Abstract
Background: Food-based dietary patterns emphasizing plant protein that were evaluated in the Dietary Approaches to Stop Hypertension (DASH) and OmniHeart trials are recommended for the treatment of metabolic syndrome (MetS). However, the contribution of plant protein to total protein in these diets is proportionally less than that of animal protein.
Objective: This study compared 3 diets varying in type (animal compared with plant) and amount of protein on MetS criteria.
Design: Sixty-two overweight adults with MetS consumed a healthy American diet for 2 wk before being randomly allocated to either a modified DASH diet rich in plant protein (18% protein, two-thirds plant sources, n = 9 males, 12 females), a modified DASH diet rich in animal protein (Beef in an Optimal Lean Diet: 18.4% protein, two-thirds animal sources, n = 9 males, 11 females), or a moderate-protein diet (Beef in an Optimal Lean Diet Plus Protein: 27% protein, two-thirds animal sources, n = 10 males, 11 females). Diets were compared across 3 phases of energy balance: 5 wk of controlled (all foods provided) weight maintenance (WM), 6 wk of controlled weight loss (minimum 500-kcal/d deficit) including exercise (WL), and 12 wk of prescribed, free-living weight loss (FL). The primary endpoint was change in MetS criteria.
Results: All groups achieved ∼5% weight loss at the end of the WL phase and maintained it through FL, with no between-diet differences (WM compared with WL, FL, P < 0.0001; between diets, P = NS). All MetS criteria decreased independent of diet composition (main effect of phase, P < 0.01; between diets, P = NS). After WM, all groups had a MetS prevalence of 80–90% [healthy American diet (HAD) compared with WM, P = NS], which decreased to 50–60% after WL and was maintained through FL (HAD, WM vs WL, FL, P < 0.01).
Conclusions: Weight loss was the primary modifier of MetS resolution in our study population regardless of protein source or amount. Our findings demonstrate that heart-healthy weight-loss dietary patterns that emphasize either animal or plant protein improve MetS criteria similarly. This study was registered at clinicaltrials.gov as NCT00937638.
Keywords: dietary protein, metabolic syndrome, lean beef, weight loss, body composition
INTRODUCTION
Metabolic syndrome (MetS)6 is characterized by a clustering of cardiovascular disease (CVD) risk factors, and as the number and severity of these increase, so does the risk of CVD, type II diabetes, and all-cause mortality (1, 2). Treatment of MetS includes weight loss to reduce abdominal obesity, a healthy dietary pattern, and regular physical activity (3). A weight loss of 5–10% is associated with substantial improvements in blood glucose, triglycerides, and blood pressure (BP), as well as LDL cholesterol and HDL cholesterol (4). Because weight loss and especially maintenance of weight loss are challenging for many individuals, a dietary pattern that improves MetS criteria independent of weight loss could beneficially affect risk of MetS comorbidities. A Dietary Approaches to Stop Hypertension (DASH) dietary pattern is recommended for LDL cholesterol and BP lowering (5). Variations in the macronutrient profile of the DASH diet that emanate from the OmniHeart trial are recommended for the treatment of MetS criteria (6).
The DASH dietary pattern decreased BP (7) and LDL cholesterol (8, 9) compared with a control diet (which was lower in total protein and higher in total fat and SFAs). The OmniHeart trial demonstrated that diets low in SFAs and higher in unsaturated fat or protein improved BP and beneficially affected HDL cholesterol and triglycerides, which typically are adversely affected by a lower fat/higher carbohydrate diet (6). Both the DASH (7–9) and OmniHeart trials (6) support cardiovascular benefits of a plant-based diet, including an emphasis on plant protein (5). Of note, the DASH dietary pattern includes substantial quantities of animal protein from reduced-fat dairy products, seafood, and white meats, and although increased, the contribution of plant protein to overall protein is proportionally less (10). Moreover, the moderate-protein diet used in the OmniHeart trial consisted of >50% animal protein (6, 11). We have shown recently that when SFAs remain low (<7% of total calories), average and moderate-protein diets containing lean beef [Beef in an Optimal Lean Diet (BOLD)] also can be included in a heart-healthy dietary pattern that lowers LDL cholesterol and BP (12, 13).
The purpose of this study was to compare 3 diets controlled for SFAs with varying amounts of protein from plant and animal (predominantly lean beef) sources on MetS criteria (primary endpoint): a modified-DASH (M-DASH) diet rich in plant protein (18% protein, two-thirds plant sources), an M-DASH diet rich in animal protein (BOLD: 18.4% protein, two-thirds animal sources), and a moderate-protein diet [Beef in an Optimal Lean Diet Plus Protein (BOLD+): 27% protein, two-thirds animal sources]. These diets were compared at 3 phases of energy balance: controlled weight maintenance (WM), controlled weight loss with an exercise component (WL), and prescribed free-living weight loss (FL). Secondary outcomes were endothelial function, LDL cholesterol, and adiposity.
METHODS
Participants
Overweight and obese [BMI (in kg/m2): 27–42] men and women 30–60 y of age with MetS were recruited. MetS was defined according to National Cholesterol Education Program Adult Treatment Panel III criteria (14) with participants having ≥3 of the following criteria: abdominal obesity [waist circumference (WC) >102 cm (40 inches) in men and >88 cm (35 inches) in women], elevated blood glucose [>100 mg/dL (5.6 mmol/L)], elevated triglycerides [>150 mg/dL (1.7 mmol/L)], low HDL cholesterol [<40 mg/dL (1.03 mmol/L) in men and <50 mg/dL (1.29 mmol/L) in women], and hypertension [systolic blood pressure (SBP) >130 mm Hg and/or diastolic blood pressure (DBP) >85 mm Hg]. Pharmacologic treatment of any of these criteria, except for abdominal obesity, was considered a MetS criterion.
Participants taking a single oral BP-lowering drug were eligible for the study as long as their screening BP was <160/100 mm Hg. BP medication was allowed throughout the study. Participants taking a single cholesterol or glucose medication were eligible for screening, but, in consultation with their primary care physician, they discontinued use of these medications before beginning the study.
All participants were nonsmokers and free of established CVD, stroke, diabetes, or liver, kidney, or autoimmune disease. Exclusion criteria included continued use of glucose and cholesterol/lipid-lowering medication or supplements (psyllium, fish oil, soy lecithin, and phytoestrogens), pregnancy or lactation, weight loss of ≥10% of body weight within the 6 mo before enrolling in the study, high alcohol consumption (≥14 drinks/wk), participation in regular physical activity (>1 formal session/wk) with the intention of losing weight or increasing fitness, inability to complete the exercise testing protocol as determined by the clinic physician, orthopedic or other health issues that precluded treadmill exercise or involvement in the pedometer-based walking program, vegetarianism, and lactose intolerance. All participants were informed that 2 of the diets contained lean beef.
The institutional review board at The Pennsylvania State University approved the experimental protocol, and all participants provided written informed consent. This study was registered at clinicaltrials.gov as NCT00937638.
Study design
This was a 6-mo, randomized, parallel-arm, open-label, controlled-feeding trial comparing the effects of different sources and amounts of dietary protein on MetS prevalence. All participants completed a 2-wk controlled-feeding (all food and drinks were prepared by a Metabolic Kitchen and provided to participants) healthy American diet (HAD) run-in where weight was held stable. Energy requirements for this phase were initially estimated with the Harris-Benedict equation (15) multiplied by an activity factor of 1.5 for men and 1.3 for women and modified as required based on changes in body weight as determined by daily weigh-ins at the Metabolic Diet Study Center. The end of the HAD phase was considered the baseline. Participants were then blocked in groups of 3 by BMI, sex, and age and randomly allocated by computer-generated assignment to one of 3 experimental treatments (diets): M-DASH, BOLD, and BOLD+. These diets were compared at different levels of energy balance. Participants first consumed one experimental diet for 5 wk at energy equilibrium (controlled-feeding weight maintenance; WM). If desired, a short compliance break (1 wk) was taken before completing a 6-wk controlled-feeding weight-loss phase (WL) where an energy deficit was induced by calorie reduction (minimum 500-kcal/d deficit through dietary changes) and increased physical activity via a walking program. Participants consumed the same experimental diets during the WM and WL phases. Participants then completed a 12-wk free-living weight-loss phase (FL), during which time they were asked to continue their assigned hypocaloric diets and physical activity, but the provision of food and drinks was discontinued. Three 90-min one-on-one nutrition education sessions with a registered dietitian were conducted for all participants during the controlled-feeding weight-loss phase in preparation for the free-living phase. Participants were educated on the unique features of their assigned diets—namely, the incorporation of increased vegetable or animal protein from specific food sources. They also were educated about the principles of healthy eating, recommended portion sizes for food groups (using food models), and provided with practical guidelines to assist them in selecting foods that were appropriate for their diet. Strategies to achieve their target calorie amount—for example, the contribution of discretionary foods and beverages (including alcohol) to energy intake—also were discussed. To assist participants with adhering to both their calorie amount and their experimental diet, they were provided with the menus and recipes used by the Metabolic Kitchen during their final week of controlled weight loss.
Participants completed a series of clinical and physical assessments on 2 consecutive days at baseline (end of HAD) and at the end of the WM, WL, and FL phases. At each visit, participants arrived in the fasting state (12 h water only, 48 h no alcohol, and 12 h without vigorous exercise) at the Clinical Research Center where body weight, WC, vascular function (by EndoPAT; Itamar Medical), BP, and blood samples (∼30 mL on each day) were obtained. Height was measured at baseline (after HAD). Body composition was measured by dual-energy X-ray absorptiometry (DXA) at baseline (end of HAD) and at the end of the WL and FL phases. Two single-day visits were completed during weeks 4 and 8 of the 12-wk FL phase. The purpose of these visits was to monitor weight (not included in the analyses) and address any concerns that subjects had about their diet and physical activity.
Participants could not be blinded to their dietary assignment. An unblinded study coordinator blocked participants and conducted all data analyses. Outcome assessors (i.e., nurses and technicians) were blinded.
