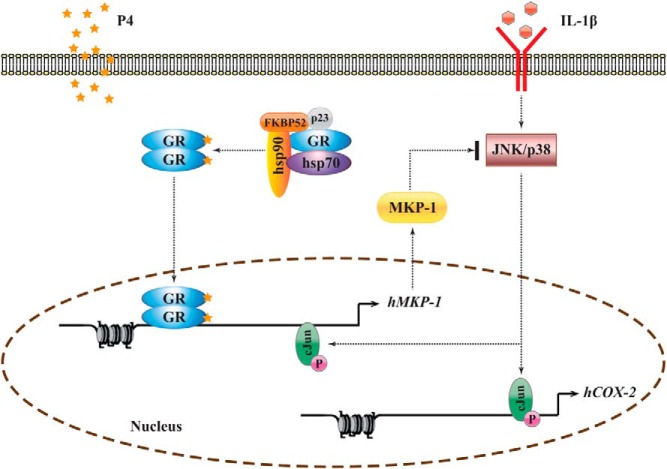
Abstract
Progesterone (P4) maintains uterine quiescence during pregnancy and its functional withdrawal is associated with increased prostaglandin synthesis and the onset of labor. In primary human myometrial cells, the glucocorticoid receptor (GR) rather than the P4 receptor mediates P4 antagonism of IL-1β-induced cyclooxygenase-2 (COX-2) expression, the rate-limiting enzyme in prostaglandin synthesis. We now report that P4 also acts via GR to induce MAPK phosphatase (MKP)-1 and knockdown of MKP-1 impairs the ability of P4 to repress IL-1β-dependent COX-2 induction. Microarray analysis revealed that P4 repressed preferentially activator protein-1-responsive genes in response to IL-1β. Consistent with these observations, we found that the ability of P4 to reduce c-Jun activation was lost upon GR as well as MKP-1 knockdown. Interestingly, c-Jun levels in human myometrial cells declined upon GR and MKP-1 knockdown, which suggests the presence of an activator protein-1 feedback loop. This is supported by our observation that c-Jun levels declined after an initial rise in primary myometrial cells treated with phorbol 12-myrisatate 13-acetate, a potent activator of c-Jun N-terminal kinase. Finally, we show that MKP-1 is an intermediate in P4-mediated repression of some but not all IL-1β-responsive genes. For example, P4 repression of IL11 and IRAK3 was maintained upon MKP-1 knockdown. Taken together, the data show that P4 acts via GR to drive MKP-1 expression, which in turn inhibits IL-1β-dependent c-Jun activation and COX-2 expression.
Both term and preterm parturition are associated with a marked inflammatory response (1, 2). The release of key cytokines, including IL-1β, activates a number of transcription factors, including nuclear factor κB (NF-κB), activator protein (AP)-1, and CCAAT enhancer-binding protein, which enhance the expression of labor-associated genes such as cyclooxygenase-2 (COX-2), oxytocin receptor, and connexin 43 (3–5). Elevated COX-2 activity increases the synthesis of stimulatory prostaglandins E2 and F2α, which induce cervical ripening, fetal membrane remodeling and uterine contractions (6–8). COX-2 is the rate-limiting enzyme in the process of prostaglandin biosynthesis, and consequently, its transcriptional regulation has been closely studied (9–12).
Progesterone (P4) plays an important role in normal human physiology, primarily in the uterus, where it is essential for the initiation and maintenance of pregnancy (13–16). During pregnancy, P4 represses COX-2 expression in the myometrium and amnion (17, 18). The underlying mechanism has been studied in breast cancer cell lines, in which P4 was shown to act via its receptor (P4 receptor [PR]) to inhibit NF-κB (19, 20). However, previous studies suggested that the glucocorticoid receptor (GR) is also involved in regulating labor-associated gene expression (21, 22), and although the affinity of P4 for GR is much less than that of cortisol, P4 can bind GR directly (21). Further, we recently reported that in human myometrial cells, PR knockdown almost had no effect (only 0.025% genes) on medroxyprogesterone acetate's antiinflammatory effect (23), and P4 actually acted via GR to modulate the expression of P4-responsive genes, such as FKBP5 and HSD11B1, and to repress IL-1β-induced COX-2 expression (18).
Several mechanisms have been implicated in GR-mediated antiinflammatory effects. GR has been shown to interact directly with NF-κB, delaying the nuclear translocation of activated NF-κB p65 subunit (p65) (24). It has also been shown to inhibit NF-κB activity by recruiting histone deacetylases (HDACs) (25) and by inducing the incorporation of GR and GR-interacting protein 1 into the transcriptional complex (26). Further, liganded GR induces MAPK phosphatase (MKP)-1 (26–32), which is responsible for dephosphorylating tyrosine and threonine residues of MAPK, limiting the duration of inflammation-induced MAPK activation (31, 33). As an immediate-early response gene, MKP-1 (also known as DUSP1) is controlled mainly at the transcriptional level and both its mRNA and protein have very short half-lives (40–60 minutes) (34, 35). Based on tissue-specific expression, posttranslational regulation, substrate specificity, and subcellular localization, 11 members of the MKP family have been identified to date (36). Among these, MKP-1 is the only one induced by P4 in human myometrial cells as shown in our microarray study and also in human endometrial stromal cells (37), suggesting a role for this phosphatase in mediating the antiinflammatory actions of P4. Indeed, there is evidence that MKP-1 is also involved in the antiinflammatory effect of glucocorticoids (38, 39).
In this study, we have investigated how P4 acts to repress COX-2 expression and shown that the antiinflammatory actions of P4 in primary human myometrial cells are in part mediated through a GR-dependent induction of MKP-1, resulting in attenuated AP-1 signaling.
Materials and Methods
Tissue specimens
Myometrial biopsies (0.5 × 0.5 × 0.5 cm3) of term human myometrium were collected at the time of elective caesarean section from the upper margin of the incision made in the lower segment of the uterus from women not in labor. Samples were then put into DMEM (Invitrogen) medium containing L-glutamine and 100-mU/mL penicillin and 100-μg/mL streptomycin and were stored at 4°C for no more than 3 hours before cell preparation for culture. All specimens were obtained after fully informed, written patient consent. The Riverside Ethics committee approved the study.
Primary cell culture
Primary human myometrial cells were isolated using a mixture of collagenases (1 mg/mL of collagenase 1A and 1 mg/mL of collagenase XI [Sigma]) and cultured in DMEM containing phenol red 7.5% fetal calf serum, L-glutamine, and 100-mU/mL penicillin and 100-μg/mL streptomycin in an atmosphere of 5% CO2, 95% air at 37°C. Myometrial cells grown in this manner have previously been characterized (40). Cells from passage 1–4 were trypsinized in 0.25% trypsin containing 0.02% EDTA and cultured in 6-well culture plates or flasks depending on the requirement. In some cases at the end of the specified time, medium was removed and cells were frozen at −80°C for the extraction of RNA or protein. In other cases, such as coimmunoprecipitation (co-IP), and cytosolic/nuclear protein extraction, cells were harvested and processed directly after treatment. Before treating the cells with different stimuli, old medium was removed and replaced with 2 mL of freshly stripped medium (1% charcoal and dextran-stripped fetal calf serum, supplemented with L-glutamine, 100-mU/mL, penicillin and 100-μg/mL streptomycin). In some cases, cells were preincubated with 1μM phorbol 12-myrisatate 13-acetate (PMA) (AP-1 activator) (Sigma-Aldrich Co Ltd) for 1 hour before other stimuli, such as IL-1β (5ng/mL) and P4 (10μM), either alone or in combination. The AP-1 inhibitor, SR11302, at the final concentration of 1μM was also used, and cells were exposed for 2 hours. The dose of P4 used in this study was determined by the dose-responsive curve on several P4-responsive genes and the P4 repression on IL-1β-driven COX-2 expression (Supplemental Figure 1). Ethanol or dimethyl sulfoxide was used as vehicle.
Gene silencing and overexpression
Both small interfering RNAs (siRNAs) (Table 1) and gene expression constructs were transfected using the Amaxa Nucleofector technology (Lonza Sales AG). Cells were harvested by trypsinization as described above. Approximately 1 × 106 cells were resuspended in 100-μL room-temperature Nucleofector Solution and mixed with different amount of either siRNA (30 pmol of siGR [siGENOME SMARTpool; Thermo Fisher Scientific]; 300 pmol of siMKP-1 [Sigma]) or 2-μg plasmid DNA (pCMV [parental control plasmid] and pCMV-TAM67 [dominant negative c-Jun; DN c-Jun] were kindly given from Dr Michael J. Birrer, Harvard Medical School, Boston, MA). The cell/siRNA or cell/DNA suspension was then transferred into certified cuvette and electroporated in the Nucleofector Cuvette Holder with the Nucleofector Program A-033. Immediately after electroporation, cells were suspended with 500-μL prewarmed culture medium and gently transferred into 6-well culture plates. The cells were then incubated in an atmosphere of 5% CO2, 95% air at 37°C until analysis.
