide (pali) vs. dox plus placebo for patients (pts) in first-line for metastatic soft tissue sarcoma (mSTS). Presented at the European Cancer Congress; September 27-October 1, 2013; Amsterdam, the Netherlands. (abstr 3802) [Google Scholar]
- 27.Batchelder RM, Wilson WR, Hay MP, et al. Oxygen dependence of the cytotoxicity of the enediyne anti-tumour antibiotic esperamicin A1. Br J Cancer. 1996;27:S52–S56. [PMC free article] [PubMed] [Google Scholar]
- 28.Teicher BA, Lazo JS, Sartorelli AC. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981;41:73–81. [PubMed] [Google Scholar]
- 29.Tannock I. Response of aerobic and hypoxic cells in a solid tumor to adriamycin and cyclophosphamide and interaction of the drugs with radiation. Cancer Res. 1982;42:4921–4926. [PubMed] [Google Scholar]
- 30.Bennewith KL, Dedhar S. Targeting hypoxic tumour cells to overcome metastasis. BMC Cancer. 2011;11:504. doi: 10.1186/1471-2407-11-504. [DOI] [PMC free article] [PubMed] [Google Scholar]