Diets
The nutrient composition of the experimental diets is shown in Table 1. The 3 experimental diets (M-DASH, BOLD, and BOLD+) were matched for total fat, SFAs, MUFAs, PUFAs, cholesterol (<300 mg/d), sodium, potassium, calcium, and magnesium. The M-DASH diet was higher in fiber (55 compared with 38 g), a consequence of incorporating more plant protein from whole-food sources such as pulses (beans) and whole grains, which also were rich in fiber. The HAD was higher in total fat, SFAs, cholesterol, and sodium and was lower in total fiber, potassium, calcium, and magnesium. The BOLD and M-DASH diets were matched for macronutrient composition but differed in the relative contribution of plant and animal protein to total protein. Animal protein (from lean beef, chicken, tuna, eggs, and dairy) contributed two-thirds of the total protein on the BOLD diet, whereas two-thirds of the total protein was from plant sources (pulses, grains, soy, nuts, and seeds were substituted for lean beef protein) on the M-DASH diet. To isolate the effect of removing red meat and SFAs rather than the substitution of functional, cholesterol-lowering foods on LDL cholesterol, we limited soy protein to <10% of total protein, and psyllium and margarines containing sterols/stanols were not included. The BOLD+ diet was higher in protein (27% of total energy) compared with HAD (17.4%), M-DASH (18%), and BOLD (18.4%) diets and thus lower in carbohydrate (45% compared with 49–55%). Animal protein contributed two-thirds of the total protein on the BOLD+ diet.
TABLE 1.
Nutritional composition of experimental diets1
| HAD | M-DASH | BOLD | BOLD+ | |
| Energy, kcal | 2104 | 2100 | 2097 | 2105 |
| Protein | 17.4 (91.4)2 | 17.9 (99.7) | 18.4 (102.6) | 27 (149.2) |
| Plant source | 42.0 (38.4) | 64.5 (64.3) | 37.9 (38.9) | 31.6 (47.1) |
| Animal source | 58.0 (53.1) | 35.5 (35.4) | 62.1 (63.7) | 68.4 (102.2) |
| Carbohydrate | 49.4 (260.0) | 55 (288.8) | 54 (283.0) | 45 (236.8) |
| Fat | 33.2 (77.6) | 27 (63) | 27 (62.9) | 27 (63) |
| Cholesterol, mg | 233 | 89 | 152 | 195 |
| SFAs | 13.3 (31.1) | 6.6 (15.3) | 6.1 (14.3) | 6.1 (14.3) |
| PUFAs | 5.4 (12.7) | 5.8 (13.5) | 6.3 (14.7) | 6.3 (14.7) |
| MUFAs | 11.3 (26.3) | 11.5 (26.9) | 10.7 (24.8) | 11.6 (27) |
| Fiber, g | 21 | 55 | 38 | 38 |
| Sodium, mg | 3640 | 2700 | 2666 | 2789 |
| Potassium, mg | 2380 | 4112 | 4241 | 4328 |
| Calcium, mg | 450 | 1282 | 1436 | 1380 |
| Magnesium, mg | 227 | 462 | 464 | 459 |
| Lean beef, g (oz/d) | 39.7 (1.4)3 | 11.7 (0.4) | 139 (4.9) | 196.2 (6.9) |
Based on 2100 kcal/d, averaged across a 6-d menu cycle. All values were determined with NUTRTIONST PRO (Axxya Systems LLC). BOLD, Beef in an Optimal Lean Diet; BOLD+, Beef in an Optimal Lean Diet Plus Protein; HAD, healthy American diet; M-DASH, modified Dietary Approaches to Stop Hypertension Diet.
Values for macronutrients are percentage of calories, except for protein sources, which are percentage of total protein; values in parentheses are grams (all such values).
Values are grams; ounces in parentheses.
The HAD contained full-fat cheese and dairy products, more vegetable oil and butter, and refined grains. The M-DASH, BOLD, and BOLD+ diets contained low-fat or nonfat versions of these foods, less oil and butter, and more whole grains. All diets were rich in fruits, vegetables, and lean meats, consistent with food-based dietary recommendations. The BOLD and BOLD+ diets included a serving of lean beef each day. More specifically, of the 14 lunch/dinner meals provided for a week, 11 meals included lean beef, 2 contained chicken, and 1 contained fish. However, the BOLD+ diet contained more lean beef than did the BOLD diet (196 compared with 139 g/d). The amount of lean beef in the BOLD diet is consistent with the recommendations from the Third Adult Treatment Panel for the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults for lean beef consumption for the Step 2 diet (16). In comparison, the M-DASH included 1 lean beef–based, 3 chicken-based, 1–2 fish-based, 7 pulse/vegetable-based, and 1–2 soy-based meals per week. The HAD and M-DASH diet contained 40 and 12 g lean beef/d, respectively. The contribution of protein from various whole-food sources for each of the experimental diets (M-DASH, BOLD, and BOLD+) is shown in Table 2. Protein from lean beef provided ∼7%, 62%, and 56% of total animal protein for the M-DASH, BOLD, and BOLD+ diets, respectively. The BOLD+ diet provided more lean beef as well as more protein, and therefore lean beef contributed proportionally less to animal protein than the BOLD diet.
TABLE 2.
Dietary protein sources in the experimental diets1
| M-DASH | BOLD | BOLD+ | |
| Total protein, g | 99.7 | 102.6 | 149.2 |
| Animal source | 35.4 (35.5)2 | 63.7 (62.1) | 102.2 (68.4) |
| Lean beef protein | 2.5 (7.2) | 39.6 (62.2) | 57.1 (55.8) |
| Other meat protein | 12.6 (35.5) | 8.3 (13.1) | 19.7 (19.3) |
| Dairy protein | 20.3 (57.3) | 15.7 (24.7) | 25.4 (24.9) |
| Plant source | 64.3 (64.5) | 38.9 (37.9) | 47.1 (31.6) |
| Soy protein | 7.1 (11.0) | 0.0 (0) | 6.5 (13.8) |
| Pulses | 8.1 (12.6) | 3.3 (8.5) | 3.7 (7.8) |
| Nuts and seeds | 11.2 (17.4) | 6.3 (16.3) | 9.5 (20.2) |
| Fruits and vegetables | 11.0 (17.1) | 12.8 (33.0) | 10.4 (22.2) |
| Grains | 26.9 (41.8) | 16.4 (42.3) | 17.0 (36.1) |
Based on 2100 kcal/d, averaged across a 6-d menu cycle. All values were determined with NUTRTIONST PRO (Axxya Systems LLC). BOLD, Beef in an Optimal Lean Diet; BOLD+, Beef in an Optimal Lean Diet Plus Protein; HAD, healthy American diet; M-DASH, modified Dietary Approaches to Stop Hypertension Diet.
Grams; percentage of total animal or plant source in parentheses (all such values).
The lean beef used in the study was purchased from The Pennsylvania State University Meats Laboratory and primarily included select grade top round, ribeye, chuck shoulder pot roast, and 95% lean ground beef. The meat was prepared via braising, grilling, or frying (95% lean ground beef only) and never over an open flame to prevent charring.
A 2100-kcal menu served as the basis for the other menus that provided a range of calorie amounts (1800–3900 kcal/d in 300-kcal increments); all foods were increased or decreased proportionally such that no single food was removed, and the foods provided were similar across all calorie amounts. A 1600-kcal menu was developed for some female participants during the WL phase. Menus were created for a 6-d cycle that was repeated throughout the controlled-feeding phases. The 6-d rather than the 7-d menu cycle ensured that the same menu was not consumed on the same day of the week throughout the controlled-feeding phases (e.g., every Monday was pot roast for dinner for participants in the BOLD+ group). To induce a calorie deficit during the controlled WL phase, we gave individuals a lower calorie menu. The dietary changes were designed to induce a minimum 500-kcal/d deficit. Sample daily menus for each of the diets are provided in Table 3. All meals and snacks were prepared for the controlled-feeding phases of the study at the Metabolic Diet Study Center at The Pennsylvania State University. Participants ate one meal per day (Monday–Friday) in the diet center, and their other meals were prepared and packed for off-site consumption for the remaining weekday and weekend meals. Participants were allowed one “free meal” on holidays (e.g., July 4th); for these meals, they were given guidance about sensible eating, including avoiding overconsumption and excessive alcohol intake.
TABLE 3.
Example of 1-d menus for the test diets1
| HAD | M-DASH | BOLD | BOLD+ | |
| Breakfast | Pancakes with butter and light syrup | Pancakes with butter and light syrup | Bran flakes with raisins and skim milk | Bran flakes with raisins and skim milk |
| Peaches, canned in juice | Blueberries | Whole-wheat mini-bagel and margarine | Cottage cheese (1%) | |
| Cottage cheese (1%) | Skim milk | Orange juice | Orange juice | |
| Apple juice | Orange juice | Banana | ||
| Lunch | Turkey, provolone cheese, and lettuce sandwich on white bread with mayonnaise | Spinach/baby greens salad with cherry tomatoes, mandarin oranges, grilled chicken breast, and dressing | Barbeque beef sandwich on whole-wheat bun | Beef chili with shredded cheddar cheese (low fat) and whole-wheat crackers |
| Granola bar | Edamame beans | Spinach salad with cherry tomatoes and dressing | Peaches, canned in juice | |
| Whole-wheat dinner roll with butter | Thin pretzels | |||
| Pistachios | Pear | |||
| Dinner | Szechuan stir-fry entrée with pork and white rice | Ratatouille (eggplant/peppers) with pasta | Spinach and beef skillet with ribeye steak | Pot roast with mashed potatoes and gravy |
| White dinner roll with butter | Spinach salad with carrots, cherry tomatoes, red bell pepper, chickpeas, and dressing | Brown rice | White dinner roll with margarine | |
| Romaine lettuce salad with carrots and Italian dressing | Mixed baby greens salad with carrots, cherry tomatoes, and dressing | Broccoli and edamame beans | ||
| Romaine salad with cherry tomatoes and dressing | ||||
| Snack | Plain bagel with cream cheese | Light yogurt | Light yogurt | Hummus with whole wheat pita and baby carrots |
| High-fiber cereal | Orange | Trail mix | ||
| Almonds | Almonds |
BOLD, Beef in an Optimal Lean Diet; BOLD+, Beef in an Optimal Lean Diet Plus Protein; HAD, healthy American diet; M-DASH, modified Dietary Approaches to Stop Hypertension.