Table 1.
siRNA Sequences (ON-TARGET Plus SMART Pool and Sigma siRNA Pool)
| Name | Target sequences |
|---|---|
| GR | GAACUUCCCUGGUCGAACA GGAAACAGACUUAAAGCUU UGACAAAACUCUUGGAUUC GCAUGUACGACCAAUGUAA |
| PR | GGACGUGGAGGGCGCAUAU GAGAUGAGGUCAAGCUACA ACAUAUUGAUGACCAGAUA GCACCUGAUCUAAUACUAA |
| MKP-1 | CACAUUCGGGACCAAUAUA UAUAUUGGUCCCGAAUGUG GGCAUUUCCCGGUCAGCCA UGGCUGACCGGGAAAUGCCGCAUAACUGCCUUGAUCAA UUGAUCAAGGCAGUUAUGC |
Quantitative real-time PCR
Total RNA was extracted and purified from myometrial cells grown in 6-well culture plates using RNAeasy mini kit (QIAGEN Ltd). After quantification 1.5 μg was reverse transcribed with oligo deoxy-thymine nucleotides random primers using Murine Leukemia Virus reverse transcriptase (Applied Biosystems Ltd). Primer sets (Table 2) were designed and obtained from Invitrogen. Quantitative PCR (qPCR) was performed in the presence of SYBR Green (Roche Diagnostics Ltd), and amplicon yield was monitored during cycling in a RotorGene Sequence Detector (Corbett Research Ltd) that continually measures fluorescence caused by the binding of the dye to double-stranded DNA. Pre-PCR cycle was 10 minutes at 95°C followed by up to 45 cycles of 95°C for 20 seconds, 58°C–60°C for 20 seconds, and 72°C for 20 seconds followed by an extension at 72°C for 15 seconds. The final procedure involves a melt over the temperature range of 72°C–99°C rising by 1° steps with a wait for 15 seconds on the first step followed by a wait of 5 seconds for each subsequent step. The cycle at which the fluorescence reached a preset threshold (cycle threshold) was used for quantitative analyses. The cycle threshold in each assay was set at a level where the exponential increase in amplicon abundance was approximately parallel between all samples. All mRNA abundance data were expressed relative to the amount of the constitutively expressed GAPDH.
Table 2.
Primer Pair Sequences With Gene Accession Numbers
| Name | F and R primer sequence (5′-3′) | GenBank/EMBL accession number |
|---|---|---|
| COX-2 | F, tgtgcaacacttgagtggct | AY151286 |
| R, actttctgtactgcgggtgg | ||
| GAPDH | F, tgatgacatcaagaaggtggtgaag | BC014085 |
| R, tccttggaggccatgtaggccat | ||
| MKP-1 | F, cagctgctgcagtttgagtc | NM_004417 |
| R, aggtagctcagcgcactgtt | ||
| c-Jun | F, cagctgctgcagtttgagtc | NM_004417 |
| R, aggtagctcagcgcactgtt | ||
| CXCL1 | F, ccatattcctcggacaccac | NM_002985 |
| R, tgtactcccgaacccatttc | ||
| MCP-1 | F, tctgtgcctgctgctcatag | NM_001151 |
| R, gcctctgcagctgtgtctct | ||
| RANTES | F, ccatattcctcggacaccac | NM_002985 |
| R, tgtactcccgaacccatttc | ||
| IL-8 | F, ggacaagagccaggaagaaacc | NM_000584 |
| R, ggcatcttcactgattcttggat | ||
| IL-1α | F, aatgacgccctcaatcaaag | NM_000575 |
| R, tgggtatctcaggcatctcc | ||
| IL-1β | F, gctgaggaagatgctggttc | NM_000576 |
| R, tccatatcctgtccctggag | ||
| IL7R | F, taccgtgagcgacaaagatg | NM_000206 |
| R, gctgaatcattgggtcacct | ||
| PLA2G4A | F, cccgacttatttggaagcaa | NM_024420 |
| R, gagcctctgctttgtgaacc | ||
| CD274 | F, gtaccttggctttgccacat | NM_001267706 |
| R, ccaacaccacaaggaggagt | ||
| HAS2 | F, gcctcatctgtggagatggt | NM_005328 |
| R, tcccagaggtccactaatgc | ||
| NR4A3 | F, gccctgactgtccagctatc | NM_006981 |
| R, tcctgctgatttgcatgttc | ||
| TNFRSF11B | F, ggcaacacagctcacaagaa | NM_002546 |
| R, ctgggtttgcatgcctttat | ||
| IL11 | F, ggttccacaagtcaccctgt | NM_000632 |
| R, tccttagcctccctgaatga | ||
| CCL8 | F, tcacctgctgctttaacgtg | NM_005623 |
| R, atccctgacccatctctcct | ||
| CXCL10 | F, aaggatggaccacacagagg | NM_001565 |
| R, agcagggtcagaacatccac | ||
| CXCL11 | F, agaggacgctgtctttgcat | NM_005409 |
| R, taagccttgcttgcttcgat | ||
| CCL11 | F, gcctccaacatgaaggtctc | NM_002986 |
| R, tatccttggccagtttggtc | ||
| IRAK3 | F, atctggaggagccaggattt | NM_001142523 |
| R, gggtgcctgtagcagagaag | ||
| 11βHSD | F, accttcgcagagcaatttgt | NM_005525 |
| R, gccagagaggagacgacaac |
F, forward; R, reverse.
Whole-cell and cytosolic/nuclear protein extraction
For the whole-cell protein extraction, protein samples were prepared from monolayer myometrial cells by being lysed in Cell Lysis buffer (New England Biolabs). The supernatant was separated from cell debris by centrifugation at 13 000g for 20 minutes at 4°C. In order to isolate the nuclear fractions, cells were harvested by trypsinization using a standard method as described before. The cell pellet was resuspended with buffer A and incubated on ice for 5–20 minutes. Lysates were vortexed for 10 seconds at a high speed followed by centrifugation at 16 000g for 30 seconds at 4°C. The supernatants were retained as the cytosolic protein extracts. The pellets were resuspended in buffer B, incubated for 15 minutes on ice with shaking (∼200 rpm), and then centrifuged for 5 minutes at 16 000g for at 4°C. After the last centrifugation, the supernatant (nuclear extract) was transferred to a separate tube. Protein concentrations were determined by Protein assay (Bio-Rad Laboratories Ltd) and BSA reference standards. Samples were then aliquot and stored at −80°C.
Western blot analysis
Electrophoresis was carried out on 20-μg aliquots of protein samples that were denatured by adding NuPAGE loading buffer (Invitrogen) and heating for 10 minutes at 70°C. Western blotting was performed after an electrophoretic transfer onto a Polyvinylidene fluoride membrane with Trans-Blot Turbo Transfer System (Bio-Rad). Membranes were blocked in blocking buffer (1× tris-buffered saline and tween 20 and 5% milk) for 1 hour at room temperature, washed in 1× Tris-buffered saline and Tween 20, and hybridized with the primary antibody (antibody for nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha [IκB-α], phospho-c-Jun [p-c-Jun, Ser63], and α-tubulin used at 1:5000), antibody for p65, COX-2, and GR used at 1:1000, antibody for MKP-1 used at 1:500 (Santa Cruz Biotechnology, Inc); antibody for phospho-p65, total, and p-c-Jun (Ser73) used at 1:1000 (Cell Signaling); antibody for TATA-binding protein used at 1:2000, antibody for DN c-Jun (targeting around Ser243) used at 1:1000 (Abcam); antibody for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin used at 1:20 000 (Millipore) overnight at 4°C. Membranes were washed again and then incubated with corresponding secondary antibody (New England Biolabs) at a dilution of 1:2000 for 2 hours at room temperature. ECL Plus (GE Healthcare Life Sciences) was used for detection. Protein band size was determined using Novex Sharp Prestained Protein Standard (Invitrogen).