Compliance with the experimental diets during the controlled-feeding phases was monitored via daily questionnaires (Supplemental Material) asking about the consumption of study and nonstudy foods and beverages, as well as weigh-ins. Participants were classified as noncompliant on any day when they consumed a nonstudy food or beverage or did not consume a study food or beverage. Compliance to each controlled-feeding phase was determined by dividing the number of noncompliant days by the number of days reported for each diet group; this excluded the free meals allowed (e.g., holiday meals). This method did not capture the degree to which participants were noncompliant because any deviation was simply tabulated as a noncompliant day; for example, a participant who replaced the entire study meal with pizza and breadsticks and a participant who did not consume the tahini dressing on a study salad at lunch were both classified as noncompliant on that day. For this reason, we considered changes in body weight as an additional measure of compliance.
The term adherence is used to describe how well participants followed the advice to continue to lose weight via dietary changes and exercise during the FL phase. This term implies that participants were in agreement with the treatment regimen and goals, but unlike the controlled-feeding phases, there was some flexibility in how participants implemented strategies to achieve their goals. To reproduce the daily monitoring implemented during the controlled-feeding phases would have placed substantial burden on participants during this phase of the study. Therefore, we based our interpretation of adherence during the FL phase on changes in body weight and MetS criteria.
Physical activity
All participants underwent a 12-lead electrocardiogram-controlled graded submaximal walking test as part of the prescreening for entry to the study. Treadmill speed and gradient were modified each minute until participants reached 85% of their age-predicted maximum heart rate (HR) (220 beats/min − age). During the walking test, participants were under permanent electrocardiogram monitoring, and BP was taken at rest and every 3 min. Oxygen uptake at 85% maximal HR was determined via the following calculation: 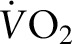 (mL/kg per minute) = (0.1 × S) + (1.8 × S × G) + 3.5 mL/kg per minute (where S = speed in m/min and G = percent grade expressed as a fraction) (17). At the end of the WL and FL phases, participants underwent the same fitness assessment to evaluate physiologic changes induced by exercise.
(mL/kg per minute) = (0.1 × S) + (1.8 × S × G) + 3.5 mL/kg per minute (where S = speed in m/min and G = percent grade expressed as a fraction) (17). At the end of the WL and FL phases, participants underwent the same fitness assessment to evaluate physiologic changes induced by exercise.
Participants were provided with a pedometer (SW-700 Digi-walker; New Lifestyles) and a personalized pedometer-based walking program that was intended to increase their amount of physical activity to 10,000 steps/d or more by the end of the WL phase. To establish their baseline, participants recorded their daily steps during the last 2 wk of the controlled-feeding WM phase. These step counts were used to guide the development of a personalized walking program, which increased progressively in volume (steps/d) and intensity (steps performed at moderate-vigorous intensity) throughout the 6-wk WL phase. Participants recorded their daily steps throughout the WL phase (energy expenditure via physical activity was not determined). Participants were advised to maintain 10,000 steps/d during the FL phase and, if desired, to incorporate additional physical activities.
Clinical assessments
Body weight was measured at each clinical visit at the Clinical Research Center (in addition to daily weigh-ins at the diet center). WC was measured according to the NHANES Anthropometry Procedures Manual as defined by the CDC (18). Body composition was determined by DXA: participants weighing <157 kg were measured with Hologic DXA (QDR-4500W; Hologic Corporation), and those weighing >157 kg were measured with a GE Lunar iDXA (General Electric). Participants were scanned while wearing cotton shorts and T-shirt and while in the supine position in accordance with the manufacturer’s instructions. Abdominal fat was calculated by inserting a 50-cm2 region of interest around the center point of the midline between the lateral iliac crests and the lowest rib margins. The abdominal region of interest could not be measured with iDXA; therefore, abdominal fat was not assessed for one individual from each of the diets (i.e., M-DASH, BOLD, and BOLD+, n = 3). Three participants with incomplete data also were not analyzed. The DXA scanners were calibrated according to the standard procedures recommended by the manufacturer. BP (3 repeat measurements spaced 1 min apart) was measured with participants in a seated position after a minimum 5-min rest period, at baseline, and at the end of each phase. Endothelial function [reactive hyperemia index (RHI), Framingham RHI] and vascular stiffness (augmentation index, augmentation index normalized to an HR of 75 beats/min) were measured with pulse amplitude tonometry (Itamar Medical) as previously described (12). HR was measured by the pulse amplitude tonometry device as beats per minute.
Biochemical assessments
Serum and plasma aliquots from fasting blood samples were stored at −80°C until time of analysis. For the first 19 enrolled participants, samples were shipped frozen and analyzed in the core endocrine laboratory at the Milton S. Hershey Medical Center (Hershey, PA). Total cholesterol (TC) and triglycerides were measured by using enzymatic procedures with commercially available kits (Alfa Wassermann). HDL cholesterol was quantified according to the modified heparin-manganese precipitation procedure of Warnick and Albers (19). LDL cholesterol was calculated with the Friedewald equation: LDL cholesterol = TC – HDL cholesterol – (triglycerides ÷ 5) (20). Glucose was determined by an immobilized enzyme biosensor for glucose with the YSI 2300 STAT Plus Glucose & Lactate Analyzer (Yellow Springs Instruments). For the subsequent participants, lipids and glucose were measured in fresh samples by Quest Diagnostics by enzymatic procedures and spectrophotometry. The CVs for TC, HDL cholesterol, and triglycerides were <2%. For all participants, insulin was quantified by radioimmunoassay (Quest Diagnostics). Serum C-reactive protein was measured by latex-enhanced immunonephelometry (Quest Diagnostics; assay CV <8%).
Statistical analysis
All statistical analyses were performed with SAS (version 9.2; SAS Institute). Screening values (means ± SDs) for the treatment groups were compared with a nonparametric 2-sided t test (PROC NPAR1WAY). Differences between treatment groups after the HAD run-in diet (means ± SEMs) were assessed with linear-mixed models (PROC MIXED). Normality of the variables at each time point was assessed, and variables were log-transformed if skewed. Means ± SEMs are presented for normally distributed variables; nonnormally distributed variables are presented as medians and 95% CIs. Repeated-measure ANCOVA (repeated for phase) was used to test the effects of treatment (diet) and phase (WM, WL, and FL) on the outcome variables, adjusting for age and sex. A doubly repeated-measure ANCOVA (repeated for phase and day of blood draw) was used to determine the effects of treatment and phase on lipids and lipoproteins, adjusting for age and sex. The model fit was determined by selecting the best covariance structure (compound symmetry, autoregressive 1, and unstructured) for each endpoint as determined by the lowest Bayesian information criterion and the normality of the model residuals. Interaction and main effects were considered statistically significant at P < 0.05 and trends at P < 0.1. Tukey-Kramer adjusted P values were used to determine where the post hoc differences occurred within statistically significant interaction or main effects, with significance set at P < 0.05. Multiple models comparing group differences were analyzed (raw values at all time points, raw values adjusted for baseline, and change scores), and all provided similar results. Adjustment of P values for multiple outcome variables by using the Benjamini-Hochberg-Yekutieli procedure (21) did not change results; therefore, unadjusted P values are presented. Multivariate logistic regression analysis was used to evaluate the relation between the independent variables diet, weight loss (percent change from HAD or WM to end WL or end FL), age, and sex and the dependent variable resolution of MetS. In this analysis, presence of MetS was coded as 0, and resolution of MetS was coded as 1.
Power calculations were based on studies comparing the effects of different amounts or sources of dietary protein on BP in hypertensive populations (6, 7, 22) and triglycerides in hypercholesterolemic populations (23). The estimated effect sizes for the M-DASH and BOLD diets (7, 22) were reductions of 5.5–10.7 mm Hg SBP and 3.0–4.7 mm Hg DBP. The estimated effect size for the BOLD+ diet (6) was a reduction of 9.5 mm Hg SBP and 5.2 mm Hg DBP. The effect size of the higher animal protein diets (BOLD, BOLD+) for triglycerides was calculated to be a reduction of 19–25% (23). Because the experimental diets had not been compared before in the literature, we could not estimate between-group differences. For 80% power to detect statistically significant within-group changes from HAD (baseline) to WM for BP and triglycerides at an α level of 0.05, a final sample size of 84 participants (28 per group) was needed. The recruitment goal was 90 participants (30 per group) to account for dropouts. Cohen’s d effect sizes were calculated for BP data (change scores, WM-HAD) by using the procedures outlined by Thalheimer and Cook (24).
RESULTS
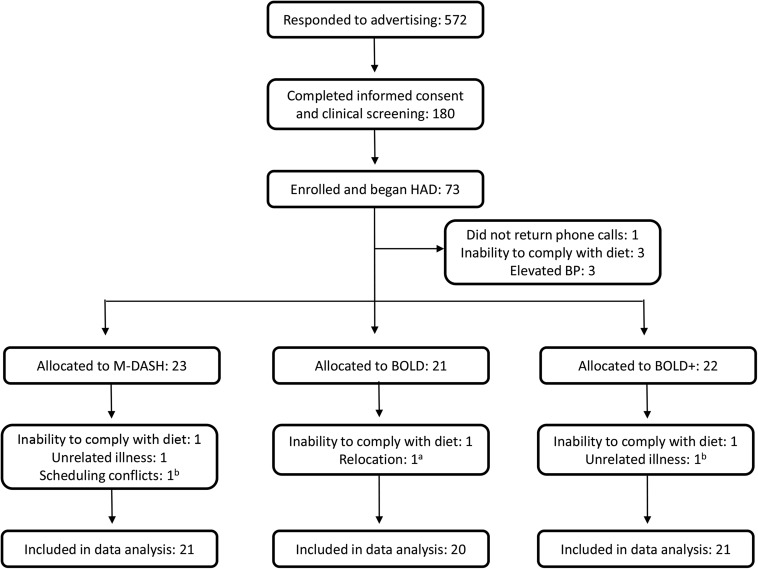
Recruitment of study participants took 2.5 y because of the strict study eligibility criteria. Of the 572 respondents, 180 (31%) completed the informed consent and were screened at the clinic for eligibility. Between November 2008 and March 2011, 73 (13%) were enrolled and began the HAD diet (Figure 1). Seven participants did not complete the HAD run-in diet. The remaining 66 individuals were randomly allocated, after which 7 participants did not complete the study because of an inability to comply with the diet (n = 3), unrelated illness (n = 2), relocation (n = 1), and scheduling conflicts (n = 1). Of these 7 participants, 3 completed an entire feeding phase before dropping out, and their data are included in the final analysis (n = 62). The retention rates for the 3 diet groups were similar: 95% (n = 20/21) for BOLD, 95% (n = 21/22) for BOLD+, and 91% (n = 21/23) for M-DASH. There were no differences among groups at screening (Table 4). One participant (M-DASH) was taken off BP medications before starting the study and maintained acceptable BP to remain in the study; 7 participants (BOLD, n = 2; BOLD+, n = 2; M-DASH, n = 3) were taking BP medications during the study. Because of difficulties in recruitment for a 6-mo dietary intervention study, recruitment goals were not met.