Coimmunoprecipitation
All immunoprecipitation procedures were performed at 4°C. Cells were harvested and washed twice with ice-cold PBS. Cells were lysed in lysis buffer (1% nonyl phenoxypolyethoxylethanol-40, 150mM NaCl, 50mM Tris-HCl [pH 7.4], 1mM EDTA, and 10% glycerol). The lysate was precleared by protein G-Agarose beads before the incubation with the antibody against the protein of interest (antibody for c-Jun [Cell Signaling]; antibody for GR [Abnova]) or preimmune mouse IgG for 1–2 hours with rotation; 10% of the lysate was kept as input. This lysate/antibody mixture was subsequently incubated with protein G-Agarose beads for 1 hour with rotation. The beads were pelleted by centrifugation at 1000g for 30 seconds and washed 4 times in IP buffer (1% nonyl phenoxypolyethoxylethanol-40, 150mM NaCl, 50mM Tris-HCl [pH 7.4], and 1mM EDTA). The protein-antibody complexes that were released from beads by adding the loading buffer and heating at 70°C for 10 minutes were subjected to Western blot analysis after separation by SDS-PAGE.
Affymetrix human genome U133 plus 2.0 array processing and analysis
Total RNA was extracted as previously described and all the samples were profiled by UCL Cancer Institute (University College London, London, UK) using the Affymetrix Human Genome U133 plus 2.0 GeneChip (Affymetrix, Inc). Briefly, cDNA generated from 2 μg of total RNA using the GeneChip Expression 3′-Amplification One-Cycle cDNA Synthesis kit, in conjunction with the GeneChip Eukaryotic PolyA RNA Control kit (Affymetrix, Inc). The cDNA was cleaned up using the GeneChip Sample Cleanup Module and subsequently processed to generate biotin-labeled cRNA using the GeneChip Expression 3′-Amplification IVT Labeling kit (Affymetrix, Inc). A total of 25 μg of labeled cRNA was fragmented using 5× fragmentation buffer and ribonuclease-free water at 94°C for 35 minutes. A total of 15 μg of the fragmented, biotin-labeled cRNA was made up in a hybridization cocktail and hybridized to the HgU133 Plus 2.0 array at 45°C for 16 hours. After hybridization, the arrays were washed and stained using the Affymetrix Fluidics Station 450 and scanned using the Affymetrix GeneChip Scanner 3000. All steps of the process were quality controlled by measuring yield (μg), concentration (μg/L) and 260:280 ratios via spectrophotometry using the Nanodrop ND-1000 and sample integrity using the Agilent 2100 bioanalyser (Agilent Technologies, Inc). After general array Quality Control and data export, the data were normalized using Robust Multichip Average and imported into Partek Genomic Suite. Array data were then identified for any potential outliers and overall grouping/separation using Principal Component Analysis. The gene lists were generated by a sequential filtering approach based on the fold change (normalization to the control group) and the confidence (fold change P value). The less-stringent gene list was resulted from the fold change greater than 1.5 by comparing the treatment group with the control group. The stringent gene list was retained by further filtering with fold change P < .05.
Statistical data analysis
All data were initially tested for normality using a Kolmogorov-Smirnoff test. Normally distributed data were analyzed using a paired t test for 2 groups and repeated measures ANOVA followed by a Dunnett's multiple comparison post hoc test for 3 groups or more. Data that were not normally distributed were analyzed using a Wilcoxon matched pairs test for 2 groups and when comparing 3 groups or more a Friedman's test, with a Dunn's multiple comparison post hoc test was applied. P < .05 was considered statistically significant. In some cases, δ-changes were calculated in order to discern the effect of a specific stimulus on a target gene.
Results
P4-induced and GR-mediated MKP-1 activation represses IL-1β-driven COX-2 expression
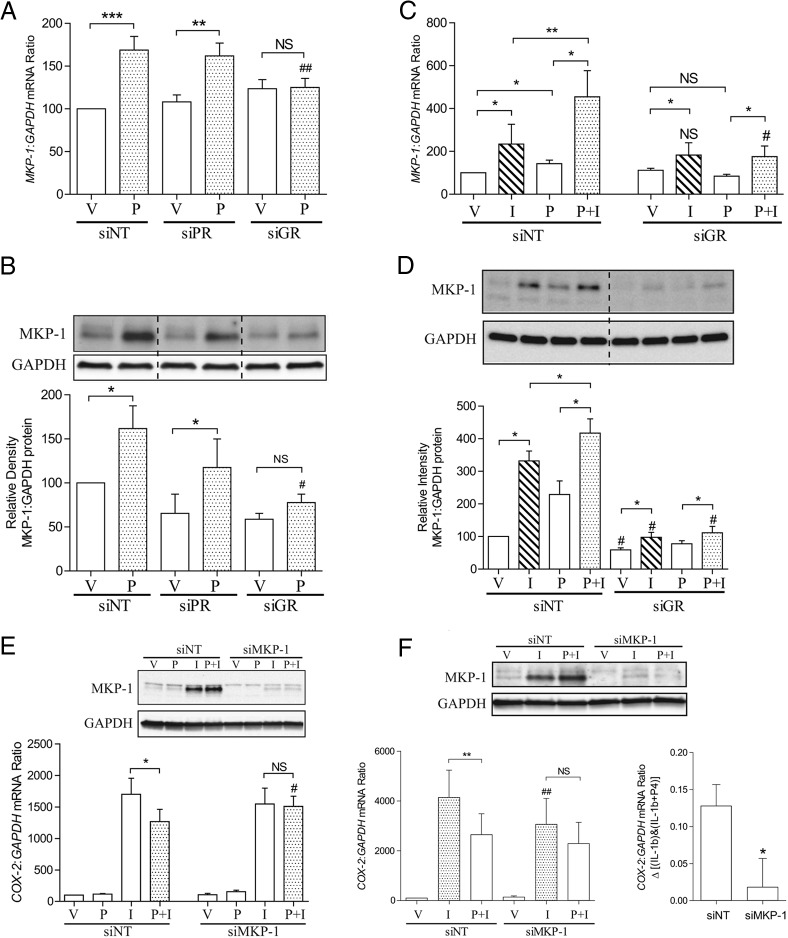
MKP-1 has been shown to be a P4-dependent gene in human endometrial stromal cells (37). Similarly, MKP-1 was also shown to be a P4- and IL-1β-responsive gene in human myometrial cells (Figure 1, A–D). The 2 stimuli together showed an additive effect compared with either P4 or IL-1β alone (Figure 1, C and D). The induction of MKP-1 by P4 was exclusively GR-dependent at both transcriptional and translational level (Figure 1, A and B) and GR knockdown completely inhibited the ability of P4 to further enhance MKP-1 expression (Figure 1, C and D), perhaps explaining why P4 fails to repress IL-1β-driven COX-2 expression upon GR knockdown or inhibition (18). Interestingly, lack of GR attenuated accumulation of MKP-1 protein in IL-1β-treated cultures (Figure 1D). Strikingly, knockdown of MKP-1 completely abolished the repressive effect of P4 at 1 hour (Figure 1E). This effect was maintained at 6 hours (Figure 1F), and MKP-1 knockdown also lowered the induction of COX-2 in cells treated with only IL-1β at this time point.
Figure 1.
P4-induced and GR-mediated MKP-1 activation represses IL-1β-driven COX-2 expression. Human myometrial cells were transfected with siRNA against PR, GR, and MKP-1 (siPR, siGR, and siMKP-1). Nontargeting siRNA (siNT) was used as control. After transfection, cells were incubated for 96 hours before being exposed to IL-1β (I), P4 (P) either alone or in combination; ethanol was used as the vehicle control (V) for 6 hours. A and B, mRNA and protein were extracted, and the MKP-1 levels were measured using qPCR and Western blotting, respectively. Data from qPCR are expressed as mean ± SEM and were analyzed using paired t test or repeated measures ANOVA followed by a Dunnett's multiple comparison post hoc test (bars with the same pattern). **, significant difference of P < .01; ***, P < .001; ##, Dunnett's P < .01 comparing with control; NS, no statistic significant difference. Data from densitometry are expressed as mean ± SEM and were analyzed using Wilcoxon matched pairs test or Friedman's test, with a Dunn's multiple comparison post hoc test (bars with the same pattern). *, significant difference of P < .05; #, Dunn's P < .05 comparing with control; NS, no statistic significant difference (n = 6–12). C and D, mRNA and protein were extracted, and the MKP-1 levels were measured using qPCR and Western blotting, respectively. Data are expressed as mean ± SEM and were analyzed using Wilcoxon matched pairs test. *, significant difference of P < .05; **, P < .01; #, P < .05 between samples exposed to the same stimuli but with or without GR knockdown; NS, no statistic significant difference between samples exposed to the same stimuli but with or without GR knockdown (n = 6). E and F, After siRNA transfection, cells were incubated for 96 hours before being exposed to different stimuli for 1 hour (E) and 6 hours (F). mRNA was then extracted, and the COX-2 mRNA levels were measured using qPCR. A representative Western blotting to show the effect of gene knockdown is at the top of the figures. Data are expressed as mean ± SEM and were analyzed using Wilcoxon matched pairs test (E) or paired t test (F). *, significant difference of P < .05; **, P < .01; #, P < .05; ##, P < .01 between samples exposed to the same stimuli but with or without MKP-1 knockdown; NS, no statistic significant difference (n = 6–12).