FIGURE 1.
Participant flow diagram. aPartial data, completed weight-maintenance and weight-loss phases. bPartial data, completed weight-maintenance phase. BP, blood pressure; BOLD, Beef in an Optimal Lean Diet; BOLD+, Beef in an Optimal Lean Diet Plus; HAD, healthy American diet; M-DASH, modified Dietary Approaches to Stop Hypertension.
TABLE 4.
Screening characteristics of participants in the different diet groups1
| M-DASH (n = 21) | BOLD (n = 20) | BOLD+ (n = 21) | |
| Age, y | 45.3 ± 6.72 | 46.2 ± 9.4 | 46.4 ± 8.5 |
| Sex, M:F, n | 9:12 | 9:11 | 10:11 |
| Caucasian race, n (%) | 21 (100) | 19 (95) | 21 (100) |
| BMI, kg/m2 | 34.7 ± 3.6 | 34.6 ± 3.7 | 35.1 ± 4.5 |
| Weight, kg | 102.1 ± 15.5 | 101.8 ± 15.6 | 104.8 ± 17.7 |
| WC, cm | 113.5 ± 9.5 | 113.6 ± 9.2 | 117.2 ± 10.3 |
| HDL cholesterol, mg/dL | 40.4 ± 9.2 | 42.1 ± 7.9 | 41.7 ± 10.8 |
| TG, mg/dL | 190.7 ± 56.8 | 182.6 ± 89.3 | 181.3 ± 75.2 |
| Glucose, mg/dL | 104.0 ± 21.6 | 101.2 ± 18.7 | 103.1 ± 13.0 |
| SBP, mm Hg | 130.0 ± 11.8 | 125.8 ± 13.3 | 127.5 ± 12.6 |
| DBP, mm Hg | 89.6 ± 6.9 | 86.9 ± 7.9 | 85.9 ± 7.5 |
| Taking medications, n participants | 7 | 8 | 7 |
| Blood pressure | 4 | 2 | 2 |
| Glucose medication | 0 | 1 | 1 |
| Lipid medication | 1 | 4 | 2 |
| Depression/anxiety | 5 | 3 | 4 |
No differences existed between groups at screening (nonparametric, 2-sided t test). BOLD, Beef in an Optimal Lean Diet; BOLD+, Beef in an Optimal Lean Diet Plus Protein; DBP, diastolic blood pressure; M-DASH, modified Dietary Approaches to Stop Hypertension; SBP, systolic blood pressure; TG, triglycerides; WC, waist circumference.
Mean ± SEM (all such values).
Dietary and exercise compliance
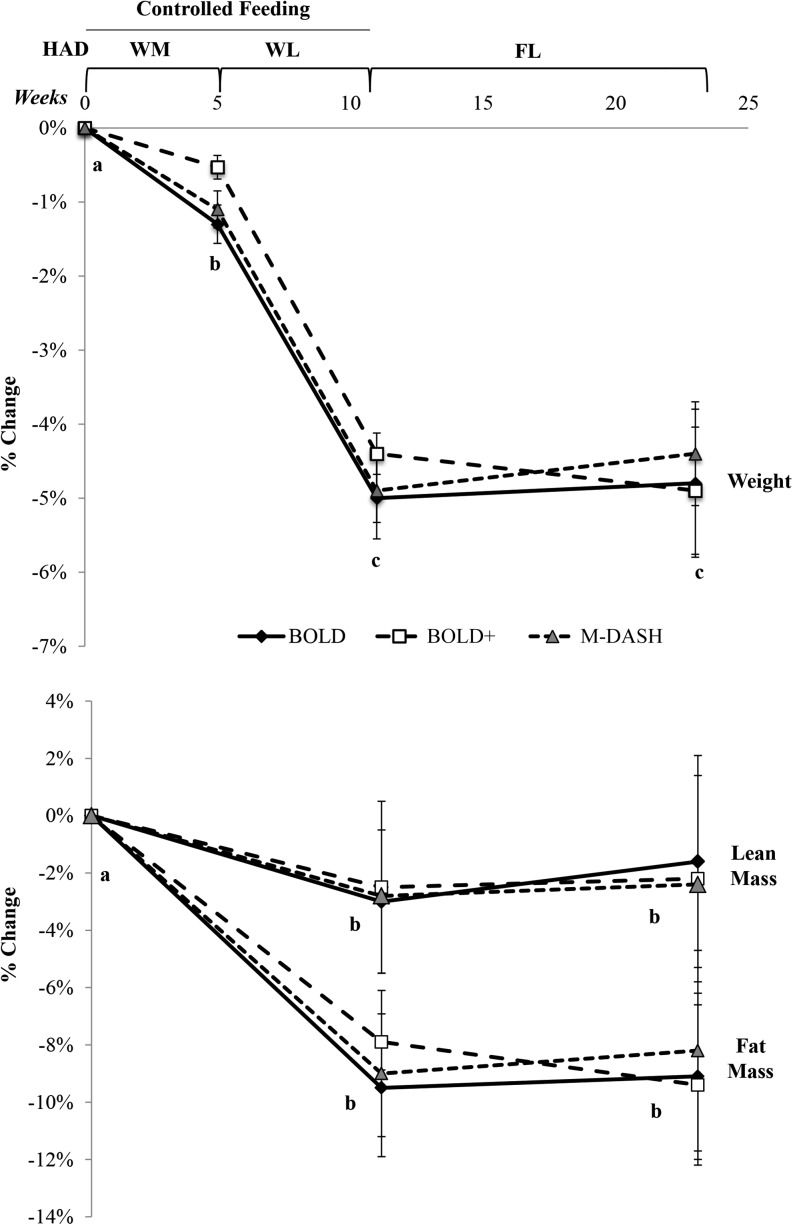
Daily compliance records for the controlled-feeding HAD phase indicated that there was total dietary compliance on 74% ± 2%, 81% ± 3%, and 84% ± 1% of reported study days for the BOLD, BOLD+ and M-DASH groups, respectively. During the WM phase (when participants were consuming the experimental diets), participants in the BOLD, BOLD+, and M-DASH groups reported being compliant on 70% ± 1%, 77% ± 1%, and 82% ± 1% of study days, respectively. Compliance during the WL phase was 75% ± 1%, 80% ± 1%, and 90% ± 1% for the BOLD, BOLD+, and M-DASH groups, respectively. Weight changes corresponding to the varying energy levels (i.e., WM or WL) during the controlled-feeding diet phases demonstrate an overall high amount of compliance to the study protocol during the controlled-feeding phases (see Weight and body composition, Figure 2). Participants did not achieve further weight loss during the FL phase; body weight losses were maintained during this phase.
FIGURE 2.
Mean ± SEM weight and body composition changes in BOLD (n = 20), BOLD+ (n = 21), and M-DASH (n = 21) diet groups after WM, WL, and FL phases. Different letters denote differences at time points from linear-mixed models adjusted for age and sex; phase, P < 0.0001; Tukey-adjusted P < 0.05. BOLD, Beef in an Optimal Lean Diet; BOLD+, Beef in an Optimal Lean Diet Plus Protein; FL, free-living weight-loss phase; HAD, healthy American diet; M-DASH, modified Dietary Approaches to Stop Hypertension; WL, weight loss (minimum 500-kcal/d deficit) including exercise phase; WM, weight-maintenance phase.
During the last 2 wk of the WM phase, participants reported walking a mean of 6303 ± 382 steps/d (self-reported pedometer data). During the last week of the WL phase, participants reported walking a mean of 10,536 ± 463 steps/d (self-reported pedometer data), which represented a significant increase in physical activity (WM compared with WL, P < 0.0001). Predicted 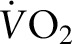 was 18.5 ± 0.6 mL/kg per minute after HAD, which significantly increased to 22.2 ± 0.7 mL/kg per minute after WL (HAD compared with WL, P < 0.0001), and the effect was maintained through FL (22.0 ± 0.7 mL/kg per minute; WL compared with FL, P = NS).
was 18.5 ± 0.6 mL/kg per minute after HAD, which significantly increased to 22.2 ± 0.7 mL/kg per minute after WL (HAD compared with WL, P < 0.0001), and the effect was maintained through FL (22.0 ± 0.7 mL/kg per minute; WL compared with FL, P = NS).
Weight and body composition
Weight and body composition mean ± SEM values are presented in Table 5. Weight was significantly reduced from HAD by the end of each phase (Figure 2, P < 0.0001), with no between-group differences. Significant weight loss (<1.5% of body weight) occurred during the WM phase but was within the acceptable range for a weight maintenance diet (HAD compared with WM, P < 0.0001). Loss of ∼5% of body weight occurred during the WL phase (WM compared with WL, P < 0.0001), which was maintained during the FL phase on all diets (WL compared with FL, P = NS).
TABLE 5.