P4 represses AP-1-regulated genes
To further investigate the antiinflammatory actions of P4 in human myometrial cells, total RNA from 3 independent primary cell cultures treated for 6 hours with IL-1β and P4, either alone or in combination, was subjected to microarray analysis. The microarray data have been deposited in NCBI's Gene Expression Omnibus (Lei et al, 2015) and are accessible through GEO Series accession number GSE68171 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE68171). P4 altered the expression of 358 genes by more than 1.5-fold, including 302 up-regulated and 56 down-regulated, respectively. A total of 773 IL-1β-responsive genes (>1.5-fold) were also identified, 439 of which were induced and 334 repressed (Figure 2A). Both P4 and IL-1β had the same effect on 20 genes, of which 18 up-regulated and 2 down-regulated genes. As expected, MKP-1 was in this gene list and closely studied in this article. Two others genes, HSD11B1 and IRAK3, are also implicated in antiinflammatory responses (41, 42). Seventeen additional genes were regulated differently by these 2 stimuli. Among them, 14 genes were induced by P4 but repressed by IL-1β, and conversely, 3 genes were induced by IL-1β but repressed by P4 (Figure 2A). Next, we compared IL-1β-driven genes with the list of genes that we previously found to be increased upon p65 overexpression in primary myocytes maintained in similar culture conditions (43). Seven IL-1β-driven genes (>1.5-fold) (Supplemental Table 1) were found to be increased by p65 overexpression (>3-fold), but none of these were repressed by the addition of P4. These findings were validated by qPCR of the 4 chemokine genes (Figure 2B). The same genes were also reported by others to be NF-κB-regulated genes in different cell types (44–51). Conversely, of the 42 IL-1β-driven genes (Supplemental Table 2) that were repressed by P4, none were induced upon p65 overexpression (Supplemental Figure 2). IL-1β and CCL11 are potential exceptions to this rule, because they are only expressed with overexpression of p65 (43).
Figure 2.
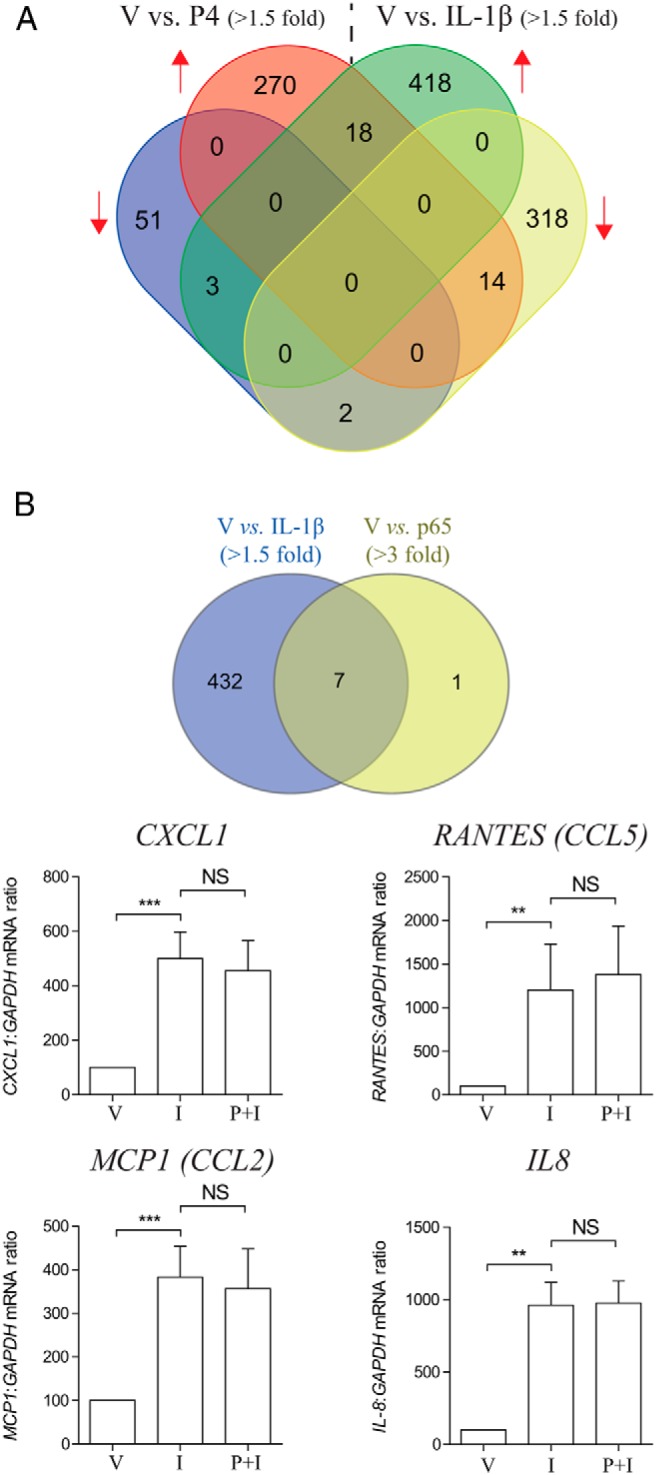
p65-regulated genes were not repressed by P4. A, Venn diagram shows numbers of P4 and IL-1β up- and down-regulated genes in human myometrial cells. B, Human myometrial cells were treated with IL-1β (I) with and without P4 (P) for 6 hours; ethanol was used as the vehicle control (V). mRNA was then extracted, and mRNA levels of 4 p65-responsive genes were measured using qPCR. Data are expressed as mean ± SEM and were analyzed using paired t test or Wilcoxon matched pairs test depending on the data normality. **, significant difference of P < .01; ***, P < .001; NS, no statistic significant difference (n = 9–12). A Venn diagram on the top showing genes that induced by both IL-1β and p65 overexpression.
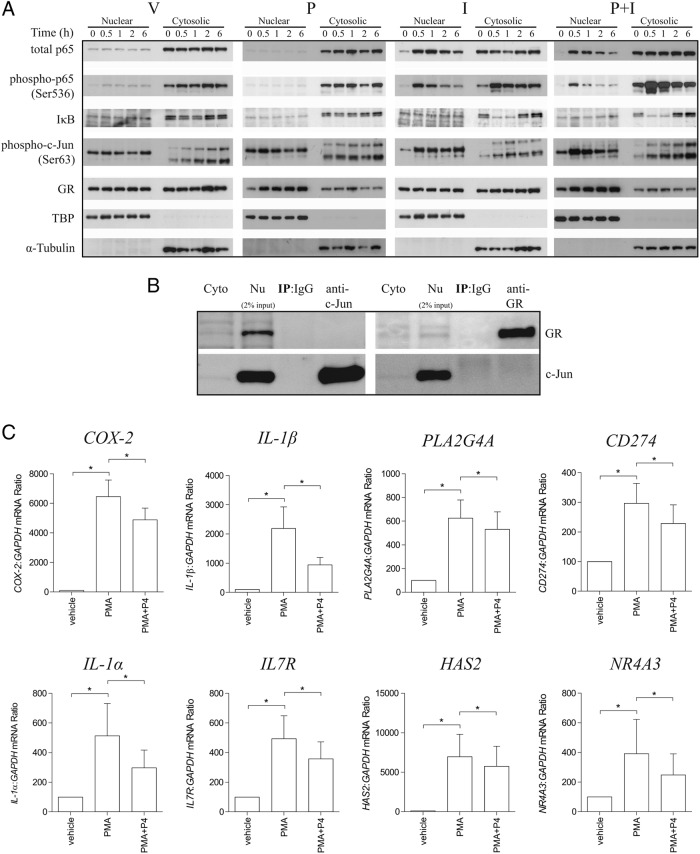
In order to investigate whether other proinflammatory transcription factors are involved in P4-mediated repression on IL-1β signaling, we extracted nuclear and cytosolic protein from myometrial cells after P4 and IL-1β treatment at different time points. P4 did not delay p65 nuclear translocation in IL-1β-treated cells (30 min after stimuli), and conversely, IL-1β did not impact on P4-induced nuclear translocation of GR (30 min after stimuli) (Figure 3A). Further, we found that the kinetics of IκB-α (upper band) in response to IL-1β did not change with P4 treatment (Figure 3A). P4 also did not affect nuclear accumulation of c-Jun in IL-1β-treated cells. However, in presence of P4, the level of p-c-Jun peaked at 30 minutes and gradually decreased back to the baseline, whereas in the absence of P4, no drop in p-c-Jun level was observed within 6 hours after IL-1β treatment (Figure 3A). co-IP experiments showed that these P4 effects could not be accounted for by physical interaction between the liganded GR and c-Jun (Figure 3B).