Weight and body composition measurements after each diet phase for M-DASH, BOLD, and BOLD+ diet groups1
| M-DASH, mean ± SEM |
BOLD, mean ± SEM |
BOLD+, mean ± SEM |
P value |
||||||||||||
| HAD | WM | WL | FL | HAD | WM | WL | FL | HAD | WM | WL | FL | Phase × diet | Phase | Diet | |
| Weight, kg | 100 ± 2 | 100 ± 2 | 96 ± 2 | 97 ± 2 | 101 ± 2 | 100 ± 2 | 97 ± 2 | 97 ± 3 | 105 ± 3 | 104 ± 3 | 101 ± 3 | 100 ± 3 | 0.89 | <0.0001a | 0.49 |
| BMI, kg/m2 | 34.2 ± 0.5 | 34.1 ± 0.5 | 32.8 ± 0.6 | 32.9 ± 0.6 | 34.4 ± 0.6 | 34.0 ± 0.6 | 32.7 ± 0.6 | 32.8 ± 0.7 | 35.1 ± 0.7 | 34.9 ± 0.7 | 33.6 ± 0.7 | 33.4 ± 0.8 | 0.94 | <0.0001a | 0.63 |
| Body fat, kg | 35.8 ± 2.0 | — | 32.5 ± 2.0 | 32.7 ± 2.1 | 37.0 ± 1.7 | — | 33.6 ± 1.8 | 33.7 ± 2.0 | 38.2 ± 2.0 | — | 35.0 ± 2.1 | 34.2 ± 2.2 | 0.53 | <0.0001b | 0.50 |
| Body lean mass, kg | 59.9 ± 2.4 | — | 58.8 ± 2.4 | 58.9 ± 2.5 | 58.8 ± 2.7 | — | 57.1 ± 2.8 | 58.4 ± 2.9 | 61.0 ± 2.9 | — | 60.3 ± 3.0 | 60.4 ± 2.8 | 0.73 | <0.0001b | 0.57 |
| Percent body fat | 35.7 ± 1.6 | — | 34.2 ± 1.7 | 34.4 ± 1.8 | 37.4 ± 1.6 | — | 35.6 ± 1.8 | 35.5 ± 1.9 | 37.1 ± 1.6 | — | 35.8 ± 1.7 | 35.3 ± 1.9 | 0.72 | <0.0001b | 0.12 |
| Abdominal fat, kg | 3.9 ± 0.3 | — | 3.4 ± 0.2 | 3.5 ± 0.3 | 4.0 ± 0.2 | — | 3.4 ± 0.2 | 3.6 ± 0.2 | 4.5 ± 0.3 | — | 3.9 ± 0.2 | 3.9 ± 0.3 | 0.88 | <0.0001b | 0.24 |
| Abdominal lean mass, kg | 5.9 ± 2.2 | — | 5.5 ± 0.2 | 5.4 ± 0.2 | 5.9 ± 0.3 | — | 5.5 ± 0.2 | 5.8 ± 0.3 | 6.0 ± 0.2 | — | 5.5 ± 0.3 | 5.5 ± 0.3 | 0.73 | <0.0001b | 0.88 |
| Percent abdominal fat | 39.6 ± 1.3 | — | 37.6 ± 1.5 | 38.1 ± 1.6 | 39.9 ± 1.1 | — | 37.8 ± 1.3 | 37.7 ± 1.4 | 42.1 ± 1.2 | — | 40.6 ± 1.3 | 40.1 ± 1.4 | 0.81 | <0.0001b | 0.09 |
Linear mixed model comparing raw values between groups at the end of each diet phase, adjusted for age and sex. Different letters for P values denote differences between groups at baseline (HAD). Post hoc Tukey-adjusted P < 0.05. aHAD compared with WM, WL, FL, Tukey-adjusted P < 0.05. bHAD compared with WL, FL, Tukey-adjusted P < 0.05. BOLD, Beef in an Optimal Lean Diet; BOLD+, Beef in an Optimal Lean Diet Plus Protein; FL, free-living weight-loss phase; HAD, healthy American diet; M-DASH, modified Dietary Approaches to Stop Hypertension; WL, weight loss (minimum 500-kcal/d deficit) including exercise phase; WM, weight-maintenance phase.
Body composition measures were taken after HAD, WL, and FL phases only (Figure 2, Table 4). Body fat decreased by 8–9% in all groups during the WL phase (HAD compared with WL, P < 0.0001), and the reductions remained through the FL phase (WL compared with FL, P = NS). Lean body mass decreased by 2–3% during the WL phase (HAD compared with WL, P < 0.0001), which was maintained through the FL phase (WL compared with FL, P = NS). Abdominal fat decreased by ∼14% (HAD compared with WL, P < 0.0001) during WL and abdominal lean mass decreased by 5–7% (HAD compared with WL, P < 0.0001); both effects were maintained through the FL phase (WL compared with FL, P = NS).
MetS endpoints
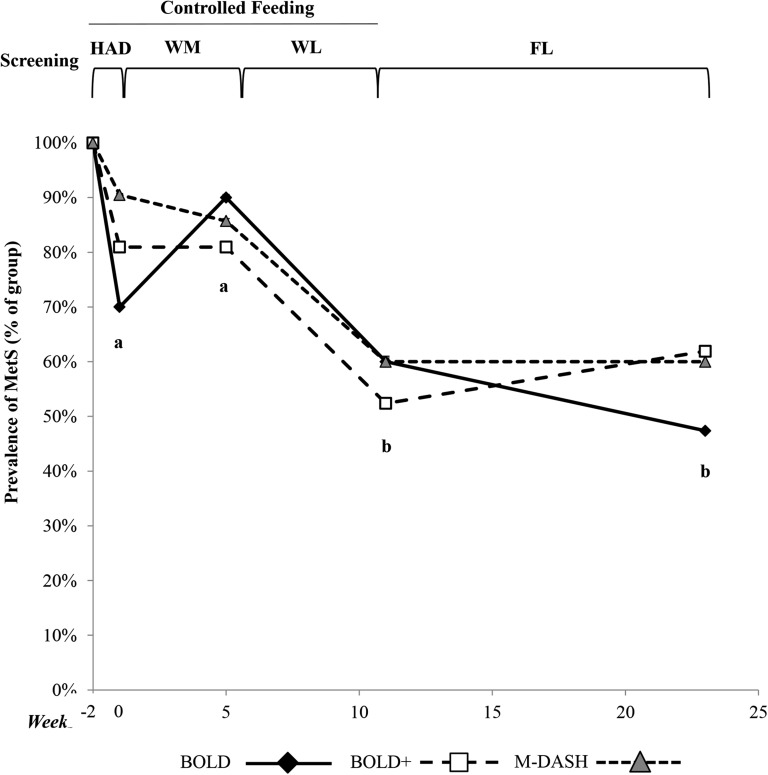
Primary and secondary endpoints are presented in Table 6. A significant main effect of phase (P < 0.01) was observed for all endpoints except for insulin. Dietary changes implemented during the WM phase did not reduce the number of MetS criteria per participant (HAD compared with WM, P = NS). MetS criteria decreased after the WL phase (HAD compared with WL, P < 0.01), and the changes were maintained during the FL phase (HAD compared with FL, P < 0.01). The prevalence of MetS was 100% in all groups at screening, but the prevalence in the BOLD group dropped to 70%, BOLD+ to 81%, and M-DASH to 90% after the HAD phase (NS between groups, Figure 3). After the WM phase, all groups had a MetS prevalence of 80–90%, which decreased significantly to 50–60% after WL and maintained through FL (χ2 for phase, P < 0.0001). Multivariate logistic regression analysis revealed that weight loss (HAD or WM to WL and HAD or WM to FL) but not diet was significantly associated with resolution of MetS (Table 7). Every 1% reduction in body weight (from HAD to WL) was associated with a 39% increase in the odds of having a resolution of MetS, holding the other independent variables constant. A stronger relation was observed for changes in body weight after the WL phase (i.e., WM to WL) and MetS resolution: every 1% reduction in body weight was associated with an 88% increase in the odds of having a resolution of MetS.
TABLE 6.
Primary and secondary endpoints after each diet phase for M-DASH, BOLD, and BOLD+ diet groups1
| M-DASH (n = 21) |
BOLD (n = 20) |
BOLD+ (n = 21) |
P value |
||||||||||||
| HAD | WM | WL | FL | HAD | WM | WL | FL | HAD | WM | WL | FL | Phase × diet | Phase | Diet | |
| Primary | |||||||||||||||
| MetS, variables, n | 3.4 ± 0.22 | 3.5 ± 0.2 | 2.9 ± 0.2 | 2.7 ± 0.2 | 3.1 ± 0.2 | 3.3 ± 0.2 | 2.8 ± 0.2 | 2.5 ± 0.3 | 3.1 ± 0.2 | 3.2 ± 0.2 | 2.6 ± 0.2 | 2.9 ± 0.2 | 0.64 | <0.0001a | 0.58 |
| WC, cm | 112 ± 2 | 112 ± 2 | 109 ± 2 | 108 ± 2 | 113 ± 2 | 112 ± 2 | 108 ± 2 | 106 ± 2 | 116 ± 3 | 115 ± 2 | 111 ± 2 | 111 ± 2 | 0.89 | <0.0001a | 0.37 |
| HDL cholesterol, mg/dL | 36.3 ± 1.2 | 33.9 ± 1 | 36.5 ± 1.3 | 40.8 ± 1.2 | 38.4 ± 1.1 | 35.2 ± 1.1 | 38.5 ± 1.4 | 43.3 ± 1.4 | 40.2 ± 1.7 | 35.6 ± 1.6 | 37.1 ± 1.5 | 41.6 ± 1.5 | 0.68 | <0.0001b | 0.18 |
| TG, mg/dL | 168 (161, 193)3 | 185 (168, 205) | 131 (132, 175) | 162 (142, 167) | 156 (144, 203) | 172 (169, 242) | 130 (123, 170) | 141 (126, 181) | 156 (147, 182) | 155 (148, 193) | 134 (119, 177) | 172 (132, 165) | 0.82 | <0.0001a | 0.34 |
| Glucose, mg/dL | 95 (91, 105) | 93 (90, 104) | 92 (89, 97) | 95 (93, 102) | 92 (88, 105) | 94 (89, 108) | 90 (89, 100) | 92 (90, 103) | 95 (90, 102) | 99 (96, 108) | 97 (92, 102) | 99 (94, 104) | 0.42 | <0.0001c | 0.75 |
| SBP, mm Hg | 127 ± 3 | 124 ± 2 | 120 ± 2 | 121 ± 2 | 122 ± 2 | 121 ± 2 | 120 ± 2 | 123 ± 3 | 127 ± 2 | 124 ± 2 | 120 ± 2 | 123 ± 3 | 0.53 | <0.0001d | 0.56 |
| DBP, mm Hg | 86.3 ± 1.8 | 85.6 ± 1.2 | 82.7 ± 1.4 | 81.9 ± 1.2 | 85.3 ± 1.3 | 83.2 ± 1.6 | 82.2 ± 1.8 | 82.1 ± 2.1 | 85.3 ± 1.6 | 83 ± 1.5 | 82.3 ± 1.6 | 82.6 ± 2 | 0.84 | <0.0001e | 0.73 |
| Secondary | |||||||||||||||
| TC, mg/dL | 198 ± 5 | 188 ± 5 | 182 ± 5 | 203 ± 4 | 198 ± 8 | 197 ± 8 | 183 ± 8 | 201 ± 7 | 191 ± 6 | 177 ± 6 | 169 ± 7 | 192 ± 7 | 0.67 | <0.0001f | 0.27 |
| LDL cholesterol, mg/dL | 126 ± 4 | 117 ± 4 | 115 ± 4 | 134 ± 4 | 126 ± 7 | 127 ± 8 | 115 ± 7 | 127 ± 7 | 118 ± 5 | 107 ± 5 | 103 ± 6 | 117 ± 5 | 0.64 | <0.0001f | 0.18 |
| Insulin, mIU/L | 7 (5, 9.3) | 7 (5.2, 11.9) | 4 (4.1, 10.2) | 5 (4.6, 9.9) | 7 (4.5, 8.9) | 5 (4.1, 9.1) | 4.5 (3.6, 7) | 5 (4.2, 9.4) | 8 (6.6, 10.8) | 6 (4.6, 9.5) | 6 (4.3, 10.6) | 5 (4, 9.9) | 0.22 | 0.22 | 0.63 |
| CRP, mg/L | 2.6 (1.8, 5.1) | 1.9 (1.6, 4.1) | 1.2 (1.1, 4.9) | 1.2 (0.9, 4.5) | 2.5 (1.9, 4.5) | 3.4 (2.5, 5) | 2.4 (1.8, 3.9) | 3.2 (2.3, 4.3) | 2.1 (1.6, 3.7) | 2.4 (1.9, 4) | 1.7 (1.3, 3.4) | 1.7 (1.2, 2.9) | 0.18 | 0.0001a | 0.4 |
Linear mixed model comparing raw values between groups from the end of each diet phase, adjusted for age and sex. Post hoc differences for the main effect of phase are represented by the letters. aHAD, WM compared with WL, FL, P < 0.05. bHAD compared with WM, FL; WM compared with WL, FL, P < 0.05. cWM compared with WL, P < 0.05. dHAD compared with WL, FL; WM compared with WL, P < 0.05. eHAD compared with WL, FL, P < 0.05. fHAD compared with WM, WL; WM, WL compared with FL, P < 0.05. BOLD, Beef in an Optimal Lean Diet; BOLD+, Beef in an Optimal Lean Diet Plus Protein; CRP, C-reactive protein; DBP, diastolic blood pressure; FL, free-living weight-loss phase; HAD, healthy American diet; M-DASH, modified Dietary Approaches to Stop Hypertension; MetS, metabolic syndrome; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; WC, waist circumference; WL, weight loss (minimum 500-kcal/d deficit) including exercise phase; WM, weight-maintenance phase.
Mean ± SEM (all such values).
Median; 95% CI in parentheses (all such values). These values are presented because of nonnormally distributed model residuals; log-transformed values are analyzed in model.
FIGURE 3.
MetS prevalence (percentage of group) in BOLD (n = 20), BOLD+ (n = 21), and M-DASH (n = 21) diet groups at screening and after a healthy run-in diet, WM, WL, and FL phases. Different letters denote differences in MetS prevalence by phase, χ2, P < 0.0001. Screening values were not included in the model. BOLD, Beef in an Optimal Lean Diet; BOLD+, Beef in an Optimal Lean Diet Plus Protein; HAD, healthy American diet; M-DASH, modified Dietary Approaches to Stop Hypertension; FL, free-living weight-loss phase; MetS, metabolic syndrome; WL, weight loss (minimum 500-kcal/d deficit) including exercise phase; WM, weight-maintenance phase.
TABLE 7.
Logistic regression model determining factors associated with resolution of MetS1
| Independent variable | Coefficient (95% CI) | P value |
| HAD to WL | ||
| Weight, % change | 0.328 (0.039, 0.618) | 0.026 |
| Diet | 0.428 (−0.303, 1.158) | 0.251 |
| Age | −0.019 (−0.099, 0.061) | 0.640 |
| Sex | −1.727 (−3.328, −0.126) | 0.034 |
| HAD to FL | ||
| Weight, % change | 0.290 (0.093, 0.488) | 0.004 |
| Diet | 0.370 (−0.417, 1.158) | 0.356 |
| Age | −0.038 (−0.130, 0.055) | 0.424 |
| Sex | −2.058 (−3.682, −0.434) | 0.013 |
| WM to WL | ||
| Weight, % change | 0.632 (0.228, 1.036) | 0.002 |
| Diet | 0.377 (−0.407, 1.162) | 0.346 |
| Age | −0.034 (−0.124, 0.056) | 0.456 |
| Sex | −2.59 (−4.5, −0.686) | 0.008 |
| WM to FL | ||
| Weight, % change | 0.361 (0.135, 0.587) | 0.002 |
| Diet | 0.309 (−0.516, 1.134) | 0.463 |
| Age | −0.038 (−0.136, 0.060) | 0.450 |
| Sex | −2.359 (−4.092, −0.626) | 0.008 |
Multivariate logistic regression analysis was used to determine associations between independent variables and resolution of MetS from end of HAD or WM to WL or FL. The dependent variable in this analysis is MetS coded so that 0 = has MetS and 1 = does not have MetS. FL, free-living weight-loss phase; HAD, healthy American diet; MetS, metabolic syndrome; WL, weight loss (minimum 500-kcal/d deficit) including exercise phase; WM, weight-maintenance phase.
WC and triglycerides did not decrease until after the WL phase and thereafter remained stable through the FL (HAD compared with WL, FL, P < 0.05). HDL cholesterol decreased during the WM phase (HAD compared with WM, P < 0.0001), returned to HAD levels after the WL phase (HAD compared with WL, P = NS), and increased during the FL phase (WL compared with FL, P < 0.0001). Glucose concentrations were not different from HAD after any diet phase, but there was a significant reduction during the WL phase from the WM phase, partially because of a slight increase during WM (WM compared with WL, P < 0.01). SBP tended to decrease during the WM phase (HAD compared with WM, P = 0.07) and decreased significantly during the WL phase (HAD, WM compared with WL, P < 0.05). During the FL phase, SBP increased slightly (although not significantly) from WL (WL compared with FL, P = NS) but remained significantly lower than HAD (HAD compared with FL, P < 0.01). DBP decreased only after the WL phase, and the effect was sustained through the FL phase (HAD compared with WL, FL, P < 0.001).
Secondary endpoints
TC and LDL cholesterol were reduced after the WM and WL phases (HAD compared with WM, WL, P < 0.05) but returned to HAD levels after FL (HAD compared with FL, P = NS). C-reactive protein decreased after the WL phase (HAD compared with WL, P < 0.05) and remained stable through the FL phase (WL compared with FL, P = NS). Insulin did not change throughout the study (phase, P = NS).
Mean values of EndoPAT measurements are presented in Table 8. Augmentation index did not change over time (phase, P = NS), but augmentation index normalized to an HR of 75 beats/min decreased during the controlled-feeding WL phase (HAD compared with WL, P < 0.01). A time × treat interaction trend for RHI (P = 0.09) and a significant effect for Framingham RHI (P = 0.04) were observed; however, post hoc analysis did not reveal where the differences occurred. Framingham RHI decreased nominally on the BOLD diet during the WM and WL phases and increased nominally on the BOLD+ diet during the WM phase, which may account for the significant interaction. Supine SBP and DBP decreased through the WL phase but returned to HAD levels after FL (HAD, FL compared with WL, P < 0.05). Baseline HR was significantly lower after the WL phase compared with all other time points (P < 0.05).
TABLE 8.
EndoPAT measurements after each diet phase for M-DASH, BOLD, and BOLD+ diet groups1
| M-DASH (n = 21) |
BOLD (n = 20) |
BOLD+ (n = 21) |
P value |
||||||||||||
| HAD | WM | WL | FL | HAD | WM | WL | FL | HAD | WM | WL | FL | Phase × diet | Phase | Diet | |
| AI | 8.7 ± 3.82 | 5.5 ± 3.1 | 9.8 ± 4.5 | 8.3 ± 4.5 | 7.7 ± 4.9 | 6.2 ± 3.9 | 3.9 ± 4.0 | 4.4 ± 4.0 | 3.2 ± 3.6 | 2.1 ± 2.5 | 2.8 ± 3.2 | 3.2 ± 3.0 | 0.35 | 0.50 | 0.28 |
| AI@75 | 3.0 ± 4.2 | 0.7 ± 3.5 | 0.6 ± 4.7 | 2.1 ± 4.3 | 3.1 ± 4.5 | 0.3 ± 3.9 | −2.5 ± 4.3 | −2.2 ± 4.1 | −1.0 ± 3.8 | −3.4 ± 2.6 | −4.6 ± 3.6 | −3.6 ± 3.4 | 0.54 | 0.02a | 0.39 |
| RHI | 2.0 (1.8, 2.3)3 | 2.0 (1.9, 2.4) | 2.1 (1.9, 2.4) | 1.9 (1.9, 2.4) | 1.9 (1.8, 2.4) | 1.8 (1.8, 2.4) | 1.8 (1.7, 2.1) | 2.2 (1.9, 2.6) | 1.9 (1.7, 2.3) | 2.2 (2.0, 2.6) | 1.9 (1.9, 2.4) | 2.1 (1.9, 2.5) | 0.09 | 0.16 | 0.67 |
| fRHI | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.04b | 0.79 | 0.88 |
| SBP, supine, mm Hg | 130 ± 3 | 125 ± 3 | 124 ± 4 | 126 ± 3 | 125 ± 3 | 124 ± 3 | 120 ± 3 | 123 ± 3 | 130 ± 3 | 126 ± 3 | 123 ± 4 | 130 ± 3 | 0.71 | 0.0006c | 0.50 |
| DBP, supine, mm Hg | 74 ± 2 | 73 ± 2 | 71 ± 2 | 73 ± 2 | 73 ± 2 | 71 ± 2 | 69 ± 2 | 73 ± 3 | 76 ± 2 | 73 ± 2 | 72 ± 2 | 76 ± 2 | 0.99 | 0.0035c | 0.65 |
| Resting HR, bpm | 66 ± 2 | 64 ± 1 | 60 ± 2 | 65 ± 2 | 67 ± 2 | 65 ± 11 | 65 ± 3 | 64 ± 2 | 66 ± 2 | 66 ± 2 | 63 ± 2 | 64 ± 2 | 0.22 | <0.0001d | 0.58 |
Linear mixed models were used to compare raw values from the end of each diet phase between groups, adjusted for age and sex. aHAD compared with WL, P < 0.01. bNo significant Tukey-adjusted post hoc differences. cHAD, FL compared with WL, P < 0.05. dHAD, WM, FL compared with WL, P < 0.05. AI, augmentation index; AI@75, augmentation index at heart rate of 75 beats/min; BOLD, Beef in an Optimal Lean Diet; BOLD+, Beef in an Optimal Lean Diet Plus Protein; DBP, diastolic blood pressure; FL, free-living weight-loss phase; fRHI, Framingham reactive hyperemia index; HAD, healthy American diet; HR, heart rate; M-DASH, modified Dietary Approaches to Stop Hypertension; RHI, reactive hyperemia index; SBP, systolic blood pressure; WL, weight loss (minimum 500-kcal/d deficit) including exercise phase; WM, weight-maintenance phase.