Figure 3.
AP-1-regulated genes are repressed by P4. A, Human myometrial cells were exposed to different stimuli, IL-1β (I) or P4 (P), either alone or in combination for 0 minutes, 30 minutes, 1 hour, 2 hours, and 6 hours. Cytosolic and nuclear extracts were prepared as described in Materials and Methods and analyzed by Western blotting using antibodies against total and phospho-p65, IκB-α, p-c-Jun, and GR. TATA-binding protein (TBP) and α-tubulin were used as the internal controls for nuclear and cytosolic fraction, respectively. B, co-IP experiment was carried out in human myometrial cells. Nuclear fractions were isolated after being exposed to IL-1β and P4 for 30 minutes. The lysates were then incubated with anti-GR or c-Jun antibody. For Western blotting of immunoprecipitates, anti-c-Jun and GR antibody was used, respectively. Input represented 2% of nuclear lysates used in the co-IP experiment, and rabbit IgG was used as negative control (n = 3). C, Human myometrial cells were treated with PMA and with and without P4 (P) for 6 hours; ethanol was used as the vehicle control (V). mRNA was then extracted, and mRNA levels of 8 genes were measured using qPCR. Data are expressed as mean ± SEM and were analyzed using Wilcoxon matched pairs test. *, significant difference of P < .05 (n = 6).
Because P4 affected nuclear translocation of p-c-Jun but not p-p65 in response to IL-1β, we then treated primary myocytes with PMA, a potent but nonselective AP-1 agonist, which also activates p65 (52, 53). Generally, as noted above, genes that were driven by IL-1β and p65 were not repressed by P4; however, genes that were driven by IL-1β and PMA but not p65 were repressed by P4 (Figure 3C). Intriguingly, we identified a subset of IL-1β-driven genes, including CXCL10, CXCL11, and CCL11, which were repressed by P4 but not responsive to PMA (Supplemental Figure 3), suggesting that different down-stream transcription factors are involved in mediating the effects of IL-1β and PMA in human myometrial cells.
The myometrial AP-1 system
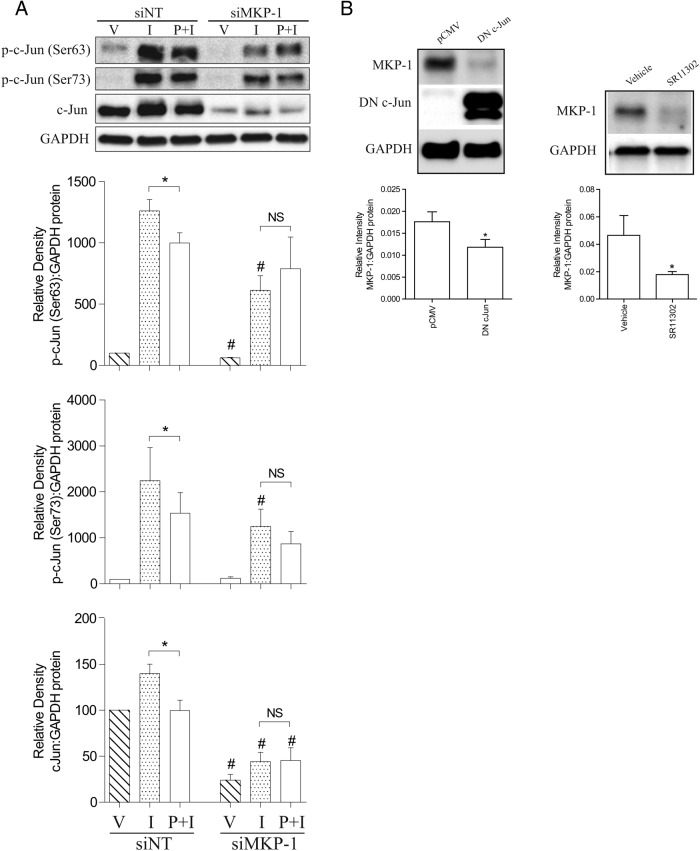
Based on the nuclear/cytosolic and microarray data, P4 appeared to preferentially repress the AP-1 system, perhaps via GR-mediated induction of MKP-1, which deactivates MAPKs and limits activation of down-stream transcription factors, such as AP-1. In line with this hypothesis, we observed that P4 failed to reduce IL-1β-induced c-Jun activation in the absence of MKP-1 (Figure 4A). Intriguingly, we found that both total and p-c-Jun levels were generally reduced upon MKP-1 knockdown (Figure 4A). Consistent with our data, others have reported that increased MKP-1 expression is associated with increased c-Jun levels (54, 55), suggesting a homeostatic feedback loop. To investigate this further, we treated myometrial cells with PMA and found that c-Jun levels were reduced for up to 72 hours (Figure 5A). In parallel, we found that the ability of IL-1β to drive COX-2 expression was also reduced and that the degree of reduction in the COX-2 response correlated with the decline in the p-c-Jun level (Figure 5, B and C). This pattern was also observed in other IL-1β-responsive genes (Supplemental Figure 4). As expected, MKP-1 expression was also induced by PMA at 6 hours and decreased in the same pattern as p-c-Jun (Figure 5B). The putative negative feedback loop is shown in Figure 5D.
Figure 4.
The myometrial AP-1 system. A, Human myometrial cells were transfected with siMKP-1, and nontargeting siRNA (siNT) was used as control. After transfection, cells were incubated for 96 hours before being exposed to IL-1β (I) and P4 (P) either alone or in combination for 6 hours; ethanol was used as the vehicle control (V). Protein was extracted, and the Western blotting was performed using antibody against total and p-c-Jun. A representative blot is shown at the top channel, with the densitometry below and on the side. Data are expressed as mean ± SEM and were analyzed using Wilcoxon matched pairs test. *, significant difference of P < .05; #, P < .05 between samples exposed to the same stimuli but with or without MKP-1 knockdown; NS, no statistic significant difference (n = 6–7). B, Human myometrial cells were transfected with DN c-Jun, and parental construct (pCMV) was used as control. After transfection, cells were incubated for 48 hours before protein was extracted (right panel). Human myometrial cells were plated and exposed to SR11302 (1μM) for 2 hours, ethanol was used as vehicle control. The Western blotting was performed using antibody against total, p-c-Jun, DN c-Jun, and MKP-1. A representative blot is shown at the top channel, with the densitometry below and on the side. Data are expressed as mean ± SEM and were analyzed using Wilcoxon matched pairs test. *, significant difference of P < .05; #, P < .05 between samples exposed to the same stimuli but with or without MKP-1 knockdown; NS, no statistic significant difference (n = 6–7).
Figure 5.
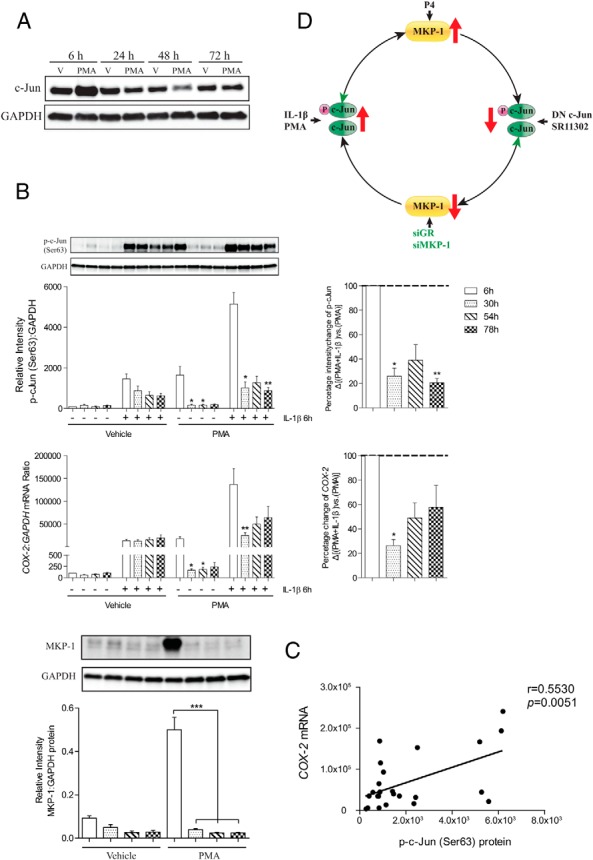
The negative feedback loop of AP-1. A, Human myometrial cells were exposed to PMA for 6, 24, 48, and 72 hours, ethanol was used as control (V). Whole-cell lysates were prepared and analyzed by Western blotting using antibodies against c-Jun, and GAPDH was used as loading control (n = 3). B, Human myometrial cells were treated with or without PMA for up to 3 days, ethanol was used as control (V). During this period, IL-1β was added at 0, 24, 48, and 72 hours and incubated for 6 hours. Protein was extracted from whole-cell lysate, and the Western blotting was performed using antibody against p-c-Jun and MKP-1. A representative blot is shown at the top of the figure, with the densitometry below. mRNA was also extracted, and the COX-2 mRNA levels were measured using qPCR. Data are expressed as mean ± SEM and were analyzed using Friedman's test, with a Dunn's multiple comparison post hoc test. *, significant difference of Dunn's P < .05; **, P < .01 comparing with 6-hour time point (n = 6). C, Correlation between the level of p-c-Jun and COX-2 mRNA expression in the presence of PMA and IL-1β. The data were derived from Figure 4B. D, This schematic diagram illustrates the negative feedback loop of AP-1 system. P4 induces MKP-1 expression to reduce AP-1 activity. This repression, in turn, leads to attenuated MKP-1 level. Reduction of MKP-1 (by MKP-1 and GR knockdown) results in a decline in both AP-1 activity and expression. When AP-1 activity is induced by IL-1β or PMA, increased MKP-1 expression is observed, and this limits AP-1 activity. However, a prolonged MKP-1 increase is associated with an induction of AP-1.
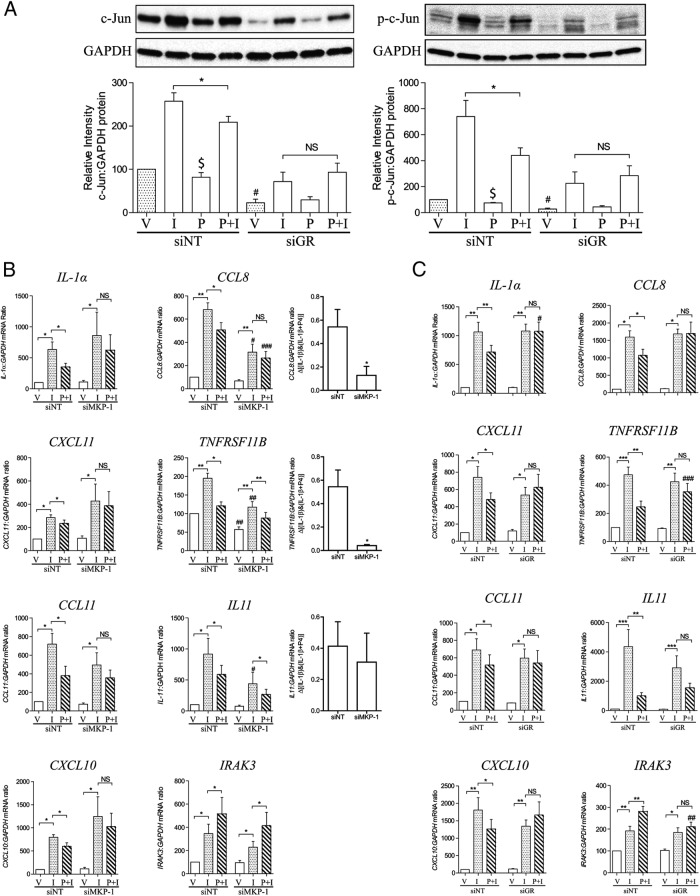
Interestingly, GR knockdown reduced the inhibitory effects of P4 on IL-1β-induced c-Jun activation and was associated with a reduction of c-Jun level (Figure 6A), but unlike knockdown of MKP-1, it did not affect IL-1β-driven COX-2 expression (18). This may be because the MKP-1 knockdown was more marked with the specific MKP-1 siRNA or it may suggest that the mechanisms involved in P4 repression and ability of IL-1β to drive gene expression are distinct, albeit that c-Jun is, at least in part, involved in both.
Figure 6.
The effect of GR and MKP-1 knockdown on IL-1β-responsive genes. Human myometrial cells were transfected with siRNA against GR and MKP-1 (siPR, siGR, and siMKP-1). Nontargeting siRNA (siNT) was used as control. After transfection, cells were incubated for 96 hours before being exposed to IL-1β (I) and P4 (P) either alone or in combination for 6 hours; ethanol was used as the vehicle control (V). A, Protein was extracted, and the Western blotting was performed using antibody against total and p-c-Jun. A representative blot is shown at the top of each figure, with the densitometry below. Data are expressed as mean ± SEM and were analyzed using Wilcoxon matched pairs test. *, significant difference of P < .05; $, P < .05 between vehicle vs P4; #, P < .05 between siNT vs siGR; NS, no statistic significant difference (n = 6). B and C, mRNA was extracted, and mRNA levels of 8 genes were measured using qPCR. Data are expressed as mean ± SEM and were analyzed using paired t test or Wilcoxon matched pairs test depending on the data normality. *, significant difference of P < .05; **, P < .01; NS, no statistic significant difference; #, P < .05; ##, P < .01; ###, P < .001 between samples exposed to the same stimuli but with or without MKP-1 or GR knockdown (n = 6).
The depletion of MKP-1 either blocked or attenuated the P4 repression of IL-1β-driven IL-1α, CXCL11, CCL11, CXCL10, CCL8, and TNFRSF11B and had no effect in the cases of IL11 and IRAK3 (for IRAK3, P4 increased the IL-1β-induced expression). The response to IL-1β was reduced in CCL8, TNFRSF11B, and IL11. GR knockdown, however, suppressed the ability of P4 to repress IL-1β-induced gene expression even in the cases where MKP-1 had no effect (IL11 and IRAK3), but it did not reduce the ability of IL-1β to drive gene expression itself (Figure 6C). These data suggest that MKP-1 is not the only mechanism mediating GR/P4 action.
Taken together, these data show that P4 acts via GR-induced MKP-1 activation to reduce IL-1β-induced-AP-1 phosphorylation and COX-2 expression. There appears to be an AP-1 negative feedback system in the myometrium, which is involves MKP-1 to limit the extent of an AP-1-mediated inflammatory stimulus (Figure 7).
Figure 7.
A schematic diagram illustrating the mechanism of P4 to repress IL-1β-induced COX-2 expression and the AP-1 system in human myometrial cells. P4 reduces IL-1β-driven COX-2 expression via GR-induced MKP-1 activation, and AP-1 has an active negative feedback system, which limits the extent of an AP-1-mediated inflammatory stimulus.
Discussion
In this study, we examined the mechanisms involved in the P4 repression of IL-1β-driven COX-2 expression in human myometrial cells. Our data indicate that P4 repression is mediated in part by an enhanced MKP-1 activity, which reduces c-Jun phosphorylation.
P4 acts via GR to increase MKP-1 and reduce IL-1β-driven COX-2 mRNA expression
Previously, we showed that P4 acted via GR to repress IL-1β-driven COX-2 expression (18). To address the mechanism, we confirmed that P4 was able to induce GR nuclear translocation in our cell model and excluded a role for the involvement of HDAC and GR-interacting protein 1 in this process (data not shown). Numerous studies have demonstrated that proinflammatory cytokines, such as IL-1β activate MAPK subfamilies to increase AP-1 phosphorylation (56). Simultaneously, IL-1β activates MKP-1, which in turn limits the IL-1β-induced MAPK activation by dephosphorylating MAPKs, extracellular-signal-regulated kinase, c-Jun N-terminal kinase, and/or p38 with variable potency in different cell types (57–59). Our findings suggest that MKP-1 may mediate the antiinflammatory effect of several steroid hormones. Indeed, glucocorticoids have been shown to act via MKP-1 to repress AP-1 activity in a variety of cell types (26, 28). In our cell model, we confirmed that P4 was able to increase MKP-1 expression via GR. Although the effect of IL-1β on MKP-1 mRNA expression was not affected by GR depletion, a reduction in the basal level of MKP-1 protein was observed. Similarly, GR knockdown did not affect c-Jun mRNA levels, but its protein levels were lower (Figure 4E and Supplemental Figure 5). These changes in protein levels may be due to accelerated protein turnover (60) or the effect of microRNAs (61).
The existence of an AP-1 negative feedback loop
The knockdown of MKP-1 reduced the ability of P4 to repress IL-1β-driven COX-2 expression. It was noted that the effect of IL-1β was attenuated at 6 hours. This is probably due to the existence of a negative feedback loop, whereby activation of AP-1 is followed by a transient repression, manifested in our studies by a reduced c-Jun protein levels. Two studies showed that increasing MKP-1 levels was associated with an increase in c-Jun levels, consistent with the existence of a feedback loop (54, 55). Indeed, when we knocked MKP-1 down, total and p-c-Jun levels were reduced. When DN c-Jun was overexpressed or the cells treated with AP-1 inhibitor (SR11302), a reduction on MKP-1 was consistently observed. Further, treatment of myometrial cells with the AP-1 activator, PMA, increased c-Jun phosphorylation and MKP-1 level at 6 hours, and this was followed by a reduction in both p-c-Jun and MKP-1 for up to 72 hours and a reduction in the ability of IL-1β to drive COX-2 expression.