Mean ± SEM (all such values).
Median; 95% CI in parentheses (all such values). These values are presented because of nonnormally distributed model residuals; log-transformed values are analyzed in model.
DISCUSSION
To our knowledge, this is the first study to compare different sources and amounts of protein under controlled-feeding conditions in both weight-stable and weight-loss phases in individuals with MetS. Weight loss was the primary driver of MetS resolution in our study population regardless of protein source or amount. Weight, fat, and lean body mass changes did not differ between diets, with reductions in weight primarily attributed to a loss of body fat. The lack of additional changes in body weight or MetS criteria in the FL phase suggests that participants in this study found it difficult to adhere to the weight-loss recommendations irrespective of the dietary profile. Second, the moderate-protein diet did not confer any advantage (or disadvantage) in improving MetS criteria in either the controlled or FL settings compared with the standard protein diets. Total and LDL cholesterol concentrations responded to the reduction in SFAs in all energy states, whereas HDL cholesterol decreased during WM and increased during the WL and FL phases, possibly as a result of increased physical activity (25).
Diets higher in protein (>25% of calories) are thought to enhance weight loss via a variety of mechanisms: enhanced satiety (26, 27), maintenance of lean body mass and metabolic rate during weight loss (28, 29), and the higher thermic effect of food for protein (30, 31). A meta-analysis of short-term weight-loss studies (32) compared the effects of moderate-protein (25–30% of total calories) with standard-protein diets (12–18% of total calories) matched for energy intake on weight, body composition, metabolic rate, and MetS criteria (32). Beneficial effects (weighted mean difference) on body weight (−0.79 kg; 95% CI: −1.5, −0.08 kg), fat mass (−0.84 kg; 95% CI: −1.26, −0.48 kg), fat-free mass (0.43 kg; 95% CI: 0.09, 0.78 kg) and triglycerides [−0.23 mmol/L (−20 mg/dL); 95% CI: −0.33, −0.12 mmol/L] were observed for the moderate-protein compared with a standard-protein diet; however, the effect sizes were small, and the analysis excluded studies with a prescribed exercise component. We did not observe any statistical differences between the moderate- and standard-protein groups in any of these endpoints during WL or FL phases, which may be because of the following reasons. First, the controlled-feeding design of this study required participants to consume all foods provided; therefore, a mechanism by which protein enhances weight loss (i.e., the reduced intake of food as a result of enhanced satiation) could not affect outcomes. Second, the high quality of the carbohydrates (rich in fiber and plant protein) in our standard-protein diets may mask the triglyceride decrease that is usually attributed to a reduction in refined carbohydrates (33).
Epidemiologic evidence regarding the impact of red meat intake and CVD remains mixed, with some studies showing an adverse association and others showing none, especially when processed and unprocessed meat are separately categorized (34–39). Micha et al. (40) cited that differences in sodium between processed and unprocessed meat may explain most of the observed higher risk. Several studies have found a link between red meat and MetS (41–43); however, the associations are not always consistent (42, 44), and Damião et al. (45) found that adjusting for SFAs eliminated the association between red meat and MetS. Importantly, the red meat in this study was unprocessed, and lean beef was prepared with methods that did not include charring. Red meat is generally restricted in a heart-healthy diet because it is a source of SFAs, yet hamburgers and beef dishes contribute fewer SFAs to the US diet than full-fat cheese, pizza, and grain-based desserts (46). Collectively, the evidence to date indicates that the protein source (plant compared with animal or red meat compared with other animal proteins) is secondary to reduced energy and SFA intake for treatment of MetS or CVD risk factors.
We have shown previously that the inclusion of lean beef in a reduced SFA diet lowers TC, LDL cholesterol, and SBP in hypercholesterolemic individuals (12, 13). In the present study, however, we did not observe this in the BOLD diet group. We believe the primary reason was the relatively lower compliance of the BOLD group during WM. Moreover, the participants in the current study did not have elevated LDL cholesterol and were obese. The cholesterol reduction in response to lowering SFAs in the absence of weight loss in obese individuals is blunted compared with normal-weight individuals (47); in addition, lower baseline LDL cholesterol concentrations are correlated with a smaller reduction to a dietary intervention (47). Nonetheless, given the reduction in the BOLD+ group, we believe that the lack of compliance in the BOLD group is the most likely explanation. Nonsignificant changes in triglycerides also were observed during WM; triglycerides remained stable in the BOLD+ group but increased in the BOLD and M-DASH groups. This is consistent with the OmniHeart Trial, which showed that replacement of dietary carbohydrate with protein lowers triglycerides (6). Any differences observed between groups during the WM phase were nullified during WL, which significantly reduced cholesterol and triglyceride concentrations in all groups.
A limitation of this study is that it was not originally powered to detect differences between groups but rather differences between the HAD and the experimental diets (considered within-group changes). Power calculations were based on BP and triglyceride changes in hypertensive or hypercholesterolemic individuals rather than individuals who were obese or had MetS because of a lack of appropriate comparative studies at the time of study design. Although recruitment goals were not met, there were significant improvements for most risk factors and no strong trends in between-group comparisons of primary endpoints that presumably may have reached significance with additional participants. We observed only small changes in SBP during weight maintenance under the current experimental conditions: −3.05 mm Hg for M-DASH, −3.19 mm Hg for BOLD+, and −1.65 mm Hg for BOLD. The magnitude of these changes was similar to those reported by Appel et al. (7) in normotensive individuals following the DASH diet (SBP: −3.5 mm Hg; DBP: −2.1 mm Hg). We estimate that sample sizes in excess of 55,976 and 557 persons per group would be required for the observed between-group differences of 0.14 mm Hg and 1.4 mm Hg for SBP (BOLD+ compared with M-DASH and BOLD compared with M-DASH, respectively, during WM) to have been statistically significant (80%, P < 0.05). Furthermore, the effect size for these differences was very small (Cohen’s d was 0.02 and −0.17, respectively) (48). This suggests that a larger controlled-feeding study evaluating differences in dietary protein sources is not warranted given the small clinical differences. More substantial reductions were observed after WL (−6.9 mm Hg in M-DASH, −7.4 mm Hg in BOLD+, and −2.4 mm Hg in BOLD). These findings support the current recommendations for weight loss for the treatment of MetS in overweight/obese individuals (4), indicating that even small changes in body weight (−5%) elicit clinically meaningful improvements in metabolic outcomes. Although well matched for macronutrient composition, the M-DASH diet was considerably higher in dietary fiber, yet all diets exceeded current intake recommendations (46). Increasing dietary fiber is associated with small reductions in BP (49, 50), TC, and LDL cholesterol (51) and improved glycemic control in patients with type 2 diabetes (52, 53) but does not affect triglycerides and HDL cholesterol (51). Because we did not observe significant differences between the BOLD and M-DASH diets in key biomarkers shown to be responsive to increases in dietary fiber, it is unlikely that the increased dietary fiber beyond recommended intakes in M-DASH affected MetS outcomes. There is little research examining the effects of dietary fiber intakes far beyond recommendations. It is possible that there is a threshold effect for dietary fiber with limited effects on MetS criteria beyond that observed with current recommendations for a dietary pattern rich in fruits, vegetables, and whole grains (54). Our study coordinator and data analyst were not blinded, but the statistics were performed by an independent coauthor by using multiple models, and the same conclusions were made. Because dietary data were unavailable during the FL phase, we were limited to changes in weight and MetS criteria for evaluating adherence. Finally, a biomarker of protein intake was not measured to assess compliance; however, a controlled-feeding design lessens the necessity for this.
This study adds to the literature in that it confirms that weight loss via diet and exercise is the primary treatment for MetS. By using a tightly controlled study design, the probability of noncompliance and confounding variables affecting these results is much lower than in studies with free-living participants. A run-in phase also reduced the variability of the baseline data and culled noncompliant individuals. The diets were matched for dietary factors that affect cholesterol, such as SFA and soy, to isolate the effects of plant compared with animal protein. We used highly sensitive methods for endpoint testing to reduce the variability in the data, such as DXA scans for body composition measurements. Moreover, the exercise component was measured objectively with pedometers and predicted 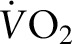 . This is one of the first studies designed to compare 2 diets rich in fruits and vegetables and low in SFAs but matched in protein from different sources (i.e., plant and animal proteins). In conclusion, this study is clinically relevant because it demonstrates that weight-loss diets low in SFAs that incorporate either plant or animal proteins (i.e., lean beef) are effective for treating MetS criteria without adverse effects on other important risk factors for CVD (i.e., LDL cholesterol and TC concentrations).
. This is one of the first studies designed to compare 2 diets rich in fruits and vegetables and low in SFAs but matched in protein from different sources (i.e., plant and animal proteins). In conclusion, this study is clinically relevant because it demonstrates that weight-loss diets low in SFAs that incorporate either plant or animal proteins (i.e., lean beef) are effective for treating MetS criteria without adverse effects on other important risk factors for CVD (i.e., LDL cholesterol and TC concentrations).
Acknowledgments
We thank our participants who made the BOLD-X study possible through their involvement and commitment. We acknowledge the work of many members of the Kris-Etherton Laboratory who were involved in recruitment and implementing the dietary intervention, including Jennifer Fleming, Melissa Hendricks, Pam Davis, and Marcella Smith. We are thankful to Tracy Lumsden, Danette Teeter, and Lisa Fuqua-Groves for performing the EndoPATs. We are also grateful to Tracey Allen, Cyndi Flanagan, Laurie Aquilino, Beth McKee, Denise Sheffield, Paula Mulhall, Becky Falsone, and Phyllis Martin of the General Clinical Research Center of The Pennsylvania State University. We thank Alvin Atlas for providing statistical advice.