P4 and NF-κB
Our data show that although IL-1β activates both NF-κB and AP-1, it seems that AP-1 is the main target of P4 repression. The nuclear-cytoplasmic fractionation studies further demonstrated that P4-activated GR did not delay p65 nuclear translocation. Another mechanism suggested to account for the reduction in p65 activity in response to P4 involves induction of IκB, a protein that blocks NF-κB nuclear translocation (20). However, we found that in our primary myometrial cells, P4 treatment did not increase IκB levels or have any effect on IL-1β-induced IκB degradation. The importance of AP-1 in inflammation-induced labor was further emphasized in our animal study, where AP-1 activation, but not NF-κB, was sufficient to drive inflammatory pathways involved in LPS-induced PTL (62). However, no physical interaction was found between c-Jun and liganded GR, which suggests that P4 represses c-Jun activity via reducing its expression and/or phosphorylation which results the temporal reduction in the IL-1β-induced nuclear translocation of c-Jun by P4 as we observed in this study.
P4 represses other IL-1β-driven genes via an MKP-1-independent mechanism
To assess whether P4 repression of the AP-1 system also modulates the expression of other proinflammatory genes, we investigated the effect of MKP-1 and GR knockdown on the ability of IL-1β to increase gene expression (we chose the most responsive genes to IL-1β that were repressed or enhanced by P4) and the ability of P4 to modulate IL-1β induced gene expression. In terms of the ability of IL-1β to increase gene expression, MKP-1 knockdown reduced the IL-1β-induced increase in 3 genes (CCL8, TNFRSF11B, and IL11) in a similar manner to the COX-2 response. GR knockdown had no effect on IL-1β-induced gene expression, again similar to its effect on the COX-2 response. This may simply be the result of a more effective knockdown of MKP-1 by its specific siRNA (siMKP-1 and siGR achieved about 30% and 60%, respectively, on the reduction of MKP-1) and therefore greater effect on the AP-1 system. In terms of the ability of P4 to modulate the IL-1β-driven increase in gene expression, MKP-1 knockdown inhibited this completely in 5 cases and partially in a sixth (TNFRSF11B). In 2 cases, IL11 and IRAK3, the P4 response was unaffected. GR knockdown, in contrast, inhibited the ability of P4 to modulate the IL-1β-induced increase in gene expression in all cases. These data suggest that P4 modifies the IL-1β-induced increase in gene expression through other GR dependent mechanisms, not only MKP-1. If P4 acted only via MKP-1, then the P4 effect would be consistently reversed by siMKP-1 (which is more effective in reducing MKP-1 levels than siGR). As it is, the effect of P4 is consistently reversed by siGR and not siMPK-1. Possible alternatives include a physical interaction between GR and other transcription factors, HDACs and alterations of cochaperone proteins.
In conclusion, we used COX-2 as a target gene to investigate the antiinflammatory action of P4 in human myometrial cells. Our data show that P4 reduces IL-1β-driven AP-1 phosphorylation via GR-induced MKP-1 activation and that myometrial AP-1 has an active negative feedback system, which limits the extent of an AP-1-mediated inflammatory stimulus. Overall, our findings indicate an important role for the P4/GR signaling pathway in the control of human parturition mediated through regulation of the AP-1 system.
Acknowledgments
This work was supported by Action Medical Research, Beneficentia Stiftung, and the Chelsea and Westminster Health Charity, Borne. This work was also supported by the National Institute for Health Research (NIHR) Biomedical Research Centre. J.J.B. is supported by the Biomedical Research Unit, a joint initiative between University Hospitals Coventry and Warwickshire and the University of Warwick.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AP
- activator protein
- co-IP
- coimmunoprecipitation
- COX-2
- cyclooxygenase-2
- DN c-Jun
- dominant negative c-Jun
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- GR
- glucocorticoid receptor
- HDAC
- histone deacetylase
- IκB-α
- nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- MKP
- MAPK phosphatase
- NF-κB
- nuclear factor κB
- p65
- nuclear factor NF-kappa-B p65 subunit
- P4
- progesterone
- p-c-Jun
- phospho-c-Jun
- PMA
- phorbol 12-myrisatate 13-acetate
- PR
- P4 receptor
- qPCR
- quantitative PCR
- siRNA
- small interfering RNA.
References
- 1. Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–1123. [DOI] [PubMed] [Google Scholar]
- 2. Cox SM, Casey ML, MacDonald PC. Accumulation of interleukin-1β and interleukin-6 in amniotic fluid: a sequela of labour at term and preterm. Hum Reprod Update. 1997;3:517–527. [DOI] [PubMed] [Google Scholar]
- 3. Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734–741. [DOI] [PubMed] [Google Scholar]
- 4. Chow L, Lye SJ. Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am J Obstet Gynecol. 1994;170:788–795. [DOI] [PubMed] [Google Scholar]
- 5. Soloff MS, Cook DL, Jr, Jeng YJ, Anderson GD. In situ analysis of interleukin-1-induced transcription of cox-2 and il-8 in cultured human myometrial cells. Endocrinology. 2004;145:1248–1254. [DOI] [PubMed] [Google Scholar]
- 6. Gibb W. The role of prostaglandins in human parturition. Ann Med. 1998;30:235–241. [DOI] [PubMed] [Google Scholar]
- 7. Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition–a review. Placenta. 2003;24(suppl A):S33–S46. [DOI] [PubMed] [Google Scholar]
- 8. Slater DM, Zervou S, Thornton S. Prostaglandins and prostanoid receptors in human pregnancy and parturition. J Soc Gynecol Investig. 2002;9:118–124. [PubMed] [Google Scholar]
- 9. Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. Human labour is associated with nuclear factor-κB activity which mediates cyclo-oxygenase-2 expression and is involved with the 'functional progesterone withdrawal'. Mol Hum Reprod. 2001;7:581–586. [DOI] [PubMed] [Google Scholar]
- 10. Johnson RF, Mitchell CM, Giles WB, Walters WA, Zakar T. The in vivo control of prostaglandin H synthase-2 messenger ribonucleic acid expression in the human amnion at parturition. J Clin Endocrinol Metab. 2002;87:2816–2823. [DOI] [PubMed] [Google Scholar]
- 11. Dixon DA, Kaplan CD, McIntyre TM, Zimmerman GA, Prescott SM. Post-transcriptional control of cyclooxygenase-2 gene expression. The role of the 3′-untranslated region. J Biol Chem. 2000;275:11750–11757. [DOI] [PubMed] [Google Scholar]
- 12. Tamura M, Sebastian S, Yang S, Gurates B, Fang Z, Bulun SE. Interleukin-1β elevates cyclooxygenase-2 protein level and enzyme activity via increasing its mRNA stability in human endometrial stromal cells: an effect mediated by extracellularly regulated kinases 1 and 2. J Clin Endocrinol Metab. 2002;87:3263–3273. [DOI] [PubMed] [Google Scholar]
- 13. Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18:502–519. [DOI] [PubMed] [Google Scholar]
- 14. Graham JD, Clarke CL. Expression and transcriptional activity of progesterone receptor A and progesterone receptor B in mammalian cells. Breast Cancer Res. 2002;4:187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li X, Lonard DM, O'Malley BW. A contemporary understanding of progesterone receptor function. Mech Ageing Dev. 2004;125:669–678. [DOI] [PubMed] [Google Scholar]
- 16. Mote PA, Graham JD, Clarke CL. Progesterone receptor isoforms in normal and malignant breast. Ernst Schering Found Symp Proc. 2007;77–107. [PubMed] [Google Scholar]
- 17. Loudon JA, Elliott CL, Hills F, Bennett PR. Progesterone represses interleukin-8 and cyclo-oxygenase-2 in human lower segment fibroblast cells and amnion epithelial cells. Biol Reprod. 2003;69:331–337. [DOI] [PubMed] [Google Scholar]
- 18. Lei K, Chen L, Georgiou EX, et al. Progesterone acts via the nuclear glucocorticoid receptor to suppress IL-1β-induced COX-2 expression in human term myometrial cells. PLoS One. 2012;7:e50167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009;23:947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-κB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20:2724–2733. [DOI] [PubMed] [Google Scholar]
- 21. Karalis K, Goodwin G, Majzoub JA. Cortisol blockade of progesterone: a possible molecular mechanism involved in the initiation of human labor. Nat Med. 1996;2:556–560. [DOI] [PubMed] [Google Scholar]
- 22. Mitchell C, Johnson R, Bisits A, Hirst J, Zakar T. PTGS2 (prostaglandin endoperoxide synthase-2) expression in term human amnion in vivo involves rapid mRNA turnover, polymerase-II 5′-pausing, and glucocorticoid transrepression. Endocrinology. 2011;152:2113–2122. [DOI] [PubMed] [Google Scholar]
- 23. Lee Y, Sooranna SR, Terzidou V, et al. Interactions between inflammatory signals and the progesterone receptor in regulating gene expression in pregnant human uterine myocytes. J Cell Mol Med. 2012;16:2487–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Widén C, Gustafsson JA, Wikström AC. Cytosolic glucocorticoid receptor interaction with nuclear factor-κ B proteins in rat liver cells. Biochem J. 2003;373:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1β-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol. 2000;20:6891–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cho IJ, Kim SG. A novel mitogen-activated protein kinase phosphatase-1 and glucocorticoid receptor (GR) interacting protein-1-dependent combinatorial mechanism of gene transrepression by GR. Mol Endocrinol. 2009;23:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bhattacharyya S, Brown DE, Brewer JA, Vogt SK, Muglia LJ. Macrophage glucocorticoid receptors regulate Toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood. 2007;109:4313–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu W, Pew T, Zou M, Pang D, Conzen SD. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem. 2005;280:4117–4124. [DOI] [PubMed] [Google Scholar]
- 29. King EM, Holden NS, Gong W, Rider CF, Newton R. Inhibition of NF-κB-dependent transcription by MKP-1: transcriptional repression by glucocorticoids occurring via p38 MAPK. J Biol Chem. 2009;284:26803–26815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shipp LE, Lee JV, Yu CY, et al. Transcriptional regulation of human dual specificity protein phosphatase 1 (DUSP1) gene by glucocorticoids. PLoS One. 2010;5:e13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abraham SM, Lawrence T, Kleiman A, et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203:1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen CC, Hardy DB, Mendelson CR. Progesterone receptor inhibits proliferation of human breast cancer cells via induction of MAPK phosphatase 1 (MKP-1/DUSP1). J Biol Chem. 2011;286:43091–43102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176:1899–1907. [DOI] [PubMed] [Google Scholar]
- 34. Charles CH, Abler AS, Lau LF. cDNA sequence of a growth factor-inducible immediate early gene and characterization of its encoded protein. Oncogene. 1992;7:187–190. [PubMed] [Google Scholar]
- 35. Kuwano Y, Kim HH, Abdelmohsen K, et al. MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol Cell Biol. 2008;28:4562–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal. 2004;16:769–779. [DOI] [PubMed] [Google Scholar]
- 37. Leitao B, Jones MC, Fusi L, et al. Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. FASEB J. 2010;24:1541–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Newton R, Holden NS. Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Mol Pharmacol. 2007;72:799–809. [DOI] [PubMed] [Google Scholar]
- 39. Clark AR, Martins JR, Tchen CR. Role of dual specificity phosphatases in biological responses to glucocorticoids. J Biol Chem. 2008;283:25765–25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mosher AA, Rainey KJ, Bolstad SS, et al. Development and validation of primary human myometrial cell culture models to study pregnancy and labour. BMC Pregnancy Childbirth. 2013;13(suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Doig CL, Bashir J, Zielinska AE, Cooper MS, Stewart PM, Lavery GG. TNFα-mediated Hsd11b1 binding of NF-κB p65 is associated with suppression of 11β-HSD1 in muscle. J Endocrinol. 2014;220:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hulsmans M, Geeraert B, De Keyzer D, et al. Interleukin-1 receptor-associated kinase-3 is a key inhibitor of inflammation in obesity and metabolic syndrome. PLoS One. 2012;7:e30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khanjani S, Kandola MK, Lindstrom TM, et al. NF-κB regulates a cassette of immune/inflammatory genes in human pregnant myometrium at term. J Cell Mol Med. 2011;15:809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Burke SJ, Lu D, Sparer TE, et al. NF-κB and STAT1 control CXCL1 and CXCL2 gene transcription. Am J Physiol Endocrinol Metab. 2014;306:E131–E149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Amiri KI, Ha HC, Smulson ME, Richmond A. Differential regulation of CXC ligand 1 transcription in melanoma cell lines by poly(ADP-ribose) polymerase-1. Oncogene. 2006;25:7714–7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deng X, Xu M, Yuan C, et al. Transcriptional regulation of increased CCL2 expression in pulmonary fibrosis involves nuclear factor-κB and activator protein-1. Int J Biochem Cell Biol. 2013;45:1366–1376. [DOI] [PubMed] [Google Scholar]
- 47. Happel C, Kutzler M, Rogers TJ. Opioid-induced chemokine expression requires NF-κB activity: the role of PKCζ. J Leukoc Biol. 2011;89:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee H, Deng J, Xin H, Liu Y, Pardoll D, Yu H. A requirement of STAT3 DNA binding precludes Th-1 immunostimulatory gene expression by NF-κB in tumors. Cancer Res. 2011;71:3772–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chou SY, Weng JY, Lai HL, et al. Expanded-polyglutamine huntingtin protein suppresses the secretion and production of a chemokine (CCL5/RANTES) by astrocytes. J Neurosci. 2008;28:3277–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- 51. Huang SM, McCance DJ. Down regulation of the interleukin-8 promoter by human papillomavirus type 16 E6 and E7 through effects on CREB binding protein/p300 and P/CAF. J Virol. 2002;76:8710–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schindler U, Baichwal VR. Three NF-κB binding sites in the human E-selectin gene required for maximal tumor necrosis factor α-induced expression. Mol Cell Biol. 1994;14:5820–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chang MS, Chen BC, Yu MT, Sheu JR, Chen TF, Lin CH. Phorbol 12-myristate 13-acetate upregulates cyclooxygenase-2 expression in human pulmonary epithelial cells via Ras, Raf-1, ERK, and NF-κB, but not p38 MAPK, pathways. Cell Signal. 2005;17:299–310. [DOI] [PubMed] [Google Scholar]
- 54. Lornejad-Schafer M, Schafer C, Richter L, Grune T, Haussinger D, Schliess F. Osmotic regulation of MG-132-induced MAP-kinase phosphatase MKP-1 expression in H4IIE rat hepatoma cells. Cell Physiol Biochem. 2005;16:193–206. [DOI] [PubMed] [Google Scholar]
- 55. Staber PB, Linkesch W, Zauner D, et al. Common alterations in gene expression and increased proliferation in recurrent acute myeloid leukemia. Oncogene. 2004;23:894–904. [DOI] [PubMed] [Google Scholar]
- 56. Clerk A, Harrison JG, Long CS, Sugden PH. Pro-inflammatory cytokines stimulate mitogen-activated protein kinase subfamilies, increase phosphorylation of c-Jun and ATF2 and upregulate c-Jun protein in neonatal rat ventricular myocytes. J Mol Cell Cardiol. 1999;31:2087–2099. [DOI] [PubMed] [Google Scholar]
- 57. Newton R, King EM, Gong W, et al. Glucocorticoids inhibit IL-1β-induced GM-CSF expression at multiple levels: roles for the ERK pathway and repression by MKP-1. Biochem J. 2010;427:113–124. [DOI] [PubMed] [Google Scholar]
- 58. Toh ML, Yang Y, Leech M, Santos L, Morand EF. Expression of mitogen-activated protein kinase phosphatase 1, a negative regulator of the mitogen-activated protein kinases, in rheumatoid arthritis: up-regulation by interleukin-1β and glucocorticoids. Arthritis Rheum. 2004;50:3118–3128. [DOI] [PubMed] [Google Scholar]
- 59. Jang BC, Lim KJ, Suh MH, Park JG, Suh SI. Dexamethasone suppresses interleukin-1β-induced human β-defensin 2 mRNA expression: involvement of p38 MAPK, JNK, MKP-1, and NF-κB transcriptional factor in A549 cells. FEMS Immunol Med Microbiol. 2007;51:171–184. [DOI] [PubMed] [Google Scholar]
- 60. Claus R, Raab S, Dehnhard M. Glucocorticoid receptors in the pig intestinal tract and muscle tissue. Zentralbl Veterinarmed A. 1996;43:553–560. [DOI] [PubMed] [Google Scholar]
- 61. Yang Z, Wang L. Regulation of microRNA expression and function by nuclear receptor signaling. Cell Biosci. 2011;1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. MacIntyre DA, Lee YS, Migale R, et al. Activator protein 1 is a key terminal mediator of inflammation-induced preterm labor in mice. FASEB J. 2014;28:2358–2368. [DOI] [PubMed] [Google Scholar]