The authors’ contributions were as follows—AMH and MAR: conceived and designed the study; AMH, KAHJ, MAR, SGW, and PMK-E: analyzed or interpreted the data; AMH, KAHJ, MAR, and PMK-E: wrote the article; AMH: had full access to all of the data and took responsibility for the integrity of the data and the accuracy of the data analysis; and all authors: read and approved the final manuscript. The authors declared no conflicts of interest.
Footnotes
Abbreviations used: BOLD, Beef in an Optimal Lean Diet; BOLD+, Beef in an Optimal Lean Diet Plus Protein; BP, blood pressure; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; DXA, dual-energy X-ray absorptiometry; FL, free-living weight-loss phase; HAD, healthy American diet; HR, heart rate; M-DASH, modified Dietary Approaches to Stop Hypertension; MetS, metabolic syndrome; RHI, reactive hyperemia index; SBP, systolic blood pressure; TC, total cholesterol; WC, waist circumference; WL, weight loss (minimum 500-kcal/d deficit) including exercise phase; WM, weight-maintenance phase.
REFERENCES
- 1.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 2005;28:1769–78. [DOI] [PubMed] [Google Scholar]
- 2.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol 2010;56:1113–32. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005;112:2735–52. [DOI] [PubMed] [Google Scholar]
- 4.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, et al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and The Obesity Society. Circulation 2014;129(Suppl 2):S102–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, et al. AHA/ACC Prevention Guideline 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk. Circulation 2014;129:579–99. [Google Scholar]
- 6.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the Omniheart randomized trial. JAMA 2005;294:2455–64. [DOI] [PubMed] [Google Scholar]
- 7.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 8.Moore TJ, Conlin PR, Ard J, Svetkey LP; DASH Collaborative Research Group. DASH (Dietary Approaches to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension 2001;38:155–8. [DOI] [PubMed] [Google Scholar]
- 9.Obarzanek E, Sacks FM, Vollmer WM, Bray GA, Miller ER, Lin P-H, Karanja NM, Most-Windhauser MM, Moore TJ, Swain JF, et al. Effects on blood lipids of a blood pressure–lowering diet: the Dietary Approaches to Stop Hypertension (DASH) trial. Am J Clin Nutr 2001;74:80–9. [DOI] [PubMed] [Google Scholar]
- 10.Sacks FM, Obarzanek E, Windhauser MM, Svetkey LP, Vollmer WM, McCullough M, Karanja N, Lin P-H, Steele P, Proschan MA, et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH): a multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol 1995;5:108–18. [DOI] [PubMed] [Google Scholar]
- 11.Swain JF, McCarron PB, Hamilton EF, Sacks FM, Appel LJ. Characteristics of the diet patterns tested in the Optimal Macronutrient Intake Trial to Prevent Heart Disease (OmniHeart): options for a heart-healthy diet. J Am Diet Assoc 2008;108:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roussell MA, Hill AM, Gaugler TL, West SG, Ulbrecht JS, Vanden Heuvel JP, Gillies PJ, Kris-Etherton PM. Effects of a DASH-like diet containing lean beef on vascular health. J Hum Hypertens 2014;28:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roussell MA, Hill AM, Gaugler TL, West SG, Vanden Heuvel JP, Alaupovic P, Gillies PJ, Kris-Etherton PM. Beef in an Optimal Lean Diet study: effects on lipids, lipoproteins, and apolipoproteins. Am J Clin Nutr 2012;95:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- 15.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Washington (DC): Carnegie Institute of Washington; 1919. (Pub. No. 279.) [Google Scholar]
- 16.NIH. Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Bethesda (MD): NIH; 2001. (NIH Pub. 01-3670.) [Google Scholar]
- 17.Whaley MH, Brubaker PH, Otto RM, Armstrong LE, editors. ACSM’s guidelines for exercise testing and prescription. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 18.CDC. National Health and Nutrition Examination Survey (NHANES) anthropometry procedures manual. Rockville (MD): Department of Health and Human Services; 2007. [Google Scholar]
- 19.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res 1978;19:65–76. [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat 2001;29:1165–88. [Google Scholar]
- 22.Conlin PR, Chow D, Miller ER, Svetkey LP, Lin P-H, Harsha DW, Moore TJ, Sacks FM, Appel LJ. The effect of dietary patterns on blood pressure control in hypertensive patients: results from the Dietary Approaches to Stop Hypertension (DASH) trial. Am J Hypertens 2000;13:949–55. [DOI] [PubMed] [Google Scholar]
- 23.Beauchesne-Rondeau É, Gascon A, Bergeron J, Jacques H. Plasma lipids and lipoproteins in hypercholesterolemic men fed a lipid-lowering diet containing lean beef, lean fish, or poultry. Am J Clin Nutr 2003;77:587–93. [DOI] [PubMed] [Google Scholar]
- 24.Thalheimer W, Cook S How to calculate effect sizes from published research: a simplified methodology [Internet]. 2002 [cited 2015 Jun 17]. Available from: http://www.bwgriffin.com/gsu/courses/edur9131/content/Effect_Sizes_pdf5.pdf.
- 25.Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, Suzuki E, Shimano H, Yamamoto S, Kondo K, et al. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med 2007;167:999–1008. [DOI] [PubMed] [Google Scholar]
- 26.Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr 2003;133:411–7. [DOI] [PubMed] [Google Scholar]
- 27.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007;15:421–9. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert JA, Bendsen NT, Tremblay A, Astrup A. Effect of proteins from different sources on body composition. Nutr Metab Cardiovasc Dis 2011;21:B16–31. [DOI] [PubMed] [Google Scholar]
- 29.Soenen S, Martens EAP, Hochstenbach-Waelen A, Lemmens SGT, Westerterp-Plantenga MS. Normal protein intake is required for body weight loss and weight maintenance, and elevated protein intake for additional preservation of resting energy expenditure and fat free mass. J Nutr 2013;143:591–6. [DOI] [PubMed] [Google Scholar]
- 30.Fine EJ, Feinman R. Thermodynamics of weight loss diets. Nutr Metab (Lond) 2004;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr 2004;23:373–85. [DOI] [PubMed] [Google Scholar]
- 32.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2012;96:1281–98. [DOI] [PubMed] [Google Scholar]
- 33.Layman DK, Clifton P, Gannon MC, Krauss RM, Nuttall FQ. Protein in optimal health: heart disease and type 2 diabetes. Am J Clin Nutr 2008;87:1571S–5S. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010;122:876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaluza J, Akesson A, Wolk A. Processed and unprocessed red meat consumption and risk of heart failure: a prospective study of men. Circ Heart Fail 2014;7:552–7. [DOI] [PubMed] [Google Scholar]
- 36.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121:2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 2012;172:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohrmann S, Overvad K, Bueno-de-Mesquita H, Jakobsen M, Egeberg R, Tjonneland A, Nailler L, Boutron-Ruault M-C, Clavel-Chapelon F, Krogh V, et al. Meat consumption and mortality—results from the European Prospective Investigation into Cancer and Nutrition. BMC Med 2013;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med 2009;169:562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Micha R, Michas G, Lajous M, Mozaffarian D. Processing of meats and cardiovascular risk: time to focus on preservatives. BMC Med 2013;11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azadbakht L, Esmaillzadeh A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J Nutr 2009;139:335–9. [DOI] [PubMed] [Google Scholar]
- 42.Babio N, Sorlí M, Bulló M, Basora J, Ibarrola-Jurado N, Fernández-Ballart J, Martínez-González MA, Serra-Majem L, González-Pérez R, Salas-Salvadó J. Association between red meat consumption and metabolic syndrome in a Mediterranean population at high cardiovascular risk: cross-sectional and 1-year follow-up assessment. Nutr Metab Cardiovasc Dis 2012;22:200–7. [DOI] [PubMed] [Google Scholar]
- 43.Cocate PG, Natali AJ, Oliveira AD, Alfenas RD, Peluzio MD, Longo GZ, Santos EC, Buthers JM, de Oliveira LL, Hermsdorff HH. Red but not white meat consumption is associated with metabolic syndrome, insulin resistance and lipid peroxidation in Brazilian middle-aged men. Eur J Prev Cardiol 2015;22:223–30. [DOI] [PubMed] [Google Scholar]
- 44.Alvarez León E, Henríquez P, Serra-Majem L. Mediterranean diet and metabolic syndrome: a cross-sectional study in the Canary Islands. Public Health Nutr 2006;9(8A):1089–98. [DOI] [PubMed] [Google Scholar]
- 45.Damião R, Castro TG, Cardoso MA, Gimeno SG, Ferreira SR; Japanese-Brazilian Diabetes Study Group. Dietary intakes associated with metabolic syndrome in a cohort of Japanese ancestry. Br J Nutr 2006;96:532–8. [PubMed] [Google Scholar]
- 46.USDA and US Department of Health and Human Services. Dietary guidelines for Americans. 7th ed. Washington (DC): US Government Printing Office; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flock MR, Green MH, Kris-Etherton PM. Effects of adiposity on plasma lipid response to reductions in dietary saturated fatty acids and cholesterol. Adv Nutr 2011;2:261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen J. A power primer. Psychol Bull 1992;112:155–9. [DOI] [PubMed] [Google Scholar]
- 49.Streppel MT, Arends LR, van ’t Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med 2005;165:150–6. [DOI] [PubMed] [Google Scholar]
- 50.Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens 2005;23:475–81. [DOI] [PubMed] [Google Scholar]
- 51.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 1999;69:30–42. [DOI] [PubMed] [Google Scholar]
- 52.Post RE, Mainous AG, King DE, Simpson KN. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med 2012;25:16–23. [DOI] [PubMed] [Google Scholar]
- 53.Silva FM, Kramer CK, de Almeida JC, Steemburgo T, Gross JL, Azevedo MJ. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Nutr Rev 2013;71:790–801. [DOI] [PubMed] [Google Scholar]
- 54.Jenkins DJ, Kendall CW, Popovich DG, Vidgen E, Mehling CC, Vuksan V, Ransom TP, Rao AV, Rosenberg-Zand R, Tariq N, et al. Effect of a very-high-fiber vegetable, fruit, and nut diet on serum lipids and colonic function. Metabolism 2001;50:494–503. [DOI] [PubMed] [Google Scholar]